Abstract
Objective:
The aim of this study was to assess the accuracy of 1.5-T MRI in the pre-operative local T and N staging of colon cancer and identification of extramural vascular invasion (EMVI).
Methods:
Between 2010 and 2012, 60 patients with adenocarcinoma of the colon were prospectively recruited at 2 centres. 55 patients were included for final analysis. Patients received pre-operative 1.5-T MRI with high-resolution T2 weighted, gadolinium-enhanced T1 weighted and diffusion-weighted images. These were blindly assessed by two expert radiologists. Accuracy of the T-stage, N-stage and EMVI assessment was evaluated using post-operative histology as the gold standard.
Results:
Results are reported for two readers. Identification of T3 disease demonstrated an accuracy of 71% and 51%, sensitivity of 74% and 42% and specificity of 74% and 83%. Identification of N1 disease demonstrated an accuracy of 57% for both readers, sensitivity of 26% and 35% and specificity of 81% and 74%. Identification of EMVI demonstrated an accuracy of 74% and 69%, sensitivity 63% and 26% and specificity 80% and 91%.
Conclusion:
1.5-T MRI achieved a moderate accuracy in the local evaluation of colon cancer, but cannot be recommended to replace CT on the basis of this study.
Advances in knowledge:
This study confirms that MRI is a viable alternative to CT for the local assessment of colon cancer, but this study does not reproduce the very high accuracy reported in the only other study to assess the accuracy of MRI in colon cancer staging.
INTRODUCTION
In 2012, there were an estimated 1.4 million new cases of colorectal cancer and 693,900 deaths worldwide.1 The global incidence of colorectal cancer is increasing, although it appears to have peaked in North America and some European countries. Colorectal cancer is the second most common form of malignancy in Europe and North America and has a 5-year mortality of 50.7%.2 It is, therefore, a major global health problem.
The primary role of pre-operative staging in colon cancer has historically been to identify patients with widespread metastatic disease who would not benefit from primary surgery. Recent developments in treatment pathways have made it increasingly important to provide patients and clinicians with accurate pre-operative local staging. The fluoropyrimidine, oxaliplatin and targeted receptor pre-operative therapy (FOXTROT) trial is currently recruiting patients to investigate whether neoadjuvant chemotherapy improves the outcome for operable colon cancer, following encouraging results in the pilot phase of the trial3 and other small studies.4 This requires patients to be accurately stratified into high- and low-risk groups by their local staging, so as to identify those who may benefit from neoadjuvant therapy who are eligible for the study.
There is also an increasing range of surgical treatment options available for patients with colon cancer, including minimally invasive techniques. However, these may not be suitable for patients with locally advanced tumours and adjacent organ involvement, who may need more radical surgery. Patients with T4 disease have a high incidence of conversion from a laparoscopic to an open procedure, and the colon carcinoma laparoscopic or open resection (COLOR) trial report recommends pre-operative assessment with CT or MRI to identify patients with locally advanced disease who are not suitable for a laparoscopic approach.5 Pre-operative imaging in this setting can help to provide accurate operative planning and to inform the consent process for patients.
The current standard for the pre-operative local staging of colon cancer is contrast-enhanced CT of the thorax, abdomen and pelvis. However, MRI is well established in rectal cancer for providing an accurate assessment of circumferential resection margin and adjacent organ involvement. A recent study has suggested that 1.5-T MRI may also provide very precise local staging of colon cancer.6
The purpose of this study was to assess the diagnostic accuracy of 1.5-T MRI in the assessment of colon cancer.
METHODS AND MATERIALS
Patients
This was a prospective study evaluating the accuracy of 1.5-T MRI in the local staging of colon cancer, designed according to the Standards for Reporting of Diagnostic Accuracy (STARD) guidelines for the reporting of studies of diagnostic accuracy.7 The study was reviewed by the Royal Marsden Research Ethics Committee and was granted ethical approval (reference number: 10/H0801/21). All patients provided written informed consent before recruitment. Consecutive patients presenting with colon cancer to Croydon University Hospital and Epsom and St Heliers University Hospitals between September 2010 and October 2012 were included in the study. Patients were considered eligible if they had a biopsy-confirmed adenocarcinoma of the colon (arising >15 cm from the anal verge on colonoscopy), were over the age of 18 years and were planned for resection of their primary tumour. Patients were excluded if they were unable or unwilling to consent, were scheduled to receive neoadjuvant chemotherapy, had contraindications to MRI scanning, or had contraindications to the administration of gadolinium contrast agent. Patients were withdrawn from the study if they withdrew consent, were unable to tolerate MRI scanning or were unable to undergo resection of their primary tumour including those patients in whom MRI identified metastatic disease requiring neoadjuvant chemotherapy.
MRI technique
Patients underwent MRI scanning on a Siemens MAGNETOM Avanto 1.5 T (Siemens Healthcare, Erlangen, Germany). Bowel-cleansing preparation was not performed. Scans were performed with patients in the prone position where tolerated using a phased-array body coil. The prone position was adopted as this exerts a mild uniform pressure on the abdominal wall, reduces intra-abdominal volume8 and is associated fewer scan terminations owing to claustrophobia.9 Patients fasted for 4 h before scanning. 30–45 min prior to the MRI scan, patients imbibed 1 l of oral contrast media for small bowel distension (225 ml 4.9% weight/volume barium sulfate diluted in water to 1 l E-Z-Cat, Bracco UK Limited, Milan, Italy). Intraluminal contrast helps to separate bowel loops and is helpful in imaging other extrahepatic abdominal malignancies.10,11 Rectal contrast was not administered, as we felt that this would limit patient acceptance. Just before the commencement of imaging, patients received 20 mg of intramuscular hyoscine butylbromide (Boehringer Ingelheim Limited, Bracknell, UK) to reduce bowel motility.
