Abstract
Objective:
This meta-analysis was performed to compare radioiodine therapy with antithyroid drugs in terms of clinical outcomes, including development or worsening of ophthalmopathy, hyperthyroid cure rate, hypothyroidism, relapse rate and adverse events.
Methods:
Randomized controlled trials (RCTs) published in PubMed, Embase, Web of Science, SinoMed and National Knowledge Infrastructure, China, were systematically reviewed to compare the effects of radioiodine therapy with antithyroid drugs in patients with Graves' disease. Results were expressed as risk ratio with 95% confidence intervals (CIs) and weighted mean differences with 95% CIs. Pooled estimates were performed using a fixed-effects model or random-effects model, depending on the heterogeneity among studies.
Results:
17 RCTs involving 4024 patients met the inclusion criteria and were included. Results showed that radioiodine treatment has increased risk in new ophthalmopathy, development or worsening of ophthalmopathy and hypothyroidism. Whereas, compared with antithyroid drugs, radioiodine treatment seems to have a higher hyperthyroid cure rate, lower recurrence rate and lower incidence of adverse events.
Conclusion:
Radioiodine therapy is associated with a higher hyperthyroid cure rate and lower relapse rate compared with antithyroid drugs. However, it also increases the risk of ophthalmopathy and hypothyroidism.
Advances in knowledge:
Considering that antithyroid drug treatment can be associated with unsatisfactory control of hyperthyroidism, we would recommend radioiodine therapy as the treatment of choice for patients with Graves' disease.
INTRODUCTION
Graves' disease is a common autoimmune disease, and the annual incidence is approximately 14 cases per 100,000 people.1 It generally occurs in females and individuals living in iodine-sufficient areas.2,3 There are four common therapies used for this disease, which include radioiodine therapy, radioiodine + prednisone, antithyroid drugs and thyroidectomy. Treatment with antithyroid drugs is frequently recommended as an initial treatment.3 In the members of the European Thyroid Association, two-third of patients with Graves' disease choose antithyroid drugs as first approach.4 Whereas, in the members of the American Thyroid Association, more than two-third prefer radioiodine therapy.5
It is reported that antithyroid drug treatment is not associated with the development or worsening of pre-existing eye disease,6 but it would lead to a 20–70% recurrence rate of hyperthyroidism following discontinuation of treatment.7–11 In contrast, radioiodine therapy is of low remission rate and effectiveness,11,12 but it might increase the risk of developing or worsening of Graves' ophthalmopathy. The question of whether radioiodine therapy is superior to antithyroid drug treatment is debated. To address this question, we conducted a meta-analysis of randomized controlled trials (RCTs) to compare the effects of radioiodine therapy with that of antithyroid drug treatment in patients with Graves' disease.
METHODS AND MATERIALS
Search strategy
Two authors conducted literature search in the databases of PubMed, Web of Science, Embase, SinoMed (Chinese BioMedical Literature Service System, China) and the National Knowledge Infrastructure, China. The selected key search terms were: (“Graves disease”[MeSH Terms] OR (“graves”[All Fields] AND “disease”[All Fields]) OR “Graves disease”[All Fields] OR (“graves'”[All Fields] AND “disease”[All Fields]) OR “graves' disease”[All Fields]) AND (radioiodine[All Fields] AND (“therapy”[Subheading] OR “therapy”[All Fields] OR “therapeutics”[MeSH Terms] OR “therapeutics”[All Fields])) AND (“antithyroid agents”[Pharmacological Action] OR “antithyroid agents”[MeSH Terms] OR (“antithyroid”[All Fields] AND “agents”[All Fields]) OR “antithyroid agents”[All Fields] OR (“antithyroid”[All Fields] AND “drugs”[All Fields]) OR “antithyroid drugs”[All Fields]) AND Clinical Trial[ptyp]. The search was limited to human subjects and no language restriction was imposed. Our search was further strengthened by searching the reference lists of the included studies.
Inclusion criteria and study selection
We included all the clinical trials that met the following inclusion criteria: (1) study design: RCT; (2) study population: patients with Graves' disease; (3) intervention: radioiodine treatment; (4) comparison intervention: antithyroid drugs; (5) outcome measures: ophthalmopathy, hyperthyroid cure rate, incidence of hypothyroidism, recurrence and adverse events; (6) sample size of patients: >50; and (7) duration of follow-up: more than 12 months.
