Abstract
Objective:
Adjuvant chest wall radiotherapy is used in patients with high-risk histological features post-mastectomy to reduce the risk of locoregional recurrence. Treatment can be given with or without a tissue-equivalent bolus to increase skin surface dose. The additional benefit of using a bolus remains unclear; however, it is known to be associated with a higher incidence of skin toxicity. This study compared chest wall recurrence and skin toxicity in patients treated with and without a bolus.
Methods:
This retrospective cohort study reviewed 314 consecutive patients who received chest wall radiotherapy between 2005 and 2010. Data were collected on histological, demographic and treatment parameters and on the incidence and grade of acute skin reactions. Treatment outcomes analyzed included chest wall recurrence, disease-free survival and overall survival (OS).
Results:
101 patients received treatment with a bolus; 213 patients received treatment without a bolus. A significantly higher incidence of acute skin toxicity was seen in the bolus treatment group (p = 0.002). One patient treated with a bolus developed chest wall recurrence compared with four patients treated without a bolus. No statistically significant difference could be shown between the two groups. 66 (21%) patients had metastatic relapse. Median time to relapse was 29.5 months and OS was 76% in both treatment groups.
Conclusion:
No statistically significant difference in chest wall recurrence can be demonstrated between patients treated with and without a bolus.
Advances in knowledge:
This study is consistent with limited previous literature and invites further evaluation of the role of a bolus in post-mastectomy chest wall radiotherapy, especially considering the increased toxicity that the use of a bolus generates.
INTRODUCTION
Breast cancer is the most common cancer affecting females and the leading cause of cancer mortality.1 Although the majority of patients are offered breast conservation therapy, audit data from the Royal College of Surgeons in the UK in 2009 showed that around 40% of patients still undergo mastectomy.2 More recently, analysis of trends in Europe shows a decline in mastectomy rates from the period 2005–2010 by 4% a year, to the current rate of <20% a year.3 This trend is not reflected in the USA, where studies have shown mastectomy rates remaining between 30 and 40%, with a greater percentage of patients choosing to undergo mastectomy in spite of being suitable for breast-conserving surgery.4 Common indications for mastectomy include patients with multifocal invasive or in situ cancer, those in whom breast conservation would have a suboptimal cosmetic outcome or patient preference for mastectomy.5 Radiotherapy is offered to patients with breast cancer with risk factors for chest wall recurrence to reduce the risk of locoregional recurrence after mastectomy. Risk factors for locoregional recurrence include a large tumour size, a positive deep margin, lymph node involvement, grade 3 tumour and the presence of lymphovascular invasion.6,7 The use of chest wall radiotherapy has been shown to reduce the risk of local recurrence in the chest wall to 5–10% at 10 years in various studies.6,7 A meta-analysis of several randomized controlled trials shows that approximately 75% of locoregional recurrences occur within 5 years of initial treatment.8