A breath-hold true fast imaging with steady-state free-precession sequence was first taken through the abdomen and pelvis to identify the tumour. Respiratory navigator echo-triggered T2 weighted turbo spin-echo (TSE) sequences were then acquired perpendicular to the long axis of the tumour. Where scan time and patient comfort permitted, further TSE sequences were acquired in one or two complimentary planes. In cases where adequate respiratory triggering could not be achieved, breath-hold half-Fourier acquisition single-shot turbo spin-echo sequences were acquired instead of the TSE sequences. Coronal gadolinium contrast-enhanced fast low-angle shot sequences were also obtained through the abdomen, and transaxial diffusion-weighted imaging (DWI) sequences were obtained through the tumour volume. DWI was acquired using a spin-echo sequence with echoplanar imaging and spectral attenuated inversion recovery fat suppression. Repetition time was 4800 ms and echo time was 68 ms. Four b-values were used: 0, 100, 500 and 750 s mm−2.
For the TSE sequences, the field of view ranged from 170 × 170 mm to 420 × 420 mm. This was dependent on the tumour location, imaging plane and patient size. The voxel size was maintained with an in-plane resolution of between 0.66 × 0.66 mm2 and 1.3 × 1.3 mm2 by varying the matrix size from 256 × 256 pixels to 320 × 320 pixels. Slice thickness was 3 mm. Repetition time ranged from 3750 ms to 5469 ms owing to respiratory triggering, and echo time was 100 ms.
Image evaluation
MRI scans were prospectively reported by two consultant gastrointestinal (GI) radiologists with more than 10 years' experience in reporting colon cancers. MRI scans were reported using a standard case record form. The reporting radiologists were informed of the location of the tumour according to colonoscopy as is normal clinical practice, but were blinded to the results of other imaging investigations and histology.
The following clinical features were reported: T-stage, depth of extramural tumour invasion, N-stage, presence of extramural vascular invasion (EMVI) and any distant metastases.
T1 tumours include those limited to the submucosa. These tumours were identified by replacement of the normally high signal in the submucosal layer by an abnormal intermediate signal which did not extend into the circular muscle layer. T2 tumours extend through the submucosal, but are limited to the muscularis propria. These tumours were identified by an abnormal intermediate signal intensity (higher than normal muscle signal, lower than normal submucosal signal) extending into the muscularis propria or replacing the muscularis propria but not extending into the pericolonic fat. An example is shown in Figure 1. T3 tumours invade through the muscularis propria into the subserosa or pericolonic fat, but do not invade the serosa or adjacent organs. These tumours were identified by a broad-based bulge or nodular projection of intermediate tumour intensity projecting beyond the outer muscle coat. An example is shown in Figure 2. T3 tumours were further classified depending on the depth of extramural tumour invasion measured in millimetres; T3a—1-mm or less extramural invasion, T3b—>1–5-mm extramural invasion, T3c—>5–15-mm extramural invasion and T3d >15-mm extramural invasion. T4 tumours include those that invade the serosa or adjacent organs. These were identified by extension of the intermediate tumour signal into adjacent organs or through the peritoneal surface. An example is shown in Figure 3. Assessments were made using T2 weighted imaging. These criteria were adapted from those used in the evaluation of rectal cancer.12
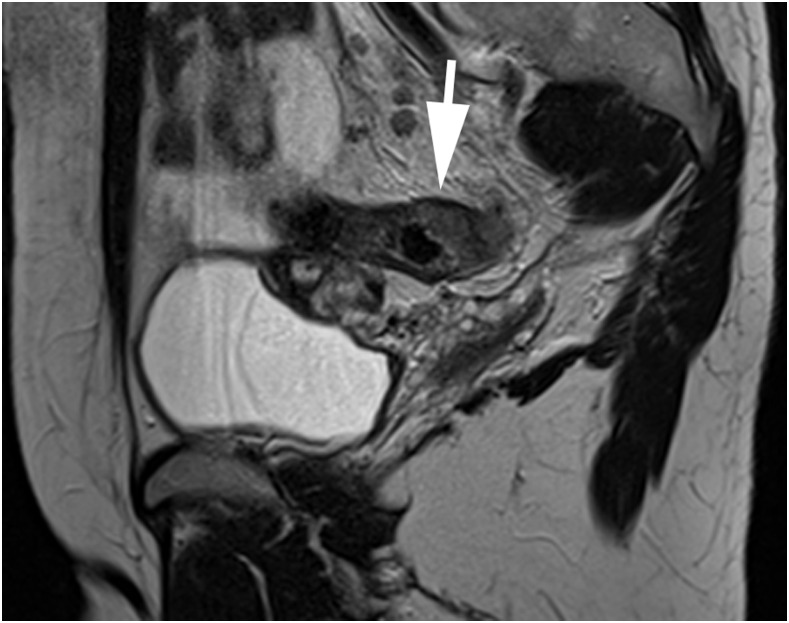
Figure 1.
A sagittal T2 weighted turbo spin-echo image showing a T2 sigmoid tumour confirmed on histopathology (arrow), correctly reported by one reader but overstaged as T3 by the second reader.
Figure 2.
An axial T2 weighted turbo spin-echo image showing a T3c sigmoid tumour confirmed on histopathology (arrow), correctly reported by both readers.
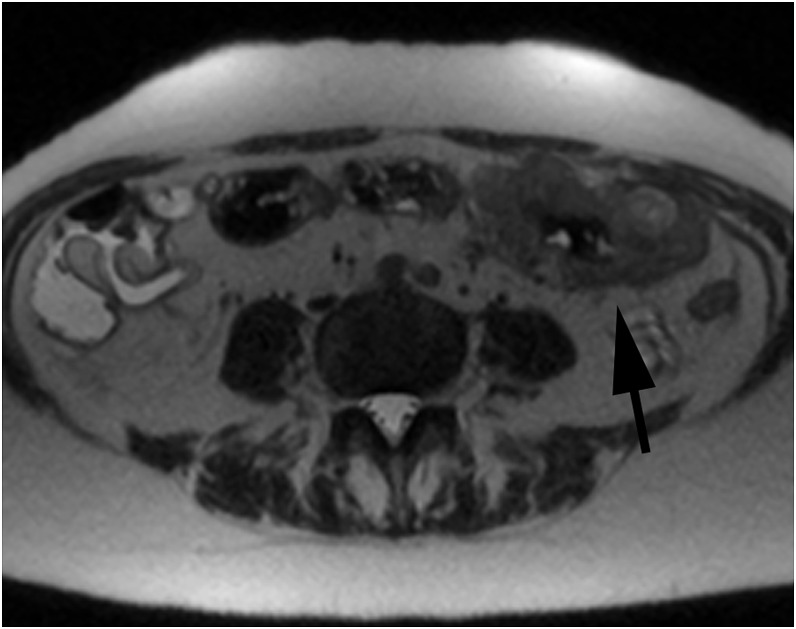
Figure 3.
