Abstract
Objective:
To determine the reproducibility of the quantitative chemical shift-based water–fat separation method with a multiecho gradient echo sequence [iteraterative decomposition of water and fat with echo asymmetry and least-squares estimation quantitation sequence (IDEAL-IQ)] for assessing bone marrow fat fraction (FF); to evaluate variation of FF at different bone sites; and to investigate its association with age and menopause.
Methods:
31 consecutive females who underwent pelvic iterative decomposition of water and fat with echo asymmetry and least-squares estimation at 3-T MRI were included in this study. Quantitative FF using IDEAL-IQ of four bone sites were analyzed. The coefficients of variance (CV) on each site were evaluated repeatedly 10 times to assess the reproducibility. Correlations between FF and age were evaluated on each site, and the FFs between pre- and post-menopausal groups were compared.
Results:
The CV in the quantification of marrow FF ranged from 0.69% to 1.70%. A statistically significant correlation was established between the FF and the age in lumbar vertebral body, ilium and intertrochanteric region of the femur (p < 0.001). The average FF of post-menopausal females was significantly higher than that of pre-menopausal females in these sites (p < 0.05). In the greater trochanter of the femur, there was no significant correlation between FF and age.
Conclusion:
In vivo IDEAL-IQ would provide reliable quantification of bone marrow fat.
Advances in knowledge:
IDEAL-IQ is simple to perform in a short time and may be practical for providing information on bone quality in clinical settings.
INTRODUCTION
Bone marrow is a dynamic organ which is composed of fat, water and protein. The haematopoietic marrow of the adult is approximately composed of 40% fat, 40% water and 20% protein, whereas fatty marrow is composed of 80% fat, 15% water and 5% protein.1 The proportion of both marrows is thought to be related to the remodelling capacity of the bone. Moreover, bone strength has been shown to depend not only on bone mineral density (BMD) but also on marrow quality, and these factors have important implications in osteoporosis.2
Since the marrow transformation process is seen earlier on T1 weighted MR images than on macroscopic analysis, MRI has become the non-invasive imaging modality of choice in diagnosing bone marrow disorders.3 By contrast, relatively few MRI studies4,5 have examined quantitative measurements of bone marrow. This is typically performed with MR spectroscopy whereby fat–water content is most accurately assessed. However, MR spectroscopy has some drawbacks, such as long scan time, small imaging range and a substantial amount of post-processing and may be impractical in some clinical settings.6,7
Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) imaging is a new method that can steadily separate fat and water by using three asymmetric echo times and the three-point Dixon method.8 The use of IDEAL as a fat quantification technique has been increasingly discussed in the literature,9 and Meisamy et al10 recently reported that IDEAL can accurately quantify fat by using MR spectroscopy as the reference standard in the liver. Our purpose in this study was to determine the reproducibility of the quantitative chemical shift-based water–fat separation method with a multiecho gradient echo sequence [iterative decomposition of water and fat with echo asymmetry and least-squares estimation quantitation sequence (IDEAL-IQ)] for assessing bone marrow fat content at 3-T MRI, to evaluate variation of bone marrow fat content at different bone sites and to investigate its association with age and menopause.
METHODS AND MATERIALS
Study population
Our institutional review board approved this study and informed consent was waived. 45 consecutive female patients who had undergone pelvic MR study including IDEAL-IQ at 3-T MRI from April 2013 to December 2013 were retrospectively evaluated. The medical records of each patient were reviewed by one of the authors (SY) who did not analyze quantitative fat content using IDEAL-IQ. Participants with malignant tumours, haematopoietic disease, anaemia, diabetes mellitus, autoimmune disease and those with severe liver and renal function, as well as any fracture and advanced degenerative change on screening X-rays, were excluded from this study. Finally, 31 (20 pre-menopausal and 11 post-menopausal) females were included in this study. There were no patients with a body mass index >35 kg m−2 or <18.5 kg m−2 and none with a history of BMD in the osteoporotic range.