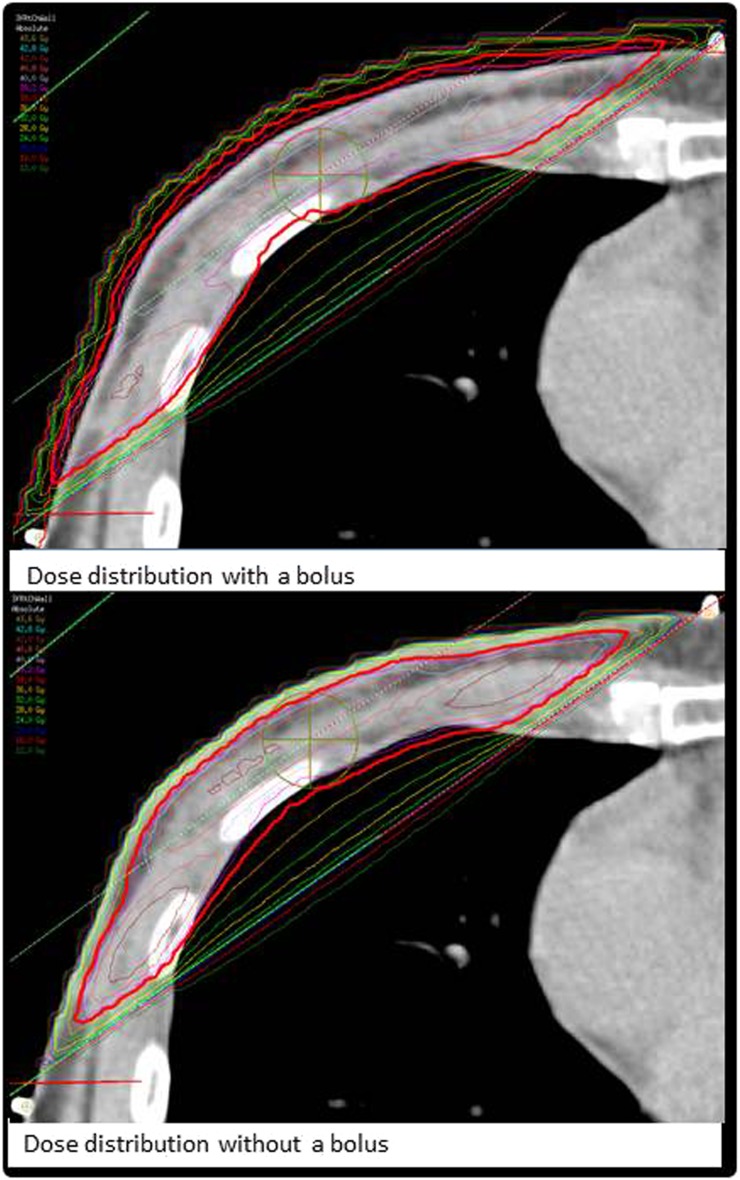
Chest wall radiotherapy can be delivered with the use of a “bolus”, a tissue-equivalent material on the skin surface. The purpose of a bolus is to increase the proportion of chest wall that is covered by the 95% isodose, by increasing the surface radiation dose.9 An example of a chest wall plan with and without a bolus can be seen in Figure 1. The use of a bolus is at the discretion of individual clinicians, with wide variations in practice.10 This is in part owing to the lack of published evidence as to the efficacy of a tissue bolus in reducing chest wall recurrence.11 In addition, use of a bolus is known to be associated with greater acute and late skin reaction.12 There is also a dosimetric uncertainty in the delivered dose at the skin surface, which adds to the complexity of treatment planning.13
Figure 1.
Pictorial distribution of dose distribution in a chest wall with and without bolus.
This retrospective single-centre study includes all patients who received adjuvant radiotherapy post-mastectomy for invasive breast cancer between 2005 and2010 at the Kent Oncology Centre (KOC), Maidstone Hospital. The study assessed the dosimetric effects of the use of a tissue-equivalent bolus and evaluated whether the use of a bolus reduced the incidence of chest wall recurrence.
METHODS AND MATERIALS
Patient selection
Patients who had received chest wall radiotherapy at the KOC between January 2005 and December 2010 were identified from radiotherapy planning records. No fixed protocol was in place for the use of a bolus and the decision to give radiotherapy with a bolus was at the individual clinician discretion.
Data collection
Data relating to demographics, radiotherapy treatment details, dosimetry and outcomes were collected retrospectively from patient records, from electronic records at the KOC and from surgical letters held at local hospitals. Information was also collected on any factors likely to impact the outcome of treatment. These included tumour stage and histology, surgical margins, the human epidermal growth factor receptor 2 status, the use of adjuvant chemotherapy, endocrine therapy and radiotherapy dose.
For patients in whom chest wall recurrence was identified, the notes were reviewed in detail to establish the site of chest wall recurrence in relation to radiotherapy fields and the coverage of the 95% isodose in patients treated with and without a bolus.
Information on acute and late toxicity, and clinical outcome, was collected from a combination of surgical letters, oncology letters and general practice records.
Primary end points and statistical methods
The primary end point was local recurrence in the chest wall. Disease-free survival was a secondary end point. The follow-up period was calculated from date of diagnosis to last medical review. Where a date of diagnosis was not available, the date of referral to the oncology department was substituted instead. Survival was calculated from completion of radiotherapy to date of relapse.
The incidence of recurrence in the two groups of patients undergoing radiotherapy with and without a bolus was analyzed with Fisher's exact test to accommodate the low recurrence rate. Fisher's exact test was also used to analyze skin toxicity, log rank analysis was used for analyzing survival and Z-test or an independent t-test was used for analyzing the differences in histological data.