An axial T2 weighted half-Fourier acquisition single-shot turbo spin-echo image showing a T4 transverse colon tumour confirmed on histopathology (arrow), correctly reported by both readers.
Lymph nodes were assessed based on their border and homogeneity of signal intensity.13,14 Lymph nodes were considered to be involved if they had an irregular border or inhomogeneous signal intensity. This was assessed on the T2 weighted TSE imaging or the T2 weighted half-Fourier acquisition single-shot turbo spin-echo images where the TSE images were inadequate. An example is shown in Figure 4. Specific size criteria were not used to identify involved lymph nodes, as previous studies in rectal cancer had demonstrated that there is a wide overlap between the size of the reactive benign lymph nodes and the lymph nodes containing small metastatic deposits.15 Tumours with no involved nodes were recorded as N0, tumours with 1–3 involved nodes were registered as N1 and tumours with four or more involved lymph nodes were recorded as N2.
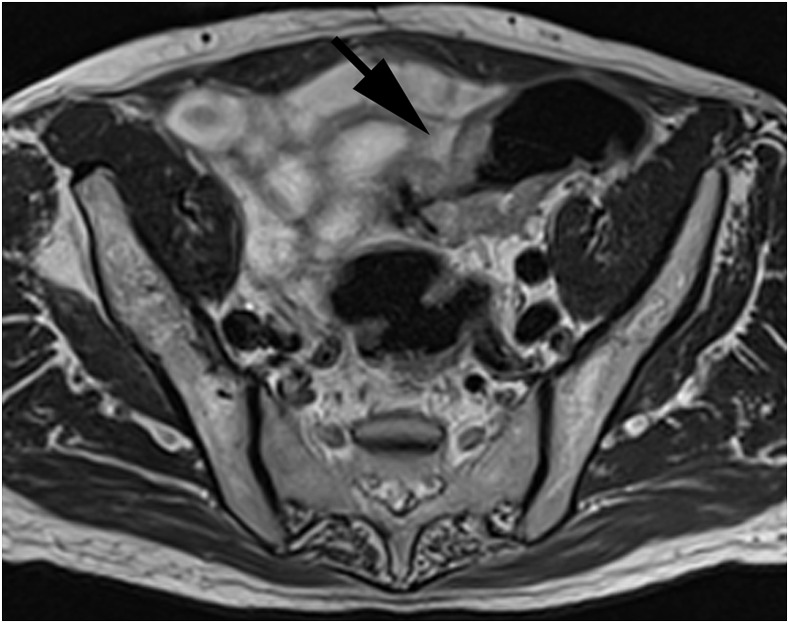
Figure 4.
An axial T2 weighted turbo spin-echo image showing an involved lymph node confirmed on histopathology (arrow), correctly reported by both readers.
EMVI was identified by the intermediate signal intensity of the tumour growing into or along a vessel recognized on MRI. Blood vessels were identified on T2 weighted MRI by a linear signal void in continuity on adjacent slices.16 An example is shown in Figure 5. EMVI was reported as small-, medium- or large-vessel EMVI on MRI. Any size of EMVI identified on MRI was considered positive for comparison with histopathology, as EMVI is reported as positive or negative on histopathology.
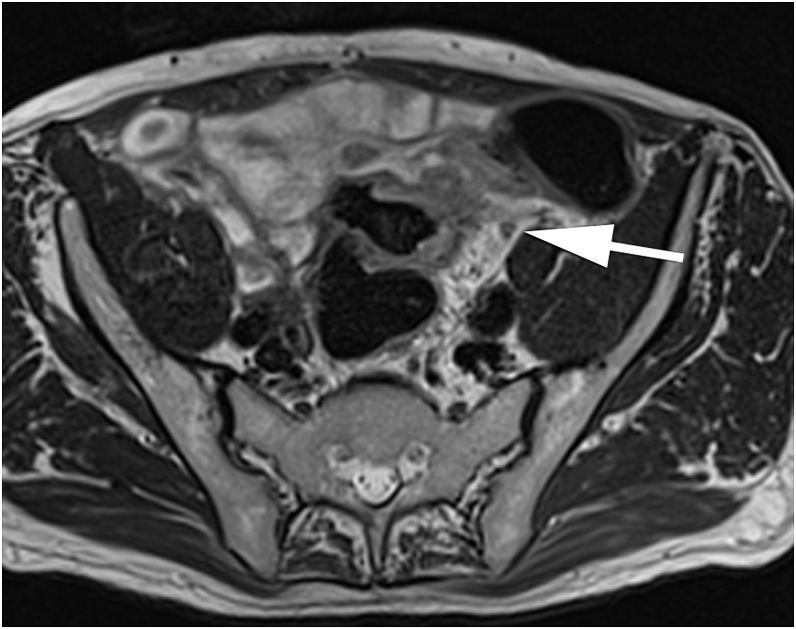
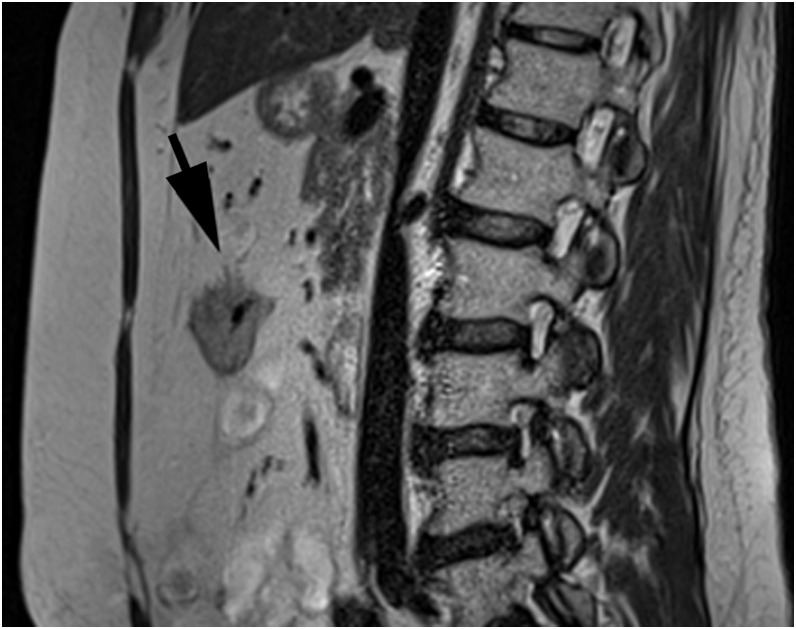
Figure 5.
