Abstract
Objective:
To compare electrocardiographic (ECG)-triggered high-pitch (HP) dual-source CT angiography (CTA) with non-ECG-triggered HP CTA of the aorta, particularly the ascending aorta, with regard to image quality, motion artefacts, contrast-to-noise ratio (CNR), signal-to-noise ratio (SNR) and radiation dose.
Methods:
59 consecutive patients who had been referred for CTA for known or suspected aortic disease, previous aortic intervention or planned transapical or transfemoral aortic valve implantation were prospectively included. Patients underwent CTAs with HP, using a dual-source CTA system, with [control group (Group A); n = 30] or without (Group B; n = 29) ECG triggering after randomization. For evaluation, image quality and a motion artefact score (MAS) were assessed in a blinded fashion at different predefined anatomic regions. CNR and SNR were measured at the same levels. Radiation dose estimates and contrast enhancement were compared between the two groups.
Results:
There were no significant differences for image quality and MAS. The intra-arterial contrast resolution was significantly higher at the level of the aortic arch and descending aorta in the non-triggered group (CNR values, p = 0.002–0.018). No significant differences in the radiation dose were found.
Conclusion:
Non-triggered HP dual-source CTA provided comparable results with regard to image quality, MAS, CNR, SNR and radiation doses compared with ECG-triggered HP CTA. Therefore, ECG triggering of the ascending aorta might be obviated when HP scanning is available.
Advances in knowledge:
HP dual-source CTA might obviate ECG triggering in the ascending aorta. Non-triggered HP CTA of the ascending aorta provides an excellent image quality.
INTRODUCTION
The visualization of the ascending aorta, aortic arch and adjacent vessels with CT angiography (CTA) is anatomically complicated by the proximity to the heart and, consequently, the risk of pulsation artefacts. Electrocardiographic (ECG)-triggering techniques were incorporated into modern CT examination protocols in order to overcome these limitations in the diagnosis of pathological changes of the ascending aorta and are recommended to avoid additional scans due to motion artefacts.1–4
Through the introduction of second-generation dual-source scanners with faster acquisition times and an improvement in temporal resolution, image quality in aortic imaging has become less dependent on heart rate5,6 and pulsation artefacts seem to occur less frequently.6 Further, it has been reported that the use of the high-pitch (HP) mode provides the possibility of image acquisition within one cardiac cycle, with less radiation exposure and less motion artefacts, but with the potential risk of increased image noise, compared with acquisitions using single-source systems and commonly used pitch factors below 1.5.5,7–9
As a result, ECG triggering of the aorta might no longer be required when using HP dual-source CT techniques. And this would obviate the need for complex patient preparations and longer in-room times,6 which may represent a critical factor in an emergency setting;1 e.g. aortic dissection.
While a number of studies have claimed that ECG triggering is essential for visualizing the ascending aorta with sufficient diagnostic image quality,2–4,10 other studies have described a similar or even lower radiation dose and excellent image quality for non-ECG-triggered protocols at HP values compared with both ECG-triggered and slow-pitch retrospectively gated protocols.5,6
The diagnostic image quality obtained by using the HP mode without ECG triggering in the visualization of the ascending aorta, aortic arch and subsequent vessels has not been described extensively yet, and since the application of higher pitch values leads to an increase of noise,8,9 it is all the more important to evaluate the image quality at HP values.
The purpose of this prospective study, therefore, was to compare non-ECG-triggered HP dual-source CTA of the aorta with ECG-triggered HP dual-source CTA, with regard to image quality, motion artefacts, contrast-to-noise ratio (CNR), signal-to-noise ratio (SNR) and radiation dose, with a special focus on the ascending aorta.
METHODS AND MATERIALS
Study population
This study was performed as a prospective, randomized, monocentric, double-blinded study between December 2010 and March 2011. Based on a sample size analysis to provide a power of 80% and an alpha level of 5% to detect a difference of ≥0.3 in the distribution of the image quality assessments, a sample size of 30 patients for each group was estimated.
59 consecutive patients (female : male=25 : 34), with a mean age of 66.68 ± 14.38 years (range, 31–91 years; n = 59) and a mean body mass index (BMI) of 26.41 ± 4.70 (range, 14.26–40.81; n = 58), referred to CTA for known and suspected aortic disease or known history of previous aortic intervention, or for a planned transapical or transfemoral aortic valve implantation procedure, were prospectively enrolled and randomized into two groups (Group A with ECG triggering, n = 30; and Group B without ECG triggering, n = 29). The mean heart rate in the group with ECG triggering was 67.17 ± 12.76 beats per minute (bpm) (52–100 bpm, n = 30) compared with 71.29 ± 12.40 bpm (57–95 bpm, n = 7) in the non-triggered group. No premedication for heart rate control was added to the patient's baseline medication prior to examination by the radiologists.
Indications for CTA of the aorta included the evaluation of known aortic aneurysms (n = 10; Group A = 6 and Group B = 4), suspected aneurysms (n = 4; Group A = 2 and Group B = 2), evaluation of suspected Type A/B aortic dissection (n = 2; Group A = 1 and Group B = 1), control examination of known Type A/B dissection (n = 2; Group A = 1 and Group B = 1), post-operative evaluation after aortic replacement (n = 16; Group A = 7 and Group B = 9), control examination after stent-graft implantation (n = 4; Group A = 3 and Group B = 1), and evaluation prior to a transapical or transfemoral aortic valve implantation (TAVI) (n = 15; Group A = 9 and Group B = 6), evaluation post TAVI (n = 1; Group A = 0 and Groop B = 1) a search for the origin of emboli (n = 1; Group A = 0 and Group B = 1) and suspected aortic valve disease (n = 4; Group A = 1 and Group B = 3).