RESULTS
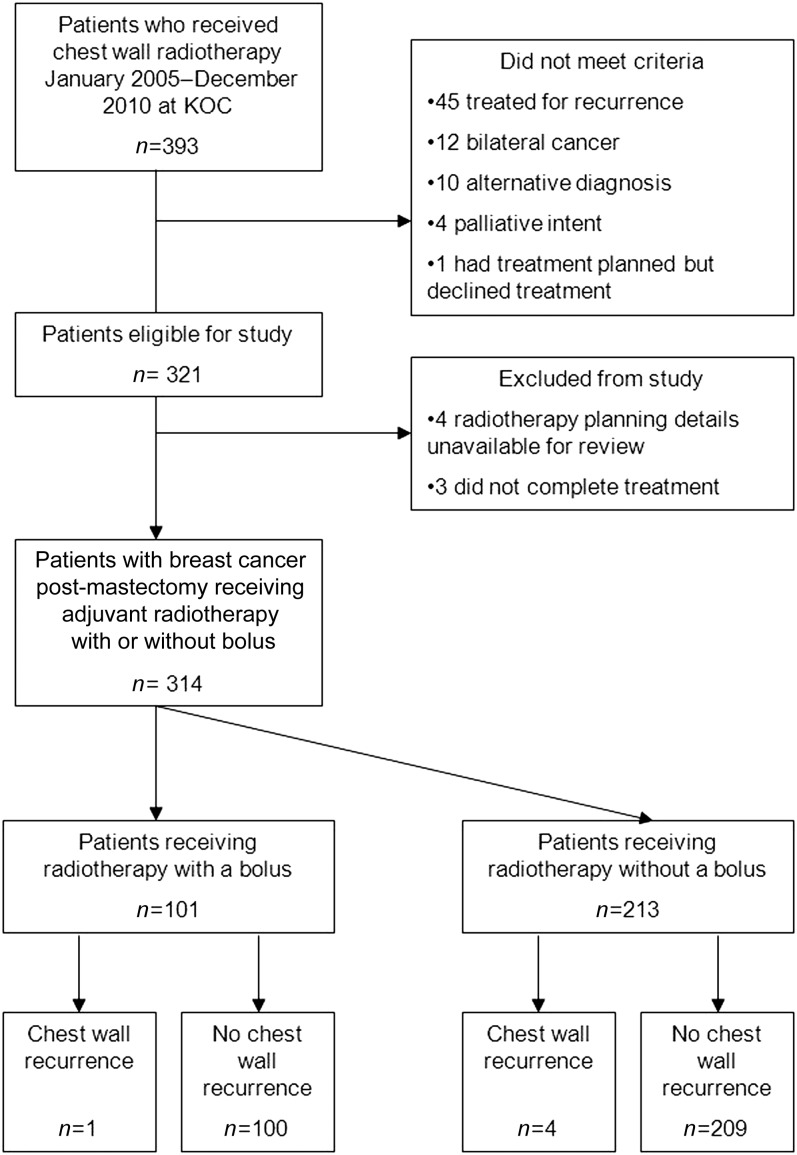
An initial screen of the radiotherapy records showed that a total of 393 patients had received chest wall radiotherapy at the KOC between January 2005 and December 2010. The following patients did not meet the criteria for inclusion in this study: 45 patients treated for recurrence, 12 patients with bilateral cancer, 10 patients treated for alternative diagnosis other than epithelial breast cancer, 4 patients treated with palliative dose and 1 patient who was planned and then declined treatment. This left a total of 321 patients eligible for inclusion in the retrospective study. A further three patients who did not complete radiotherapy owing to excessive toxicity and four patients whose paper radiotherapy records were unavailable for review were also excluded from analysis, as the field arrangement, bolus use and chest wall coverage could not be assessed.
314 patients were included for analysis in this study. Of these patients, 101 patients received radiotherapy with a bolus and 213 patients received radiotherapy without a bolus. This breakdown of patients is shown in the CONSORT diagram in Figure 2. The histological features, including hormone receptor status, of the two groups of patients are displayed in Tables 1 and 2.
Figure 2.
CONSORT diagram of study inclusion. KOC, Kent Oncology Centre.
Table 1.