A sagittal T2 weighted turbo spin-echo image showing extramural vascular invasion confirmed on histopathology (arrow), correctly reported by one reader but not by the second reader.
End points
T-stage and depth of extramural invasion were grouped into good prognosis tumours (T1, T2 or T3 tumours with 5-mm or less extramural invasion) and poor prognosis tumours (T3 tumours with >5 mm of extramural spread or T4 tumours—T3c or greater). Diagnosis with a poor prognosis tumour defined in this way is the entry criteria for the FOXTROT trial for patients who are older and less fit.17 This distinction has been demonstrated to have a greater prognostic significance than the distinction between T2 and T3 tumours both on pathology18 and to have a comparable prognostic significance on CT evaluation of colon cancer as on histopathological examination.19
T-stage was grouped into T1 and T2 tumours compared with T3 and T4 tumours to assess the sensitivity and specificity of MRI in identifying advanced T-stage. This distinction between T2 or less and T3 or greater tumours is the entry criteria for recruitment into the FOXTROT study of neoadjuvant chemotherapy in colon cancer for patients who are younger and in good general health17 and is the most commonly reported end point for the assessment of T-staging accuracy.
The accuracy of MRI in reporting a separate T-stage is also reported, with T1 and T2 tumours grouped together.
Nodal involvement is reported as not involved (N0) or involved (N1 or N2). This is a clinically significant end point, as patients with pathological nodal involvement (Stage III disease) demonstrate the most benefit from adjuvant chemotherapy in clinical trials.20,21
EMVI is reported as present or absent. This feature is increasing recognized as being prognostically significant; patients with MRI-detected EMVI in rectosigmoid cancer have poor 3-year recurrence-free survival similar to those with pathologically detected EMVI.16
Reference standard
Post-operative histopathological examination of the colon cancer specimen was used as the gold standard, against which the accuracy of the MRI scans was assessed. Histopathological examination was performed by one of two consultant GI pathologists with >10 years' experience in reporting colon cancers. Specimens were again prospectively reported using the Royal College of Pathologist minimum data set, which includes the T-stage, depth of extramural tumour invasion, the number of involved lymph nodes, N-stage and the presence or absence of EMVI.22
Sample size
The trial aimed to recruit 61 patients. This sample size was calculated to give a confidence interval (CI) of ±10% in estimating the accuracy of MRI in the assessment of T-stage with a 95% confidence level, assuming an observed accuracy of 80%.
Statistical analysis
All patients with evaluable results (MRI scan and histopathology report) were included for analysis. Statistical analysis was performed using R-3.2.1 (R Core Team, Vienna, Austria),23 including packages pROC24 and psych.25 Patient and tumour characteristics are reported using means and standard deviation for normally distributed variables, and using medians with interquartile ranges for other continuous variables. Categorical variables are reported as a number and percentage of positive patients.
The concordance between radiological and histopathological stage for ordinal outcomes (stage-for-stage T-stage) is estimated with weighted kappa coefficient and associated 95% CIs. This is reported with the accuracy of radiological assessment (percentage of radiological results which are correct using histopathology as the gold standard). The concordance for categorical outcomes (T1/T2 vs T3/T4, T1–T3b vs T3c–T4, N0 vs N1/N2 and EMVI− vs EMVI+) is estimated with Cohen's kappa coefficient and associated 95% CIs. This is reported along with the accuracy (percentage of radiological results which is correct), sensitivity (percentage of true positives detected) and specificity (percentage of true negatives detected). 95% CIs are calculated using the Wilson method.26
Interobserver reliability is reported using the unweighted Cohen's kappa coefficient for categorical outcomes.27 Interobserver reliability for ordinal outcomes is reported using weighted kappa, which takes into account the magnitude of disagreement between observations for ordinal outcome measures.28 The extent of the agreement was quantified as follows: a kappa of <0.2 denotes a slight agreement; 0.2–0.4, a fair agreement; 0.4–0.6, a moderate agreement; 0.6–0.8, a substantial agreement; and >0.8, an almost perfect agreement.
RESULTS
Patient recruitment
Recruitment started in November 2010 and finished in November 2012. 60 (38%) of 158 eligible patients were recruited. Patients were not recruited for the following reasons: they did not consent to the trial (41 patients, 26% of eligible patients), it was not possible to contact the patient with sufficient time before surgery (35 patients, 22% of eligible patients), it was not feasible to arrange the MRI scan before surgery (16 patients, 10% of eligible patients) or for other reasons (6 patients, 4% of eligible patients).
Of the 60 patients recruited into the study, 5 (8%) patients were withdrawn from the trial. Patients were withdrawn owing to failure to attend for MRI scan [1 (1.6%) patient], because no tumour was identified on histopathological assessment [1 (1.6%) patient] or because they did not undergo surgery [3 (5%) patients]. 55 patients are therefore included for final analysis. One patient was, at the time of surgery, found to have an inoperable tumour due to duodenal involvement. This patient is included in the analysis for T-stage, but not for other end points as histopathological results are not available.
Patient and tumour characteristics
The mean age of patients recruited to the study was 69.7 years (standard deviation 13.6 years). 36 (65%) patients were male. Tumours were located in the caecum [8 (15%) patients], ascending colon [14 (25%) patients], hepatic flexure [5 (9%) patients], transverse colon [6 (11%) tumours], descending colon [4 (7%) tumours] and sigmoid colon [18 (33%) tumours].
