Abstract
Objective:
To assess the effect of axial traction during MR arthrography (MRA) of the elbow joint on joint space widening, contrast dispersion between opposing cartilage surfaces and cartilage surface visibility.
Methods:
11 patients with elbow MRA with and without axial traction were prospectively studied. Two radiologists independently measured the elbow joint space width and semi-quantitatively graded contrast material dispersion between the opposing cartilage surfaces as well as the articular cartilage surface visibility before and after traction. The detection and visibility of articular cartilage defects were also compared before and after traction. Patients were instructed to report on pain or any other symptoms during elbow traction.
Results:
No patient reported discomfort, pain or any other symptoms related to traction on immediate and intermediate-term follow-up. Joint space width increased, more at the radiocapitellar joint space (Δ = 0.63 mm, p = 0.005) than at the ulnotrochlear joint space (Δ = 0.17 mm, p = 0.012), with contrast dispersion into the radiocapitellar joint and cartilage visibility of the radiocapitellar joint space significantly improving after traction (all p < 0.05). All of these parameters also improved at the ulnotrochlear joint, although this did not reach statistical significance. Traction improved the visibility of cartilage defects.
Conclusion:
This is the first study to evaluate the effect of traction on MRA of the elbow joint. This technique is safe and technically feasible. Traction MRA improves the cartilage surface visibility and cartilage defect visibility.
Advances in knowledge:
This technique is safe and technically feasible. Traction MRA improves cartilage surface visibility and cartilage defect visibility.
INTRODUCTION
Direct MR arthrography (MRA) of the elbow is primarily used to detect cartilage injury, although it may also help in the evaluation of collateral ligament injury.1–7 Evaluation of the elbow joint articular cartilage with MRA remains challenging, with a sensitivity of about 80% for the detection of all cartilage defects, although it is <50% for superficial defects.8 Improving the visibility of the articular cartilage is the first step to a more accurate evaluation of an articular cartilage injury, which will in turn improve patient management.
Direct MRA distends the joint capsule with the aim of enhancing differentiation between intra-articular structures.9,10 Most cartilage injuries involve the surface of the articular cartilage and good cartilage surface visibility requires adequate separation of articular cartilage surfaces.8 Such separation is not always attainable with standard MRA, as intra-articular contrast injection, while distending the joint and articular recesses, does not necessarily distract opposing cartilage surfaces.8–10 To take full advantage of intra-articular contrast injection, performing MRA with traction should allow better separation of opposing articular cartilages and thus improve cartilage visibility.
MRA with axial traction has been evaluated with success in the shoulder,11–13 hip,14–21 knee,22 wrist,23–30 ankle31,32 and metatarsophalangeal33 and metacarpophalangeal joints.34 To the best of our knowledge, there is no study comparing MRA of the elbow with and without traction. The purpose of this study was to determine the feasibility of elbow MRA with axial traction and to investigate whether elbow traction affects joint space width, contrast dispersion between opposing cartilage surfaces and cartilage surface visibility as well as the detection and visibility of articular cartilage defects and collateral ligament tear.
METHODS AND MATERIALS
This prospective study was approved by the local New Territory East Cluster-Prince of Wales Hospital (NTEC-PWH) Ethics Review Board, and all patients provided signed informed consent.
Study population
11 elbows from 11 consecutive patients (5 males, 6 females; mean age: 30 years, range: 13–69 years) between June 2014 and December 2015 were referred for elbow MRA. All patients had elbow pain and were clinically suspected, by one of four orthopaedic surgeons (8–20 years' experience), of having a cartilage injury, collateral ligament injury or intra-articular loose bodies. No patient had a fracture radiographically. All patients underwent MRA with and without traction within 2 months of clinical assessment.
Ultrasound-guided contrast injection
Ultrasound-guided intra-articular injection was performed immediately prior to MRA. An aseptic injection technique was employed including the use of a sterile transducer probe cover. Intra-articular injection of a 12-ml dilute (0.1 ml of gadoteric acid diluted in 20 ml of a solution made up of saline) gadolinium-based contrast agent (gadoteric acid, Dotarem; Guerbet, Roissy, France) was performed by one of two musculoskeletal radiologists (AWHN or RKLL) under ultrasound guidance using a posterior approach with the patient in a prone position and the elbow flexed to 90° at the edge of the bed. A local anaesthetic (3–4 ml of 0.2% lignocaine) was injected into the skin and outer margin of the joint capsule. A 22-gauge spinal needle was advanced into the olecranon fossa from either the medial or lateral side of the olecranon. 12 ml of contrast medium solution was slowly injected into the joint under real-time ultrasound imaging. The patient was then transferred to the MRI suite.
MR arthrography of elbow
MRA was performed on a 3.0-T MR whole-body system (Achieva TX-series; Philips Medical Systems, Best, Netherlands) using a commercially available dual-flex receiver coil, which incorporates paired medium and small flex coils working in synergy to provide uniform signal acquisition around the elbow (SENSE flex; Philips Medical Systems, Best, Netherlands). Finger traps (ConMed Linvatec, Largo, FL) were applied to the index and ring fingers before the patient entered the MR room. Any metal parts of the finger traps were removed so that they were fully MRI compatible. The elbow traction device was also set up in the MR room before imaging was performed (Figure 1a,b). The patient was positioned supine on the MR table with the affected upper limb in a fully extended position at the side of the body. The elbow was centred parallel to the long axis of the gantry.