After obtaining the written informed consent, patients were randomized into two groups using QuickCalcs© (Graph Pad, San Diego, CA). Subsequently, patients underwent CTAs of the aorta using the new HP mode (flash) either with ECG triggering (Group A) or without ECG triggering (Group B). To be included in this prospective study, patients had to have been 18 years of age or older with a referral for a CTA of the thoracic aorta at least. Patients with renal impairment, defined as serum creatinine levels over 1.3 mg dl−1 or an estimated creatinine clearance <60 ml per min (estimated glomerular filtration rate ≤60 ml min−1/1.73 m2), known allergy to iodinated contrast media, untreated hyperthyroidism, left heart failure, pregnancy or breast feeding were not included.
The local institutional review board approved the study.
CT acquisition and post-processing
All examinations were performed on a second-generation dual-source CT system (SOMATOM Definition Flash; Siemens Medical Systems, Erlangen, Germany). Patients underwent CTA from the level of the carotid bifurcation down to the femoral bifurcation. Iopamidol, a non-ionic iodinated contrast medium, was used at a concentration of 400-mg iodine per millilitre for all CT studies (Iomeron 400; Bracco, Milan, Italy) (blinded) and was injected using a power injector (Angiomat CT, Digital Injection System; Liebel-Flarsheim Company, Cincinnati, OH).
The detailed examination protocol for Group A consisted of an initial flash scan, which was performed from the level of the carotid bifurcation to the celiac trunk at 60% of the RR interval. A prospective ECG-triggered algorithm with a fixed pitch factor of 3.4 was used, with a slice collimation of 2 × 128 × 0.6 mm and a gantry rotation time of 280 ms. The tube potential was set to 120 kV. Patients with an indication for complete aortic assessment received a second scan (28 out of 29 patients). With a delay of 4 s, a second scan was performed from the celiac trunk to the femoral bifurcation, with a fixed pitch value of 2.5 and 120 kV. This delay of 4 s is provided and defined by the scanner and represents the minimal possible delay between two scans in different (with and without ECG synchronization) modes. All patients received an injection of a total of 120-ml contrast material, as described above, at a flow rate of 4 ml s−1, followed by 40 ml of saline solution.
Patients in Group B were investigated with a single acquisition from the level of the carotid bifurcation up to the femoral bifurcation, with a pitch factor fixed at 3.2 and a slice collimation of 2 × 128 × 0.6 mm. The gantry rotation time was 280 ms and the tube potential was 120 kV. Because of the decreased duration of the examination, the contrast injection protocol used was slightly different from that used in Group A and consisted of a total volume of 110 ml at a flow rate of 5 ml s−1, followed by 40 ml of saline solution.
In both groups, dose reduction techniques, including online dose modulation (automatic exposure control CARE dose 4D; Siemens Medical Systems, Erlangen, Germany), were applied. The monitoring delay in both groups was 10 s, with reference scans obtained every second up to a predefined trigger threshold (ECG-triggered group: 130 HU; non-ECG-triggered group: 150 HU), and after a post-threshold delay (ECG-triggered group: 10 s; non-ECG-triggered group: 15 s), delineation of the aorta was initiated at the level of the supra-aortic vessels.
Images were exported to a picture archiving and communication system (Impax ES; Agfa, Mortsel, Belgium).
Analysis
Contrast-to-noise ratio, signal-to-noise ratio and figure of merit
CNR and SNR were assessed as image quality parameters, as described previously.8,11,12
The following equation11 was used to calculate CNR and SNR values:
where HUves describes the attenuation value within the vessel and HUmuscle describes the attenuation value within the muscle at the same level. The standard deviation background noise (SD BN) was the average standard deviation of the surrounding air at the right, left and anterior positions.
Intravascular attenuation values (Hounsfield units) were measured on axial slices reconstructed to a slice thickness of 3 mm, with 2-mm increments at four predefined anatomic levels, including the level of the aortic valve, aortic arch and descending aorta (celiac artery) and above the aortic bifurcation. At the same levels, CT attenuation values were obtained from the muscles (the sternocleidomastoid muscle or erector spinae muscle), and background noise was obtained from three areas of the surrounding air (right, left and anterior) (Figure 1). The size and area of all region-of-interest measurements were kept as large as possible (area ≥0.76 cm2) for all measurements while avoiding the inclusion of plaques and calcifications.
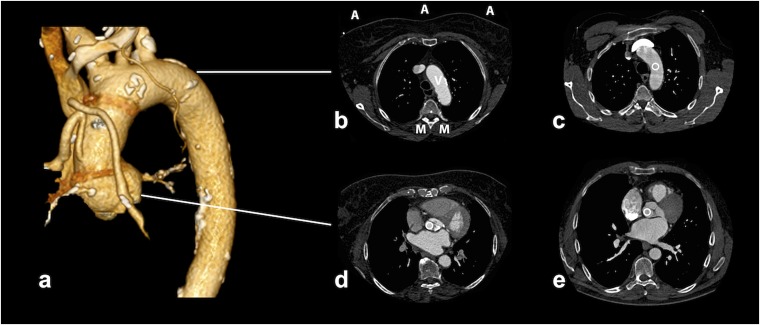
Figure 1.
Examples of CT angiography attenuation measurements at different levels. (a) The three-dimensional volume-rendered image reconstruction showing an excellent image quality in a non-triggered examination; images (b, c) were acquired using the non-gated protocol, whereas images (d, e) were acquired using the electrocardiographic-triggered mode. The anatomic levels represent those where the vessel enhancement [Hounsfield units (HU)] and parameters for contrast-to-noise ratio (CNR) and signal-to-noise ratio (SNR) calculation were assessed. As described in image (b), the CNR and SNR were calculated based on the assessed HU of the muscles (M) at three points in the air (A) and within the vessels (V) (c-e; circles).
One radiologist (AW) performed the CNR and SNR measurements in a single session and was blinded to the scan technique used.