Baseline characteristics in treatment groups with and without a bolus
| Characteristics | Radiotherapy delivered with a bolus (n = 101) | Radiotherapy delivered without a bolus (n = 213) | Total (n = 314) | p-value (Z-test) (*independent t-test) |
|---|---|---|---|---|
| Histological grade n (%) | ||||
| 1 | 7 (6.9) | 19 (8.9) | 26 (8.3) | 0.55 |
| 2 | 36 (35.6) | 91 (42.7) | 127 (40.4) | 0.23 |
| 3 | 58 (57.4) | 100 (46.9) | 157 (50.0) | 0.08 |
| Unknown | 0 (0) | 3 (1.4) | 3 (1.0) | 0.23 |
| Size (mm) n (%) | ||||
| 1–20 | 15 (14.8) | 43 (20.2) | 58 (18.5) | 0.25 |
| 21–50 | 66 (65.3) | 131 (61.5) | 197 (62.7) | 0.51 |
| >50-80 | 20 (19.8) | 39 (18.3) | 59 (18.8) | 0.75 |
| Lymph node involvement n (%) | ||||
| 0 | 33 (32.7) | 80 (37.6) | 113 (36.0) | 0.40 |
| 1–3 | 63 (62.4) | 110 (51.6) | 173 (55.1) | 0.01 |
| 4–9 | 5 (4.9) | 22 (10.3) | 27 (8.6) | 0.11 |
| ≥10 | 0 (0) | 1 (0.5) | 1 (0.3) | 0.49 |
| Closest margin n (%) | ||||
| Closest margin distance (median/mean) (range) (mm) | 3/5.1 (0–37) | 5/8.2 (0–45) | 5/7.0 (0–45) | 0.002* |
| Deep margin distance (median/mean): mm (range) | 3/5.8 (0–37) | 5/8.7 (0–45) | 5/8.3 (0–45) | 0.002* |
| Lymphovascular space invasion n (%) | ||||
| Yes | 45 (44.6) | 80 (37.5) | 124 (39.5) | 0.24 |
| No | 51 (50.5) | 126 (59.2) | 177 (56.4) | 0.15 |
| Unknown | 4 (4.0) | 5 (2.3) | 7 (2.2) | 0.42 |
| Equivocal | 1 (1.0) | 2 (0.9) | 3 (1.0) | 0.97 |
Table 2.
Hormone receptor status and systemic treatment delivered in treatment groups with and without a bolus
| Characteristics | Radiotherapy delivered with a bolus (n = 101) | Radiotherapy delivered without a bolus (n = 213) | Total (n = 314) | p-value (Z-test) (*independent t-test) |
|---|---|---|---|---|
| Hormone receptor status | ||||
| Oestrogen receptor positive | 75 (78.1) | 167 (81.1) | 242 (80.1) | 0.41 |
| Progesterone receptor positive | 55 (57.3) | 127 (61.7) | 182 (60.3) | 0.38 |
| HER2 positive | 18 (18.8) | 44 (21.4) | 62 (20.5) | 0.56 |
| Chemotherapy n (%) | ||||
| Neoadjuvant | 4 (4.0) | 14 (6.6) | 18 (5.7) | 0.14 |
| Adjuvant | 70 (69.3) | 114 (53.5) | 184 (58.6) | 0.008 |
| Adjuvant hormone treatment n (%) | ||||
| Tamoxifen | 44 (45.8) | 80 (37.6) | 124 (41.1) | 0.31 |
| Aromatase inhibitor | 33 (32.7) | 85 (39.9) | 118 (37.6) | 0.21 |
HER2, human epidermal growth factor receptor 2.
The median age of patients was 58 years (30–87 years) at the time of treatment. The majority of patients had an axillary node clearance (74.5%), in keeping with the standard surgical technique in the study period. A further 13.4% patients had sentinel node biopsy and 10.8% patients had sentinel node biopsy followed by axillary node clearance. 11.1% of patients underwent immediate reconstruction after mastectomy.
Systemic therapies are illustrated by treatment group in Table 2. A total of 202 (64.3%) patients received adjuvant chemotherapy. The majority of patients (184 patients) received chemotherapy in the adjuvant setting, but 18 patients received neoadjuvant chemotherapy. Of these, 14 patients were treated without a bolus and 4 patients were treated with a bolus. The most common regime was 5 flurouracil, epirubicin and cyclophosphamide (62.6%) or 5 flurouracil, epirubicin and cyclophosphamide followed by docetaxel (35.0%). The remaining 2.4% of patients received non-standard regimes. There is a statistically significant difference in the number of patients who received adjuvant chemotherapy in the treatment groups, with 70 (69.3%) patients receiving adjuvant chemotherapy in the group treated with bolus compared with 114 (53.5%) patients treated without a bolus (p = 0.008).
The radiotherapy dose given was 40 Gy in 15 fractions over 3 weeks to (58.6%) 184 patients, 45 Gy in 20 fractions over 4 weeks to 113 (36%) patients and 50 Gy in 25 fractions over 5 weeks to 11 (3.5%) patients. The remaining 6 (1.9%) patients received alternative fractionations. Of these, four patients received 36 Gy in six fractions treating twice weekly and one patient received 30 Gy in six fractions treating twice weekly. The clinical reason documented for this variance was frailty. One patient received 46 Gy in 23 fractions for over 4.5 weeks owing to a non-healing surgical wound.