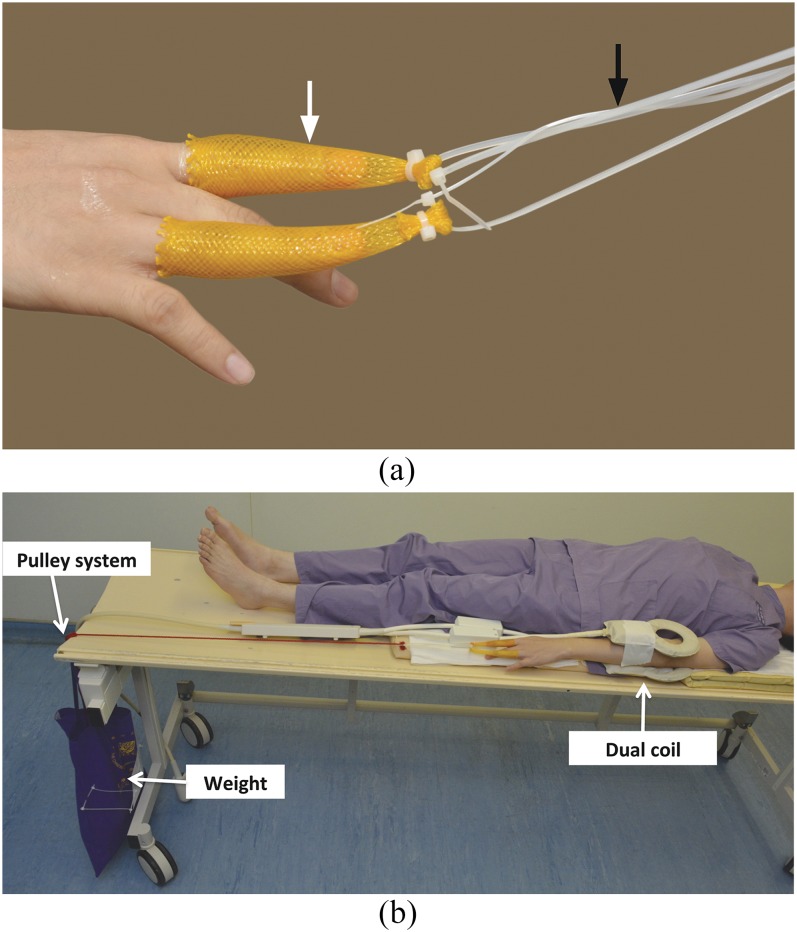
Figure 1.
(a) Finger traps applied to index and ring fingers (white arrow). The finger traps are connected to the weight by using a non-elastic cord (black arrow). (b) The non-elastic cord is routed through an in-house developed pulley system to a weight to give traction force to the elbow. We put the dual coils (medium/small flex) over the elbow region.
MRI sequences used before and after traction are listed in Table 1. A standard non-traction MRI study was performed initially (Table 1). The finger traps were then clipped to the freely hanging traction weight using a non-elastic cord routed over the edge of the MR gantry table (Figure 2).24,25 The traction weight used was 7 kg for males and 5 kg for females. MR examination of the elbow was then repeated (Table 1). Overall mean time for MRA with and without traction was 29 min. The non-traction sequences took 23 min to complete. The traction sequences took 6 min to complete. The time to set up the traction prior to MRI scanning was less than 5 min. Patients were instructed to report on discomfort, pain or any other symptoms related to traction during the MRI examination time immediately after the examination. In February 2016 (median 15 months, range 2–20 months, following traction MRI examination), a phone interview was conducted with all 11 patients specifically enquiring about any delayed discomfort, pain or other symptoms following the MR examination with traction.
Table 1.
MRI sequences before and after traction are listed
| MRI sequence | Plane | Thickness (mm) | TR (ms) | TE (ms) | Flip angle (°) | FOV (mm) | Matrix |
|---|---|---|---|---|---|---|---|
| Before traction | |||||||
| Fat-sat T1W TSE | Axial | 3 | 621 | 20 | 90 | 120 × 99 | 304 × 202 |
| Fat-sat T1W TSE | Coronal | 2.5 | 578 | 20 | 90 | 120 × 55 | 268 × 211 |
| Fat-sat T1W TSE | Sagittal | 2.5 | 575 | 20 | 90 | 66 × 120 | 268 × 214 |
| Fat-sat T2W TSE | Axial | 3 | 4983 | 60 | 90 | 120 × 100 | 268 × 170 |
| Intermediate-weighted TSE | Coronal | 2.5 | 1935 | 30 | 90 | 120 × 55 | 344 × 268 |
| Intermediate-weighted TSE | Sagittal | 2.5 | 5431 | 30 | 90 | 66 × 120 | 304 × 233 |
| After traction | |||||||
| Intermediate-weighted TSE | Coronal | 2.5 | 1935 | 30 | 90 | 120 × 55 | 344 × 268 |
| Intermediate-weighted TSE | Sagittal | 2.5 | 5431 | 30 | 90 | 66 × 120 | 304 × 233 |
Fat-sat, fat-saturated; FOV, field of view; T1W, T1 weighted; T2W, T2 weighted; TE, echo time; TR, repetition time; TSE, turbo spin echo.