Data extraction and study quality assessment
Two investigators (JQW and LQ) independently extracted the following data from each RCT: first author, year of publication, number of patients (intervention/control), duration of follow-up, study design, incidence of hypothyroidism, hyperthyroid cure rate, relapse rate, complications, concentration of free T3, free T4 and thyroid stimulating hormone and other important clinical outcome data. We used a standardized Excel® (Microsoft, Redmond, WA) file to extract the data. When the same population appeared in several publications, we only included the most informative article or complete study. Disagreements between the investigators were resolved by discussion and consensus.
The methodological quality of RCT was assessed by using Jadad scale.13 This tool used the following three items to define the quality of an RCT: (1) randomization; (2) double-blinding; (3) description of withdrawals and dropouts. A score of 1 point is given for each of the items mentioned above. A further point is obtained when the method of randomization or double-blinding is given and is appropriate. The total score is 5 points and studies with a score >2 points are said to be of high quality.14
Data synthesis and statistical analysis
We used STATA software v. 12.0 (Stata Corporation, College Station, TX) for data analysis. For dichotomous outcomes, they were expressed as risk ratios (RRs) with 95% confidence intervals (CIs); for continuous outcomes, they were expressed as weighted mean differences. Before the original data were synthesized, the heterogeneity across studies was tested by using Cochrane Q χ2 test and I2 statistic. A p-value <0.1 or I2 > 50%15 was defined to have significant heterogeneity. If substantial heterogeneity existed, a random-effects model (DerSimonian–Laird method)16 was used; otherwise, the fixed-effects model (Mantel–Haenszel method)17 was preferred to summarize the pooled data. Whenever heterogeneity was present, sensitivity analysis and subgroup analysis were conducted to identify the potential sources. Publication bias was assessed by using Begg18 and Egger's19 tests. A p-value <0.05 was judged as statistically significant.
RESULTS
Study identification and selection
As shown in Figure 1, the initial search identified 2248 relevant publications, of which 1354 publications were excluded for duplicate studies. After manually reviewing the title and abstracts, 791 publications were excluded based on various reasons (reviews, abstracts, animal experiments, letters or unrelated to our topic). Then, the remaining 103 articles were screened for the full-text review and 86 of them were removed owing to the single-arm study design (15 articles), lack of interest data (11 articles), sample size <50 (23 articles) or assessing other treatment rather than radioiodine and antithyroid drugs (37 articles). Finally, 17 RCTs met the inclusion criteria and were included in this meta-analysis.20–36
Figure 1.
Eligibility of studies for inclusion in meta-analysis. CNKI, National Knowledge Infrastructure, China.
Study characteristics and quality assessment
The main characteristics of the 17 RCTs in this meta-analysis are presented in Table 1. These studies were published between 1992 and 2015. The sample size of these RCTs ranged from 65 to 640 (total 4024). Maximum follow-up ranged from 1 to 11.1 years. Of these 17 RCTs, 13 RCTs were conducted in China, 2 RCTs in Sweden and 1 RCT in Iran. Among the 17 RCTs, 16 RCTs involved adult patients30 and the remaining 1 study involved paediatric patients.21 All of these patients received radioiodine treatment and antithyroid drugs and completed a follow-up of more than 12 months. The median Jadad scale of the RCTs was 3 (ranging from 3 to 4).
Table 1.