MRI protocol
MRI was performed with a 3-T MRI unit (Discovery MR750W 3.0 T scanner; GE Healthcare, Waukesha, WI) and a 32-channel phased-array torso coil. The patients underwent imaging in a supine position. All patients underwent MRI covering the vertebral body of L4 to the lesser trochanter of the femur, including T2 weighted axial and coronal imaging and IDEAL-IQ. For IDEAL-IQ, the following pulse sequences were used: 6 echoes per repetition time, repetition time: 5.8 ms, echo time min: 0.8 ms, echo time spacing: 0.66 ms, slice thickness: 8 mm, field of view: 45 × 45 cm, matrix: 128 × 128, scan time: 16 s.
Quantitative image assessment
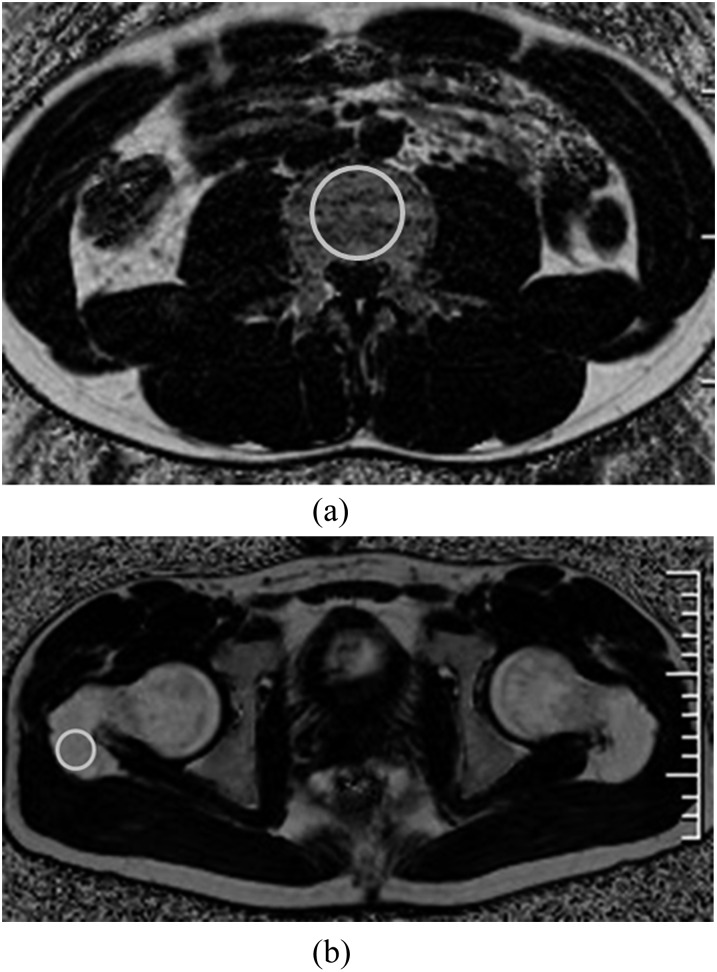
Quantitative fat fraction (FF) using IDEAL-IQ of 4 sites (lumbar vertebral body of L4, ilium, intertrochanteric region and greater trochanter of the femur) were analyzed. Maximum circular regions of interest (ROIs) were drawn on the bone marrow of these sites (Figure 1). T2 weighted axial and coronal images were used for the reference. The selection of ROIs was performed manually by one musculoskeletal radiologist (TA; with 20 years' experience in interpreting musculoskeletal MRI). The ROI sizes varied slightly owing to different thickness of cortex among the patients but were similar and specific for the anatomic structure. In lumbar vertebral body, maximum circular ROI was drawn on the centre carefully. The coefficients of variance (CV) on each site were evaluated repeatedly 10 times to assess the reproducibility.
Figure 1.
Maximum circular regions of interest were drawn on the lumbar vertebral body (a), ilium, intertrochanteric region and greater trochanter of the femur (b) for measurements of fat fraction using iterative decomposition of water and fat with echo asymmetry and least-squares estimation quantitation sequence (IDEAL-IQ) image.
Statistical method
Statistical analysis of the relationship between FF and age was evaluated on each site and was performed using Pearson's correlation. The FFs between pre- and post-menopausal groups were compared with an unpaired t-test. All calculations were performed by using the StatView software v. 5.0 (SAS® Institute Inc., Cary, NC). For reproducibility estimations, the CV (standard deviation divided by mean) was calculated across the 10 repetitions for each anatomic location and each subject.