Further, the “figure of merit” (FOM) was calculated to evaluate the CNR independent of the tube current–time product according to a formula previously applied by others:6
Image quality and motion artefact score
One experienced radiologist (CL) analyzed the images of the thoracic series in two independent readout sessions with an interval of 6 months in between. The radiologist was blinded to the patient information and to the acquisition parameters used. The analysis was performed based on axial slices reconstructed as described above (3-mm slice thickness with 2-mm increments). Image quality was assessed through six predefined anatomic structures, including the aortic valve (sinus valsalva), right coronary artery (RCA) and left marginal artery (LMA) (origin of coronary arteries), supra-aortic vessels, aortic wall (mid ascending segment) and descending aorta. Image quality was rated using a four-point scale (0 = excellent, 1 = good, 2 = moderate and 3 = non-diagnostic image quality). In addition, a motion artefact score was assessed using a four-point scale (0 = all details clear and assessable, no or minimal motion artefacts, 1 = mild motion artefacts, 2 = severe motion artefacts and 3 = non-diagnostic image quality).
Intraobserver variability was also calculated. Image examples are provided in Figure 2.
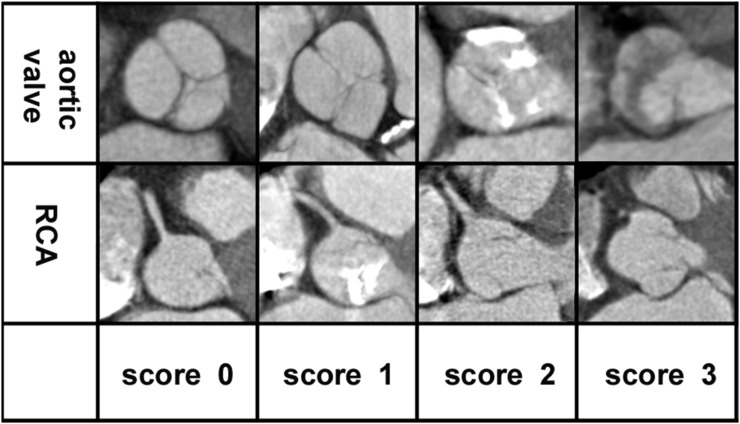
Figure 2.
Image quality: axial CT angiography images at the height of the aortic valve and at the right coronary artery (RCA) ostium, given as examples of the evaluation of motion artefacts at the aortic valve and RCA; 0 = excellent (no to minimal motion artefacts), 1 = good (mild motion artefacts), 2 = moderate (severe motion artefacts) and 3 = non-diagnostic. None of the aortic valves assessed were evaluated as non-diagnostic.
Radiation exposure
To estimate the radiation dose, the dose–length product (DLP) and the effective dose (EDLP) were determined. The DLP was obtained from an automatically generated protocol, based on the CT dose index. The ED was calculated according to the method and conversion factors described in the “European guidelines on quality criteria for computed tomography”.13
As a conversion factor for the entire aorta (kAORTA), we used 0.017 mSv/(mGy × cm).14 The following equation was used to calculate the ED:11,14
Statistical analysis
All statistical analyses were performed using SPSS (SPSS® Mac, v. 20.0; IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). Continuous data were expressed as mean ± standard deviation and discrete data as frequencies and percentages. A p-value below 0.05 was considered statistically significant.
To evaluate the distribution of the biological gender between both groups, χ2 test was used. Differences in patient characteristics (BMI and age) were compared between both protocols using an unpaired t-test. Shapiro–Wilk and Kolmogorov–Smirnov tests were performed to assess the distribution of the data. The Mann–Whitney U-test for continuous variables and the unpaired t-test were used to assess the differences between the ECG-triggered and the non-ECG-triggered protocol with regard to CNR, SNR, DLP and ED. A difference with a p-value of <0.05 was considered significant. Intraobserver agreement for subjective image quality assessment was determined by performing a Cohen's kappa analysis and was interpreted as described elsewhere5 (“κ-value >0.81 = excellent interobserver agreement; 0.61–0.80 = good; 0.41–0.60 = moderate; 0.21–0.40 = fair; and <0.20 = poor agreement”).5 Differences in image quality and motion artefacts were assessed using the χ2 test and Mann–Whitney U-test.
RESULTS
Patient characteristics
Patient characteristics are summarized in Table 1. Both groups were similar with respect to age (p = 0.68), sex (p = 0.19), BMI (p = 0.616) and heart rates (p = 0.43). One examination was documented twice and therefore, 59 patients were ultimately enrolled in the study. In addition, for one patient, height and weight were not recorded. To avoid miscalculations based on missing data, the image and radiation dose assessments of even these patients were included in the statistical analysis.
Table 1.
Patient characteristics
| Total | Group A (ECG triggered) | Group B (non triggered) | |
|---|---|---|---|
| Number of patients | 59 | 30 | 29 |
| Sex (female/male) | 25/34 | 10/20 | 15/14 |
| Age (years) ± SD (range) | 66.68 ± 14.38 (31–91) | 67.43 ± 13.74 (32–87) | 65.90 ± 15.21 (31–91) |
| BMI (kg/m2) (range) (n) | 26.41 ± 4.70 (14.26–40.81); (58) | 26.10 ± 5.32 (14.26–40.81); (29) | 26.73 ± 4.07 (19.91–36.98); (29) |
BMI, body mass index; ECG, electrocardiographic; SD, standard deviation.
Statistics revealed no significant differences among the groups with regard to sex, age and BMI. Height and body weight were not documented in one patient and, therefore, the BMI could not be obtained.