Baseline characteristics of patients in the trials included in the meta-analysis
| Study (years) | Country | Treatment regimen | Number of patients | Male/female | Age (mean ± SD) (years) | Maximum follow-up (years) | Jadad score |
|---|---|---|---|---|---|---|---|
| Abraham-Nordling et al (2010)20 | Sweden | Radioiodine | 163 | NR | 35–69 | 4 | 3 |
| Antithyroid drug | 145 | NR | 35–69 | 4 | |||
| Azizi et al (2005)21 | Iran | Radioiodine | 41 | NR | 59 ± 7 | 11.1 | 3 |
| Methimazole | 26 | NR | 57 ± 6 | 11.1 | |||
| Kung et al (1994)22 | China | Radioiodine | 57 | 14/43 | 46 ± 19 | 2 | 3 |
| Methimazole + L-T4 | 57 | 16/41 | 45 ± 9 | 2 | |||
| Kung et al (1995)23 | China | Radioiodine | 79 | 20/59 | 47.5 ± 10.2 | 10 | 3 |
| Methimazole + L-T4 | 80 | 21/59 | 46.1 ± 9.9 | 10 | |||
| Tallstedt et al (1992)24 | Sweden | Radioiodine | 39 | 6/33 | 45 ± 5 | 5 | 4 |
| Methimazole | 27 | 3/24 | 28 ± 4 | 5 | |||
| Bartalena et al (1998)25 | Italy | Radioiodine | 150 | 29/121 | 16–85 | 1 | 4 |
| Methimazole | 148 | 28/120 | 17–77 | 1 | |||
| Zhao (2015)26 | China | Radioiodine | 50 | 15/35 | 44.5 ± 1.25 | 3 | 3 |
| Methimazole | 50 | 16/34 | 45.25 ± 1.5 | 3 | |||
| Ye (2015)27 | China | Radioiodine | 150 | 68/82 | 42.68 ± 4.16 | 1 | 3 |
| Antithyroid drug | 150 | 70/80 | 41.26 ± 3.32 | 1 | |||
| Huang et al (2009)28 | China | 131I | 280 | 63/217 | 38 ± 7.9 | 5 | 4 |
| Antithyroid drug | 240 | 52/188 | 30.2 ± 8.3 | 5 | |||
| Liu et al (2008)29 | China | 131I | 115 | 21/94 | 36.5 ± 8.1 | 2 | 3 |
| Antithyroid drug | 125 | 22/103 | 33.2 ± 7.3 | 2 | |||
| Chen et al (2005)30 | China | 131I | 40 | 14/26 | 10.8 ± 2.3 | 2 | 4 |
| Antithyroid drug | 40 | 14/26 | 10.7 ± 2.1 | 2 | |||
| Tao (2015)31 | China | Radioiodine | 69 | NR | 56.3 ± 5.6 | 5 | 3 |
| Antithyroid drug | 69 | NR | 56.3 ± 5.6 | 5 | |||
| Xu et al (2006)32 | China | 131I | 120 | 37/83 | 18–75 | 3 | 3 |
| Antithyroid drug | 112 | 27/85 | 15–73 | 3 | |||
| Yang (2009)33 | China | 131I | 100 | NR | 30–84 | 2 | 3 |
| Methimazole | 100 | NR | 30–84 | 2 | |||
| Deng et al (2003)34 | China | 131I | 230 | 43/187 | 36 ± 8 | 2 | 3 |
| Methimazole | 250 | 44/206 | 33 ± 7 | 2 | |||
| Li (2010)35 | China | 131I | 310 | 70/240 | 35 ± 7.2 | 3 | 4 |
| Methimazole | 330 | 72/258 | 32 ± 6.9 | 3 | |||
| Ge (2015)36 | China | 131I | 41 | 13/28 | 32.7 ± 10.5 | 1 | 3 |
| Methimazole | 41 | 15/26 | 33.0 ± 11.2 | 1 | 3 |
L-T4, L-thyroxine; NR, not reported; SD, standard deviation.
Effects of the treatments on ophthalmopathy
12 studies reported the data of ophthalmopathy.20,22,24,25,28–35 New ophthalmopathy developed in 266 out of 1138 patients who received radioiodine treatment compared with 187 out of 1158 patients who received antithyroid drugs. Pooled results showed that radioiodine treatment was associated with increased risk of developing new ophthalmopathy compared with antithyroid drugs (RR = 1.36, 95% CI: 1.04, 1.77; p = 0.024) (Figure 2). The test for heterogeneity was significant (heterogeneity p = 0.005, I2 = 50.2%). Therefore, we conducted a sensitivity analysis to explore the potential source of heterogeneity. When we excluded the trial which involved paediatric patients,30 the results changed slightly (RR = 1.35, 95% CI: 1.02, 1.78; p = 0.033), yet heterogeneity was still present (heterogeneity p = 0.032, I2 = 56.5%). Further, exclusion of any single study at one time changed the overall estimate a little (data not shown), but the heterogeneity did not disappear.
Figure 2.
Comparison of new ophthalmopathy between radioiodine therapy and antithyroid drugs for patients with Graves' disease. CI, confidence interval; RR, risk ratio.