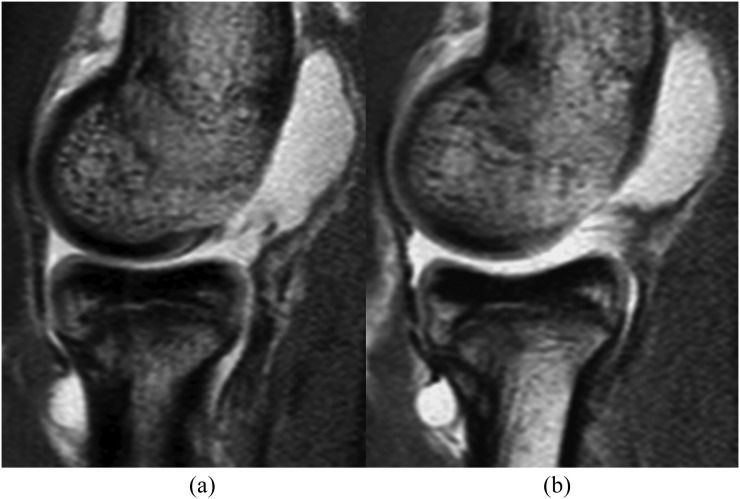
Figure 2.
Intermediate-weighted sagittal MR arthrography without traction (a) and with traction (b). After traction, the radiocapitellar joint space has increased. Contrast material dispersion between the opposing cartilage surfaces has improved from “partial” to complete. Opposing cartilage surface visibility at the capitellum and radial head has improved from “intermediate” to “good”. It can be noted that the cartilage at the anterior part of the capitellum is covered and partially obscured by the anterior capsule on both pre- and post-traction images. Although not specially addressed in this study, this capsular apposition is not likely to be affected by traction and may potentially be improved by increased contrast injection into the joint.
MR arthrography image analysis
Three musculoskeletal radiologists (AWHN, RKLL and BTYY) with 19, 9 and 4 years' experience in MR reporting, respectively, independently evaluated all of the MRI examinations. All images were interpreted on a dedicated picture archiving and communication system workstation (Carestream solution working station, v. 11.0; Carestream Health, Rochester, NY). All images were initially zoomed and the greyscale contrast was adjusted to optimize visualization of the structures being assessed. Pre-traction and then post-traction images were reviewed separately 1 week apart, with each observer blinded to the initial assessment.
Joint space width
Two radiologists (RKLL and BTYY) independently measured the minimum joint space width of the ulnohumeral and radiocapitellar articulations on intermediate-weighted coronal and sagittal images before and after traction. The minimum joint space width was defined as the shortest distance between two opposing articular cartilage surfaces. If the articular cartilage surface was poorly seen, the shortest distance of contrast material contained within the joint space was measured. Joint space width was measured before and after traction separately in the same sitting. One of these two radiologists (RKLL) repeated all of the joint space width measurements 2 weeks later blinded to the initial assessment.
Amount of contrast between opposing cartilage surfaces
Two radiologists (RKLL and AWHN) independently graded the amount of contrast material in the ulnohumeral and radiocapitellar joint spaces using a three-point scale: 0 = absent, when no contrast material was present between opposing cartilage surfaces on any sequential sagittal and coronal image; 1 = partial, when no contrast material was present between opposing cartilage surfaces on at least one sequential sagittal or coronal image; and 2 = complete, when contrast material was present between opposing cartilage surfaces on all sequential sagittal and coronal images. One of these two radiologists (RKLL) repeated the same assessment 2 weeks later.
Visibility of articular cartilage contour
Two radiologists (RKLL and AWHN) independently graded the visibility of opposing articular cartilage contours as good, intermediate or poor. “Good” visibility was a clear articular contour with an unambiguously sharp outline; “poor” referred to non-visibility of the articular cartilage contour, while “intermediate” referred to the articular cartilage contour being visible but not sharply demarcated. Each opposing articular cartilage contour visibility at the trochlea, trochlear notch of ulna, capitellum and radial head was separately assessed. One of these two radiologists (RKLL) repeated the same assessment 2 weeks later.
Presence and improved visibility of cartilage defect
Regarding the presence or absence of a cartilage defect, two radiologists (RKLL and AWHN) independently reviewed all images before and after traction for cartilage defects and graded these cartilage defects as partial or full-thickness (Grade 1: partial thickness chondral loss <50% of total chondral thickness, Grade 2: partial thickness chondral loss >50% of total chondral thickness and Grade 3: full-thickness chondral loss). The final diagnosis of a cartilage defect being present on MRI was then reached by consensus. Any improved visibility of any cartilage defect on MR images before and after traction was then determined by reading the pre- and post-traction images synchronously and grading the visibility of the cartilage defects as being “better”, “similar” or “worse” on post-traction images when compared with pre-traction images. One of these two radiologists (RKLL) repeated this same grading assessment 2 weeks later.
Statistical analysis
All analyses were performed using SPSS (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). All data were tested for normal distribution. The mean of both observer measurements was used to determine changes in the joint space width, contrast medium dispersion and articular cartilage contour visibility pre- and post-traction. The Wilcoxon matched-pairs signed-ranks test was used to assess the difference in joint space width before and after traction. The Stuart–Maxwell test was used to test for differences in contrast material dispersion between opposing cartilage surfaces and visibility of articular cartilage contour. Intraobserver and interobserver agreements of joint space width were calculated using the intraclass correlation coefficient, with the following criteria applied to these agreements: r >0.8 excellent, 0.6–0.8 good, 0.4–0.6 moderate, 0.2–0.4 fair and <0.2 poor. Interobserver agreement for the semi-quantitative analysis of contrast material dispersion between opposing cartilage surfaces and cartilage contour visibility was assessed by calculating Cohen's kappa coefficient and the results were interpreted according to the criteria proposed by Landis et al.35 For all tests, a probability level ‘p’ of <0.05 was regarded as statistically significant.