In this single-centre study, all patients who were treated with a bolus were treated with a standard 5-mm tissue-equivalent sheet for all fractions of treatment. All patients were treated with 6-MV photons. The time period in question incorporates a period of transition between two-dimensional (2D) and three-dimensional (3D) planning at the KOC, and as such data on the type of planning were also collected. 52.5% of patients, treated up to July 2008, had 2D radiotherapy planning and 47.5% of patients had CT-planned 3D radiotherapy planning.
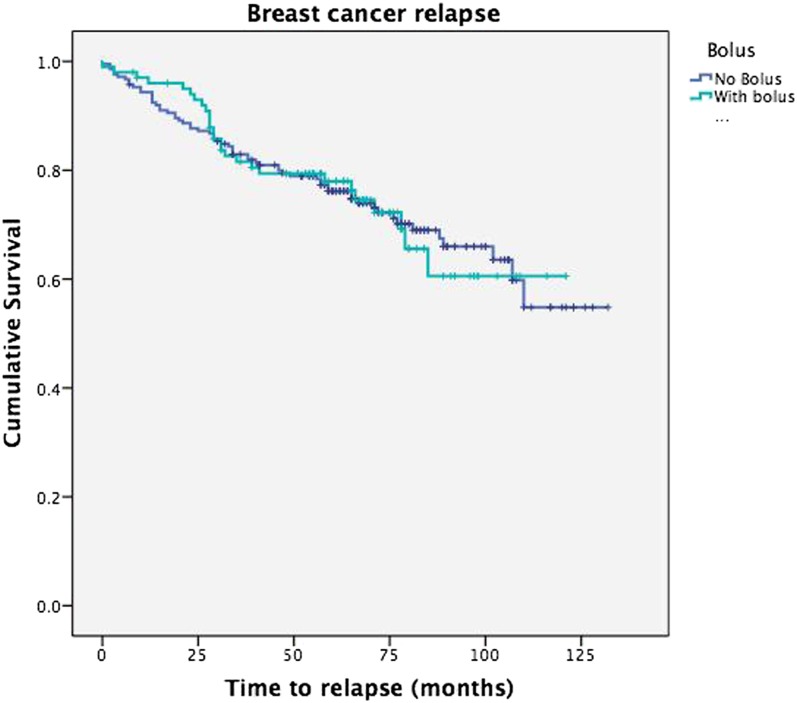
The median time to last follow-up across all groups is 66 months [range 2–132 months; interquartile (IQ) range 53–82 months]. Patients who remain disease free have a median follow-up of 69 months (range 7–132 months; IQ range 60–89 months). 25 patients were identified as having less than 24 months of follow-up; of these, 21 patients had died, of whom 13 had died of disease. 4 (1.3%) patients were therefore lost to follow-up. At the last follow-up, 66.2% of patients had no evidence of disease, 5.4% of patients were alive with disease, 9.6% of patients had died with no evidence of disease and 18.8% of patients had died of disease. The proportion of patients who failed to achieve 2 years of follow-up is similar across both treatment groups. In the bolus treatment group 4 (3.96%) patients and in the no bolus treatment group 8 (3.76%) patients failed to complete 2 years of follow-up. Data were also collected on axillary and metastatic recurrence. 6 (1.9%) patients had axillary recurrence and 66 (21.0%) patients had metastatic recurrence. The median time to axillary recurrence was 7.5 months (range 2–47 months; IQ range 2.75–20.75 months) and the median time to metastatic recurrence was 29.5 months (range 0–102 months; IQ range 15–46.5 months). The disease-free survival is displayed as a Kaplan–Meier curve in Figure 3, comparing the two treatment groups (p = 0.995).
Figure 3.
Kaplan–Meier survival curve of breast cancer recurrence.
The skin toxicity data are shown in Table 3. 64 (30%) patients treated without a bolus had no skin toxicity compared with 14 (10%) patients treated with a bolus. Grade 1–2 toxicity according to the Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer criteria was seen in 122 (57.3%) patients treated without a bolus compared with 76 (75%) patients in the bolus treatment group. Grade 3 toxicity was rare in both groups; it was seen in two patients treated without a bolus and in one patient treated with a bolus. The acute skin toxicity was not formally graded in 35 patients. The data were analyzed using Fisher's exact test and were found to be significant (p = 0.002).
Table 3.