The median delay from MRI scan to surgery was 9 days (interquartile range 5.5–13.5 days).
The T-stage for tumours on final pathology was T1 for 2 (4%) patients, T2 for 10 (18%) patients, T3 for 28 (51%) patients and T4 for 15 (27%) patients. The N-stage on final pathology was N0 for 31 (57%) patients, N1 for 19 (35%) patients and N2 for 4 (7%) patients. EMVI was present on pathological assessment in 19 (35%) patients.
There were no adverse events reported as a result of the performing of the MRI scans.
Accuracy of MRI for the identification of poor prognosis disease
The agreement between each observer and pathology for the identification of poor prognosis disease on the basis of extramural invasion is shown in Table 1. T3 tumours with >5 mm of extramural spread and T4 tumours were considered poor prognosis. The overall accuracy for Reader 1 in the identification of poor prognosis (T3c or greater) disease was 75% (95% CI 63–84%), with an unweighted kappa value of 0.46 (95% CI 0.22–0.7). The sensitivity was 67% (95% CI 45–83%) and specificity was 79% (95% CI 63–90%).
Table 1.
Summary statistics of assessment of poor prognosis (T3c or greater) of disease with MRI for both observers
| Histopathology | MRI |
|||||
|---|---|---|---|---|---|---|
| Observer 1 (number of patients) |
Observer 2 (number of patients) |
|||||
| T3b or less | T3c or greater | Total | T3b or less | T3c or greater | Total | |
| T3b or less | 27 | 7 | 34 | 32 | 2 | 34 |
| T3c or greater | 7 | 14 | 21 | 12 | 9 | 21 |
| Total | 34 | 21 | 55 | 44 | 11 | 55 |
T3b or less—T1, T2 or T3 with 5-mm or less extramural invasion (good prognosis).
T3c or greater—T3 with >5-mm extramural invasion or T4 (poor prognosis).
Observer 1: kappa 0.46 (0.22–0.7), accuracy 75% (62–84%), sensitivity 67% (45–83%), specificity 79% (63–90%), positive-predictive value (PPV) 67% (45–83%) and negative-predictive value (NPV) 79% (63–90%).
Observer 2: kappa 0.41 (0.17–0.64), accuracy 75% (62–84%), sensitivity 43% (24–63%), specificity 94% (81–98%), PPV 82% (52–95%) and NPV 73% (58–84%).
The overall accuracy for Reader 2 in the identification of poor prognosis disease was also 75% (95% CI 62–84%), with an unweighted kappa value of 0.41 (95% CI 0.17–0.64). The sensitivity was 43% (95% CI 24–63%) and specificity was 94% (95% CI 81–98%).
Interobserver reliability between Reader 1 and Reader 2 was moderate with a Cohen's kappa value of 0.41 (95% CI 0.17–0.64).
Accuracy of MRI for T-stage assessment
The overall accuracy for Reader 1 for the identification of T3 or greater disease on MRI was 71% (95% CI 58–81%), with an unweighted kappa value of 0.28 (95% CI 0.01–0.54). The sensitivity was 74% (95% CI 60–85%) and specificity was 58% (95% CI 32–81%).
The overall accuracy for Reader 2 for the identification of T3 or greater disease was 51% (95% CI 38–64%), with an unweighted kappa value of 0.15 (95% CI −0.02 to 0.32). The sensitivity was 42% (95% CI 28–57%) and specificity was 83% (95% CI 55–95%). Interobserver reliability between Reader 1 and Reader 2 was fair with a Cohen's kappa value of 0.3 (95% CI 0.1–0.5).
The agreement between each reader and pathology for the identification of individual T-stage is shown in Table 2, with T1 and T2 grouped together. Concordance between MRI and histopathology for the determination of stage-for-stage T-stage (T1/T2, T3 or T4) for Reader 1 was fair, with a weighted kappa value of 0.30 (95% CI 0.08–0.52) and an accuracy of 51% (95% CI 38–64%).
Table 2.
Summary statistics of tumour staging with MRI for both observers
| Histopathology | MRI |
|||||||
|---|---|---|---|---|---|---|---|---|
| Observer 1 (number of patients) |
Observer 2 (number of patients) |
|||||||
| T1 or T2 | T3 | T4 | Total | T1 or T2 | T3 | T4 | Total | |
| T1 or T2 | 7 | 5 | 0 | 12 | 10 | 1 | 1 | 12 |
| T3 | 8 | 18 | 2 | 28 | 18 | 9 | 1 | 28 |
| T4 | 3 | 9 | 3 | 15 | 7 | 3 | 5 | 15 |
| Total | 18 | 32 | 5 | 55 | 35 | 13 | 7 | 55 |
Observer 1: weighted kappa 0.30 (0.08–0.52) and accuracy 51% (38–64%).
Observer 2: weighted kappa 0.24 (0.02–0.45) and accuracy 44% (31–57%).
Concordance for Reader 2 was also fair, with a weighted kappa value of 0.24 (95% CI 0.02–0.45) and an accuracy of 44% (95% CI 31–57%). The weighted kappa value for interobserver reliability between Reader 1 and Reader 2 was 0.32 (95% CI 0.23–0.52), indicating moderate agreement.
Accuracy of MRI for N-stage assessment
The agreement between each reader and pathology for the identification of N-stage is shown in Table 3. The overall accuracy for Reader 1 in the identification of lymph node-positive disease (N1 or greater) was 57% (95% CI 44–70%), with an unweighted kappa value of 0.07 (95% CI −0.17 to 0.31). The sensitivity was 26% (95% CI 13–46%) and specificity was 81% (95% CI 64–91%).
Table 3.