RESULTS
No patient reported discomfort, pain or any other symptoms related to the traction immediately after the MR examination and on medium-term (median: 15 months) follow-up after the MRI examination.
Joint space width
With traction, the joint space width significantly increased at both the radiocapitellar and, to a lesser degree, ulnohumeral joint spaces (Table 2). Both the interobserver correlation (r: 0.60–0.75) and intraobserver correlation (r: 0.64–0.79) for the measurement of joint space width were good. Figures 2–5 illustrate an increase in the elbow joint space after traction.
Table 2.
Comparison of the mean narrowest joint space widths (± standard deviation) before and after traction on MR arthrography
| Joint spaces | Without traction (mm) | With traction (mm) | p-value |
|---|---|---|---|
| Radiocapitellar joint space | 0.23 ± 0.32 | 0.86 ± 0.52 | 0.005 |
| Ulnohumeral joint space | 1.31 ± 1.28 | 1.48 ± 1.57 | 0.012 |
Measurements to the 1/100th of a millimetre are recorded.
Significant p-values are in bold.
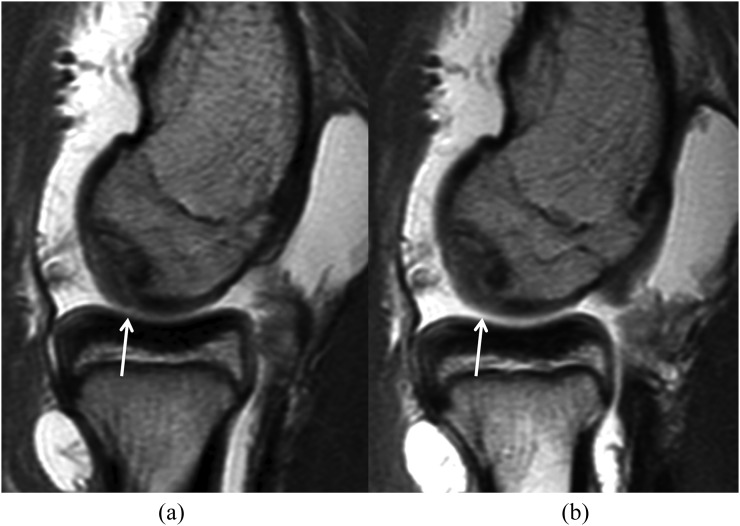
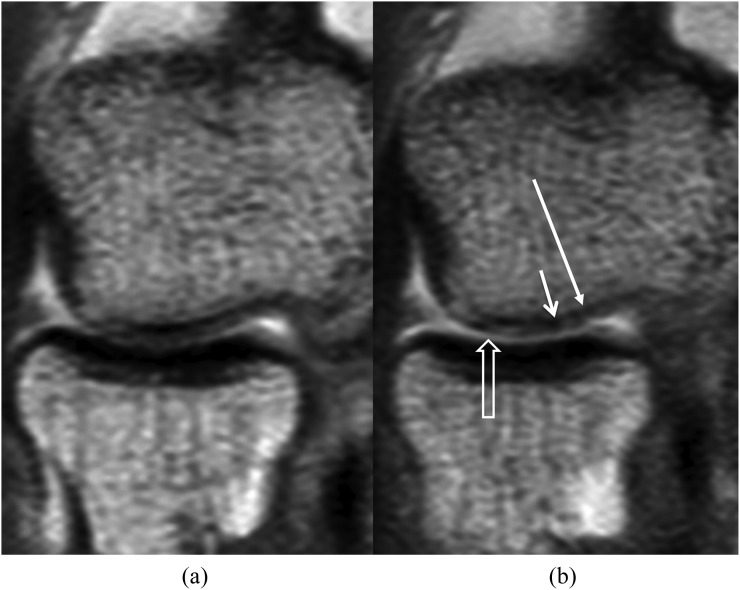
Figure 5.
Intermediate-weighted sagittal MR arthrography without traction (a) and with traction (b). After traction, the radiocapitellar joint space has widened. Contrast material dispersion between the cartilage surfaces has improved from “partial” to “complete”. Cartilage surface visibility at the capitellum and radial head has improved from “intermediate” to “good”. The visibility of the surface of the osteochondral injury of the capitellum (arrows) has also improved.
Figure 3.
Intermediate-weighted coronal MR arthrography without traction (a) and with traction (b). After traction, the joint spaces at both radiocapitellar and ulnotrochlear joint spaces have increased. Contrast material dispersion between the cartilage surfaces at both joint spaces has improved from “partial” to “complete”. Cartilage surface visibility at the capitellum, radial head, trochlea and proximal ulna has improved from “intermediate” to “good”. The small superficial chondral defect (Grade 1) (arrow) at the trochlear articular surface is better seen in the traction image, particularly the extent of the lesion (open arrow). All of the partial thickness defects we encountered were superficial, with this example being quite typical of those seen.
Dispersion of contrast between opposing cartilage surfaces
The degree of contrast dispersion between opposing articular surfaces is shown in Table 3. There was a significant increase in contrast material dispersion at the radiocapitellar joint space following traction. Contrast dispersion at the ulnohumeral joint space also tended to increase following traction, but this did not reach statistical significance. Interobserver and intraobserver correlations of grading for contrast medium dispersion were good to excellent (k: 0.6–0.8; p < 0.05). Figures 2–5 illustrate an improvement in contrast medium dispersion between opposing cartilage surfaces after traction.
