Abstract
Vertebral fracture (VF) is a common condition with >160,000 patients affected every year in North America and most of them with affected lumbar vertebrae. The management of VF is well known and defined by many protocols related to associated clinical neurological symptoms, especially in case of the presence or absence of myelopathy or radicular deficit. In this article, we will explore the percutaneous stabilization of the lumbar spine by showing the newest approaches for this condition.
INTRODUCTION
Vertebral fracture (VF) can be secondary to high- or low-energy trauma. Low-energy trauma occurs more frequently in females, with an increased prevalence related with the ages, whereas high-energy traumas are more frequent in males and are not related with older age.1 Pathological VF is secondary to osseous involvement by a localized debilitating condition, mainly tumours. Most are due to malignancies such as myeloma and primary bone tumours. The spine is the most affected target by metastases.1 Anatomically and functionally, the thoracic and lumbar spine can be divided into three regions—thoracic spine (T1–T10), thoracolumbar junction (T10–L2) and the lumbar spine (L3–L5).2
The narrow spinal canal in this region predisposes to spinal cord damage resulting in a high incidence of neurological deficit. The relatively lesser incidence of neurological injury in lumbar fractures can be attributed to the large size of the neural canal.3 In North America, the incidence of spinal injuries is >160,000 every year.4 Among the thoracolumbar injuries, 50–60% affected the transitional zone (T11–L2), 25–40% affected the thoracic spine and 10–14% involved the lower lumbar spine and sacrum.5 Neurological injury complicates 20–36% of fractures at the thoracolumbar junction in different studies.6–8
Conventional radiographs with anteroposterior and lateral radiographs are usually the first technique used to study patients suspected for VF. Radiographic evaluation should include spinal alignment, the presence of any rotation or translation, assessment of the kyphosis, loss of vertebral height and widened interpedicular or interspinous distance.9–11 CT scan provides further information on the extent of bony injury, and MRI scan shows injury to the spinal cord and soft-tissue structures.3
The classification of thoracolumbar fractures has evolved over the years. In 1929, Jones12 had described thoracolumbar fractures classification based only on radiographs. In 1970, Holdsworth had introduced the “two column concept”.13 Denis et al,14 in 1976, proposed the “three column concept. The most recent classification system is the AOSpine Knowledge Forum classification of thoracolumbar trauma. Based on the AO thoracic and lumbar fracture classification, fracture dislocation injuries could be B1.2, B2.3, B3.3 and C types.6,15,16
It is possible to classify vertebral traumatic fractures as stable or unstable related and in the past years several classifications have been suggested: the most commonly used is the Magerl's classification that considers trauma in compression, rotation and distraction injuries.
Magerl A1 type is considered a main indication for percutaneous vertebroplasty (PVP), percutaneous balloon kyphoplasty (BKP) or vertebral augmentation (VA). However, it is important to underline that these subjects can be treated also with orthosis devices, bed rest as well as medical and/or physical therapy.
Anatomy and physiopathology
The spine (also known as the vertebral column or spinal column) is a structure with 26 bones in an adult body: 24 separate vertebrae interspaced with cartilage and, then additionally, the sacrum and coccyx. Prior to adult age, the spine has 33 bones because the sacrum and coccyx bones do not fuse together until adolescence.
In each vertebra, there are the following parts: the body, vertebral foramen, spinous process and transverse process. The vertebral body is the main part of the vertebra.
A fundamental part in the spinal column is the intervertebral disc that is made of two different parts: the annulus fibrosus and the nucleus pulposus. The annulus fibrosus is made of tough fibrocartilage, whereas the nucleus pulposus absorbs the body's weight.
A VF occurs after a trauma that is usually quite big, but in some cases, VFs can occur in people with some pathologies such as osteoporosis or cancer. These conditions reduce the bone strength of the spine and can break them with little or no force. Generally, the most commonly fractured vertebrae are those in the lower back.17,18
Three major types of VF are considered: osteoporosis, trauma and pathological fracture. The first one usually occurs in females who have gone through menopause or in elderly people under steroid medication. In this condition, the normal bone density is lost and the resulting abnormally porous bone is more compressible, and bone fractures are at increased risk due to the weakened skeletal bones. When affected by osteoporosis, the bones can fracture with relatively minor injury that would normally not cause a bone to fracture.19,20 Another cause of fracture is the trauma; an injury that is severe enough to cause vertebrae to fracture can occur as a result of many incidents. VF can occur in people who have been involved in car accidents.
The third category is the so-called pathological fracture that refers to a VF that occurs because of some preexisting disease at the site of fracture such as metastasis, bone infection, osteomyelitis or diabetes. Pathological fractures are usually caused by cancer in the bone (primary or metastasis), especially by prostate, breasts or lungs.21
Percutaneous stabilization method 1: vertebroplasty
The first case of percutaneous vertebral procedure (PVP) was described in France, by Deramond and Galibert in 1984 who injected polymethylmethacrylate (PMMA) bone cement at the C2 level in a patient with an aggressive haemangioma with relief of pain.22 This technique became popular among interventional radiologists for rapid pain relief.23 In 1988, PVP was widely used in primary and secondary painful osteoporotic vertebral fractures around the world. The application of PVP in VF was first published in 1989,24 and the first case series was published in 1997. However, PVP is not effective in restoring vertebral height.
According to the guidelines published in 2003 by the Society of Interventional Radiology (SIR), common indications for PVP include osteoporotic VF of more than 2 weeks and refractory by medical therapy, painful vertebra with extensive osteolysis or invasion secondary to benign or malignant tumour.25 Any patient with improvement of symptoms with conservative treatment, asymptomatic VF, the presence of osteoblastic metastasis, tumour mass with spinal canal involvement, pregnancy, uncorrectable coagulopathy, severe cardiorespiratory disease, cement allergy, and systemic and especially local infection cannot be eligible. Injection of PMMA cement into an injured vertebral body can be performed using a needle that is placed percutaneously using a transpedicular approach.