Skin toxicity by treatment group
| Skin toxicity | Bolus |
||
|---|---|---|---|
| No | Yes | Total | |
| Not specified | 26 | 9 | 35 |
| None | 64 | 14 | 78 |
| Grades 1–2 | 122 | 76 | 198 |
| Grade 3 | 1 | 2 | 3 |
| Total | 213 | 101 | 314 |
During the follow-up period, 5 (1.6%) patients were identified to have developed chest wall recurrence. All of these patients were planned using a 2D technique. No chest wall recurrences were found in patients who were treated with a 3D radiotherapy planning technique (p = 0.062) (Table 4). In the group of patients who received treatment with a bolus, the recurrence was 0.99% (n = 1) and in the group of patients treated without a bolus, the recurrence was 1.88% (n = 4) (p = 1.0). The median time to chest wall recurrence was 34 months (range 3–53 months; IQ range 29.5–35 months). In this patient group, no statistical reduction was shown in the incidence of chest wall recurrence with the use of a bolus.
Table 4.
Chest wall recurrence by treatment group
| Planning technique | Chest wall recurrence |
||
|---|---|---|---|
| No | Yes | Total | |
| 2D plan | 160 | 5 | 165 |
| 3D CT plan | 149 | 0 | 149 |
| Total | 309 | 5 | 314 |
2D, two-dimensional; 3D, three-dimensional.
The case notes of all five patients with chest wall recurrence were reviewed and the planning dosimetry details were examined. The patient treated with a bolus had full skin coverage treated with a 95% isodose. She had an out-of-field recurrence, 4 months after treatment, in an inferolateral location over a previous surgical drain site. Review of the case notes of the remaining four patients who received treatment without a bolus revealed that Patient 1 had superficial coverage by the 95% isodose between 4- and 5-mm depth from the chest wall surface, Patient 2 had 95% isodose coverage at 4-mm depth from the chest wall surface and Patients 3 and 4 had 95% isodose coverage between 3- and 4-mm depth from the chest wall surface. Of the four patients who recurred after treatment without a bolus, all had recurrence in the mastectomy scar or in close proximity to the scar at the lateral borders of the treatment field. One recurrence was at the sternal border and three were at the axillary border.
In this non-randomized comparison, the patients in the group treated with a bolus had narrower closest margins than the patients treated without a bolus—a 3-mm median average compared with a 5-mm median average (p = 0.004). There was a difference in the deep margins again, with a closer margin in the bolus group—a 4-mm median average compared with a 6-mm median average (p = 0.005).
DISCUSSION
There is no established consensus regarding the use of a bolus for the delivery of chest wall radiotherapy post-mastectomy. There is a significant clinical variation in the use of a bolus, often at the discretion of the individual clinician. Vu et al10 found in an international survey of clinicians in the Americas, Europe and Australasia that there were wide variations in regional practice. Their respondents showed a picture of extensive bolus use in the USA, with 82% of clinicians always using a bolus, and a less frequent use in Europe (31%) and Australasia (65%). More recently, regimes have been developed with bolus application for a proportion of the radiotherapy fractions. These have been shown to be effective in increasing surface and skin dose in the overall treatment, even though a bolus is not used for every fraction.9 However, we know from other studies that the use of a bolus is associated with greater acute and late effects including moist desquamation and skin telangiectasia.12 Lilla et al14 also demonstrated in their cohort study the association between severe acute effects of radiation treatment and subsequent development of telangiectasia. We have been able to replicate these data at the KOC by also demonstrating a statistically significant increase of skin toxicity in the group treated with a bolus.
This analysis did not demonstrate a statistical significance in chest wall recurrence between the patients who were treated with a bolus and those treated without a bolus. Another cohort study by Tieu et al,11 looking at the effect of adjuvant post-mastectomy radiotherapy on local recurrence, also demonstrated a lack of statistically significant increase in chest wall recurrence between groups treated with and without a bolus. However, they did find that 20 of the 146 patients treated with a bolus had to stop treatment early owing to severe skin toxicity. The team then went on to perform a multivariant analysis which, after adjusting for other risk factors, gave a hazard ratio of 4.8 for incomplete radiotherapy treatment on the risk of recurrence.
The overall rate of recurrence in our cohort is much lower than the published figures, which range from around 5 to 10%, with a median follow-up of 69 months.6 However, it is worth noting that some of the previously published studies used an extended follow-up period of up to 10 years.
There are uncertainties around the delivered surface dose in chest wall radiotherapy, especially at the periphery of the field.13 Studies have shown that treatment planning systems, even with advanced algorithms, show variations at a shallow depth.15 This makes accurate prescription at a shallow depth close to the skin surface difficult. Studies have also demonstrated that planning target volume coverage, especially at the edges of the treatment field, is reduced with a 2D plan compared with a 3D CT plan.16,17 In this data set, just over half of the patients were planned using a 2D technique. Now, all patients are planned using 3D CT planning and some more complex plans are forward planned using intensity-modulated radiotherapy. Chest wall recurrences were seen only in the patients planned using a 2D planning technique (Table 4). Although not statically significant in this cohort, there does appear to be an emerging trend towards fewer chest wall recurrences with 3D CT planning. It is also interesting to note that in three of the four patients with recurrences after treatment without a bolus, recurrence was on the periphery of the treatment field. With 2D planning, these areas may have been particularly vulnerable to dosimetry discrepancies.