CT attenuation values
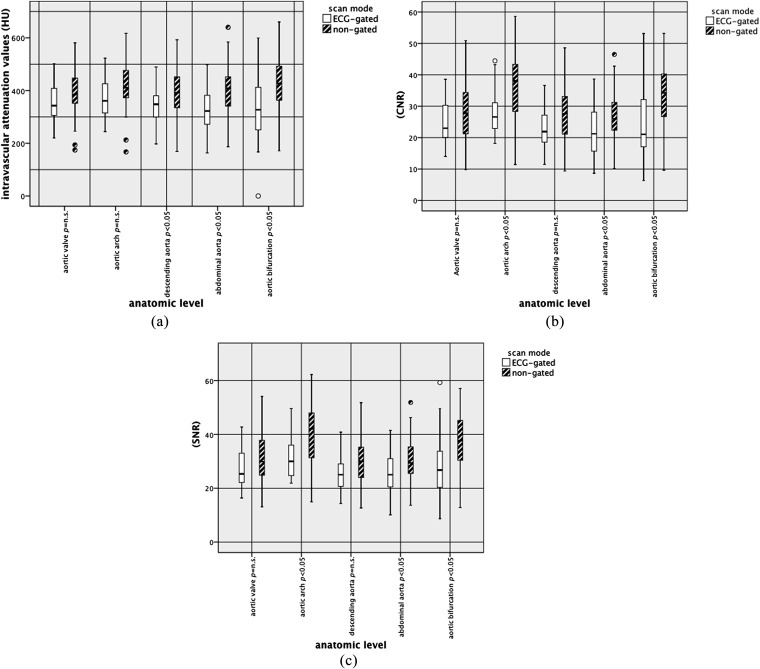
The vessel attenuation measured in Hounsfield units was significantly higher in the non-triggered group in the descending aorta (level of the aortic valve, p = 0.017; celiac artery, p = 0.002). Attenuation values for the muscles, and also for the background noise (BN), did not reveal significant differences in any region other than in the descending aorta at the level of the celiac artery (BN, p < 0.05). The CNR, SNR values and FOM values were significantly higher only at the level of the aortic arch (CNR, p = 0.002; SNR, p = 0.003) and descending aorta (celiac artery CNR = 0.013, SNR = 0.018; aortic bifurcation CNR = 0.002, SNR = 0.002). The CTA attenuation values are presented in detail in Table 2 and Figure 3a–c.
Table 2.
Results of image noise, estimated radiation dose and scan range
| Region | Parameter | Group A (ECG triggered) | Group B (non-triggered) | Differences in % | Group A vs Group B (p-level) |
|---|---|---|---|---|---|
| Aortic valve | Vessel (HU) | 355.74 ± 78.09 | 389.29 ± 99.30 | 9.4 | n.s. |
| Muscle (HU) | 35.16 ± 11.25 | 35.29 ± 9.86 | 0.3 | n.s. | |
| BN (SD) | 13.22 ± 1.76 | 12.97 ± 1.68 | −1.9 | n.s. | |
| CNR | 25.82 ± 8.52 | 27.78 ± 9.46 | 7.5 | n.s. | |
| SNR | 28.58 ± 9.22 | 30.54 ± 9.23 | 6.8 | n.s. | |
| FOM | 108.59 ± 71.87 | 130.31 ± 97.52 | 20 | n.s. | |
| Aortic arch | Vessel (HU) | 370.17 ± 75.59 | 417.58 ± 104.55 | 12.8 | n.s. |
| Muscle (HU) | 40.72 ± 13.35 | 38.93 ± 11.41 | −4.4 | n.s. | |
| BN | 11.77 ± 1.76 | 10.70 ± 2.33 | −9 | n.s. | |
| CNR | 28.50 ± 7.59 | 36.37 ± 10.92 | 27.6 | 0.002 | |
| SNR | 32.07 ± 8.24 | 40.17 ± 11.18 | 25 | 0.003 | |
| FOM | 140.87 ± 90.98 | 219.68 ± 137.51 | 55.94 | 0.012 | |
| Descending aorta (level of the aortic valve) | Vessel (HU) | 335.41 + 72.05 | 389.83 ± 95.55 | 16 | 0.017 |
| Muscle (HU) | 35.16 ± 11.25 | 35.29 ± 9.86 | 0.37 | n.s. | |
| BN | 13.22 ± 1.76 | 12.97 ± 1.68 | −1.9 | n.s. | |
| CNR | 24.22 ± 8.93 | 27.77 ± 8.84 | 14.6 | n.s. | |
| SNR | 27.07 ± 9.57 | 30.52 ± 8.63 | 10.2 | n.s. | |
| FOM | 76.69 ± 48.41 | 128.44 ± 90.65 | 61.17 | 0.011 | |
| Descending aorta (level of the celiac artery) | Vessel (HU) | 325.17 ± 83.80 | 404.34 ± 99.66 | 24.3 | 0.002 |
| Muscle (HU) | 45.78 ± 10.51 | 45.90 ± 12.79 | 0.3 | n.s. | |
| BN | 25.23 ± 5.07 | 22.05 ± 2.91 | −12.6 | 0.005 | |
| CNR | 21.98 ± 7.62 | 27.27 ± 8.17 | 24 | 0.013 | |
| SNR | 25.65 ± 8.11 | 30.79 ± 8.12 | 20 | 0.018 | |
| FOM | 77.79 ± 59.17 | 122.76 ± 85.09 | 57.80 | 0.027 | |
| Descending Aorta (level of the aortic bifurcation) | Vessel (HU) | 333.14 ± 122.86 | 426.76 ± 104.96 | 28.1 | 0.003 |
| Muscle (HU) | 43.44 ± 11.82 | 40.2 ± 11.52 | −7.5 | n.s. | |
| BN | 13.04 ± 2.70 | 11.98 ± 3.03 | −8.8 | n.s. | |
| CNR | 24.39 ± 11.10 | 33.48 ± 10.09 | 37.2 | 0.002 | |
| SNR | 27.87 ± 11.43 | 37.02 ± 10.37 | 32.8 | 0.002 | |
| FOM | 107.27 ± 129.32 | 189.85 ± 121.77 | 76.98 | 0.014 |
BN, background noise; CNR, contrast-to-noise ratio; ECG, electrocardiographic; FOM, figure of merit; HU, Hounsfield units; n.s., not significant; SD, standard deviation; SNR, signal-to-noise ratio.
Figure 3.