Table 3.
Comparison of contrast dispersion between the cartilage surfaces before and after traction on MR arthrography
| Joint | Contrast dispersion | Without traction (number of cases) | With traction (number of cases) | p-value |
|---|---|---|---|---|
| Ulnohumeral joint | Absence | 0 | 0 | 0.5 |
| Partial | 11 | 9 | ||
| Complete | 0 | 2 | ||
| Radiocapitellar joint | Absence | 1 | 0 | 0.03 |
| Partial | 9 | 4 | ||
| Complete | 1 | 7 |
The amount of contrast dispersion significantly improved at the radiocapitellar joint space after traction (p < 0.05, in bold text).
Visibility of opposing articular cartilage surfaces
Opposing articular cartilage surface visibility significantly improved with traction at the radiocapitellar joint (Table 4). There was better cartilage surface visibility at the ulnohumeral joint, although this did not reach statistical significance. Interobserver and intraobserver correlations of grading for visibility of the articular cartilage surface was good to excellent (k: 0.6–0.8; p < 0.05). Figures 2–5 illustrate an improvement in cartilage surface visibility after traction.
Table 4.
Comparison of articular cartilage surface visibility at different joint spaces before and after traction
| Cartilage surface | Cartilage surface visibility | Without traction (number of cases) | With traction (number of cases) | p-value |
|---|---|---|---|---|
| Trochlea | Poor | 2 | 2 | 0.317 |
| Intermediate | 7 | 6 | ||
| Good | 2 | 3 | ||
| Trochlear notch of ulna | Poor | 1 | 1 | 0.317 |
| Intermediate | 8 | 7 | ||
| Good | 2 | 3 | ||
| Capitellum | Poor | 0 | 0 | 0.023 |
| Intermediate | 10 | 4 | ||
| Good | 1 | 7 | ||
| Radial head | Poor | 1 | 1 | 0.014 |
| Intermediate | 8 | 2 | ||
| Good | 2 | 8 |
Articular cartilage surface visibility significantly improved at radiocapitellar joint space after traction (p < 0.05, in bold text).
Presence and visibility of cartilage defect
In total, there were 6 cartilage defects in 3 of the 11 patients both before and after traction. These cartilage defects comprised two Grade 1 partial thickness defects at the ulnar trochlear notch, capitellum and trochlea on consensus. All cartilage defects seen after traction were also identified before traction. Visibility of all six partial thickness cartilage defects was improved after traction (Table 5). Interobserver and intraobserver correlations for the detection and visibility of cartilage defect was good to perfect (k: 0.6–1.0; p < 0.05). Figures 4 and 5 illustrate an improvement in the visibility of a cartilage defect after traction.
Table 5.
Comparison of visibility of articular cartilage defect in different joint surfaces before and after traction
| Cartilage surface | Type of cartilage defect | Without traction (number of cases) | With traction (number of cases) | Visibility of defect |
|---|---|---|---|---|
| Trochlea | None | 9 | 9 | All improved |
| Partial thickness | 2 | 2 | ||
| Full thickness | 0 | 0 | ||
| Trochlear notch of ulna | None | 9 | 9 | All improved |
| Partial thickness | 2 | 2 | ||
| Full thickness | 0 | 0 | ||
| Capitellum | None | 9 | 9 | All improved |
| Partial thickness | 2 | 2 | ||
| Full thickness | 0 | 0 | ||
| Radial head | None | 11 | 11 | All improved |
| Partial thickness defect | 0 | 0 | ||
| Full thickness | 0 | 0 |
The visibility of all articular cartilage defects was improved after traction.
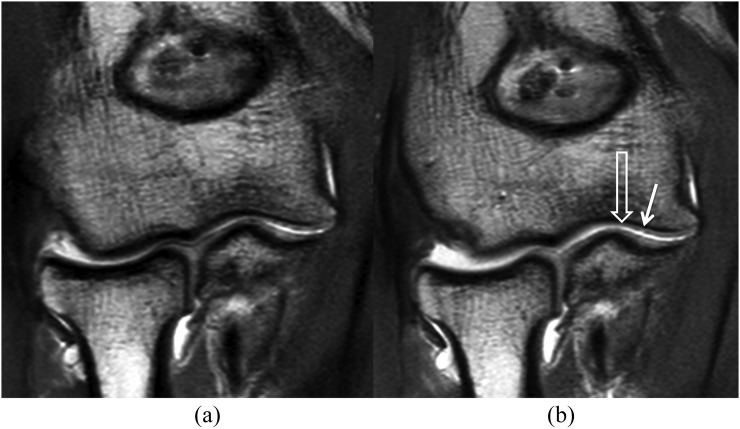
Figure 4.
Intermediate-weighted coronal MR arthrography without traction (a) and with traction (b). After traction, the radiocapitellar joint space has slightly increased with increased contrast material dispersion between the opposing cartilage surfaces (open arrow). Visibility of the small partial thickness cartilage defect (Grade 1) (long arrow) at the capitellum has improved after traction, particularly the extent of the cartilage thinning (short arrow).