High-quality fluoroscopy equipment is essential but a hybrid technique using both fluoroscopy and CT has been described by some authors.26,27 PVP was carried out under local anesthesia. Needles used in PVP procedures are standard bone marrow biopsy-type needles. For lumbar levels, 10- or 11-gauge needles are used, but for the thoracic levels, 13- or 14-gauge needles are preferred.28 The transpedicular approach is most often used in the lumbar spine and the extrapedicular approach in the thoracic spine.29 The standard bipediculate approach has been used to fill each vertebral half separately.
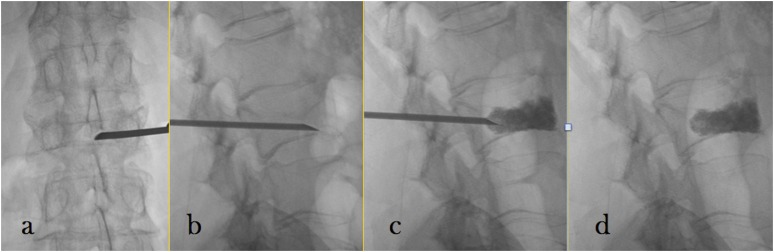
The patient is placed in the prone position, and using fluoroscopic or CT guidance, a needle was placed into the anterior third of the damaged vertebral body. In order to facilitate filling of the fracture area, typically, anterosuperior route is selected and this also allows to avoid injection into the larger vascular sinuses of the basivertebral venous plexus.30 The injection of PMMA has to be forced to surpass the local pressure of the trabecular bone of the treated vertebra, so the bone cement is transferred under pressure. During injection of the cement, continuous observation is necessary to prevent excessive bone cement leakage (Figure 1).
Figure 1.
Vertebroplasty: the steps of percutaneous vertebroplasty: introduction of the needle in anteroposterior (a) and laterolateral (b) view, injection of polymethylmethacrylate under continuous fluoroscopic guidance (c) and final result (d).
PVP and BKP are associated with some potential peri-operative and post-operative adverse events: cement leakage, pulmonary embolism, haematoma, adjacent vertebral fracture, spinal cord compression, radiculopathy and infection.31–36 Cement leakage constitutes the most common complication of these minimally invasive procedures (27–75%). Fortunately, most of the cement extravasation phenomena are clinically asymptomatic.37–39 Cement may leak into the intervertebral disc space (most common), anterior paravertebral area, throughout the needle tract, venous system, intervertebral foramen or even epidural space (spinal canal).39–41 Pulmonary embolisms occur, with rates ranging from 0.6% (for PVP) to 0.01% (for BKP).42
Walker et al43 reported osteomyelitis a rare complication, which requires corpectomy. Adjacent-level vertebral fracture is a major post-operative complication of PVP or BKP. In a comparative study on adjacent-level vertebral fractures after VA procedures, Movrin et al44 found that the rate of adjacent level fractures widely varied for both PVP (8–52%).
In the recent years, surgeons showed an increasing interest for the use of a synthetic bone substitute capable of remodelling or integrating into the surrounding bone; in particular, calcium phosphate cement. This is expected to work as a carrier for osteoinductive proteins.45–49 Recent studies showed that few new-generation bioactive bone cements have been found to induce new bone formation and also have good mechanical stability.50,51 Bioactive composite materials prepared from acrylic cement in conjunction with ceramics showed good radio-opacity.52 Cortoss, a composed material-based bone cement currently undergoing clinical trials for PVP and BKP is composed of terpolymer resin with combeite glass-ceramics.
Percutaneous stabilization method 2: balloon kyphoplasty
BKP technique was proposed some years ago. It was introduced in 1990s with the aim of stabilizing the vertebral fracture and restoring the vertebral height as close as possible to the pre-fracture level and minimizing the associated kyphotic deformity.53 The term “Kyphoplasty®” was coined by Kyphon, the company that markets the balloons, to emphasize that correcting kyphosis is among the main objectives of the procedure. BKP is a slightly more complicated technique then PVP and is involved in the introduction of an inflatable bone tamp into the compressed vertebral body with the aim of elevate the end plates. This is carried out by creating a cavity inside the vertebral body that is filled with cement. PMMA is the most frequent bone cement used in these procedures. BKP has been suggested in the treatment of osteoporotic VF, in the vertebral collapse fractures caused by malignancies or in traumatic VF in young patient. The initial experience of BKP for use in patients with myeloma with multiple fractures has been favourable. In 18 patients who underwent 55 kevel of BKP, there were no major complications at a mean follow-up time of 7.4 months.54 Posterolateral approach is preferred; an alternative approach could be the transpedicular route.55 A 10-gauge cannula is inserted into the vertebral body under fluoroscopic or CT guidance. The inner cannula is removed, and a trocar is inserted through the cannula and is used to create a path into the vertebral body.
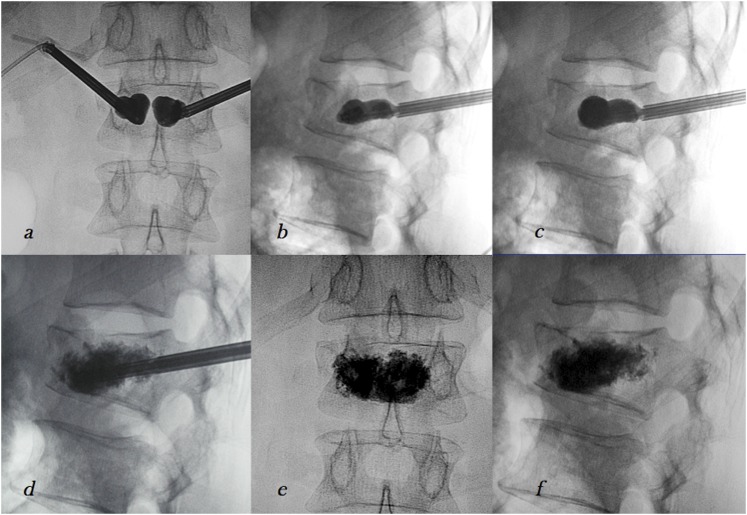
Then, the trocar is removed and a balloon is inserted into the vertebral body via the tube, under fluoroscopic guidance. An appropriate position of the inflatable balloon is identified placing distal and proximal radiographic markers. The balloon is inflated under manometric control to restore the collapsed end plate to its normal position and to create a cavity within the vertebra. The contralateral balloon is placed before inflation to allow symmetric elevation of the vertebral end plates (Figure 2). The balloon is filled with radiographic contrast medium to evaluate the size of balloon expansion. When the height is restored through an en masse reduction, the balloon is deflated and removed. The bone cement is injected into the cavity under a low-pressure delivery system, thus reducing the risk of cement leakage. The rate of neurological complications with BKP as reported by Hulme et al56 was 0.03%. In BKP, rupture of the bone tamp also rarely occurs and usually does not carry any adverse effect.57 Phillips et al58 reported significantly less contrast extravasation-related complications in BKP than PVP [odds ratio (95% confidence interval): 0.04 (0.00–0.68) p = 0.03]. In a comparative study on an adjacent-level VF after VA procedures, Movrin et al44 found that the rate of adjacent level fractures widely varied for BKP (3–29%). Limited BPK literature is available compared with PVP.
