Abstract
Objective:
To investigate the need for elective neck irradiation (ENI) to nodal Level IB in patients with nasopharyngeal carcinoma (NPC) with negative Level IB lymph nodes (IB-negative) treated by intensity-modulated radiotherapy (IMRT).
Methods:
We conducted a Phase 2 prospective study in 123 newly diagnosed IB-negative patients with NPC treated by IMRT, who met at least 1 of the following criteria: (1) unilateral or bilateral Level II involvement with 1 of the following: Level IIA involvement or any Level II node ≥2 cm/with extracapsular spread; (2) ≥2 unilateral node-positive regions. Bilateral Level IB nodes were not contoured as part of the treatment target and treated electively. Level IB regional recurrence rate; pattern of treatment failure; 3-year overall survival (3y-OS), 3-year local control (3y-LC) and 3-year regional control (3y-RC) rates; toxicities; and dosimetric data for planning target volumes, organs at risk, Level IB and submandibular glands (SMGs) were evaluated.
Results:
Two patients developed failures at Level IB (1.6%). The 3y-LC, 3y-RC and 3y-OS rates were 93.5%, 93.5% and 78.0%, respectively. Bilateral Level IB received unplanned high-dose irradiation with a mean dose (Dmean) ≥50 Gy in 60% of patients. The average Dmean of bilateral SMGs was approximately 53 Gy.
Conclusion:
ENI to Level IB may be unnecessary in IB-negative patients with NPC treated by IMRT. A further Phase 3 study is warranted.
Advances in knowledge:
Based on the results of this first Phase 2 study, we suggest omitting ENI to Level IB in Ib-negative patients with NPC with extensive nodal disease treated by IMRT.
INTRODUCTION
Intensity-modulated radiotherapy (IMRT) has been shown to be an effective and safe treatment modality for nasopharyngeal carcinoma (NPC).1–4 The most common side effect of IMRT is salivary gland damage with xerostomia. The parotid glands (PGs) produce most of the stimulated saliva, whereas most unstimulated saliva is secreted by the submandibular glands (SMGs) and contains mucins, which influence the degree of sensation of mouth dryness.5,6 The risk of xerostomia would thus be significantly reduced if the SMGs, as well as the PGs, could be spared during the treatment of head and neck cancer.7,8 Level IB lymphadenopathy may occur when the nasopharyngeal tumour invades surrounding tissues such as the anterior two thirds of the nasal cavity, the hard and soft palate, or the alveoli.9 Retrograde metastases to superficial cervical lymph nodes and Level IB may occur when cervical lymphatic vessels are either blocked by deep cervical nodal metastases or are damaged by radical neck dissection or radiation.10 However, Ng et al11 found that the risk of Level IB involvement was rare in NPC, with an incidence of 2.2%.
Treatment centres have developed their own guidelines for target delineation and dose definition for IMRT in patients with NPC, including using different rules for elective neck irradiation (ENI) of nodal Level IB in patients with negative Level IB lymph nodes (IB-negative).1–3,12,13 Based on our internal data, Level IB has not been included in the clinical target volume (CTV) in our practice guidelines for IB-negative NPC cases since the 1990s (the three-dimensional conformal radiation therapy era). Although some studies demonstrated an incidence of Level IB metastasis of <5%,14–16 studies of omitting ENI to Level IB in patients with NPC treated by IMRT are rare. We therefore conducted a Phase 2 prospective study to investigate the need for ENI to Level IB in IB-negative patients with NPC treated by IMRT.
METHODS AND MATERIALS
Patient eligibility
Previously untreated patients with a pathological diagnosis of NPC, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate liver, renal and bone marrow functions were considered. In addition to being bilateral Level IB-negative by MRI, patients were eligible if they met at least one of the following criteria: (1) unilateral or bilateral Level II involvement with one of the following: Level II A involvement [LN-IIA(+)], any Level II node ≥2 cm or any Level II node with extracapsular spread (DLN-II ≥2 cm/ES) and (2) ≥2 unilateral node-positive regions. Bilateral Level IB nodes were not contoured as part of the treatment target and treated electively. Exclusion criteria were: (1) distant metastases (DM); (2) enlarged neck nodes involving the skin; (3) previous neck surgery; and (4) nasopharyngeal primary tumours involving the anterior two thirds of the nasal cavity, the soft and hard palates or the alveoli. This study was approved by institutional review board of Cancer Hospital of Shantou University Medical College, and all patients signed written informed consent before the initiation of therapy.
Pre-treatment evaluations
Pre-treatment evaluations consisted of medical history and physical, dental and laboratory studies. MRI of the nasopharynx/neck was required unless there was a contraindication. Additional tests to evaluate the extent of disease included chest radiography, abdominal ultrasound, liver and renal function tests and CT scans of the chest and abdomen and bone scans, when indicated. Data collection for this study began in October 2008, and patients were therefore initially staged using the American Joint Committee on Cancer (AJCC) 2002 staging system. After the publication of the 2010 AJCC system, all patients were restaged using the new system.