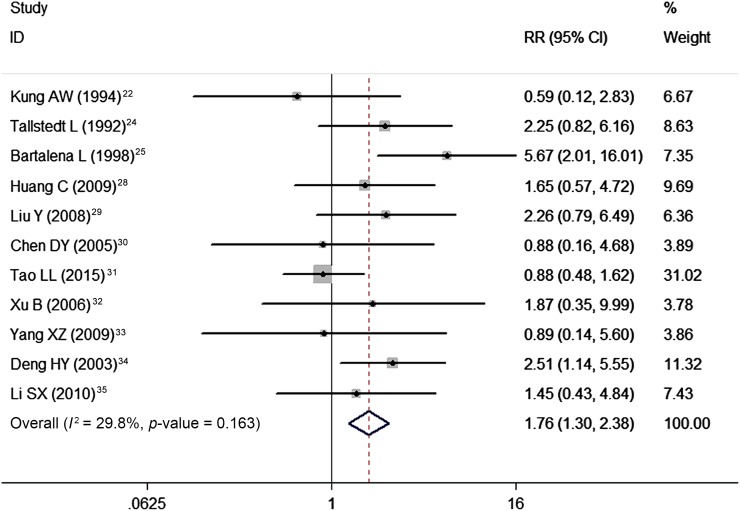
Pre-existing ophthalmopathy developed or worsened in 101 out of 606 patients who were treated with radioiodine compared with 54 out of 557 patients who were treated with antithyroid drugs. Pooling of these data using a fixed-effects model showed that radioiodine treatment was associated with an increased risk of development or worsening of ophthalmopathy compared with antithyroid drugs (RR = 1.76, 95% CI: 1.30, 2.38; p < 0.001) (Figure 3). The test for heterogeneity was not significant (heterogeneity p = 0.163, I2 = 29.8%).
Figure 3.
Comparison of the development or worsening of ophthalmopathy between radioiodine therapy and antithyroid drugs for patients with Graves' disease. CI, confidence interval; RR, risk ratio.
Hyperthyroid cure rate
Nine studies reported the data of hyperthyroid cure rate.22,27–32,34,35 The hyperthyroid cure rate in the radioiodine therapy group and antithyroid drug group was 77.8% and 45.6%, respectively. Meta-analysis of these studies using a random-effects model showed that radioiodine therapy was associated with a significantly higher hyperthyroid cure rate than antithyroid drugs (RR = 1.66, 95% CI: 1.49, 1.86; p < 0.001) (Figure 4). The test for heterogeneity was significant (heterogeneity p = 0.01, I2 = 60.2%).
Figure 4.
Comparison of hyperthyroid cure rate between radioiodine therapy and antithyroid drugs for patients with Graves' disease. CI, confidence interval; RR, risk ratio.
Subsequently, we conducted sensitivity analysis. Exclusion of the trial which involved paediatric patients30 yielded similar results (RR = 1.63, 95% CI: 1.63, 1.86; p < 0.001), with moderate heterogeneity (heterogeneity p = 0.007, I2 = 63.7%). Exclusion of the trial that was conducted before 200022 changed the pooled results slightly (RR = 1.75, 95% CI: 1.64, 1.87; p < 0.001), yet almost no evidence of heterogeneity was observed among the remaining studies (heterogeneity p = 0.255, I2 = 21.9%).
Effects of the treatments on hypothyroidism
10 studies reported the data of hypothyroidism.20,23,25,28–34 The hypothyroidism rate in the radioiodine therapy group and antithyroid drug group was 19.7% and 9.3%, respectively. Meta-analysis of these studies using a random-effects model showed that radioiodine treatment was associated with an increased risk of hypothyroidism compared with antithyroid drugs (RR = 1.99, 95% CI: 1.34, 2.98; p = 0.001) (Figure 5). The test for heterogeneity was significant (heterogeneity p < 0.001, I2 = 71.7%).
Figure 5.
Comparison of hypothyroidism between radioiodine therapy and antithyroid drugs for patients with Graves' disease. CI, confidence interval; RR, risk ratio.