DISCUSSION
Injury to the articular cartilage of the elbow is less common than in other joints such as the knee, hip, ankle and wrist.6 Nevertheless, articular cartilage injury can be a source of troublesome elbow pain and is the main indication for MRA of the elbow. For example, osteochondritis dissecans of the capitellum is one of the leading causes of long-term elbow disability in young athletes.36–38 Trochlear and trochlear notch chondromalacia occurs in throwing athletes with medial instability,39 while osteochondral injury of the capitellum is a feature of posterolateral rotatory instability.40 Capitellar cartilage injury also occurs in about 10% of patients with radial head fracture.41 The type, size and stability are the key elements of any focal cartilage injury that needs to be assessed when deciding on whether to pursue conservative or arthroscopic treatment. These features are generally assessed by MRI.37,38,42 Direct MRA of the elbow improves the diagnostic performance of conventional MRI in detecting and grading cartilage injuries.3,8 However, MRA of the elbow still needs to be improved, with regard to the assessment of articular cartilage.
This study is the first to investigate the effect of traction during elbow MRA. Applying traction significantly increased joint space at the radiocapitellar and ulnohumeral articulations. Radiocapitellar joint space width increased by at least 270%, while ulnohumeral joint space width increased by about 10%. This led to an appreciable increase in contrast material dispersion between opposing cartilage surfaces, which improved cartilage surface visibility, particularly at the radiocapitellar joint. The visibility of all cartilage defects was improved after traction. Although not tested in this study, traction may be particularly useful for CT arthrography of the elbow, where the delineation of chondral lesions is largely dependent on the contrast outlining the chondral surface.
The use of finger traps for applying traction to the elbow will indiscriminately distract the small joints of the hand as well as the wrist, elbow and shoulder joints of the ipsilateral limb. The elbow traction system used was well tolerated by patients with no pain or other symptoms being reported. Our previous study, which used an identical traction system as the one in this study for the wrist, showed that this degree of upper limb traction is well tolerated by patients.23 We used these weights (5 kg for females and 7 kg for males) for elbow traction as no previous study has investigated elbow traction.
This study has some limitations. First, the number of patients and number of cartilage defects was small as relatively few patients are referred for investigation of chondral injury. Despite the small number of patients, significant differences were still seen for most features assessed, emphasizing the benefit of traction MRA of the elbow. Second, blinding the assessor as to whether the study being assessed was performed before or after traction was not possible, as the degree of joint space widening made it readily apparent whether or not traction had been applied. To minimize this bias, reviewers assessed the pre- and post-traction studies 1 week apart blinded to the initial assessment. Third, no arthroscopic correlation was available against which to confirm cartilage defects. Following MR examination, elbow arthroscopy was deemed to be not clinically indicated in any patient. As arthroscopy of the elbow is technically the most difficult of the larger joints to perform, being able to provide a clear evaluation of cartilage integrity and unambiguously demonstrate any cartilage injury is of particular clinical relevance in this joint.
In conclusion, elbow MRA with axial traction is feasible and safe. This technique widens the radiocapitellar and ulnohumeral joint spaces, allowing better coverage of the articular cartilage surface by contrast material and improved visibility of articular cartilage surfaces at the radiocapitellar joint space. The visibility of articular cartilage defects also improved following traction.
Contributor Information
Ryan K L Lee, Email: leekalok2909@yahoo.com.hk.
James F Griffith, Email: griffith@cuhk.edu.hk.
Brian T Y Yuen, Email: yty1120@hotmail.com.
Alex W H Ng, Email: alex@sunghim.com.
David K W Yeung, Email: dkyeung@cuhk.edu.hk.
REFERENCES
- 1.Shahabpour M, Kichouh M, Laridon E, Gielen JL, De Mey J. The effectiveness of diagnostic imaging methods for the assessment of soft tissue and articular disorders of the shoulder and elbow. Eur J Radiol 2008; 65: 194–200. doi: 10.1016/j.ejrad.2007.11.012 [DOI] [PubMed] [Google Scholar]
- 2.Sampath SC, Sampath SC, Bredella MA. Magnetic resonance imaging of the elbow: a structured approach. Sports Health 2013; 5: 34–49. doi: 10.1177/1941738112467941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LiMarzi GM, O'Dell MC, Scherer K, Pettis C, Wasyliw CW, Bancroft LW. Magnetic resonance arthrography of the wrist and elbow. Magn Reson Imaging Clin N Am 2015; 23: 441–55. doi: 10.1016/j.mric.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 4.Delport AG, Zoga AC. MR and CT arthrography of the elbow. Semin Musculoskelet Radiol 2012; 16: 15–26. doi: 10.1055/s-0032-1304298 [DOI] [PubMed] [Google Scholar]