Intensity-modulated radiotherapy planning and treatment
Planning was carried out based on CT with intravenous contrast using a 3-mm slice from the head to 2-cm below the sternoclavicular joint. The neck node levels including Level IB were contoured according to the consensus guidelines jointly published by the Danish Head and Neck Cancer Group (DAHANCA), the European Organisation for Research and Treatment of Cancer (EORTC), the Groupe d' Oncologie Radioth (GORTEC), the National Crime Information Center (NCIC) and the Radiation Therapy Oncology Group (RTOG) in 2003.17 Cervical lymphadenopathy was determined according to the diagnostic criteria proposed by van den Brekel et al.18 The nasopharyngeal gross tumour volume (GTVnx) included all known primary tumour gross disease and retropharyngeal lymphadenopathy, based on the CT image, MRI and endoscopic findings. Enlarged neck lymph nodes were localized as a separate GTV (GTVnd). CTV1 was defined as the CTV at high risk for involvement, including GTVnx, GTVnd, the entire nasopharynx, retropharyngeal nodal regions, skull base, clivus, pterygoid fossae, parapharyngeal space, sphenoid sinus, the posterior one third of the nasal cavity/maxillary sinuses, part of the posterior ethmoid sinus and the electively prophylactic irradiated cervical nodal regions. CTV2 was defined as the CTV at low risk for involvement. Electively prophylactic irradiated cervical nodal regions were defined as follows: (1) cases with unilateral neck lymph node involvement; CTV1 included ipsilateral neck Levels II–V and contralateral upper neck Levels II, III and VA; (2) cases with enlarged neck lymph nodes noted by CT/MRI that did not meet the diagnostic criteria; CTV1 included bilateral upper neck Levels II, III, and VA and CTV2 included ipsilateral neck Levels IV–VB; and (3) cases with bilateral neck lymph node involvement; CTV1 included bilateral neck Levels II–V. Bilateral Level IB nodes were not contoured as CTV1 or CTV2 and treated electively for the entire cohort. A planning target volume (PTV) was added (i.e. additional margin of 3–5 mm) to each of the above GTVs and CTVs. That is, PTVnx, PTVnd, PTV1 and PTV2 refer to the planning target volumes of the GTVnx, GTVnd, CTV1 and CTV2, respectively.
A simultaneous integrated boost IMRT technique with static fields was applied. Dose prescribing was as follows: 69.96–70.68 Gy in 2.12–2.28 Gy/fraction for PTVnx, 66 Gy in 2–2.13 Gy/fraction for PTVnd, 60 Gy for PTV1 and 54 Gy for PTV2 in 31–33 fractions and 5fractions per week. The dose to 50% volume of the PGs was limited to <30 Gy, whereas the mean dose (Dmean) of the oral cavity and larynx/laryngeal pharynx were limited to <40 Gy, where possible. There was no dose restriction to Level IB and the SMGs; however, we used the dose-limiting ring structure to form the dose gradients surrounding the PTV, resulting in the reduced dose to Level IB and the SMGs.
Chemotherapy
Concurrent chemotherapy was prescribed to patients with Stages III–IVB without major medical comorbidities precluding its use. The chemotherapy regimens were TP (docetaxel 75 mg m−2 Day 1 plus cisplatin 75 mg m−2 Day 1) or PF (cisplatin 75 mg m−2 Day 1 plus tegafur 1000 mg m−2 Days 1–3) on Days 1 and 22 for two cycles. Two cycles of induction chemotherapy (regimen as above) were given to patients with bulky tumours, a close margin between the target volumes and the critical structures, or an intracranial invasion. If induction chemotherapy was applied, the concurrent regimen was changed to cisplatin alone (cisplatin 75 mg m−2 Day 1).
Toxicity assessment and follow-up
Systemic/acute radiotherapy adverse effects were scored using the National Cancer Institute Common Toxicity Criteria v. 3.0, and late radiotherapy effects were scored according to the RTOG/EORTC criteria. Patients were assessed every 3 months for the first 3 years after treatment, followed by every 6 months for the subsequent 2 years and annually thereafter. Routine evaluations included a complete physical examination, nasopharyngoscopy, a biochemical profile and annual chest radiography, abdominal ultrasonography and head-and-neck CT/MRI. Further investigations included chest/abdomen CTs, bone scans and/or positron emission tomography scans, as indicated. Management of residual tumours or relapses was determined on a case-by-case basis.
Evaluation of failure patterns
Recurrence in the nasopharynx and nodal regions (including parotid lymph node) were defined as local and regional failures, respectively, and were pathologically confirmed by biopsy. Skull base recurrence, defined as local failure as well, and DM (i.e., liver, bone and lung) were identified by CT, MRI or positron emission tomography scans.
End points and statistical analysis
The primary end point was Level IB regional recurrence rate. Other end points were patterns of treatment failure, 3-year overall survival (3y-OS) rate, 3-year local control (3y-LC) rate, 3-year regional control (3y-RC) rate, the incidence and severity of acute mucositis and late xerostomia, and dosimetric analysis of the PTVs, OARs, Level IB and the SMGs. The dose–volume of the targets and OARs were calculated by dose–volume histogram. Statistical analyzes were performed using SPSS® v. 20.0 software (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). Overall survival (OS) rates were calculated using Kaplan–Meier analysis.