Summary statistics of nodal staging with MRI for both observers
| Histopathology | MRI |
|||||
|---|---|---|---|---|---|---|
| Observer 1 (number of patients) |
Observer 2 (number of patients) |
|||||
| Node negative | Node positive | Total | Node negative | Node positive | Total | |
| Node negative | 25 | 6 | 31 | 23 | 8 | 31 |
| Node positive | 17 | 6 | 23 | 15 | 8 | 23 |
| Total | 42 | 12 | 54 | 38 | 16 | 54 |
Observer 1: kappa 0.07 (−0.17 to 0.31), accuracy 57% (44–70%), sensitivity 26% (13–46%), specificity 81% (64–91%), positive-predictive value (PPV) 50% (25–75%) and negative-predictive value (NPV) 60% (44–73%).
Observer 2: kappa 0.09 (−0.16 to 0.35), accuracy 57% (44–70%), sensitivity 35% (19–55%), specificity 74% (57–86%), PPV 50% (28–72%) and NPV 61% (45–74%).
The overall accuracy for Reader 2 in the identification of lymph node positive disease was 57% (95% CI 44–70%), with an unweighted kappa value of 0.09 (95% CI −0.16 to 0.35). The sensitivity was 35% (95% CI 19–55%) and specificity was 74% (95% CI 57–86%).
Interobserver reliability between Readers 1 and 2 was moderate with a Cohen's kappa value of 0.49 (95% CI 0.23–0.75).
Accuracy of MRI for identification of extramural vascular invasion
The agreement between each reader and pathology for the identification of EMVI is shown in Table 4. The overall accuracy for Reader 1 in the identification of EMVI was 74% (95% CI 61–84%), with an unweighted kappa of 0.43 (95% CI 0.18–0.68). The sensitivity was 63% (95% CI 41–81%) and specificity was 80% (95% CI 64–90%).
Table 4.
Summary statistics for extramural vascular invasion (EMVI) assessment with MRI for both observers
| Histopathology | MRI |
|||||
|---|---|---|---|---|---|---|
| Observer 1 (number of patients) |
Observer 2 (number of patients) |
|||||
| EMVI negative | EMVI positive | Total | EMVI negative | EMVI positive | Total | |
| EMVI negative | 28 | 7 | 35 | 32 | 3 | 35 |
| EMVI positive | 7 | 12 | 19 | 14 | 5 | 19 |
| Total | 35 | 19 | 54 | 46 | 8 | 54 |
Observer 1: kappa 0.43 (0.18–0.68), accuracy 74% (61–84%), sensitivity 63% (41–81%), specificity 80% (64–90%), positive-predictive value (PPV) 63% (41–81%) and negative-predictive value (NPV) 80% (64–90%).
Observer 2: kappa 0.2 (−0.04 to 0.45), accuracy 69% (55–79%), sensitivity 26% (12–49%), specificity 91% (78–97%), PPV 62% (31–86%) and NPV 70% (55–81%).
The overall accuracy for Reader 2 in the identification of EMVI was 69% (95% CI 55–79%), with an unweighted kappa of 0.2 (95% CI −0.04 to 0.45). The sensitivity was 26% (95% CI 12–49%) and specificity was 91% (95% CI 78–97%).
Interobserver reliability for Reader 1 and Reader 2 was slight, with a Cohen's kappa value of 0.07 (95% CI −0.17–0.3)
DISCUSSION
The accuracy of MRI in the identification of poor prognosis disease (T4 tumours and tumours which have invaded >5 mm outside the bowel wall) in this study was good at 75% (95% CI 62–84%) for both readers. This distinction has been demonstrated to be of greater prognostic significance than the distinction between T2 and T3 tumours on histopathology,18 and the distinction between good prognosis and poor prognosis tumours on CT has been demonstrated to be as prognostically relevant as this distinction on histopathology.19 This was the initial entry criteria for the FOXTROT study and remains the entry criteria for patients who are older and frailer. Accuracy for the identification of T3 or greater disease has previously been reported for CT by Dighe et al29 at 70%, and by Rollven et al6 at 79% for Observer 1 and 76% for Observer 2. The Rollven et al study also reports that the accuracy of 1.5-T MRI is very high, with an accuracy of 90% (95% CI 74–96%) for Observer 1 and 93% (95% CI 78–98%) for Observer 2 in a study of 28 patients. The accuracy of MRI in this study in identifying poor prognosis disease is, therefore, comparable with the reported accuracy of CT, but with a trend towards being less accurate than the only other study to report the accuracy of MRI in the assessment of this outcome measure.
The more commonly reported end point for the evaluation of local staging accuracy is the distinction between tumours confined to the bowel wall (T1 and T2 tumours) and those invading through the bowel wall (T3 and T4). This end point was assessed in a meta-analysis by Dighe et al30 in 2010, which included 19 studies from 1986 to 2008, 17 studies of which had sufficient data to be included in a meta-analysis. The overall sensitivity was 86% (95% CI 78–92%), with a specificity of 78% (95% CI 71–84%) and diagnostic odds ratio (DOR) of 22.4 (95% CI 11.9–42.4). In this study, Reader 1 had a sensitivity of 74% (95% CI 60–85%), specificity of 58% (95% CI 32–81%) and DOR of 4.1 (95% CI 1.1–15.5). Reader 2 had a sensitivity of 42% (95% CI 28–57%), specificity of 83% (95% CI 55–95%) and DOR of 3.6 (95% CI 0.7–18.5). Therefore, although there is an overlap in the 95% CIs, the trend for MRI in this study is to have a lower sensitivity, specificity and DOR for the identification of invasion through the muscularis propria than CT in the recent meta-analysis.
The accuracy of 1.5-T MRI for correctly identifying individual T-stage category was moderate for both readers (51% and 44%). Two articles report the stage-for-stage accuracy for the prediction of T-stage on CT; Smith et all report 60% and 61% accuracy for two observers19 and Burton et al31 report 35% and 51% accuracy for two observers. The accuracy of MRI in this study for the assessment of stage-for-stage T-stage is, therefore, comparable with CT in the Burton study, although it is slightly lower than the accuracy reported for CT in the Smith study.
Interobserver reliability for the reporting of T-stage was slightly disappointing, with a Cohen's kappa value of 0.3. Criteria for reporting T-stage was based on MRI T-staging criteria12 and was agreed before reporting of the trial scans commenced. Both observers were experienced GI radiologists who are part of the core colorectal multi-disciplinary team at their institutions and regularly report rectal MRI and colon CT. It, therefore, seems likely that the relatively high interobserver variability is related to the MRI quality.