- 5.Johnson D, Stevens KJ, Riley G, Shapiro L, Yoshioka H, Gold GE. Approach to MR imaging of the elbow and wrist: technical aspects and innovation. Magn Reson Imaging Clin N Am 2015; 23: 355–66. doi: 10.1016/j.mric.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bancroft LW, Pettis C, Wasyliw C, Varich L. Osteochondral lesions of the elbow. Semin Musculoskelet Radiol 2013; 17: 446–54. doi: 10.1055/s-0033-1360665 [DOI] [PubMed] [Google Scholar]
- 7.Ramnath RR. 3T MR imaging of the musculoskeletal system (part II): clinical applications. Magn Reson Imaging Clin N Am 2006; 14: 41–62. doi: 10.1016/j.mric.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 8.Waldt S, Bruegel M, Ganter K, Kuhn V, Link TM, Rummeny EJ, et al. Comparison of multislice CT arthrography and MR arthrography for the detection of articular cartilage lesions of the elbow. Eur Radiol 2005; 15: 784–91. doi: 10.1007/s00330-004-2585-9 [DOI] [PubMed] [Google Scholar]
- 9.Elentuck D, Palmer WE. Direct magnetic resonance arthrography. Eur Radiol 2004; 14: 1956–67. doi: 10.1007/s00330-004-2449-3 [DOI] [PubMed] [Google Scholar]
- 10.Steinbach LS, Palmer WE, Schweitzer ME. Special focus session. MR arthrography. Radiographics 2002; 22: 1223–46. doi: 10.1148/radiographics.22.5.g02se301223 [DOI] [PubMed] [Google Scholar]
- 11.Izadpanah K, Winterer J, Vicari M, Jaeger M, Maier D, Eisebraun L, et al. A stress MRI of the shoulder for evaluation of ligamentous stabilizers in acute and chronic acromioclavicular joint instabilities. J Magn Reson Imaging 2013; 37: 1486–92. doi: 10.1002/jmri.23853 [DOI] [PubMed] [Google Scholar]
- 12.Chan KK, Muldoon KA, Yeh L, Boutin R, Pedowitz R, Skaf A, et al. Superior labral anteroposterior lesions: MR arthrography with arm traction. AJR Am J Roentgenol 1999; 173: 1117–22. doi: 10.2214/ajr.173.4.10511190 [DOI] [PubMed] [Google Scholar]
- 13.Becce F, Richarme D, Omoumi P, Djahangiri A, Farron A, Meuli R, et al. Direct MR arthrography of the shoulder under axial traction: feasibility study to evaluate the superior labrum-biceps tendon complex and articular cartilage. J Magn Reson Imaging 2013; 37: 1228–33. doi: 10.1002/jmri.23824 [DOI] [PubMed] [Google Scholar]
- 14.Llopis E, Cerezal L, Kassarjian A, Higueras V, Fernandez E. Direct MR arthrography of the hip with leg traction: feasibility for assessing articular cartilage. AJR Am J Roentgenol 2008; 190: 1124–8. doi: 10.2214/AJR.07.2559 [DOI] [PubMed] [Google Scholar]
- 15.Wettstein M, Guntern D, Theumann N. Direct MR arthrography of the hip with leg traction: feasibility for assessing articular cartilage. AJR Am J Roentgenol 2008; 191: W206; author reply W207. doi: 10.2214/AJR.08.1214 [DOI] [PubMed] [Google Scholar]
- 16.Suter A, Dietrich TJ, Maier M, Dora C, Pfirrmann CW. MR findings associated with positive distraction of the hip joint achieved by axial traction. Skeletal Radiol 2015; 44: 787–95. doi: 10.1007/s00256-015-2099-3 [DOI] [PubMed] [Google Scholar]
- 17.Nishii T, Nakanishi K, Sugano N, Naito H, Tamura S, Ochi T. Acetabular labral tears: contrast-enhanced MR imaging under continuous leg traction. Skeletal Radiol 1996; 25: 349–56. doi: 10.1007/s002560050094 [DOI] [PubMed] [Google Scholar]
- 18.Schmaranzer F, Klauser A, Kogler M, Henninger B, Forstner T, Reichkendler M, et al. Diagnostic performance of direct traction MR arthrography of the hip: detection of chondral and labral lesions with arthroscopic comparison. Eur Radiol 2015; 25: 1721–30. doi: 10.1007/s00330-014-3534-x [DOI] [PubMed] [Google Scholar]
- 19.Schmaranzer F, Klauser A, Kogler M, Henninger B, Forstner T, Reichkendler M, et al. Improving visualization of the central compartment of the hip with direct MR arthrography under axial leg traction: a feasibility study. Acad Radiol 2014; 21: 1240–7. doi: 10.1016/j.acra.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 20.Nishii T, Nakanishi K, Sugano N, Masuhara K, Ohzono K, Ochi T. Articular cartilage evaluation in osteoarthritis of the hip with MR imaging under continuous leg traction. Magn Reson Imaging 1998; 16: 871–5. doi: 10.1016/S0730-725X(98)00009-5 [DOI] [PubMed] [Google Scholar]
- 21.Cerezal L, Carro LP, Llorca J, Fernández-Hernando M, Llopis E, Montero JA, et al. Usefulness of MR arthrography of the hip with leg traction in the evaluation of ligamentum teres injuries. Skeletal Radiol 2015; 44: 1585–95. doi: 10.1007/s00256-015-2210-9 [DOI] [PubMed] [Google Scholar]
- 22.Palhais NS, Guntern D, Kagel A, Wettstein M, Mouhsine E, Theumann N. Direct magnetic resonance arthrography of the knee: utility of axial traction. Eur Radiol 2009; 19: 2225–31. doi: 10.1007/s00330-009-1389-3 [DOI] [PubMed] [Google Scholar]