Figure 2.
Balloon kyphoplasty: inflation of the balloons in anteroposterior (a) and laterolateral view (b, c), injection of the bone cement after deflation and delivery of the balloons (d) and final result (e, f).
In 2003, Ledlie et al59 concluded that BKP was efficacious in restoring vertebral body height in 26 patients (41 fractures) when monitored for 1 year after procedure. In 2009, a multicentre study assessed the effectiveness and safety of BKP and followed up patients up to 1 year post-operatively. The authors concluded that BKP was effective and safe in treating acute VF.60
In 2005, Masala61 treated 11 patients with VF occurred up to 3 months earlier and due to osteoporosis or myeloma, and the authors found that BKP proved to be a safe and effective method for the treatment of intractable pain due to VF.
In a study recently published, Niu et al62 have concluded that BKP is an effective treatment strategy for osteoporotic VF.
Percutaneous stabilization method 3: vertebral augmentation systems
As an alternative to BKP, VA mechanical devices were introduced in the past few years,63–71 in order to reach the best long-term vertebral height restoration. In fact, clinical experience has demonstrated a potential limitation of BKP with loss of restored height after balloon deflation due to vertebral elastic recoil.72,73
Thanks to the capacity of VA to restore the physiological vertebral height, this treatment is considered a good indication in patients affected by Magerl A1 fractures. Also, subjects having Magerl lesions A2 and A3 type can also be considered for this treatment in selected cases (polytrauma with comorbidities, cases which the surgical and anaesthesiologist risk is too high or in elderly people not suitable for surgical intervention and in all).
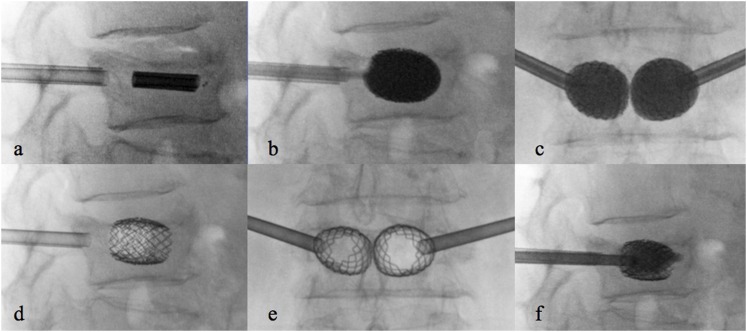
To the best of our knowledge, four VA systems were described in literature: the VerteLift® (Spine Align Medical Inc., San Jose, CA)64 (Figure 3), a nitinol cage; the OsseoFix® (Alphatec Spine Inc., Carlsbad, CA)69–71 (Figure 4), a titanium self expandable mesh cage; the Vertebral Body Stenting® (Synthes, Soletta, Switzerland) (Figure 5),66,67 a titanium balloon-expandable stent; and the Spine Jack® (Vexim, Balma, France),74 a titanium endovertebral jack (Figure 6). Procedures can be performed in an operating room equipped with a C arm or in an angiography room. Usually, local anaesthesia64 or deep sedation (fentanest and propofol) is used, but some surgeons described general anaesthesia.63
Figure 3.
VerteLift® (Spine Align Medical Inc., San Jose, CA) device: final position of the implant (a, b) and after injection of polymethylmethacrylate (c, d).
Figure 4.
OsseoFix® (Alphatec Spine Inc., Carlsbad, CA) device: introduction (a), expansion (b), and delivery (c) of the implantand final result after injection of the bone cement (d, e).
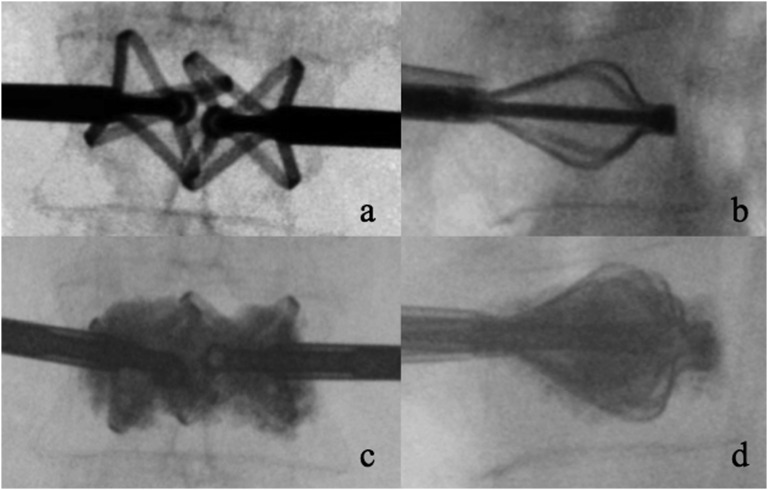
Figure 5.
Steps of the vertebral body stenting device: insertion of the device (a), inflation of the balloons (b, c), deflation of the balloons and delivery of the two stents (d, e) and injection of polymethylmethacrylate (f).
