Abstract
Objective:
To report a novel approach for craniospinal irradiation (CSI) using a supine isocentric technique.
Methods:
Patients were treated in the supine position using CT simulation. Half-beam-blocked lateral cranial fields and superior spinal fields have the same isocentre, and their beam divergences match. Tangential irradiation provides a non-divergent junction for the other two full-beam spinal fields. Shielding for cranial fields was generated, and dose distribution was calculated using a three-dimensional planning system. When sacral spinal fields were required, two lateral opposite fields were designed to protect the urogenital organs. All treatment portals were filmed once per week.
Results:
At a median follow-up of 49.8 months, 5 relapses and no cases of radiation myelitis developed in 26 consecutive patients. In the junctions of the brain–spine or spine–spine field, no failure occurred. Three failures occurred in the primary site alone, two in the spinal axis alone.
Conclusion:
The results of our study have shown that our novel approach for CSI was not associated with increased failures at the field junction and deaths. In addition, no radiation myelitis, pneumonia, severe damage to the heart and gastrointestinal tract, and second cancers occurred in our study.
Advances in knowledge:
This new approach is an optimal alternative in cancer centre without tomotherapy because of its convenience for immobilization, repeatability, optimal dose distribution and satisfactory clinical outcome.
INTRODUCTION
When the clinical target volume (CTV) includes the entire central nervous system (CNS) subarachnoid space, craniospinal irradiation (CSI) is indicated and commonly used. Traditionally, CSI is delivered with the patient in the prone position, using lateral opposed fields covering the whole brain and upper cervical spine matched to a direct posterior field that extends inferiorly to cover the caudal extent of the thecal sac with a source–skin distance (SSD) set-up.1 Most patients who require CSI are young children who have recently undergone surgery and require anaesthesia for immobilization and prone positioning; this causes patient discomfort and reduces the accuracy of the treatment. Furthermore, to avoid over- or underdosing, weekly shifts of the cranial–spinal and spinal–spinal field junctions are required, which is complicated and time consuming.
Several CSI techniques employing the supine position have been reported in recent years,2–6 but numerous issues must be considered. One relates to SSD irradiation and the need for more parameters, another concerns limitations to the plan when the length of the spinal field exceeds the maximal length of the collimator jaw (40 cm) and a third involves couch rotation, which adds to the complexity of the treatment. Mostly importantly, problems with cranial–spinal matching and the spinal field junction can easily lead to overdose. However, these concerns can be eliminated with the use of our novel CSI approach involving immobilization in the supine position, CT simulation, source–axis distance (SAD) irradiation instead of traditional SSD irradiation and the combination of half- and full-beam irradiation. Our clinical experience indicates that this new approach is better than traditional treatment because of its convenience for immobilization, repeatability and optimal dose distribution and may be recommended for clinical application in the treatment of CNS cancer.
METHODS AND MATERIALS
The equipment used for this novel technique includes a linear accelerator with an independent collimator and asymmetric jaws that can open to provide a half-beam field, CT simulator, treatment planning system, record-and-verify system, plastic mask and vacuum bag.
Patient position and immobilization
Simulation is conducted in the supine position using a CT simulator. The patient's head and neck are immobilized with a custom thermoplastic mask. Maximum neck extension is needed to avoid inclusion of the mandible in the exit of the posterior field used to treat the spine. A slice thickness of 3 mm extending from the vertex to the inferior limit of the S4 vertebral body is used.
Treatment field definition and virtual simulation
The entire brain and spine are carefully contoured on every CT slice to define the CTV. Post-operative diagnostic MRI studies are performed to contour the target volume for the boost treatment that is normally given after the completion of CSI.