- 23.Lee RK, Griffith JF, Ng AW, Nung RC, Yeung DK. Wrist traction during MR arthrography improves detection of triangular fibrocartilage complex and intrinsic ligament tears and visibility of articular cartilage. AJR Am J Roentgenol 2016; 206: 155–61. doi: 10.2214/AJR.15.14948 [DOI] [PubMed] [Google Scholar]
- 24.Guntern D, Becce F, Richarme D, Palhais NS, Meuli R, Theumann N. Direct magnetic resonance arthrography of the wrist with axial traction: a feasibility study to assess joint cartilage. J Magn Reson Imaging 2011; 34: 239–44. doi: 10.1002/jmri.22615 [DOI] [PubMed] [Google Scholar]
- 25.Leventhal EL, Moore DC, Akelman E, Wolfe SW, Crisco JJ. Conformational changes in the carpus during finger trap distraction. J Hand Surg Am 2010; 35: 237–44. doi: 10.1016/j.jhsa.2009.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerny M, Marlois R, Theumann N, Bollmann C, Wehrli L, Richarme D, et al. 3-T direct MR arthrography of the wrist: value of finger trap distraction to assess intrinsic ligament and triangular fibrocartilage complex tears. Eur J Radiol 2013; 82: e582–9. doi: 10.1016/j.ejrad.2013.04.039 [DOI] [PubMed] [Google Scholar]
- 27.Spies CK, Unglaub F. Comment on “3-T direct MR arthrography of the wrist: value of finger trap distraction to assess intrinsic ligament and triangular fibrocartilage complex tears”, Cerny M et al. Eur J Radiol 2013; 82: e909. doi: 10.1016/j.ejrad.2013.08.022 [DOI] [PubMed] [Google Scholar]
- 28.Becce F, Bollmann C. Response to ‘Comment on: 3-T direct MR arthrography of the wrist: value of finger trap distraction to assess intrinsic ligament and triangular fibrocartilage complex tears'. Eur J Radiol 2013; 82: e910. doi: 10.1016/j.ejrad.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 29.Kirchgesner T, Pesquer L, Larbi A, Meyer P, Moreau-Durieux MH, Silvestre A, et al. Axial traction in magnetic resonance arthrography of the wrist: how to do? Diagn Interv Imaging 2015; 96: 519–22. doi: 10.1016/j.diii.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 30.Dallaudière B, Meyer P, Larbi A, Moinard M, Moreau-Durieux MH, Poussange N, et al. Magnetic resonance arthrography of the wrist with axial traction: an iconographic review. Diagn Interv Imaging 2015; 96: 1307–12. doi: 10.1016/j.diii.2014.11.028 [DOI] [PubMed] [Google Scholar]
- 31.Jungmann PM, Baum T, Schaeffeler C, Sauerschnig M, Brucker PU, Mann A, et al. 3.0T MR imaging of the ankle: axial traction for morphological cartilage evaluation, quantitative T2 mapping and cartilage diffusion imaging-a preliminary study. Eur J Radiol 2015; 84: 1546–54. doi: 10.1016/j.ejrad.2015.04.023 [DOI] [PubMed] [Google Scholar]
- 32.Baer TE, Stolley MP, Thedens DR, Brown TD, Saltzman CL. Clinical tip: development of an ankle distraction device compatible with MRI and radiography. Foot Ankle Int 2006; 27: 472–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepage-Saucier M, Linda DD, Chang EY, Huang BK, Fliszar EA, Trudell D, et al. MRI of the metatarsophalangeal joints: improved assessment with toe traction and MR arthrography. AJR Am J Roentgenol 2013; 200: 868–71. doi: 10.2214/AJR.12.9164 [DOI] [PubMed] [Google Scholar]
- 34.Shepherd SM, Chang EY, Rutledge JL, Huang B, Trudell D, Resnick DL. Cartilage assessment of the metacarpophalangeal joints: cadaveric study with magnetic resonance arthrography and finger traction. Clin Imaging 2013; 37: 718–22. doi: 10.1016/j.clinimag.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 35.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 36.Ghahremani S, Griggs R, Hall T, Motamedi K, Boechat MI. Osteochondral lesions in pediatric and adolescent patients. Semin Musculoskelet Radiol 2014; 18: 505–12. doi: 10.1055/s-0034-1389268 [DOI] [PubMed] [Google Scholar]
- 37.Yadao MA, Field LD, Savoie FH, 3rd. Osteochondritis dissecans of the elbow. Instr Course Lect 2004; 53: 599–606. [PubMed] [Google Scholar]
- 38.Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am 2007; 89: 1205–14. doi: 10.2106/JBJS.F.00622 [DOI] [PubMed] [Google Scholar]
- 39.Osbahr DC, Dines JS, Rosenbaum AJ, Nguyen JT, Altchek DW. Does posteromedial chondromalacia reduce rate of return to play after ulnar collateral ligament reconstruction? Clin Orthop Relat Res 2012; 470: 1558–64. doi: 10.1007/s11999-011-2132-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeon IH, Min WK, Micic ID, Cho HS, Kim PT. Surgical treatment and clinical implication for posterolateral rotatory instability of the elbow: Osborne-Cotterill lesion of the elbow. J Trauma 2011; 71: E45–9. doi: 10.1097/TA.0b013e3182095c8a [DOI] [PubMed] [Google Scholar]
- 41.Nalbantoglu U, Gereli A, Kocaoglu B, Aktas S, Turkmen M. Capitellar cartilage injuries concomitant with radial head fractures. J Hand Surg Am 2008; 33: 1602–7. doi: 10.1016/j.jhsa.2008.05.016 [DOI] [PubMed] [Google Scholar]
- 42.Iwasaki N, Kato H, Kamishima T, Minami A. Sequential alterations in magnetic resonance imaging findings after autologous osteochondral mosaicplasty for young athletes with osteochondritis dissecans of the humeral capitellum. Am J Sports Med 2009; 37: 2349–54. doi: 10.1177/0363546509341793 [DOI] [PubMed] [Google Scholar]