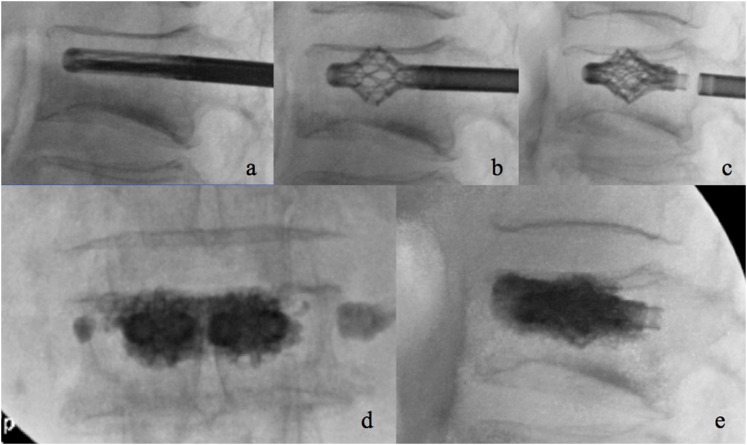
Figure 6.
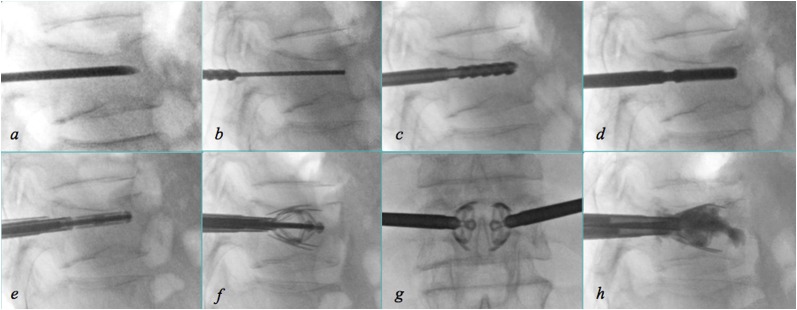
Steps of the Spine Jack device: introduction of the trocar needle (a) followed by the Kirshner wire (b), the reamer (c) and the template (d); once the contralateral approach has been performed, introduction of the implants (e), their expansion in laterolateral (f) and anteroposterior (g) view and injection of polymethylmethacrylate (h).
All these implants require the same bipedicular approach; access cannulae are inserted using the oblique projection and then advanced in the anteroposterior projection to the medial aspect of the pedicle; in the anteroposterior view, the medial margin of the pedicle is an absolute anatomical landmark to check before to pass over the posterior wall of the vertebral body in laterolateral view.
The delivery pathway for the VerteLift is obtained with trocars and a manual drill through the transpedicular cannulae. It is important to underline that the bone tissue obtained during drilling can be used for histological examination. Once the implants are properly positioned and expanded, the delivery system is detached. Injection cannulae are pre-filled with PMMA. When the cement reaches the optimal viscosity (“toothpaste consistency”) the cement injection is performed. For each level, an average value of 5 ml are injected.
The OsseoFix mesh cage implant requires two Kirshner wires inserted into the access cannulae, followed by cannulated drilling of the pathway into the anterior third of the vertebral body. An implant delivery system helps to insert the two titanium mesh cages. A mechanical actuation system is used to deploy the expandable cages in a controlled manner. The size of the cage has been previously selected according to pre-operative planning from pre-operative CT scans. Then, cement injection could be performed by means of dedicated fillers, but some authors described the implant of the mesh cages without PMMA injection.69
The vertebral body stenting device represents an evolution of BKP, having a balloon expandable titanium stent that can be delivered with balloon inflation.63,66,67 Through the working cannula, a metallic drill can be used to model the trabecular bone such as other osteotomy cannula to lead the insertion of the metallic implant without problems. The cement can be injected through a slow injection system such as bone filler or through a 1-ml syringe that lead to inject a quite high viscosity cement, with less either disk and venous leakage.
The concept of the Spine Jack implant is different from the other VA systems; the craniocaudal expansion of the device (instead of spherical, such as the other devices) allows forces to be applied only on the vertebral end plates, and in that way, height restoration can be obtained without retropulsion of bone fragments; moreover, the reduction of the bone fragments is also possible due to ligamentotaxis. Following the positioning of the trocar needles, a Kirshner wire is then inserted; a manual cannulated reamer is then used to do the pathway for the template. The procedure is then repeated through the contralateral pedicle unless both devices are inserted into the vertebral body. Owing to the craniocaudal expansion of the laminas, the optimal position of the devices is parallel to the end plates. The implants can be expanded and deployed with manual rotation of a multifunctional handle. Once the implants are delivered, the bone cement can be injected through dedicated bone fillers under fluoroscopic guidance to avoid leakages.
Evidence from literature and suggested guidelines
In the past few years, several articles have been authored to assess if VA is really effective in pain relief in patients with osteoporotic fractures and what is the best management of osteoporotic VF between non-surgical management, PVP and BKP. Taylor et al36 performed an analysis of the literature, assessed 69 articles and found pain relief is 87% with an improvement of functions.
Wardlaw et al60 published in The Lancet, an article that assessed 300 patients with 1 to 3 acute VFs in a randomized controlled trial at 21 sites in 8 countries. 149 subjects received BKP treatment, whereas 151 subjects received non-surgical care. The authors found that BKP is an effective and safe procedure for patients with acute VF and will help to inform decisions regarding its use as an early treatment option. In particular, patients in the BKP group reported a better quality of life improvement than conservative medical care at 1 month and 12 months. Patients in the BKP group reported pain relief and analgesic drug reduction superior to the control group.
In another article, Boonen et al73 assessed the effect of BKP at 24 months; 120 patients underwent BKP, whereas 112 were treated with conservative care. The authors found that patients who were treated with BKP experienced and maintained a better quality of life improvement than patients with conservative care. It is interesting to observe that the new fracture rate difference between the BKP group (47.5%) and the conservative care group (44.1%) is not statistically significant. This is probably related to the pathology (osteoporosis) and not to the procedure.
The effects of the PVP were assessed in different articles published in extremely prestigious journals.74–76 The first randomized controlled trials published in The New England Journal of Medicine in 200975,76 demonstrated that PVP did not show statistically significant difference against placebo for the attributes of pain relief and quality of life. This, expectedly, stimulated a wide array of criticism and review. There were a number of reactions for these critiques. Those authors who criticized these studies outlined a number of faults with the design including (but not limited to) the limited number of patients, high refusal rate, insufficient amount of PMMA injection, inappropriate sham procedure and others. The topic was further complicated when The Lancet published a radio-chemo-therapy study of its own that demonstrated the superiority of PVP over conservative management;74 in this study, the authors identified 431 patients who fulfilled the inclusion criteria: 50 years or older; the presence of VF on spine radiograph (minimum 15% height loss, level of fracture T5 or lower, and bone oedema on MRI); back pain of 6 weeks or less; and a visual analogue score of 5 or greater.