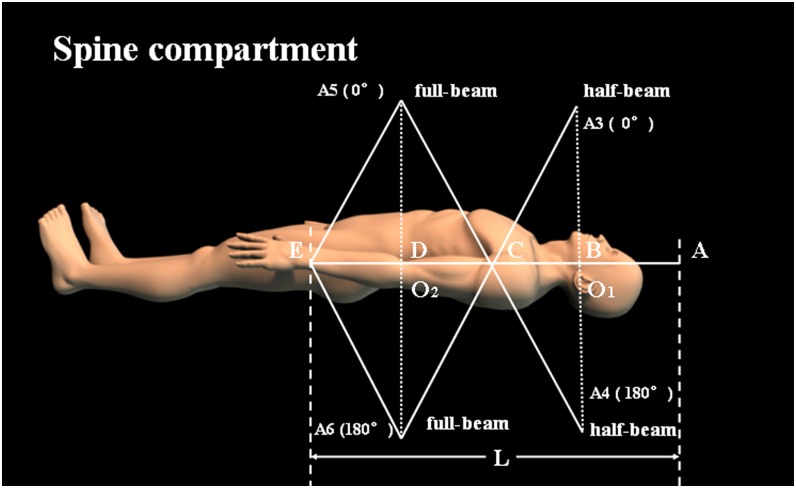
The following two planes and one line were set up as follows: (1) an isocentre sagittal plane, comprising the midline of the skull (using the nasal septum as reference) and the vertebral process line; (2) an isocentre coronal plane with optimal dose distribution; and (3) an isocentre line at the vertical intersection of the isocentre sagittal plane and isocentre coronal plane (Figure 1).
Figure 1.
Set-up of points, lines and planes with the new approach.
The reference isocentre O1 for the brain fields is defined at the isocentre line at the level of the C2 vertebral body (Figure 2). The distance from 1 cm above the top of skull to O1 is defined as L1 (≤20 cm). The spinal field in the isocentre line is first divided into three parts, each equal to L1 in length: L2 = L3 = L4 = L1. If another part, L5, is required, this is located from the posterior border of L4 to the inferior limit of the S4 vertebral body. The isocentre of the superior spinal field is also O1, and the isocentre of the inferior spinal field, O2, is always located at a point 2L1 distal to O1 (Figure 3). Special attention is required to verify that the intersection point C of points O1 and O2 should be within 5 or so centimetres from the centre of the cord but not on the cord, to avoid over- or underdosing. If L5 is not needed, six parallel opposed fields can be set up: A1 and A2 for the brain field with the half-beam technique, A3 and A4 for the spinal field with the half beam, and A5 and A6 for the spinal field with the full beam. The gantry needs to be rotated to 90° for the A1 field and 270° for the A2 field, with O1 as their isocentre, whereas the gantry rotation angles are 0° and 180° for A3 and A4 fields, respectively. These four half-blocked fields at the same isocentre provide a non-divergent junction, obviating the need for any couch rotations. For the A5 and A6 fields, the gantry rotation angles are 0° and 180°. The isocentre of the two full-beam parallel fields is located at O2. Tangential irradiation not only provides a non-divergent junction of the inferior edge of the thoracic spinal field and the superior edge of the lumber spinal field but also achieves the desired dose distribution. If L5 is required, A7 and A8 fields for the sacrum are produced with the half beam, with gantry rotation angles of 90° and 270°, respectively. The collimator is rotated to match the divergence from the inferior spinal field. To protect the urogenital organs, blocks or a multileaf collimator is necessary. Meanwhile, the A7 and A8 fields are blocked to avoid overlap with the A5 field. The length of the sacral spinal field is adjusted with the asymmetric jaws to cover the caudal extent of the thecal sac. This approach is illustrated for an adult patient on a sagittal CT reconstruction in Figure 4.
Figure 2.
Geometry of the supine craniospinal irradiation of the brain compartment in the new approach.
Figure 3.
Geometry of the supine craniospinal irradiation of the spinal compartment using the new approach.
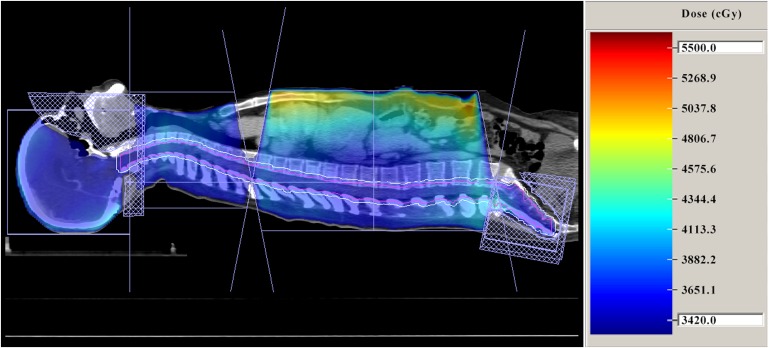
Figure 4.