Considering that this meta-analysis was performed on the basis of different sample sizes, we therefore performed a sensitivity analysis to identify whether this result was influenced by this factor. When two trials with a modest sample size (N ≤ 100) were excluded, similar results were obtained (RR = 2.14, 95% CI: 1.33, 3.43; p = 0.002) and the heterogeneity was still present (heterogeneity p < 0.001, I2 = 74.6%).
Effects of the treatments on relapse rate
10 studies reported the data of relapse rate.23,27–35 The relapse rate in the radioiodine therapy group and antithyroid drug group was 6.3% and 35.0%, respectively. The pooled analysis using a random-effects model suggested that radioiodine treatment was associated with a lower relapse rate than antithyroid drugs (RR = 0.16, 95% CI: 0.08, 0.33; p < 0.001) (Figure 6). The test for heterogeneity was significant (heterogeneity p < 0.001, I2 = 89.3%).
Figure 6.
Comparison of the relapse rate between radioiodine therapy and antithyroid drugs for patients with Graves' disease. CI, confidence interval; RR, risk ratio.
Adverse events
Six studies reported the data of adverse events,26,28,29,32–34 including leukopenia, hepatitis vasculitis and rash. Pooled results using a random-effects model showed that radioiodine treatment was associated with a lower incidence of adverse events than antithyroid drugs (RR = 0.22, 95% CI: 0.10, 0.49; p < 0.001).
Publication bias
Assessment of publication bias was conducted by using Egger and Begg's test and the results showed that no publication bias existed among the included studies (Egger's test: t = 1.71, p = 0.122; Begg's test: Z = 0.62, p = 0.533).
DISCUSSION
This is a further meta-analysis to compare the effect of radioiodine therapy with antithyroid drugs in the treatment of Graves' disease. All trials included in this meta-analysis are RCTs. These data can give greater power to assess whether radioiodine treatment is superior to antithyroid drugs in the treatment of Graves' disease. Our results suggest that radioiodine treatment is associated with an increased risk of new ophthalmopathy and development or worsening of ophthalmopathy than antithyroid drugs. In addition, radioiodine treatment seems to have a higher incidence of hypothyroidism, but a lower recurrence rate and adverse events than antithyroid drugs. Patients treated with radioiodine therapy achieved a higher hyperthyroid cure rate than those treated with antithyroid drugs.
There have been two published meta-analyses on the comparison of the effects of radioiodine treatment vs antithyroid drugs for Graves' disease.37,38 Our meta-analysis expands on these two earlier meta-analyses to provide a better evidence for an effective comparison between the two treatments. First, there are an enlarged number of included studies and sample sizes in our analysis, which gives greater power to detect the outcome differences between the two treatments. Whereas, in the network meta-analysis conducted by Ren et al,38 only 4 RCTs (in a total of 1404 patients) were included, and the comparisons on ophthalmopathy were performed based on only 2 RCTs. Similarly, in another meta-analysis conducted by Acharya et al,37 10 RCTs were included and only 2 RCTs compared the 2 treatments. Second, in this meta-analysis, we included not only English publications but also Chinese publications. Whereas, in the two meta-analyses, only English publications were included for data analysis, which would prevent their outcomes from being applied for universally practical use. Third, all of the included studies were well designed and of high quality (Jadad score ranged from 3 to 4), and no publication bias was identified. However, in the two meta-analyses, publication bias was not assessed, since the number of included studies was small (<10). Thus, their final results may be biased by unpublished studies. Furthermore, we also assessed the effects of the two treatments on other clinical outcomes, including hyperthyroid cure rate, incidence of hypothyroidism and adverse events, which were not evaluated in the other two meta-analyses. The enlarged sample size and comprehensive literature search have enhanced our statistical power to provide more precise and reliable effect estimates.