RESULTS
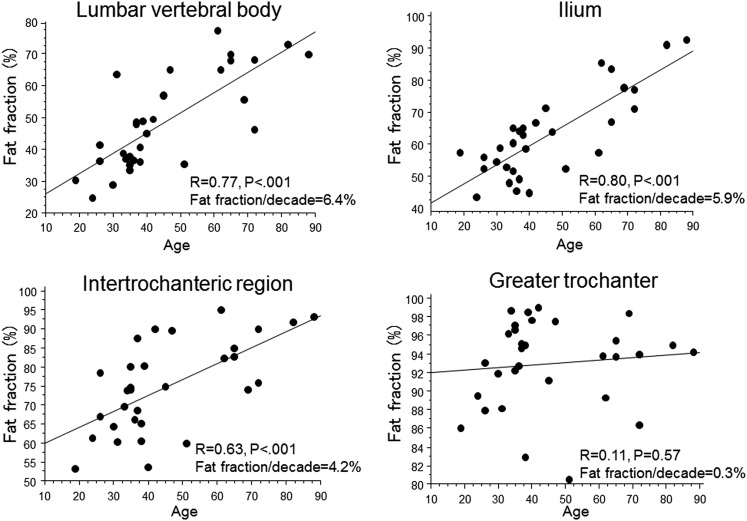
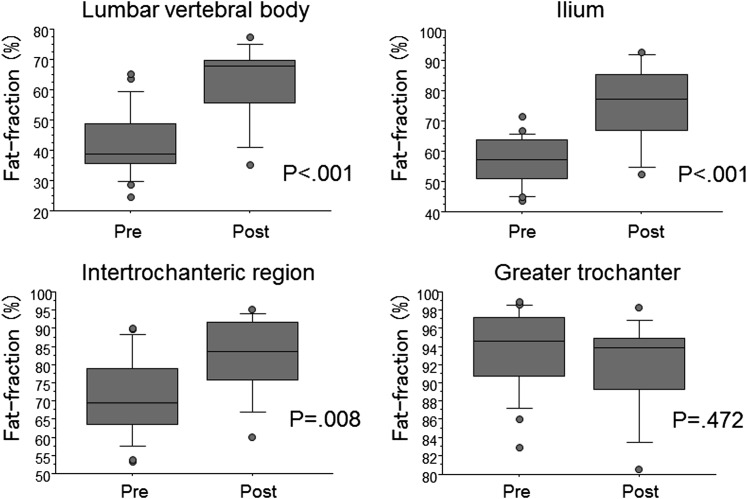
The CV in the quantification of bone marrow fat content ranged from 0.69% to 1.70%. A statistically significant correlation was established between the FF and the age in lumbar vertebral body, ilium and intertrochanteric region of the femur (r = 0.77, p < 0.001; r = 0.80, p < 0.001; r = 0.63, p < 0.001, respectively) (Figure 2). The average FF of post-menopausal females was significantly higher than that of pre-menopausal females in these sites (p < 0.05) (Figure 3). In the greater trochanter of the femur, there was no significant correlation between FF and age (Figure 2). The average FF of post-menopausal females was not significantly different from that of pre-menopausal females in this site (Figure 3).
Figure 2.
Correlation between fat fraction using iterative decomposition of water and fat with echo asymmetry and least-squares estimation quantitation sequence (IDEAL-IQ) and age in adult female on each bone site.
Figure 3.
Comparison between pre-menopausal and post-menopausal females in fat fraction using iterative decomposition of water and fat with echo asymmetry and least-squares estimation quantitation sequence (IDEAL-IQ) on each bone site.
DISCUSSION
The proportion of bone marrow fat changes dramatically with age. The age-related increase in fat content occurs first in the peripheral and later in the axial skeleton.3 In the peripheral bone, epiphyseal and apophyseal conversion, such as the greater trochanter, to fatty marrow occurs first and the last parts to convert are the proximal humeral and femoral metaphyses such as intertrochanteric region of the femur.11 In this study, significant correlation was established between the FF and the age in lumbar vertebral body, ilium and intertrochanteric region of the femur but was not observed in greater trochanter of the femur. The rate of increase in the FF in lumbar vertebral body and ilium was higher than that in intertrochanteric region of the femur. These results concur with the established age-related bone marrow fat transformation findings from the analysis of macroscopic and histological data.