Comparison of objective image quality between both scan modes: attenuation values [Hounsfield units (HU)], contrast-to-noise ratio (CNR) and signal-to-noise ratio (SNR). Box and whisker plots were used to graphically depict the attenuation values (a), CNR (b) and SNR (c). ECG, electrocardiographic; n.s., not significant.
Image quality
The image quality was comparable between the triggered (Group A) and the non-triggered groups (Group B). Most of the images were assessed as “excellent” to “good”. The following paragraph will summarize the percentages of structures rated as “excellent” to “good” and mention the structures rated as not assessable. A detailed description can be found in Table 3.
Table 3.
Results of image quality and motion artefact score
| Location | Image quality | Group A (ECG triggered) (n = 30) |
Group B (non-triggered) (n = 29) |
Agreement/n, agreement in %, kappac |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First | Second | Both in % | First | Second | Both in % | Group A | Group B | |||
| Aortic valvea | 0 | 8 | 10 | 26.7 | 13 | 13 | 41.4 | /n | 28/30 | 26/29 |
| 1 | 15 | 13 | 50 | 12 | 11 | 41.4 | % | 93.3 | 89.6 | |
| 2 | 7 | 7 | 23.3 | 4 | 5 | 17.2 | κ | 0.896 | 0.833 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Aortic walla | 0 | 25 | 29 | 83.3 | 27 | 28 | 93 | /n | 26/30 | 28/29 |
| 1 | 5 | 1 | 16.7 | 2 | 1 | 6.9 | % | 86.6 | 96.5 | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | κ | 0.294 | 0.651 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Supra-aortic vesselsa | 0 | 24 | 26 | 80 | 28 | 27 | 93.1 | /n | 28/30 | 28/29 |
| 1 | 6 | 4 | 20 | 1 | 2 | 6.9 | % | 93.3 | 96.5 | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | κ | 0.762 | 0.651 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Descending aortaa | 0 | 29 | 28 | 93.2 | 29 | 27 | 93.1 | /n | 29/30 | 27/29 |
| 1 | 1 | 2 | 6.8 | 0 | 2 | 6.9 | % | 96.6 | 93.1 | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | κ | 0.651 | d | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| LMAa | 0 | 16 | 15 | 50 | 19 | 18 | 58.6 | /n | 28/30 | 28/29 |
| 1 | 10 | 10 | 33.3 | 8 | 9 | 31 | % | 93.3 | 96.5 | |
| 2 | 4 | 5 | 16.7 | 2 | 2 | 10.3 | κ | 0.889 | 0.931 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| RCAa | 0 | 9 | 7 | 23.3 | 9 | 9 | 27.6 | /n | 26/30 | 27/29 |
| 1 | 12 | 12 | 40 | 13 | 13 | 48.3 | % | 86.6 | 93.1 | |
| 2 | 8 | 10 | 33.3 | 6 | 6 | 20.7 | κ | 0.804 | 0.895 | |
| 3 | 1 | 1 | 3.3 | 1 | 1 | 3.4 | ||||
| Motion artefact scoreb | 0 | 3 | 14 | 43.3 | 19 | 18 | 65.5 | /n | 27/30 | 28/29 |
| 1 | 13 | 13 | 46.7 | 6 | 7 | 20.7 | % | 90 | 96.5 | |
| 2 | 14 | 3 | 10 | 4 | 4 | 13.8 | κ | 0.829 | 0.934 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
ECG, electrocardiographic; LMA, left marginal artery; RCA, right coronary artery.
Image quality: 0 = excellent, 1 = good, 2 = moderate and 3 = non-diagnostic image quality. In case of disagreement between two readouts, the lower score was chosen for the overall %.
Motion artefact score: 0 = all details visible and assessable, 1 = mild motion artefacts, 2 = severe motion artefacts and 3 = non-diagnostic.
Intraobserver agreement: κ >0.81 = excellent intraobserver agreement; κ 0.80–0.61 = good; κ 0.60–0.41 = moderate; κ 0.40–0.21 = fair; and κ <0.20 = poor.
Kappa cannot be computed when one variable is a constant.
“Excellent” to “good” was applied to the images of the: aortic valve in 77.6% (Group A) compared with 82.8% (Group B); aortic wall in 100% (both groups); supra-aortic vessels in 100% (both groups); LM in 83.3% (Group A) compared with 89.6% (Group B); and the RCA in 63.3% (Group A) compared with 75.9% (Group B). Further, both groups had one patient each in whom the RCA was not assessable owing to severe motion artefacts.
There were no significant differences in the subjective image quality between the groups (p = not significant) (Table 3) (Figure 4).
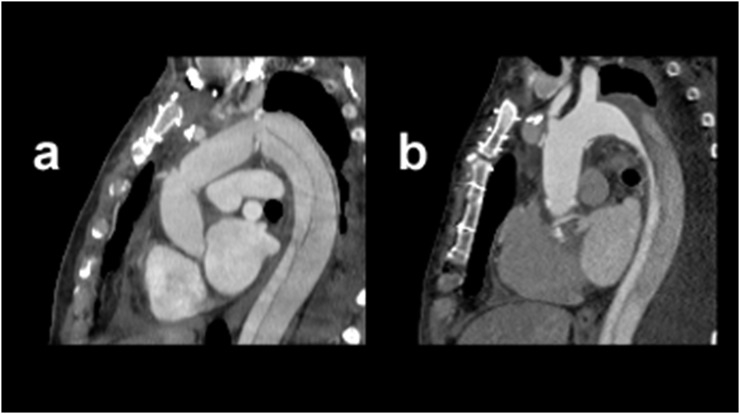
Figure 4.
Comparison of high-pitch (HP) electrocardiographic (ECG)-gated CT angiography with and without ECG triggering. Sagittal images using HP CT angiography for the delineation of aortic structures: image (a) was acquired using the ECG-triggered protocol, whereas image (b) was acquired using the non-triggered protocol.