Radioiodine is increasingly considered the treatment of choice because of its low expense, ease of administration and safety.39 However, the major problem of radioiodine therapy lies in thyroid failure; hypothyroidism may develop many years after patients have been rendered euthyroid by radioiodine.40 In the present meta-analysis, we observed that the incidence of hypothyroidism in the radioiodine therapy group was significantly higher than that in the antithyroid drug group. Our results were consistent with a previously published study,23 in which 159 patients with Graves' disease were randomly assigned to receive radioiodine I131 therapy or methimazole plus L-thyroxine for 6 months. The cumulative incidence of hypothyroidism in the patients treated with radioiodine therapy was significantly higher than that in those treated with antithyroid drugs (p = 0.0009).23 Similarly, in another study conducted by Bartalena et al,25 patients who received radioiodine therapy developed a higher incidence of hypothyroidism than those treated with antithyroid drugs at the follow-up of 10–12 months.25
Numerous studies have compared the effects of radioiodine therapy with that of antithyroid drugs on the development or worsening of ophthalmopathy; however, their results remained controversial. In the trial conducted by Kung et al,22 22.8% of patients in the radioiodine therapy group and 24.0% of patients in the antithyroid drug group had development or deterioration of ophthalmopathy during the 2-year follow-up.22 And the difference between them was not significant. However, in another clinical trial, Bartalena et al25 reported that the incidence of development or progression of ophthalmopathy was significantly higher in the radioiodine group (15.3%) than in the methimazole group (2.7%) (p < 0.001).25
In this meta-analysis, our results indicated that radioiodine therapy was associated with increased higher risk of new ophthalmopathy and development or worsening of ophthalmopathy than antithyroid drugs. These results were consistent with that of the previous two meta-analyses. In the meta-analysis conducted by Acharya et al,37 radioiodine therapy significantly increased the incidence of developing or worsening of ophthalmopathy compared with antithyroid drugs (RR = 4.23, 95% CI: 2.04, 8.77). Similarly, the other network meta-analysis by Ren et al38 suggests that treatment with antithyroid drugs significantly decreased the incidence of new or deteriorative ophthalmopathy compared with radioiodine therapy (OR = 2.958, 95% CI: 1.731, 4.735).
With regard to recurrence rate, radioiodine therapy seemed to have the advantage than antithyroid drugs. This conclusion was identified in all of the included studies except one,23 in which the two treatments had a similar effect. In that study, the relapse rate within the first year was similar between the two treatments (38.7% vs 44.5%).23 Frequent recurrence of hyperthyroidism is the major drawback of antithyroid drugs when the therapy is discontinued. Therefore, there is no need to discontinue antithyroid drug therapy unless mild adverse events occur.
This study has several limitations. First, our meta-analysis is based on 17 RCTs. And some of the included studies had a modest sample size, which would result in an overestimation of the treatment effect when compared with trials with larger sample sizes. Second, there is substantial heterogeneity among the included studies, such as patients of different age, gender, smoking status, the duration of hyperthyroidism and thyroid function. These factors contributed to the heterogeneity and might have had potential impacts on our final results. Third, the data analysis for biochemical variables was based on only three studies. Thus, the conclusion on the biochemical changes should be interpreted with caution. Fourth, owing to the limited data that compared the effects between radioiodine and radioiodine plus prednisone, we could not perform an analysis to explore whether radioiodine is more effective than radioiodine plus prednisone. Fifth, the majority of these included studies were from China. Physicians should be cautious before applying these results to other countries, since both the demographics and clinical practices in those countries may differ significantly.
In summary, our study suggests that radioiodine therapy is associated with a higher hyperthyroid cure rate and lower relapse rate than antithyroid drugs. However, it also increases the risk of ophthalmopathy and hypothyroidism. Overall, we consider that antithyroid drug treatment can be associated with unsatisfactory control of hyperthyroidism and frequent reappearance of hyperthyroidism after the discontinuation of treatment; we would recommend radioiodine therapy as the treatment of choice for patients with Graves' disease. Further well-designed, large-scale RCTs are needed to verify our findings.
Contributor Information
Junqi Wang, Email: wangjunqi_2016@163.com.
Lan Qin, Email: qinlan_ql@163.com.