Sagittal CT reconstruction of a patient treated in the supine position with the craniospinal irradiation technique and its dose distribution.
Using an autoblocking function in the virtual simulation software, shielding for the cranial fields and sacral spinal field are generated automatically. Taking into account patient set-up error, patient motion and dose distribution accuracy, the shielding is designed such that there is a 10-mm margin between the CTV and the blocks.
Treatment
The plan data are transferred to a three-dimensional treatment planning system and the optimal dose distributions calculated. Patients are treated with 6- to 8-MV photons produced by a linear accelerator, and radiotherapy is administered at a dose of 100–200 cGy per day (median fraction, 180 cGy) for a total of 2400–3600 cGy to the craniospinal axis, followed by an appropriate boost dose delivered to certain cranial regions according the patient's diagnosis. The dose distribution in the sagittal plane is shown in Figure 4. Because all of the fields are at a common SAD and there are non-divergent junctions, rotation, lateral or vertical movement of the couch is never required.
Portal imaging and quality assurance
All fields are imaged once per week and portal images are compared with the simulation digitally reconstructed radiographs. Before each patient begins radiotherapy, portal verification films of each isocentre are obtained and evaluated by the physician and therapist. The superior film is placed sagittal to the brain fields and the superior spinal fields at the level of the cranial field's isocentre to confirm the geometric and mechanical accuracy. For other spinal fields, a film must be obtained to verify beam-matching depths.
RESULTS
A total of 26 patients with newly diagnosed, biopsy-proven medulloblastoma, germ-cell tumour, ependymoma or teratoma from the brain were treated with our novel approach for CSI at the Sun Yat-Sen University Cancer Center (South China), Guangzhou, China, between June 2004 and December 2009; all of these patients were retrospectively reviewed. Of the 26 patients, 21 (80.8%) were male and 5 (19.2%) were female (male : female ratio 4.2 : 1). The median age was 13.5 years (range, 4–39 years). The diagnosis included germ-cell tumour in 11 (42.3%), medulloblastoma in 10 (38.5%), teratoma in 1 (3.8%) and ependymoma in 4 (15.4%) patients. 26 (100%) patients received surgery and 17 (65.4%) patients received chemotherapy. The clinical characteristics of 26 patients are demonstrated in the Supplementary material.
Target volume coverage and dose homogeneity were assessed as the volume of the brain or spinal cord PTV receiving at least 95% (V95%) and 107% (V107%) of the prescribed dose, respectively.7,8 The mean V95% and V107% of the brain of 26 patients in our study were 98.2% and 0.4%, respectively. Furthermore, the mean V95% and V107% of the spinal cord of 26 patients were 98.4% and 24.1%, respectively.
With a median follow-up of 49.8 months, five local failures and deaths were documented. No cases of radiation myelitis, pneumonia, severe damage to the heart and gastrointestinal tract, and second cancers had occurred. The characteristics of failure in five patients with relapse after supine isocentric CSI are presented in Table 1. No failures occurred in the junctions of the spine–spine or brain–spine fields. Three failures occurred in the primary site alone, two in the spinal axis alone.
Table 1.