In the 4th lumbar vertebra, there was a significant and gradual increase in FF, and the rate of increase in FF per decade is equivalent to 6.4% in this study. This compares favourably with previously reported values of approximately 6–7.5% by MR spectroscopy.12–14 Although MR spectroscopy is thought to be the most accurate method in quantifying fat–water content, an improvement in scan time and resolution is offered as practical quantitative imaging. Clinically, IDEAL-IQ may represent an interesting alternative for the evaluation of FF.
The incidence of osteoporosis in females is significantly increased after menopause.15,16 Post-menopausal females also have increased risks for fragility bone fracture. Recently, Patsch et al17 reported that bone marrow fat composition by MR spectroscopy is linked with fragility fractures in post-menopausal females and may serve as a novel tool for BMD-independent fracture risk assessment. This study demonstrated that IDEAL-IQ is a rapid and highly reproducible method for quantitatively assessing fat contents in bone marrow. Variations of bone marrow fat contents among the 4 myelopoietic sites and differentiation of those between pre- and post-menopausal females were also shown. These preliminary data suggest that IDEAL-IQ may be a practical tool for evaluating FF and potential fracture risk non-invasively.
Most recently, Ergen et al18 assessed the relationship between FF measured by IDEAL-IQ and BMD and found a moderate negative correlation between them (r = −0.42). Although we unfortunately could not investigate the correlation with BMD in this study, it may be possible to predict the risk of accelerated loss of BMD in osteoporotic patients with IDEAL-IQ. Further studies on multiple sites with IDEAL-IQ as a comparison combined with clinical and BMD would be necessary to derive a conclusion.
Our study had some limitations. First, our results were not compared with tissue fat content directly. A single targeted biopsy is unlikely to provide sufficient validation owing to wide-ranging sampling variability, and therefore, unambiguous validation would require comparison of MR images to large post-mortem or surgically obtained tissue. Second, this study was performed with one set of IDEAL-IQ parameters for MRI examinations. For FF using IDEAL-IQ to be fully validated as a biomarker for osteoporosis, its robustness to the types of alterations in MRI parameters such as matrix and slice thickness which may be encountered clinically should be assessed. Third, in lumbar vertebra, we estimate FF in a single vertebra. Martin et al19 calculated FF from Th10 to S2 using IDEAL sequence in 5 males and found that there was a trend of increasing FF moving inferiorly. FF at a single vertebral level may not be reflected by the marrow distribution in lumbar spine. Finally, this study is retrospective and included a rather small number of cases. Additional prospective studies with a large number of cases may be necessary to confirm the clinical usefulness of IDEAL-IQ.
In conclusion, in vivo IDEAL-IQ provides reliable quantification of bone marrow fat with good reproducibility. It may be a valuable tool for providing information on bone quality in clinical settings. Given that there is considerable variation in the fat proportion among osteopenic, osteoporotic and healthy population at various sites, a study design with various subgroups and sites would be helpful to validate the clinical implication of this method.
Contributor Information
Takatoshi Aoki, Email: a-taka@med.uoeh-u.ac.jp.
Shinpei Yamaguchi, Email: ymgchs@med.uoeh-u.ac.jp.
Shunsuke Kinoshita, Email: piko20080615@yahoo.co.jp.
Yoshiko Hayashida, Email: yhlinda@med.uoeh-u.ac.jp.
Yukunori Korogi, Email: ykorogi@med.uoeh-u.ac.jp.