REFERENCES
- 1.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev 2003; 2: 119–25. doi: 10.1016/S1568-9972(03)00006-5 [DOI] [PubMed] [Google Scholar]
- 2.Abraham-Nordling M, Bystrom K, Torring O, Lantz M, Berg G, Calissendorff J, et al. Incidence of hyperthyroidism in Sweden. Eur J Endocrinol 2011; 165: 899–905. doi: 10.1530/EJE-11-0548 [DOI] [PubMed] [Google Scholar]
- 3.Menconi F, Marcocci C, Marino M. Diagnosis and classification of Graves' disease. Autoimmun Rev 2014; 13: 398–402. doi: 10.1016/j.autrev.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 4.Wartofsky L, Glinoer D, Solomon B, Nagataki S, Lagasse R, Nagayama Y, et al. Differences and similarities in the diagnosis and treatment of Graves' disease in Europe, Japan, and the United States. Thyroid 1991; 1: 129–35. doi: 10.1089/thy.1991.1.129 [DOI] [PubMed] [Google Scholar]
- 5.Solomon B, Glinoer D, Lagasse R, Wartofsky L. Current trends in the management of Graves' disease. J Clin Endocrinol Metab 1990; 70: 1518–24. doi: 10.1210/jcem-70-6-1518 [DOI] [PubMed] [Google Scholar]
- 6.Prummel MF, Wiersinga WM, Mourits MP, Koornneef L, Berghout A, van der Gaag R. Effect of abnormal thyroid function on the severity of Graves' ophthalmopathy. Arch Intern Med 1990; 150: 1098–101. doi: 10.1001/archinte.1990.00390170124027 [DOI] [PubMed] [Google Scholar]
- 7.Weetman AP, McGregor AM, Hall R. Evidence for an effect of antithyroid drugs on the natural history of Graves' disease. Clin Endocrinol 1984; 21: 163–72. doi: 10.1111/j.1365-2265.1984.tb03456.x [DOI] [PubMed] [Google Scholar]
- 8.Pucilowska JB, Davenport ML, Kabir I, Clemmons DR, Thissen JP, Butler T, et al. The effect of dietary protein supplementation on insulin-like growth factors (IGFs) and IGF-binding proteins in children with shigellosis. J Clin Endocrinol Metab 1993; 77: 1516–21. doi: 10.1210/jcem.77.6.7505287 [DOI] [PubMed] [Google Scholar]
- 9.Allannic H, Fauchet R, Orgiazzi J, Madec AM, Genetet B, Lorcy Y, et al. Antithyroid drugs and Graves' disease: a prospective randomized evaluation of the efficacy of treatment duration. J Clin Endocrinol Metab 1990; 70: 675–9. doi: 10.1210/jcem-70-3-675 [DOI] [PubMed] [Google Scholar]
- 10.Vitti P, Rago T, Chiovato L, Pallini S, Santini F, Fiore E, et al. Clinical features of patients with Graves' disease undergoing remission after antithyroid drug treatment. Thyroid 1997; 7: 369–75. doi: 10.1089/thy.1997.7.369 [DOI] [PubMed] [Google Scholar]
- 11.Wartofsky L. Has the use of antithyroid drugs for Graves' disease become obsolete? Thyroid 1993; 3: 335–44. doi: 10.1089/thy.1993.3.335 [DOI] [PubMed] [Google Scholar]
- 12.Reynolds LR, Kotchen TA. Antithyroid drugs and radioactive iodine. Fifteen years' experience with Graves' disease. Arch Intern Med 1979; 139: 651–3. doi: 10.1001/archinte.1979.03630430031011 [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. doi: 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 14.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001; 135: 982–9. doi: 10.7326/0003-4819-135-11-200112040-00010 [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22: 719–48. [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham-Nordling M, Wallin G, Traisk F, Berg G, Calissendorff J, Hallengren B, et al. Thyroid-associated ophthalmopathy; quality of life follow-up of patients randomized to treatment with antithyroid drugs or radioiodine. Eur J Endocrinol 2010; 163: 651–7. doi: 10.1530/EJE-10-0475 [DOI] [PubMed] [Google Scholar]
- 21.Azizi F, Ataie L, Hedayati M, Mehrabi Y, Sheikholeslami F. Effect of long-term continuous methimazole treatment of hyperthyroidism: comparison with radioiodine. Eur J Endocrinol 2005; 152: 695–701. doi: 10.1530/eje.1.01904 [DOI] [PubMed] [Google Scholar]