The characteristics of failure in five patients with relapse after supine isocentric craniospinal irradiation (CSI)
| Patient number | Diagnosis | Treatment after surgery | Site and time of failure |
|---|---|---|---|
| 1 | Medulloblastoma | CSI 30 Gy, tumour bed 46 Gy plus chemotherapy | Primary site failure at 20 months |
| 2 | Medulloblastoma | CSI 30 Gy, tumour bed 55 Gy plus chemotherapy | T5–T6 spinal leptomeningeal metastasis at 24 months |
| 3 | Medulloblastoma | CSI 30 Gy, tumour bed 46.5 Gy plus chemotherapy | Primary site failure at 10 months |
| 4 | Ependymoma | CSI 30 Gy, tumour bed 60 Gy plus chemotherapy | Primary site progression at 3 months |
| 5 | Teratoma | CSI 30 Gy, tumour bed 60 Gy plus chemotherapy | T12–L1 spinal leptomeningeal metastasis at 18 months |
DISCUSSION
CSI is needed in patients with cancers who carry a risk of leptomeningeal spread, including medulloblastoma, high-grade posterior fossa ependymoma, germinomatous germ-cell tumour, CNS lymphoma and other CNS tumours that metastasize.5 Because most patients who require CSI are children, the timing of simulation and treatment is important. CT simulation has drastically decreased the time required for simulation and increased the accuracy of coverage of the target volume.4 Furthermore, the supine technique is better tolerated, more comfortable and more stable than the prone technique, which reduces the treatment time and improves the accuracy of treatment.2,3 Not only involving immobilization in the supine position and CT simulation as the traditional techniques,1–3 our approach also includes SAD irradiation and the combination of half- and full-beam irradiation which saves time and minimizes the risk of over- or underdosing at the junctions.
With the introduction of the SAD technique for all fields, the isocentre line can be quickly identified from the intersection of the isocentre sagittal plane and the isocentre coronal plane, which makes it unnecessary to calculate gantry angles using antitag methods as in the traditional approach. The SAD technique avoids field alterations, with the consequence that the dose distribution is barely affected. This is not a trivial point for young patients who have recently undergone surgery and are often in discomfort. Verification of the isocentre points O1 and O2 simplifies the portal imaging and quality assurance procedure. One of the important concerns with this technique is the long-term survival status and adverse events of patients. Our data are similar to the results of Michael South's study, which means it is quite a safe and effective technique.6 The problem of junctioning non-coplanar fields over cranial and spinal fields is crucial, and numerous different solutions have been proposed. In contrast to table or gantry and collimator rotation to match adjacent fields of the brain and spinal compartments, our approach using the half-beam technique and the same isocentre point O1 is a practical solution to the problem that effectively eliminates over- and underdosed spots at the junction. When the mandible is vertical to the table, the length from 1 cm above the top of skull to the C2 vertebra hardly ever exceeds 20 cm; therefore, the half-beam method is practical for the cranial field. The isosceles triangle method was used to solve the problem with spinal–spinal field matching, and a desirable dose distribution could be obtained with the use of complementary lateral opposite fields. When the length of the spinal field exceeds 4L1, two lateral opposite fields for the sacral spine are added to protect the urogenital organs from irradiation. The problem of shifting the junction point by about 0.5–1.0 cm every 10 Gy does not exist in our approach, although the clinical significance of the weekly shift is uncertain according to Tinkler et al.9 Although our approach is based on CT simulation, the same concepts can be applied to conventional simulation.
Helical tomotherapy (HT) represents both an innovative RT approach and a novel treatment device that merges a linear accelerator designed for IMRT with elements of a helical megavoltage CT (MV-CT) scanner, which is a highly conformal radiation technique.7,10 HT is widely used in CSI which does not need match-line junction shifts just as our approach. Furthermore, HT showed excellent target coverage and good dose homogeneity compared with other conformal radiotherapy.10 Thus, our approach may be an optimal option for cancer centres without HT.
However, there are limitations to our approach. The main disadvantage is that the part of the abdomen containing the small intestine might be irradiated due to the anterior fields in our technique, although no distinct toxicity of gastrointestinal organs such as enteritis has been observed during treatment or on follow-up. The reason might be that the total dose delivered to the intestine in the craniospinal axis is less than the TD5/5 of the intestine, and the irradiated volume of other normal tissues is limited. Another weakness is that the blocks for the A7 and A8 fields may slide in the collimator, as it is rotated to match the divergence from the inferior spinal field. The multileaf collimator is too wide (up to 1 cm), and the conformal degree is unsatisfactory to protect the urogenital organs. Furthermore, considering that this study used a relatively small number of patients with different underlying diagnosis, further studies with larger sample and an increased power are warranted to confirm the results.