Another commonly reported end point in the assessment of local staging accuracy is the identification of nodal metastases (N1 and N2 disease). This end point was also assessed by Dighe et al30 in his meta-analysis and 15 studies were included for this end point. The overall sensitivity for nodal detection in this meta-analysis was 70% (95% CI 59–80%), specificity was 78% (95% CI 66–86%) and DOR was 8.1 (95% CI 4.7–14.1). Reader 1 in this study demonstrated a sensitivity of 26% (95% CI 13–46%), specificity of 81% (95% CI 64–91%) and DOR of 1.5 (95% CI 0.4–5.3). Reader 2 had a sensitivity of 35% (95% CI 19–55%), specificity of 74% (95% CI 57–86%) and DOR of 1.5 (95% CI 0.5–5.0). These results suggest that in this study, MRI is less sensitive than CT in the identification of nodal involvement, although it is comparatively specific, with an overlap in the 95% CIs for the DOR.
The other imaging feature which has been demonstrated to be prognostically important in colorectal cancer on imaging is EMVI.16 The accuracy of MRI in this study for the identification of EMVI was again good at 74% for Reader 1 and 69% for Reader 2. The accuracy of CT for the identification of EMVI is reported in three previous studies; Burton et al reported an accuracy of 55% for Observer 1 and 61% for Reader 2,31 Dighe et al32 reported an accuracy of 70% and Rollven et al reported an accuracy of 78% for Reader 1 and 69% for Reader 2. Rollven et al also reported the accuracy of MRI of 78% and 82%. The accuracy of MRI in the identification of EMVI in this study is again comparable with the reported accuracy of CT and slightly less than the reported accuracy in the only other study of MRI.
Overall, it would appear that in this study, MRI is comparable with CT in the assessment of poor prognosis tumours with extramural invasion of >5 mm (T3c disease) and in the evaluation of EMVI, and it is slightly inferior to CT in the assessment of invasion through the muscularis propria (assessment of T3 disease) and lymph node positivity (N1–2 disease). This is in contrast to the other reported study on the 1.5-T MRI by Rollven et al, which reported a very high accuracy in the identification of locally advanced (T3c) disease of 90% and 93%. This may be because both are small studies (28 patients in the Rollven et al study and 55 patients in the present study), resulting in wide and overlapping CIs for the 2 studies. Alternatively, it may be as a consequence of the different MRI techniques which were used in the two studies. Rollven et al obtained high-resolution TSE sequences in three planes through the tumour. Although the present study also obtained high-resolution TSE sequences through the tumour, it was not always possible to get these in the three planes owing to time constraints as gadolinium-enhanced T1 sequences and DWI sequences were also obtained, which were less useful in assessing local invasion of the tumour.
One of the obstacles to acquiring high-quality images in this study was motion artefact. It is possible that imaging at a higher field strength (3.0 T or greater) might improve the accuracy of MRI staging, as image acquisition is more rapid at a higher field strength for the same resolution and signal-to-noise ratio.33
Two patients were withdrawn from the study because MRI identified liver metastases, and following discussion in the multidisciplinary meeting, these patients underwent chemotherapy rather than primary surgery. On imaging review, liver metastases were apparent on CT for one patient, but one patient had liver metastases identified on MRI which were not identified on CT. The purpose of this study was not to assess the sensitivity of MRI in identifying liver metastases, and so the liver was imaged only if it fell within the volume imaged on the T1 and T2 weighted sequences. The liver was therefore imaged partially in 47 patients and completely in 19 patients. DWI of the liver was not performed. It is therefore not possible to say from this study what proportion of patients would have had additional liver metastases identified by MRI, only that this is a potential benefit for some patients. No extrahepatic metastases were identified in this study.
This study did not assess the accuracy of MRI in restaging after neoadjuvant chemotherapy. Although this would be an interesting topic to investigate, accuracy might vary before and after neoadjuvant therapy and we felt that the utility of MRI in the assessment of primary colon cancer before treatment was the most important issue to address.
In summary, it is not possible to recommend the routine use of MRI in place of CT for the local staging of colon cancer on the basis of this study, although it does approach the accuracy of CT in some areas. MRI may have a role in specific situations, such as where CT is contraindicated as in pregnancy or where an i.v. contrast is contraindicated. The excellent results achieved with MRI in other studies suggest that the technique may yet warrant further investigation.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to thank the Bowel Disease Research Foundation, Croydon University Hospital and the National Institute for Health Research Biomedical Research Centre at the Royal Marsden National Health Service Foundation Trust for their help in funding this research project. They would also like to thank the MR radiographers at the Royal Marsden Hospital and Croydon University Hospital for their help in running this research project.
Contributor Information
Chris Hunter, Email: chris_j_hunter@hotmail.com.
Helena Blake, Email: blake_helena@hotmail.com.
Nelesh Jeyadevan, Email: nelesh.jeyadevan@croydonhealth.nhs.uk.
Muti Abulafi, Email: muti.abulafi@croydonhealth.nhs.uk.
Ian Swift, Email: Ian.Swift@croydonhealth.nhs.uk.
Paul Toomey, Email: paul.toomey@esth.nhs.uk.
Gina Brown, Email: gina.brown@rmh.nh.uk.
FUNDING
This research project was supported by a Bowel Diseases Research Foundation grant, a Croydon University Hospital bursary, and funding from the National Institute for Health Research Biomedical Research Centre at the Royal Marsden National Health Service Foundation Trust.