REFERENCES
- 1.Vogler JB, 3rd, Murphy WA. Bone marrow imaging. Radiology 1988; 168: 679–93. doi: 10.1148/radiology.168.3.3043546 [DOI] [PubMed] [Google Scholar]
- 2.Eriksson SA, Isberg BO, Lindgren JU. Prediction of vertebral strength by dual photon absorptiometry and qualitative computed tomography. Calcif Tissue Int 1989; 44: 243–50. doi: 10.1007/BF02553758 [DOI] [PubMed] [Google Scholar]
- 3.Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in the appearance at MR imaging. Radiology 1990; 175: 219–23. doi: 10.1148/radiology.175.1.2315484 [DOI] [PubMed] [Google Scholar]
- 4.Shellinger D, Lin CS, Fertikh D, Lee JS, Lauerman WC, Henderson F, et al. Normal lumbar vertebrae: anatomic, age and sex variance in subjects at proton MR spectroscopy—initial experience. Radiology 2000; 215: 910–16. [DOI] [PubMed] [Google Scholar]
- 5.Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging 2005; 22: 279–85. doi: 10.1002/jmri.20367 [DOI] [PubMed] [Google Scholar]
- 6.Bernard CP, Liney GP, Manton DJ, Turnbull LW, Langton CM. Comparison of fat quantification methods: a phantom study at 3.0T. J Magn Reson Imaging 2008; 27: 192–7. doi: 10.1002/jmri.21201 [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Taksali SE, Dufour S, Befroy D, Goodman TR, Petersen KF, et al. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point Dixon and three-point IDEAL. Magn Reson Med 2008; 59: 521–7. doi: 10.1002/mrm.21561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeder SB, Pelc NJ, Alley MT, Gold GA. Multi-coil Dixon chemical species separation with an iterative least squares method. Magn Reson Med 2004; 51: 34–45. doi: 10.1002/mrm.10675 [DOI] [PubMed] [Google Scholar]
- 9.Yu H, McKenzie CA, Shimakawa A, Vu AT, Brau AC, Beatty PJ, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging 2007; 26: 1153–61. doi: 10.1002/jmri.21090 [DOI] [PubMed] [Google Scholar]
- 10.Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 2011; 258: 767–75. doi: 10.1148/radiol.10100708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaramillo D, Laor T, Hoffer FA, Zaleske DJ, Cleveland RH, Buchbinder BR, et al. Epiphyseal marrow in infancy: MR imaging. Radiology 1991; 180: 809–12. doi: 10.1148/radiology.180.3.1871298 [DOI] [PubMed] [Google Scholar]
- 12.Kugel H, Jung C, Schulte O, Heindel W. Age and sex specific differences in the 1H-spectrum of vertebral bone marrow. J Magn Reson Imaging 2001; 13: 263–8. doi: [DOI] [PubMed] [Google Scholar]
- 13.De Bisschop E, Luypaert R, Louis O, Osteaux M. Fat fraction of lumbar bone marrow using in vivo proton nuclear magnetic resonance spectroscopy. Bone 1993; 14: 133–6. doi: 10.1016/8756-3282(93)90239-7 [DOI] [PubMed] [Google Scholar]
- 14.Liney GP, Bernard CP, Manton DJ, Manton DJ, Turnbull LW, Langton CM. Age, gender, and skeletal variation in bone marrow composition: a preliminary study at 3.0 Tesla. J Magn Reson Imaging 2007; 26: 787–93. doi: 10.1002/jmri.21072 [DOI] [PubMed] [Google Scholar]
- 15.Riggs BL, Melton LJ, 3rd. Involutional osteoporosis. N Engl J Med 1986; 314: 1676–86. doi: 10.1056/NEJM198606263142605 [DOI] [PubMed] [Google Scholar]
- 16.Mazess RB, Barden HS, Ettinger M, Johnston C, Dawson-Hughes B, Baran D, et al. Spine and femur density using dual-photon absorptiometry in US white women. Bone Miner 1987; 2: 211–19. [PubMed] [Google Scholar]
- 17.Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res 2012; 28: 1721–8. doi: 10.1002/jbmr.1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ergen FB, Gulal G, Yildiz AE, Celik A, Karakaya J, Aydingoz U. Fat fraction estimation of the vertebrae in females using the T2*-IDEAL technique in detection of reduced bone mineralization level: comparison with bone mineral densitometry. J Comput Assist Tomogr 2014; 38: 320–4. doi: 10.1097/RCT.0b013e3182aa4d9d [DOI] [PubMed] [Google Scholar]
- 19.Martin J, Nicholson G, Cowin G, Ilente C, Wong W, Kennedy D. Rapid determination of vertebral fat fraction over a large range of vertebral bodies. J Med Imaging Radiat Oncol 2014; 58: 155–63. doi: 10.1111/1754-9485.12143 [DOI] [PubMed] [Google Scholar]