The restoration of the vertebral height is also associated with pain relief. The mechanisms underlying pain relief following VA remain still debated but are likely multifactorial, owing at least in part to restoration of vertebral body height, but perhaps also to other mechanisms. In particular, reduced vertebral body compliance at microfracture sites, or such as in case of PVP or BPK direct neurotoxic effects of the PMMA could occur during cement polymerization.77
The conflicting evidence has sparked ongoing debate in the medical community. All sides have provided arguments supported by evidence of varying strength and validity, and the evidences presented by proponents of both sides of the debate appear to have validity. In a recent published article from the Everest Group,65 where >4000 patients were studied, the authors found that 88.0% of the patients reported significant pain relief within 48 h and that 13% of the subjects were retreated for a subsequent fracture. It is interesting to observe that best results are obtained in the treatment of myeloma and trauma. The authors concluded that PVP is an effective and safe procedure in the treatment of VF and that best results are obtained in the treatment of myeloma and trauma.
However, these articles for or against VA procedures have significant limitations in their validity and, for this reason, the debate cannot be concluded, until more elaborate studies are conducted involving larger numbers of patients with clear procedure methods agreed upon by the major authorities in the field.78
BKP and PVP are well-established minimally invasive procedures for the treatment of VF/compression, but other approaches have also been suggested. By considering the other approaches, in an article recently published by Spine,64 the authors assessed 40 patients, with a follow-up of 15 months, with one or more painful osteoporotic VF using VA with nitinol endoprosthesis. The authors found that this technique can be considered a safe and effective procedure. Hartmann et al66 assessed the value of the vertebral body stenting in a very small population (n = 18), and therefore this could be considered a preliminary study, but the results are similar to BKP.
Another study71 published in 2013 assessed the implantation of an expandable titanium mesh cage (OsseoFix) with a 12 month follow-up are presented. The authors assessed 24 patients with 32 osteoporotic VFs. The authors found significant pain relief. Moreover, the mean kyphotic angle showed significant improvements after 12 months.
According to the reviewed literature, we suggest to adopt the following guidelines that were proposed by Anselmetti et al77 in a recently published article in Pain Physician and that can be summarized as follows:
Non-surgical management
Non-surgical management is indicated in case of negative MRI or in case of positive MRI without other unfavourable conditions and is non-indicated when there is proof of ongoing fracture process or ≥ other unfavourable conditions.
Vertebroplasty
Vertebroplasty is indicated in case of positive MRI with time since fracture or 6 weeks and over and no spinal deformity and is non-indicated where there is negative MRI or spinal deformity.
Other vertebral augumentation procedures
Other vertebral augumentation procedures indicated in case of ongoing fracture process with positive MRI and 1 and more other unfavourable factors and is non-indicated where there is negative MRI with time since fracture ≥3 months or when there is a negative MRI with time since fracture ≥6 weeks–3 months and moderate impact of symptoms.
CONCLUSION
PVP and BKP both are effective in VA and pain relief in patients with osteoporotic or tumour-associated VF. On the basis of systematic reviews of available literature, based on indirect comparisons, very few differences in terms of clinical outcomes of these two procedures are present. Both procedures are minimally invasive, and therefore represent a relatively low-cost alternative to open surgical interventions for VF. Both procedures give immediate pain relief and improvement in physical functioning, although the effect is not long term. Based on the current literature, we can conclude that PVP and BKP may be considered in patients who sustain an acute VF and who do not adequately improve after a reasonable course of non-surgical management.
Contributor Information
Stefano Marcia, Email: stemarcia@gmail.com.
Luca Saba, Email: lucasaba@tiscali.it.
Mariangela Marras, Email: mariangela.marrasmd@gmail.com.
Jasjit S Suri, Email: jsuri@comcast.net.
Eros Calabria, Email: eros.calabria@gmail.com.
Salvatore Masala, Email: salva.masala@tiscali.it.
REFERENCES
- 1.Ruiz Santiago F, Santiago Chinchilla A, Guzmán Álvarez L, Pérez Abela AL, Castellano García Mdel M, Pajares López M. Comparative review of vertebroplasty and kyphoplasty. World J Radiol 2014; 6: 329–43. doi: 10.4329/wjr.v6.i6.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood KB, Li W, Lebl DR, Ploumis A. Management of thoracolumbar spine fractures. Spine J 2014; 14: 145–64. doi: 10.1016/j.spinee.2012.10.041 [DOI] [PubMed] [Google Scholar]
- 3.Rajasekaran S, Kanna RM, Shetty AP. Management of thoracolumbar spine trauma: an overview. Indian J Orthop 2015; 49: 72–82. doi: 10.4103/0019-5413.143914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.el-Khoury GY, Whitten CG. Trauma to the upper thoracic spine: anatomy, biomechanics, and unique imaging features. AJR Am J Roentgenol 1993; 160: 95–102. doi: 10.2214/ajr.160.1.8416656 [DOI] [PubMed] [Google Scholar]