Motion artefact score
In both groups, images were assessed as “all details visible and assessable” to “mild artefacts” in over 80% of all the images (Group A: 43.3% “all details visible and assessable”, 46.7% “mild artefacts” and 10% “severe artefacts”; Group B: 65.5% “all details visible and assessable”, 20.7% “mild artefacts” and 13.8% “severe artefacts”). There were no significant differences between both groups with regard to motion artefacts (Table 3).
Intraobserver agreement
Intraobserver agreement for image quality was excellent for both groups (Group A, k = 0.829; Group B, k = 0.934). Motion artefacts at most levels were assessed with excellent agreement (aortic valve—Group A = 93.3%, Group B = 89.6%; aortic wall—Group A = 86.6%, Group B 96.5%; LM—Group A = 93.3%, Group B = 96.5%; RCA—Group A = 86.6%, Group B = 93.1%; and supra-aortic vessels—Group A = 93.3%, Group B = 96.5%). The intraobserver agreement and Cohen's kappa for each assessment are summarized in Table 3.
Dose–length product and effective dose
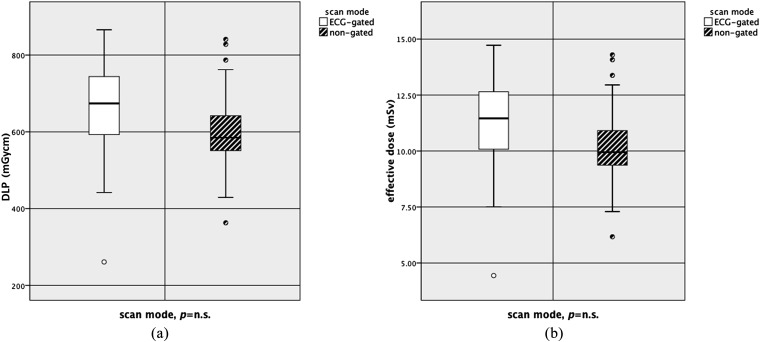
Radiation dose estimates (EDLP) in the ECG-triggered group (Group A) reached 11.19 ± 2.11 mSv compared with an ED of 10.24 ± 2.01 mSv within the non-triggered group (Group B). The median scan range in the ECG-triggered group was 778.69 ± 95.30 mm and the median scan range in the non-ECG-triggered group was 765.63 ± 137.19 mm. Differences were not statistically significant (DLP, p = 0.084; ED, p = 0.084; scan range, p = 0.675) (Table 4) (Figure 5a,b).
Table 4.
Results of scan ranges and radiation dose estimates
| Parameters | Group A (ECG triggered) | Group B (non-triggered) | Differences in % | Group A vs Group B (p-level) |
|---|---|---|---|---|
| Scan range (mm) | 778.69 ± 95.30 | 765.63 ± 137.19 | −1.7 | n.s. |
| DLP (mGy × cm) | 658.53 ± 124.26 | 602.93 ± 118.62 | −8.45 | n.s. |
| ED (mSv) | 11.19 ± 2.11 | 10.24 ± 2.01 | −8.45 | n.s. |
DLP, dose–length product; ECG, electrocardiographic; ED, effective dose; n.s., not significant.
Figure 5.
Comparison of the effective dose (EDLP) and dose–length product (DLP) between both groups. Box and whisker plots were used to visualize the ED (a) and DLP (b). ECG, electrocardiographic; mSV, millisievert; n.s., not significant.
DISCUSSION
Assessment of the ascending aorta and adjacent structures is especially prone to motion artefacts owing to the proximity of the heart, which can mimic or masquerade aortic disease.15–17 In acute aortic disease, but also for regular follow-up examinations in chronic disease, it is essential to delineate the vessel wall correctly for diagnosis, management and further treatment.17,18 Ongoing improvements in aortic and cardiac imaging have led to improved temporal and spatial resolutions and reduction of the radiation dose.2,5,19 ECG triggering is recommended to avoid motion artefacts;2,4 however, it also leads to an increased in-room time and more complex patient management.6 HP values on dual-source CTAs provide a better temporal resolution, lead to decreased scan times6,20 and might obviate the need for ECG triggering.6 Thus, the aim of the present study was to compare a non-triggered HP CTA with ECG-triggered HP CTA and to focus on the image quality, motion artefacts, CNR and SNR values and radiation dose.
In the data analysis, we did not find significant differences for the image quality or for motion artefacts between the two groups (excellent in most cases for both groups). This illustrates the high image quality, even at the level of the ascending aorta, for the non-triggered technique. An excellent image quality using HP protocols was also reported in previously published studies that assessed the aorta.5,6,21
In those studies, the authors reported a similar or even higher image quality.5,6,21 However, it should be pointed out that these previously published articles compared either the HP mode on dual-source CTA with single-source CTA techniques6,21 or had another focus and did not present data about adjacent structures that are also prone to motion artefacts, e.g. the aortic arch, and also did not report CNR and SNR.5
In contrast to previous publications,6,21 we compared two HP modes on the same CTA system—either with or without ECG triggering. This seems to be of importance for several reasons. First, the fact that the same scanner was used for all patients ensured that all patients were investigated by applying the same radiation dose-saving tools. Consequently, any difference in radiation exposure, as measured between the two groups, should reflect the direct and real difference caused by the selection of the acquisition technique, which is not necessarily the case if two different scanners are compared. Second, since it has already been shown that the use of an HP mode seems to be advantageous compared with slow-pitch protocols,6 especially for the fast acquisition of scans of the proximal aorta, it seems to be of special interest to determine whether ECG triggering provides some additional advantages when the same pitch is applied.
Our study revealed significantly higher image quality equivalents (CNR and SNR) in the non-triggered group only at the height of the aortic arch and the descending aorta. The calculation of the FOM6 supports this finding. However, these findings could be explained by differences in the arterial enhancement due to differing iodine delivery rates, which result in varying contrast material concentrations within the vessels while scanning. The faster acquisition speed allowed for higher iodine delivery rates, without increasing the total iodine application. Although a similar amount and the same concentration of contrast material were applied, different contrast administration protocols were used because of the different scanning times. However, these differences led to an iodine reduction of 10 ml (total 110 ml vs 120 ml), and even a small reduction of contrast material is beneficial for the patient.