The data from this cohort of patients treated at the KOC are from 2005 to 2010. Since 2010 a change in technique means that patients are now sometimes given a bolus for a proportion of their radiotherapy; for example, 50% of fractions, rather than throughout the course of treatment. Future analysis would be required to establish whether recent changes in technique alter the outcome in terms of the role of a bolus. Being a single-centre analysis, all patients were treated with a standard tissue-equivalent bolus; however, at other centres, different types and thicknesses of bolus are used. The results from this study may not therefore be widely transferrable.
This analysis identified narrower closest and deep margins in the bolus treatment group. Some studies have shown that tumour distance from the margin is an independent risk factor in future chest wall recurrence.18 However, a comprehensive review of radiotherapy in patients with negative lymph nodes post-mastectomy showed that the margins are not an independent predictor for locoregional recurrence in the absence of other risk factors such as age, large tumour size and lymphovascular invasion.19 Furthermore, no difference was seen in the superficial margins between the two treatment groups, which would be the more relevant margin in relation to surface dose of radiotherapy.
It is also worth considering that in this retrospective study, the size of the groups was uneven, with approximately one-third of patients treated without a bolus compared with two-third of patients treated with bolus. Apart from narrower closest and deep margins, the remaining characteristics were similar between the two groups, as seen in Table 1. The disparity in numbers is due to the lack of a fixed protocol in the department for use of a bolus in chest wall radiotherapy. Clinicians would sometimes add a bolus if the surface dose, on the review of the plan, was inadequate or if a very thin chest wall did not allow for adequate dose build-up.
In the group receiving radiotherapy without a bolus, a significantly smaller proportion of patients received adjuvant chemotherapy compared with the group treated with a bolus. In spite of this, the local recurrence rates were similar in both groups.
The small number of recurrences makes it difficult to achieve a statistical significance. However, this study is still clinically relevant, as it confirms that chest wall recurrence is a rare event after mastectomy and chest wall radiotherapy. For the clinician making a decision for an individual patient, it is important that benefit be demonstrated in using a bolus, to justify the higher risk of worse acute and late toxicity. The suggestion from this retrospective study of patients at the KOC, as well as previous studies, is that no statistically significant benefit can be demonstrated in reducing local recurrence. However, treatment with a bolus is associated with increased toxicity and likely early cessation of treatment, which itself is a risk factor for chest wall recurrence.11 The routine use of a bolus for treatment post-mastectomy may therefore be unjustified.
CONCLUSION
In the patients treated with chest wall radiotherapy post-mastectomy for breast cancer between 2005 and 2010 at the KOC, no statistical difference in chest wall recurrence could be demonstrated in patients who received treatment with or without a bolus. This raises questions about the benefit of treatment with a bolus in view of the increase in acute skin toxicity with bolus use. In the age of 3D CT-guided radiotherapy planning, the benefit of using a bolus may be outweighed by toxicity. A prospective trial in this area would be helpful in clarifying further the role of a bolus in chest wall radiotherapy in patients with breast cancer post-mastectomy.
Contributor Information
Jennifer Y Turner, Email: jennifer.turner3@nhs.net.
Anthi Zeniou, Email: a.zeniou@nhs.net.
Amanda Williams, Email: awilliams3@nhs.net.
Rema Jyothirmayi, Email: rema.jyo@nhs.net.
REFERENCES
- 1.Shah R, Rosso K, Nathanson SD. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J Clin Oncol 2014; 5: 283–98. doi: 10.5306/wjco.v5.i3.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.First Annual Report of the National Mastectomy and Breast Reconstruction Audit 2008. The NHS Information Centre, National Mastectomy and Breast Reconstruction Audit. All rights reserved—example citation National Mastectomy and Breast Reconstruction Audit 2009. The NHS Information Centre, Clinical Effectiveness Unit at the Royal College of Surgeons; 2009.