- 5.Gertzbein SD. Scoliosis Research Society. Multicenter spine fracture study. Spine (Phila Pa 1976) 1992; 17: 528–40. [DOI] [PubMed] [Google Scholar]
- 6.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J 1994; 3: 184–201. doi: 10.1007/BF02221591 [DOI] [PubMed] [Google Scholar]
- 7.Kraemer WJ, Schemitsch EH, Lever J, McBroom RJ, McKee MD, Waddell JP. Functional outcome of thoracolumbar burst fractures without neurological deficit. J Orthop Trauma 1996; 10: 541–4. doi: 10.1097/00005131-199611000-00006 [DOI] [PubMed] [Google Scholar]
- 8.Knop C, Blauth M, Bühren V, Hax PM, Kinzl L, Mutschler W, et al. Surgical treatment of injuries of the thoracolumbar transition. 1: epidemiology. [In German.] Unfallchirurg 1999; 102: 924–35. [DOI] [PubMed] [Google Scholar]
- 9.Keene JS. Radiographic evaluation of thoracolumbar fractures. Clin Orthop Relat Res 1984; 189: 58–64. [PubMed] [Google Scholar]
- 10.Harris JH, Jr. Radiographic evaluation of spinal trauma. Orthop Clin North Am 1986; 17: 75–86. [PubMed] [Google Scholar]
- 11.Dalinka MK, Kessler H, Weiss M. The radiographic evaluation of spinal trauma. Emerg Med Clin North Am 1985; 3: 475–90. [PubMed] [Google Scholar]
- 12.Jones RW. The results of postural reduction of fractures of the spine. J Bone Joint Surg Am 1938; 20: 567–86. [Google Scholar]
- 13.Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am 1970; 52: 1534–51. [PubMed] [Google Scholar]
- 14.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976) 1983; 8: 817–31. [DOI] [PubMed] [Google Scholar]
- 15.Denis F. Spinal instability as defined by the three-column spine concept in acute spinal trauma. Clin Orthop Relat Res 1984: 65–76. [PubMed] [Google Scholar]
- 16.Liu J, Shan Z, Zhao F. The anatomy of failure in lumbar disc herniation. Spine (Phila Pa 1976) 2015; 40: 1053. doi: 10.1097/BRS.0000000000000948 [DOI] [PubMed] [Google Scholar]
- 17.Davey T, Lanham-New SA, Shaw AM, Cobley R, Allsopp AJ, Hajjawi MO, et al. Fundamental differences in axial and appendicular bone density in stress fractured and uninjured Royal Marine recruits—a matched case-control study. Bone 2015; 73: 120–6. doi: 10.1016/j.bone.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 18.Changstrom BG, Brou L, Khodaee M, Braund C, Comstock RD. Epidemiology of stress fracture injuries among US high school athletes, 2005–2006 through 2012–2013. Am J Sports Med 2015; 43: 26–33. doi: 10.1177/0363546514562739 [DOI] [PubMed] [Google Scholar]
- 19.Polat SB, Evranos B, Aydin C, Cuhaci N, Ersoy R, Cakir B. Effective treatment of severe pregnancy and lactation-related osteoporosis with teriparatide: case report and review of the literature. Gynecol Endocrinol 2015; 31: 522–5. doi: 10.3109/09513590.2015.1014787 [DOI] [PubMed] [Google Scholar]
- 20.Bousson V, Bergot C, Sutter B, Thomas T, Bendavid S, Benhamou CL, et al. Trabecular bone score: where are we now? Joint Bone Spine 2015; 82: 320–5. doi: 10.1016/j.jbspin.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 21.Maccauro G, Muratori E, Sgambato A, Liuzza F, Esposito M, Grieco A, et al. Bone metastasis in hepatocellular carcinoma. A report of five cases and a review of the literature. Chir Organi Mov 2005; 90: 297–302. [PubMed] [Google Scholar]
- 22.Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. [In French.] Neurochirurgie 1987; 33: 166–8. [PubMed] [Google Scholar]
- 23.Patel AA, Vaccaro AR, Martyak GG, Harrop JS, Albert TJ, Ludwig SC, et al. Neurologic deficit following percutaneous vertebral stabilization. Spine (Phila Pa 1976) 2007; 32: 1728–34. doi: 10.1097/BRS.0b013e3180dc9c36 [DOI] [PubMed] [Google Scholar]
- 24.Amar AP, Larsen DW, Esnaashari N, Albuquerque FC, Lavine SD, Teitelbaum GP. Percutaneous transpedicular polymethylmethacrylate vertebroplasty for the treatment of spinal compression fractures. Neurosurgery 2001; 49: 1105–14; discussion 1114–15. [PubMed] [Google Scholar]
- 25.Baerlocher MO, Saad WE, Dariushnia S, Barr JD, McGraw JK, Nikolic B; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous vertebroplasty. J Vasc Interv Radiol 2014; 25: 165–70. doi: 10.1016/j.jvir.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 26.Gangi A, Kastler BA, Dietemann JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR Am J Neuroradiol 1994; 15: 83–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez L, Pérez-Higueras A, Quiñones D, Calvo E, Rossi RE. Vertebroplasty in the treatment of vertebral tumors: postprocedural outcome and quality of life. Eur Spine J 2003; 12: 356–60. doi: 10.1007/s00586-003-0525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masala S, Magrini A, Taglieri A, Nano G, Chiaravalloti A, Calabria E, et al. Chronic obstructive pulmonary disease (COPD) patients with osteoporotic vertebral compression fractures (OVCFs): improvement of pulmonary function after percutaneous vertebroplasty (VTP). Eur Radiol 2014; 24: 1577–85. doi: 10.1007/s00330-014-3165-2 [DOI] [PubMed] [Google Scholar]
- 29.Elnoamany H. Percutaneous vertebroplasty: a first line treatment in traumatic non-osteoporotic vertebral compression fractures. Asian Spine J 2015; 9: 178–84. doi: 10.4184/asj.2015.9.2.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrino JA, Chan R, Vaccaro AR. Vertebral augmentation: vertebroplasty and kyphoplasty. Semin Roentgenol 2004; 39: 68–84. doi: 10.1016/j.ro.2003.10.012 [DOI] [PubMed] [Google Scholar]
- 31.Kallmes DF, Schweickert PA, Marx WF, Jensen ME. Vertebroplasty in the mid- and upper thoracic spine. AJNR Am J Neuroradiol 2002; 23: 1117–20. [PMC free article] [PubMed] [Google Scholar]