Thus, this study showed that the shortening of the acquisition time due to the application of an HP technique enables excellent image quality, even without ECG synchronization, and higher contrast enhancement at a lower total iodine dose.
In comparison with other studies,5,6,21 our study found no significant differences for the radiation dose between ECG-triggered HP CTA and non-triggered HP CTA. The reason the dose reduction in the present study is diverse compared with that of other previously published publications is that the imaging protocols applied in other articles differed much more than those in the present article.5,6,8 For example, the fundamental dose reduction of up to 86%, as reported previously, was achieved by comparing HP and standard pitch protocols.5,8 In addition, the control group used retrospective triggering, which reportedly led, per se, to higher radiation doses compared with prospective triggering.5 Therefore, the differences shown are due to not only the application of ECG triggering, but also the control modes chosen. In contrast to that the major difference between the groups in our study was whether or not ECG triggering was applied and therefore, no major differences with regard to dose reduction were expected.
Median ED values assessed in our study (of 10.24 mSv, non-triggered HP) are lower compared with prospectively triggered CTAs at low pitch values, with comparable scan ranges (14.13–18.622). An important difference in the radiation doses reported in other articles is likely attributable to the differences in the scan ranges and conversion factors. We applied a conversion factor of 0.017 mSv/(mGy × cm) to calculate the ED, as reported previously,14 whereas other studies used conversion factors as low as 0.014 mSv/(mGy × cm).20 As a consequence, our equation might have led to a higher estimated ED than that in other studies. A factor of 0.017 mSv/(mGy × cm) appears more appropriate in a scan of the thorax, abdomen and pelvis.14
A limitation of our study is that the distribution of indications between the groups is not identical. This is because consecutive patients were prospectively randomized using a program called QuickCalcs. Therefore, the distribution of pathologies between the two groups was not influenced. Despite this, the groups seem to be comparable and thus, it seems to be unlikely that these minor differences had a major effect on the obtained results.
In our study, the radiologist did not prescribe any heart rate medication, and mean heart rates were not assessed for most of the patients in the non-triggered group. However, a recent study that addressed the necessity for beta-blocker premedication in aortic HP CTA reports that a comparable image quality was obtained without beta-blockers.23 Further, a comparable study described a good image quality in the HP CTA of the aortic root complex even at high heart rates.5
Another limitation is that two different examination protocols were used for the two groups. Group A received an ECG-triggered scan of the chest (thoracic scan) and a non-ECG-triggered scan of the abdominal aorta (second series, abdominal). Group B received one single scan (aortic flash scan). This was performed to keep the radiation dose as low as possible. And, as shown in Table 4, total scan ranges were very similar.
Another shortcoming is the use of two different contrast material administration protocols. Owing to the decreased duration of the non-ECG-triggered examination, the contrast injection protocol used was slightly different from that used in the ECG-triggered group. This might explain the differences found in the CNR and SNR. However, this was necessary in order to preclude incomplete enhancement and, consequently, a possible lack of image quality.15 Given the higher SNR and CNR levels in almost all territories for the non-triggered HP group, a reduction of the total iodine dose, together with the iodine delivery rate, seems to be possible when applying the non-triggered HP technique. This should be confirmed in further studies.
CONCLUSION
Our study showed that HP CTA scanning without ECG triggering provides an excellent image quality of the ascending aorta equal to that of ECG-triggered HP scans. Furthermore, it seems that the total iodine dose could be decreased by using this imaging technique. In conclusion, these prospectively acquired data show that the HP mode may obviate the need for ECG triggering in the assessment of the ascending aorta in CTA.
Contributor Information
Alice Wielandner, Email: alice.wielandner@meduniwien.ac.at.
Dietrich Beitzke, Email: dietrich.beitzke@meduniwien.ac.at.
Ruediger Schernthaner, Email: Ruediger.Schernthaner@meduniwien.ac.at.
Florian Wolf, Email: florian.wolf@meduniwien.ac.at.
Christina Langenberger, Email: christina.langenberger@meduniwien.ac.at.
Alfred Stadler, Email: alfred.stadler@meduniwien.ac.at.
Christian Loewe, Email: christian.loewe@meduniwien.ac.at.