REFERENCES
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prevent 2016; 25: 16–27. [DOI] [PubMed] [Google Scholar]
- 2.Sawbridge D, Probert C. Population-based screening in colorectal cancer—current practice and future developments: faecal biomarkers review. J Gastrointestin Liver Dis 2014; 23: 195–202. [DOI] [PubMed] [Google Scholar]
- 3.Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012; 13: 1152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arredondo J, Pastor C, Baixauli J, Rodriguez J, Gonzalez I, Vigil C, et al. Preliminary outcome of a treatment strategy based on perioperative chemotherapy and surgery in patients with locally advanced colon cancer. Colorectal Dis 2013; 15: 552–7. doi: 10.1111/codi.12119 [DOI] [PubMed] [Google Scholar]
- 5.Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009; 10: 44–52. [DOI] [PubMed] [Google Scholar]
- 6.Rollven E, Holm T, Glimelius B, Lorinc E, Blomqvist L. Potentials of high resolution magnetic resonance imaging versus computed tomography for preoperative local staging of colon cancer. Acta Radiol 2013; 54: 722–30. doi: 10.1177/0284185113484018 [DOI] [PubMed] [Google Scholar]
- 7.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for reporting of diagnostic accuracy. Clin Chem 2003; 49: 1–6. doi: 10.1373/49.1.1 [DOI] [PubMed] [Google Scholar]
- 8.Cronin CG, Lohan DG, Mhuircheartaigh JN, McKenna D, Alhajeri N, Roche C, et al. MRI small-bowel follow-through: prone versus supine patient positioning for best small-bowel distention and lesion detection. AJR Am J Roentgenol 2008; 191: 502–6. doi: 10.2214/AJR.07.2338 [DOI] [PubMed] [Google Scholar]
- 9.Eshed I, Althoff CE, Hamm B, Hermann KG. Claustrophobia and premature termination of magnetic resonance imaging examinations. J Magn Reson Imaging 2007; 26: 401–4. doi: 10.1002/jmri.21012 [DOI] [PubMed] [Google Scholar]
- 10.Low RN. MR imaging of the peritoneal spread of malignancy. Abdom Imaging 2007; 32: 267–83. doi: 10.1007/s00261-007-9210-8 [DOI] [PubMed] [Google Scholar]
- 11.Low RN. Magnetic resonance imaging in the oncology patient: evaluation of the extrahepatic abdomen. Semin Ultrasound CT 2005; 26: 224–36. doi: 10.1053/j.sult.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 12.Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol 2008; 191: 1827–35. doi: 10.2214/AJR.08.1004 [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 2004; 52: 78–83. doi: 10.1016/j.ejrad.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 14.Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003; 227: 371–7. doi: 10.1148/radiol.2272011747 [DOI] [PubMed] [Google Scholar]
- 15.Dworak O. Morphology of lymph nodes in the resected rectum of patients with rectal carcinoma. Pathol Res Pract 1991; 187: 1020–4. doi: 10.1016/S0344-0338(11)81075-7 [DOI] [PubMed] [Google Scholar]
- 16.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg 2008; 95: 229–36. doi: 10.1002/bjs.5917 [DOI] [PubMed] [Google Scholar]
- 17.FOXTROT Collaborative. FOXTROT protocol version 6.0. [PDF]: birmingham clinical trials unit 2012. [updated 9/7/2012; cited 2015 15/12/2015]; FOXTROT trial protocol. [Google Scholar]
- 18.Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis 2001; 16: 298–304. doi: 10.1007/s003840100309 [DOI] [PubMed] [Google Scholar]
- 19.Smith NJ, Bees N, Barbachano Y, Norman AR, Swift RI, Brown G. Preoperative computed tomography staging of nonmetastatic colon cancer predicts outcome: implications for clinical trials. Br J Cancer 2007; 96: 1030–6. doi: 10.1038/sj.bjc.6603646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27: 3109–16. doi: 10.1200/JCO.2008.20.6771 [DOI] [PubMed] [Google Scholar]
- 21.Gill S, Thomas RR, Goldberg RM. Review article: colorectal cancer chemotherapy. Aliment Pharmacol Ther 2003; 18: 683–92. doi: 10.1046/j.1365-2036.2003.01735.x [DOI] [PubMed] [Google Scholar]
- 22.Williams GT, Quirke P, Shepherd NA. Dataset for colorectal cancer. 2nd edn. London, UK: The Royal College of Pathologists; 2007. [Google Scholar]
- 23.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 24.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12: 77. doi: 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Revelle W. Psych: procedures for psychological, psychometric, and personality research. Evanston, IL: Northwestern University; 2013. [Google Scholar]
- 26.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927; 22: 209–12. doi: 10.1080/01621459.1927.10502953 [DOI] [Google Scholar]
- 27.Cohen JA. Coefficient of agreement for nominal scales. Educ Psychol Meas 1960; 20: 37–46. doi: 10.1177/001316446002000104 [DOI] [Google Scholar]
- 28.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull 1968; 70: 213–20. doi: 10.1037/h0026256 [DOI] [PubMed] [Google Scholar]
- 29.Dighe S, Swift I, Magill L, Handley K, Gray R, Quirke P, et al. Accuracy of radiological staging in identifying high-risk colon cancer patients suitable for neoadjuvant chemotherapy: a multicentre experience. Colorectal Dis 2012; 14: 438–44. doi: 10.1111/j.1463-1318.2011.02638.x [DOI] [PubMed] [Google Scholar]
- 30.Dighe S, Purkayastha S, Swift I, Tekkis PP, Darzi A, A'Hern R, et al. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol 2010; 65: 708–19. doi: 10.1016/j.crad.2010.01.024 [DOI] [PubMed] [Google Scholar]
- 31.Burton S, Brown G, Bees N, Norman A, Biedrzycki O, Arnaout A, et al. Accuracy of CT prediction of poor prognostic features in colonic cancer. Br J Radiol 2008; 81: 10–19. doi: 10.1259/bjr/19492531 [DOI] [PubMed] [Google Scholar]
- 32.Dighe S, Blake H, Koh MD, Swift I, Arnaout A, Temple L, et al. Accuracy of multidetector computed tomography in identifying poor prognostic factors in colonic cancer. Br J Surg 2010; 97: 1407–15. doi: 10.1002/bjs.7096 [DOI] [PubMed] [Google Scholar]
- 33.Soher BJ, Dale BM, Merkle EM. A review of MR physics: 3T versus 1.5T. Magn Reson Imaging Clin N Am 2007; 15: 277–90. doi: 10.1016/j.mric.2007.06.002 [DOI] [PubMed] [Google Scholar]