- 32.Martin JB, Jean B, Sugiu K, San Millán Ruíz D, Piotin M, Murphy K, et al. Vertebroplasty: clinical experience and follow-up results. Bone 1999; 25(Suppl. 2): 11S–15S. doi: 10.1016/S8756-3282(99)00126-X [DOI] [PubMed] [Google Scholar]
- 33.Hochmuth K, Proschek D, Schwarz W, Mack M, Kurth AA, Vogl TJ. Percutaneous vertebroplasty in the therapy of osteoporotic vertebral compression fractures: a critical review. Eur Radiol 2006; 16: 998–1004. [DOI] [PubMed] [Google Scholar]
- 34.Masala S, Anselmetti GC, Marcia S, Nano G, Taglieri A, Calabria E, et al. Treatment of painful Modic type I changes by vertebral augmentation with bioactive resorbable bone cement. Neuroradiology 2014; 56: 637–45. doi: 10.1007/s00234-014-1372-9 [DOI] [PubMed] [Google Scholar]
- 35.Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J 2008; 8: 488–97. doi: 10.1016/j.spinee.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 36.Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J 2007; 16: 1085–100. doi: 10.1007/s00586-007-0308-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgy BA. Clinical experience with high-viscosity cements for percutaneous vertebral body augmentation: occurrence, degree, and location of cement leakage compared with kyphoplasty. AJNR Am J Neuroradiol 2010; 31: 504–8. doi: 10.3174/ajnr.A1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Maida GA, Giarratana LS, Acerbi A, Ferrari V, Mineo GV, Misaggi B. Cement leakage: safety of minimally invasive surgical techniques in the treatment of multiple myeloma vertebral lesions. Eur Spine J 2012; (21 Suppl. 1): S61–8. doi: 10.1007/s00586-012-2221-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieuwenhuijse MJ, van Erkel AR, Dijkstra PD. Cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: identification of risk factors. Spine J 2011; 11: 839–48. doi: 10.1016/j.spinee.2011.07.027 [DOI] [PubMed] [Google Scholar]
- 40.Kao FC, Tu YK, Lai PL, Yu SW, Yen CY, Chou MC. Inferior vena cava syndrome following percutaneous vertebroplasty with polymethylmethacrylate. Spine (Phila Pa 1976) 2008; 33: E329–33. doi: 10.1097/BRS.0b013e31816f6a10 [DOI] [PubMed] [Google Scholar]
- 41.Harrington KD. Major neurological complications following percutaneous vertebroplasty with polymethylmethacrylate: a case report. J Bone Joint Surg Am 2001; 83-A: 1070–3. [DOI] [PubMed] [Google Scholar]
- 42.François K, Taeymans Y, Poffyn B, Van Nooten G. Successful management of a large pulmonary cement embolus after percutaneous vertebroplasty: a case report. Spine (Phila Pa 1976) 2003; 28: E424–5. [DOI] [PubMed] [Google Scholar]
- 43.Walker DH, Mummaneni P, Rodts GE, Jr. Infected vertebroplasty. Report of two cases and review of the literature. Neurosurg Focus 2004; 17: E6. doi: 10.3171/foc.2004.17.6.6 [DOI] [PubMed] [Google Scholar]
- 44.Movrin I, Vengust R, Komadina R. Adjacent vertebral fractures after percutaneous vertebral augmentation of osteoporotic vertebral compression fracture: a comparison of balloon kyphoplasty and vertebroplasty. Arch Orthop Trauma Surg 2010; 130: 1157–66. doi: 10.1007/s00402-010-1106-3 [DOI] [PubMed] [Google Scholar]
- 45.Verlaan JJ, Oner FC, Slootweg PJ, Verbout AJ, Dhert WJ. Histologic changes after vertebroplasty. J Bone Joint Surg Am 2004; 86-A: 1230–8. [DOI] [PubMed] [Google Scholar]
- 46.Fulmer MT, Ison IC, Hankermayer CR, Constantz BR, Ross J. Measurements of the solubilities and dissolution rates of several hydroxyapatites. Biomaterials 2002; 23: 751–5. [DOI] [PubMed] [Google Scholar]
- 47.Friedman CD, Costantino PD, Takagi S, Chow LC. BoneSource hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res 1998; 43: 428–32. doi: [DOI] [PubMed] [Google Scholar]
- 48.Chow LC, Hirayama S, Takagi S, Parry E. Diametral tensile strength and compressive strength of a calcium phosphate cement: effect of applied pressure. J Biomed Mater Res 2000; 53: 511–7. doi: [DOI] [PubMed] [Google Scholar]
- 49.Seeherman HJ, Bouxsein M, Kim H, Li R, Li XJ, Aiolova M, et al. Recombinant human bone morphogenetic protein-2 delivered in an injectable calcium phosphate paste accelerates osteotomy-site healing in a nonhuman primate model. J Bone Joint Surg Am 2004; 86-A: 1961–72. [DOI] [PubMed] [Google Scholar]
- 50.Marcia S, Boi C, Dragani M, Marini S, Marras M, Piras E, et al. Effectiveness of a bone substitute (CERAMENT™) as an alternative to PMMA in percutaneous vertebroplasty: 1-year follow-up on clinical outcome. Eur Spine J 2012; 21 Suppl. 1: S112–8. doi: 10.1007/s00586-012-2228-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masala S, Nano G, Marcia S, Muto M, Fucci FP, Simonetti G. Osteoporotic vertebral compression fractures augmentation by injectable partly resorbable ceramic bone substitute (Cerament™|SPINE SUPPORT): a prospective nonrandomized study. Neuroradiology 2012; 54: 589–96. doi: 10.1007/s00234-011-0940-5 [DOI] [PubMed] [Google Scholar]
- 52.Yimin Y, Zhiwei R, Wei M, Jha R. Current status of percutaneous vertebroplasty and percutaneous kyphoplasty—a review. Med Sci Monit 2013; 19: 826–36. doi: 10.12659/MSM.889479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belkoff SM, Mathis JM, Deramond H, Jasper LE. An ex vivo biomechanical evaluation of a hydroxyapatite cement for use with kyphoplasty. AJNR Am J Neuroradiol 2001; 22: 1212–6. [PMC free article] [PubMed] [Google Scholar]
- 54.Dudeney S, Lieberman IH, Reinhardt MK, Hussein M. Kyphoplasty in the treatment of osteolytic vertebral compression fractures as a result of multiple myeloma. J Clin Oncol 2002; 20: 2382–7. doi: 10.1200/JCO.2002.09.097 [DOI] [PubMed] [Google Scholar]