REFERENCES
- 1.Litmanovich D, Bankier AA, Cantin L, Raptopoulos V, Boiselle PM. CT and MRI in diseases of the aorta. AJR Am J Roentgenol 2009; 193: 928–40. doi: 10.2214/AJR.08.2166 [DOI] [PubMed] [Google Scholar]
- 2.Roos JE, Willmann JK, Weishaupt D, Lachat M, Marincek B, Hilfiker PR. Thoracic aorta: motion artifact reduction with retrospective and prospective electrocardiography-assisted multi-detector row CT. Radiology 2002; 222: 271–7. doi: 10.1148/radiol.2221010481 [DOI] [PubMed] [Google Scholar]
- 3.Wu W, Budovec J, Foley WD. Prospective and retrospective ECG gating for thoracic CT angiography: a comparative study. AJR Am J Roentgenol 2009; 193: 955–63. doi: 10.2214/AJR.08.2158 [DOI] [PubMed] [Google Scholar]
- 4.Chin AS, Fleischmann D. State-of-the-art computed tomography angiography of acute aortic syndrome. Semin Ultrasound CT MR 2012; 33: 222–34. doi: 10.1053/j.sult.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 5.Karlo C, Leschka S, Goetti RP, Feuchtner G, Desbiolles L, Stolzmann P, et al. High-pitch dual-source CT angiography of the aortic valve-aortic root complex without ECG-synchronization. Eur Radiol 2011; 21: 205–12. doi: 10.1007/s00330-010-1907-3 [DOI] [PubMed] [Google Scholar]
- 6.Beeres M, Schell B, Mastragelopoulos A, Herrmann E, Kerl JM, Gruber-Rouh T, et al. High-pitch dual-source CT angiography of the whole aorta without ECG synchronisation: initial experience. Eur Radiol 2012; 22: 129–37. doi: 10.1007/s00330-011-2257-5 [DOI] [PubMed] [Google Scholar]
- 7.Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J 2010; 31: 340–6. doi: 10.1093/eurheartj/ehp470 [DOI] [PubMed] [Google Scholar]
- 8.Apfaltrer P, Hanna EL, Schoepf UJ, Spears JR, Schoenberg SO, Fink C, et al. Radiation dose and image quality at high-pitch CT angiography of the aorta: intraindividual and interindividual comparisons with conventional CT angiography. AJR Am J Roentgenol 2012; 199: 1402–9. doi: 10.2214/AJR.12.8652 [DOI] [PubMed] [Google Scholar]
- 9.Mileto A, Ramirez-Giraldo JC, Nelson RC, Hurwitz LM, Choudhury KR, Seaman DM, et al. High-pitch dual-source MDCT for imaging of the thoracoabdominal aorta: relationships among radiation dose, noise, pitch, and body size in a Phantom Experiment and Clinical Study. AJR Am J Roentgenol 2015; 205: 834–9. doi: 10.2214/AJR.15.14334 [DOI] [PubMed] [Google Scholar]
- 10.Ueda T, Chin A, Petrovitch I, Fleischmann D. A pictorial review of acute aortic syndrome: discriminating and overlapping features as revealed by ECG-gated multidetector-row CT angiography. Insights Imaging 2012; 3: 561–71. doi: 10.1007/s13244-012-0195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beitzke D, Wolf F, Edelhauser G, Plank C, Schernthaner R, Weber M, et al. Computed tomography angiography of the carotid arteries at low kV settings: a prospective randomised trial assessing radiation dose and diagnostic confidence. Eur Radiol 2011; 21: 2434–44. doi: 10.1007/s00330-011-2188-1 [DOI] [PubMed] [Google Scholar]
- 12.Pontana F, Duhamel A, Pagniez J, Flohr T, Faivre JB, Hachulla AL, et al. Chest computed tomography using iterative reconstruction vs filtered back projection (part 2): image quality of low-dose CT examinations in 80 patients. Eur Radiol 2011; 21: 636–43. doi: 10.1007/s00330-010-1991-4 [DOI] [PubMed] [Google Scholar]
- 13.Bongartz G, Golding SJ, Jurik AG, Leonardi M, Persijn, van Meerten EV, Geleijns J. The European Commission's Study Group on development of quality criteria for computed tomography. 2000; Eur 16262. [Google Scholar]
- 14.Wuest W, Anders K, Schuhbaeck A, May MS, Gauss S, Marwan M, et al. Dual source multidetector CT-angiography before Transcatheter Aortic Valve Implantation (TAVI) using a high-pitch spiral acquisition mode. Eur Radiol 2012; 22: 51–8. doi: 10.1007/s00330-011-2233-0 [DOI] [PubMed] [Google Scholar]
- 15.Batra P, Bigoni B, Manning J, Aberle DR, Brown K, Hart E, et al. Pitfalls in the diagnosis of thoracic aortic dissection at CT angiography. Radiographics 2000; 20: 309–20. doi: 10.1148/radiographics.20.2.g00mc04309 [DOI] [PubMed] [Google Scholar]
- 16.Nagra K, Coulden R, McMurtry MS. A type A aortic dissection missed by non-cardiac gated contrast-enhanced computed tomography due to an aortic root dissection flap masquerading as an aortic valve apparatus: a case report. J Med Case Rep 2013; 7: 285. doi: 10.1186/1752-1947-7-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raymond CE, Aggarwal B, Schoenhagen P, Kralovic DM, Kormos K, Holloway D, et al. Prevalence and factors associated with false positive suspicion of acute aortic syndrome: experience in a patient population transferred to a specialized aortic treatment center. Cardiovasc Diagn Ther 2013; 3: 196–204. doi: 10.3978/j.issn.2223-3652.2013.12.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holloway BJ, Rosewarne D, Jones RG. Imaging of thoracic aortic disease. Br J Radiol 2011; 84 Spec No 3: S338–354. doi: 10.1259/bjr/30655825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achenbach S, Marwan M, Schepis T, Pflederer T, Bruder H, Allmendinger T, et al. High-pitch spiral acquisition: a new scan mode for coronary CT angiography. J Cardiovasc Comput Tomogr 2009; 3: 117–21. doi: 10.1016/j.jcct.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 20.Amacker NA, Mader C, Alkadhi H, Leschka S, Frauenfelder T. Routine chest and abdominal high-pitch CT: an alternative low dose protocol with preserved image quality. Eur J Radiol 2012; 81: e392–7. doi: 10.1016/j.ejrad.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 21.Bolen MA, Popovic ZB, Tandon N, Flamm SD, Schoenhagen P, Halliburton SS. Image quality, contrast enhancement, and radiation dose of ECG-triggered high-pitch CT versus non-ECG-triggered standard-pitch CT of the thoracoabdominal aorta. AJR Am J Roentgenol 2012; 198: 931–8. doi: 10.2214/AJR.11.6921 [DOI] [PubMed] [Google Scholar]
- 22.Brink M, de Lange F, Oostveen LJ, Dekker HM, Kool DR, Deunk J, et al. Arm raising at exposure-controlled multidetector trauma CT of thoracoabdominal region: higher image quality, lower radiation dose. Radiology 2008; 249: 661–70. doi: 10.1148/radiol.2492080169 [DOI] [PubMed] [Google Scholar]
- 23.Entezari P, Collins J, Chalian H, Tore HG, Carr J, Yaghmai V. Impact of beta-blockade premedication on image quality of ECG-gated thoracic aorta CT angiography. Acta Radiol 2014; 55: 1180–5. doi: 10.1177/0284185113516950 [DOI] [PubMed] [Google Scholar]