- 3.Garcia-Etienne CA, Tomatis M, Heil J, Friedrichs K, Kreienberg R, Denk A, et al. Mastectomy trends for early-stage breast cancer: a report from the EUSOMA multi-institutional European database. Eur J Cancer 2012; 48: 1947–56. doi: 10.1016/j.ejca.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 4.McGuire KP, Santillan AA, Kaur P, Meade T, Parbhoo J, Mathias M, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 2009; 16: 2682–90. doi: 10.1245/s10434-009-0635-x [DOI] [PubMed] [Google Scholar]
- 5.Pierce LJ, Lichter AS. Defining the role of post-mastectomy radiotherapy: the new evidence. Oncology (Williston Park) 1996; 10: 991–1002; discussion 6–7, 1011. [PubMed] [Google Scholar]
- 6.Truong PT, Olivotto IA, Speers CH, Wai ES, Berthelet E, Kader HA. A positive margin is not always an indication for radiotherapy after mastectomy in early breast cancer. Int J Radiat Oncol Biol Phys 2004; 58: 797–804. doi: 10.1016/S0360-3016(03)01626-2 [DOI] [PubMed] [Google Scholar]
- 7.Truong PT, Olivotto IA, Whelan TJ, Levine M; Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: 16. Locoregional post-mastectomy radiotherapy. CMAJ 2004; 170: 1263–73. doi: 10.1503/cmaj.1031000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366: 2087–106. doi: 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 9.Andic F, Ors Y, Davutoglu R, Baz Cifci S, Ispir EB, Erturk ME. Evaluation of skin dose associated with different frequencies of bolus applications in post-mastectomy three-dimensional conformal radiotherapy. J Exp Clin Cancer Res 2009; 28: 41. doi: 10.1186/1756-9966-28-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vu TT, Pignol JP, Rakovitch E, Spayne J, Paszat L. Variability in radiation oncologists' opinion on the indication of a bolus in post-mastectomy radiotherapy: an international survey. Clin Oncol (R Coll Radiol) 2007; 19: 115–9. doi: 10.1016/j.clon.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 11.Tieu MT, Graham P, Browne L, Chin YS. The effect of adjuvant postmastectomy radiotherapy bolus technique on local recurrence. Int J Radiat Oncol Biol Phys 2011; 81: e165–71. doi: 10.1016/j.ijrobp.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 12.Pignol JP, Vu TTT, Mitera G, Bosnic S, Verkooijen HM, Truong P. Prospective evaluation of severe skin toxicity and pain during postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 2015; 91: 157–64. doi: 10.1016/j.ijrobp.2014.09.022 [DOI] [PubMed] [Google Scholar]
- 13.Quach KY, Morales J, Butson MJ, Rosenfeld AB, Metcalfe PE. Measurement of radiotherapy x-ray skin dose on a chest wall phantom. Med Phys 2000; 27: 1676–80. doi: 10.1118/1.599035 [DOI] [PubMed] [Google Scholar]
- 14.Lilla C, Ambrosone CB, Kropp S, Helmbold I, Schmezer P, von Fournier D, et al. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Res Treat 2007; 106: 143–50. doi: 10.1007/s10549-006-9480-9 [DOI] [PubMed] [Google Scholar]
- 15.Akino Y, Das IJ, Bartlett GK, Zhang H, Thompson E, Zook JE. Evaluation of superficial dosimetry between treatment planning system and measurement for several breast cancer treatment techniques. Med Phys 2013; 40: 011714. doi: 10.1118/1.4770285 [DOI] [PubMed] [Google Scholar]
- 16.Thomsen MS, Berg M, Nielsen HM, Pedersen AN, Overgaard M, Ewertz M, et al. Post-mastectomy radiotherapy in Denmark: from 2D to 3D treatment planning guidelines of the Danish Breast Cancer Cooperative Group. Acta Oncol 2008; 47: 654–61. doi: 10.1080/02841860801975000 [DOI] [PubMed] [Google Scholar]
- 17.Barsoum M, Mostafa M, El Hossieny H, Nasr A, Mahmoud M, Fouda S. Dosimetric prospective study comparing 2D and 3D planning for irradiation of supraclavicular and infraclavicular regions in breast cancer patients. J Egypt Natl Canc Inst 2015; 27: 25–34. doi: 10.1016/j.jnci.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 18.Houssami N, Macaskill P, Marinovich ML, Dixon JM, Irwig L, Brennan ME, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer 2010; 46: 3219–32. doi: 10.1016/j.ejca.2010.07.043 [DOI] [PubMed] [Google Scholar]
- 19.Rowell NP. Radiotherapy to the chest wall following mastectomy for node-negative breast cancer: a systematic review. Radiother Oncol 2009; 91: 23–32. doi: 10.1016/j.radonc.2008.09.026 [DOI] [PubMed] [Google Scholar]