- 55.Hardouin P, Fayada P, Leclet H, Chopin D. Kyphoplasty. Joint Bone Spine 2002; 69: 256–61. [DOI] [PubMed] [Google Scholar]
- 56.Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine (Phila Pa 1976) 2006; 31: 1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b [DOI] [PubMed] [Google Scholar]
- 57.Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 2001; 26: 1631–8. doi: 10.1097/00007632-200107150-00026 [DOI] [PubMed] [Google Scholar]
- 58.Phillips FM, Todd Wetzel F, Lieberman I, Campbell-Hupp M. An in vivo comparison of the potential for extravertebral cement leak after vertebroplasty and kyphoplasty. Spine (Phila Pa 1976) 2002; 27: 2173–78; discussion 2178–9. doi: 10.1097/00007632-200210010-00018 [DOI] [PubMed] [Google Scholar]
- 59.Ledlie JT, Renfro M. Balloon kyphoplasty: one-year outcomes in vertebral body height restoration, chronic pain, and activity levels. J Neurosurg 2003; 98(Suppl. 1): 36–42. [DOI] [PubMed] [Google Scholar]
- 60.Wardlaw D, Cummings SR, Van Meirhaeghe J, Bastian L, Tillman JB, Ranstam J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009; 373: 1016–24. doi: 10.1016/S0140-6736(09)60010-6 [DOI] [PubMed] [Google Scholar]
- 61.Masala S, Fiori R, Massari F, Simonetti G. Kyphoplasty: indications, contraindications and technique. Radiol Med 2005; 110: 97–105. [PubMed] [Google Scholar]
- 62.Niu J, Song D, Zhou H, Meng Q, Meng B, Yang H. Percutaneous kyphoplasty for the treatment of osteoporotic vertebral fractures with intravertebral fluid or air: a comparative study. J Spinal Disord Tech 2015; 21: 535–7. doi: 10.1097/BSD.0000000000000262 [DOI] [PubMed] [Google Scholar]
- 63.Muto M, Marcia S, Guarnieri G, Pereira V. Assisted techniques for vertebral cementoplasty: why should we do it? Eur J Radiol 2015; 84: 783–8. doi: 10.1016/j.ejrad.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 64.Anselmetti GC, Manca A, Marcia S, Chiara G, Marini S, Baroud G, et al. Vertebral augmentation with nitinol endoprosthesis: clinical experience in 40 patients with 1-year follow-up. Cardiovasc Intervent Radiol 2014; 37: 193–202. doi: 10.1007/s00270-013-0623-1 [DOI] [PubMed] [Google Scholar]
- 65.Anselmetti GC, Marcia S, Saba L, Muto M, Bonaldi G, Carpeggiani P, et al. Percutaneous vertebroplasty: multi-centric results from EVEREST experience in large cohort of patients. Eur J Radiol 2012; 81: 4083–6. doi: 10.1016/j.ejrad.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 66.Hartmann F, Griese M, Dietz SO, Kuhn S, Rommens PM, Gercek E. Two-year results of vertebral body stenting for the treatment of traumatic incomplete burst fractures. Minim Invasive Ther Allied Technol 2015; 24: 161–6. doi: 10.3109/13645706.2014.962546 [DOI] [PubMed] [Google Scholar]
- 67.Disch AC, Schmoelz W. Cement augmentation in a thoracolumbar fracture model: reduction and stability after balloon kyphoplasty versus vertebral body stenting. Spine (Phila Pa 1976) 2014; 39: E1147–53. doi: 10.1097/BRS.0000000000000470 [DOI] [PubMed] [Google Scholar]
- 68.Krüger A, Oberkircher L, Figiel J, Floßdorf F, Bolzinger F, Noriega DC, et al. Height restoration of osteoporotic vertebral compression fractures using different intravertebral reduction devices: a cadaveric study. Spine J 2015; 15: 1092–8. doi: 10.1016/j.spinee.2013.06.094 [DOI] [PubMed] [Google Scholar]
- 69.Eschler A, Ender SA, Ulmar B, Herlyn P, Mittlmeier T, Gradl G. Cementless fixation of osteoporotic VCFs using titanium mesh implants (OsseoFix): preliminary results. Biomed Res Int 2014; 2014: 853897. doi: 10.1155/2014/853897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ender SA, Gradl G, Ender M, Langner S, Merk HR, Kayser R. Osseofix® system for percutaneous stabilization of osteoporotic and tumorous vertebral compression fractures—clinical and radiological results after 12 months. Rofo 2014; 186: 380–7. doi: 10.1055/s-0033-1355504 [DOI] [PubMed] [Google Scholar]
- 71.Ender SA, Wetterau E, Ender M, Kühn JP, Merk HR, Kayser R. Percutaneous stabilization system Osseofix® for treatment of osteoporotic vertebral compression fractures—clinical and radiological results after 12 months. PLoS One 2013; 8: e65119. doi: 10.1371/journal.pone.0065119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verlaan JJ, van de Kraats EB, Oner FC, van Walsum T, Niessen WJ, Dhert WJ. Bone displacement and the role of longitudinal ligaments during balloon vertebroplasty in traumatic thoracolumbar fractures. Spine (Phila Pa 1976) 2005; 30: 1832–9. doi: 10.1097/01.brs.0000173897.67839.92 [DOI] [PubMed] [Google Scholar]
- 73.Boonen S, Van Meirhaeghe J, Bastian L, Cummings SR, Ranstam J, Tillman JB, et al. Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res 2011; 26: 1627–37. doi: 10.1002/jbmr.364 [DOI] [PubMed] [Google Scholar]
- 74.Klazen CA, Lohle PN, de Vries J, Jansen FH, Tielbeek AV, Blonk MC, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet 2010; 376: 1085–92. doi: 10.1016/S0140-6736(10)60954-3 [DOI] [PubMed] [Google Scholar]
- 75.Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361: 569–79. doi: 10.1056/NEJMoa0900563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361: 557–68. doi: 10.1056/NEJMoa0900429 [DOI] [PubMed] [Google Scholar]
- 77.Anselmetti GC, Bernard J, Blattert T, Court C, Fagan D, Fransen H. Criteria for the appropriate treatment of osteoporotic vertebral compression fractures. Pain Physician 2013; 16: E519–30. [PubMed] [Google Scholar]
- 78.Hussain A, Erdek M. Vertebroplasty augmentation procedures: examining the controversy. Pain Physician 2013; 16: E483–90. [PubMed] [Google Scholar]