- 22.Kung AW, Yau CC, Cheng AC. The incidence of ophthalmopathy after radioiodine therapy for Graves' disease: prognostic factors and the role of methimazole. J Clin Endocrinol Metab 1994; 79: 542–6. doi: 10.1210/jcem.79.2.7913934 [DOI] [PubMed] [Google Scholar]
- 23.Kung AW, Yau CC, Cheng AC. The action of methimazole and L-thyroxine in radioiodine therapy: a prospective study on the incidence of hypothyroidism. Thyroid 1995; 5: 7–12. doi: 10.1089/thy.1995.5.7 [DOI] [PubMed] [Google Scholar]
- 24.Tallstedt L, Lundell G, Torring O, Wallin G, Ljunggren JG, Blomgren H, et al. Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. The thyroid study group. N Engl J Med 1992; 326: 1733–8. doi: 10.1056/NEJM199206253262603 [DOI] [PubMed] [Google Scholar]
- 25.Bartalena L, Marcocci C, Bogazzi F, Manetti L, Tanda ML, Dell'Unto E, et al. Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J Med 1998; 338: 73–8. doi: 10.1056/NEJM199801083380201 [DOI] [PubMed] [Google Scholar]
- 26.Zhao W. Effect comparison of tapazole versus radioiodine therapy in the treatment of patients with Graves hyperthyroidism. World Latest Med Inform 2015; 15: 102–3. [Google Scholar]
- 27.Ye H. Treatment analysis of I131 and ATD in 300 patients with Graves' disease. China Med Eng 2015; 123: 163–4. [Google Scholar]
- 28.Huang C, Li W, Hu B, Cao X, Wang J. The curative effects of 131I and ATD for patients with Graves hyperthyroidism. Shaanxi Med J 2009; 38: 676–7. [Google Scholar]
- 29.Liu Y, Fu L, Wei Y, Chang Y, Huang C. Curative effect evaluation of 131I and ATD therapy for patients with Graves' hyperthyroidism. J Henan Univ Sci Technol 2008; 26: 265–6. [Google Scholar]
- 30.Chen D, Chen T. Comparison of efficacy of 131I and antithyroid drugs in the treatment of Graves' disease in children. [In Chinese.] Chin J Pediatr 2005; 43: 507–9. [PubMed] [Google Scholar]
- 31.Tao L. Comparison of efficacy of 131I and antithyroid drugs in the treatment of Graves' disease. Chin J Mod Drug Appl 2015; 9: 151–2. [Google Scholar]
- 32.Xu B, Huang H, Qi J. Comparative study on the therapeutic effect of 131I and anti-thyroidism drugs on hyperthyroidism. China Trop Med 2006; 6: 2041–2. [Google Scholar]
- 33.Yang X. Therapeutic effect comparison of 131I and anti-thyroid drugs on patients with hyperthyroidism. China Pharmacist 2009; 12: 1283–4. [Google Scholar]
- 34.Deng H, Liang C, Xiao M, Li X. Comparison of 131I ATD and surgical treatment in the Graves hyperthyroidism patients. China J Mod Med 2003; 13: 24–7. [Google Scholar]
- 35.Li S. Comparison between the curative effects of 131I and anti-thyroid drugs on hyperthyroidism. J Mil Surgeon 2010; 12: 1051–2. [Google Scholar]
- 36.Ge Z. Effects comparison between the curative effects of 131I and anti-thyroid drugs on hyperthyroidism. Jilin Med J 2015; 36: 2605. [Google Scholar]
- 37.Acharya SH, Avenell A, Philip S, Burr J, Bevan JS, Abraham P. Radioiodine therapy (RAI) for Graves' disease (GD) and the effect on ophthalmopathy: a systematic review. Clin Endocrinol 2008; 69: 943–50. doi: 10.1111/j.1365-2265.2008.03279.x [DOI] [PubMed] [Google Scholar]
- 38.Ren Z, Qin L, Wang JQ, Li Y, Li J, Zhang RG. Comparative efficacy of four treatments in patients with Graves' disease: a network meta-analysis. Exp Clin Endocrinol Diabetes 2015; 123: 317–22. doi: 10.1055/s-0035-1548824 [DOI] [PubMed] [Google Scholar]
- 39.Becker DV. Choice of therapy for Graves' hyperthyroidism. N Engl J Med 1984; 311: 464–6. doi: 10.1056/NEJM198408163110710 [DOI] [PubMed] [Google Scholar]
- 40.Sridama V, McCormick M, Kaplan EL, Fauchet R, DeGroot LJ. Long-term follow-up study of compensated low-dose 131I therapy for Graves' disease. N Engl J Med 1984; 311: 426–32. doi: 10.1056/NEJM198408163110702 [DOI] [PubMed] [Google Scholar]