CONCLUSION
This novel approach for CSI offers convenient simulation, reproducibility and an optimal dose distribution and is a reliable and convenient alternative in cancer centres without tomotherapy.
FUNDING
This study was supported by grants from the General Funds of the National Natural Science Foundation of China (no. 81272575), the Science and Technology Planning Project of Guangdong Province, China (nos. 2010B080701015 and 2012A030400038) and Guangdong Provicial Medical Scientific Research fund (grant no. A2016286).
Contributor Information
Yi-Kan Cheng, Email: chengyk3@mail.sysu.edu.cn.
Lei Zeng, Email: 232404116@qq.com.
Shu-Biao Ye, Email: yeshb@sysucc.org.cn.
Jian Zheng, Email: yingjuxchzh@163.com.
Lin Zhang, Email: zhanglin@sysucc.org.cn.
Peng Sun, Email: sunpeng@sysucc.org.cn.
Xiao-Bo Jiang, Email: jiangxiaob@sysucc.org.cn.
Wen-Zhao Sun, Email: sunwzh@sysucc.org.cn.
Tao Xu, Email: asian.you@163.com.
Lei Chen, Email: 296288274@qq.com.
REFERENCES
- 1.Morgan J, Navrozov M. Central nervous system, In: Bentel GC. ed. Patient positioning and immobilization in radiation oncology. 1st edn. New York, St Louis: McGraw-Hill; 1998. pp. 71–91. [Google Scholar]
- 2.Hawkins RB. A simple method of radiation treatment of craniospinal fields with patient supine. Int J Radiat Oncol Biol Phys 2001; 49: 261–4. doi: 10.1016/S0360-3016(00)01367-5 [DOI] [PubMed] [Google Scholar]
- 3.Michalski JM, Klein EE, Gerber R. Method to plan, administer, and verify supine craniospinal irradiation. J Appl Clin Med Phys 2002; 3: 310–6. doi: 10.1120/1.1508013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker WA, Freeman CR. A simple technique for craniospinal radiotherapy in the supine position. Radiother Oncol 2006; 78: 217–22. doi: 10.1016/j.radonc.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 5.Munshi A, Jalali R. A simple technique of supine craniospinal irradiation. Med Dosim 2008; 33: 1–5. doi: 10.1016/j.meddos.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 6.South M, Chiu JK, Teh BS, Bloch C, Schroeder TM, Paulino AC. Supine craniospinal irradiation using intrafractional junction shifts and field-in-field dose shaping: early experience at Methodist Hospital. Int J Radiat Oncol Biol Phys 2008; 71: 477–83. doi: 10.1016/j.ijrobp.2007.10.029 [DOI] [PubMed] [Google Scholar]
- 7.Sharma DS, Gupta T, Jalali R, Master Z, Phurailatpam RD, Sarin R. High-precision radiotherapy for craniospinal irradiation: evaluation of three-dimensional conformal radiotherapy, intensity-modulated radiation therapy and helical tomotherapy. Br J Radiol 2009; 82: 1000–9. doi: 10.1259/bjr/13776022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seppälä J, Kulmala J, Lindholm P, Minn H. A method to improve target dose homogeneity of craniospinal irradiation using dynamic split field IMRT. Radiother Oncol 2010; 96: 209–15. doi: 10.1016/j.radonc.2010.05.018 [DOI] [PubMed] [Google Scholar]
- 9.Tinkler SD, Lucraft HH. Are moving junctions in craniospinal irradiation for medulloblastoma really necessary? Br J Radiol 1995; 68: 736–9. doi: 10.1259/0007-1285-68-811-736 [DOI] [PubMed] [Google Scholar]
- 10.Mascarin M, Giugliano FM, Coassin E, Drigo A, Chiovati P, Dassie A, et al. Helical tomotherapy in children and adolescents: dosimetric comparisons, opportunities and issues. Cancers (Basel) 2011; 3: 3972–90. doi: 10.3390/cancers3043972 [DOI] [PMC free article] [PubMed] [Google Scholar]