RESULTS
Patient characteristics and treatment
A total of 123 patients were eligible for analysis between 1 October 2008 and 31 October 2010. 112 patients (91.1%) had Stages III–IVB disease requiring chemotherapy, but only 104 (84.6%) received chemotherapy because the rest had chemotherapy contraindications. Table 1 shows the patient characteristics and treatment data.
Table 1.
Patient characteristics and treatment data
| Variable | Item | Case | (%) |
|---|---|---|---|
| Age, years | <60 | 96 | 78.0 |
| ≥60 | 27 | 22.0 | |
| Sex | Male | 97 | 78.9 |
| Female | 26 | 21.1 | |
| Tumour classification | T1 | 12 | 9.8 |
| T2 | 24 | 19.6 | |
| T3 | 53 | 43.1 | |
| T4 | 34 | 27.5 | |
| Nodal classification | N1 | 28 | 22.8 |
| N2 | 78 | 63.4 | |
| N3 | 17 | 13.8 | |
| Stage group | II | 11 | 8.9 |
| III | 64 | 52.0 | |
| IVa | 31 | 25.2 | |
| IVb | 17 | 13.9 | |
| Pathological type | Type 1 | 2 | 1.6 |
| Type 2.1 | 66 | 53.7 | |
| Type 2.2 | 55 | 44.7 | |
| Chemotherapy | Yes | 104 | 84.6 |
| Induction + concurrent | 11 | 10.6 | |
| Concurrent alone | 93 | 89.4 |
Dose–volume analysis
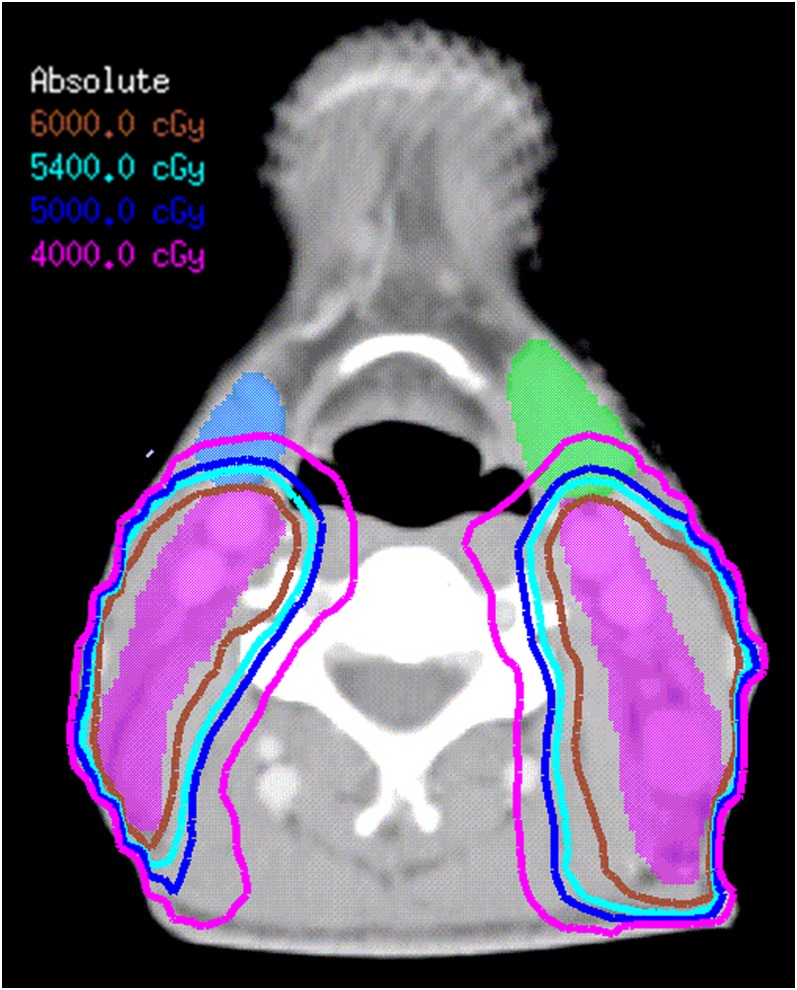
Tables 2 and 3 show the dose–volume histogram statistics for the target volumes, OARs, Level IB and SMGs, respectively. Maximum dose (Dmax) and minimum dose (Dmin) were defined as the doses received by 2% and 98% of the volume concerned, respectively (Table 2). The dose–volume of each OAR was below the tolerance limit (Table 3). Although it was not contoured as CTV1 or CTV2, Level IB received unplanned irradiation. As shown in Figure 1, the 40-Gy isodose line covered the posterior one third to one half of the SMGs and Level IB, of which the lower dose was resulted only by the dose-limiting ring structure used to form the dose gradients surrounding the PTV.
Table 2.
Dose–volume histogram statistics for target volumes, Level IB and submandibular glands (SMGs)
| Variable | Dmin (Gy) | Dmax (Gy) | Dmean (Gy) (range) |
|---|---|---|---|
| PTVnx | 65.66 ± 6.88 | 75.07 ± 1.51 | 71.17 ± 0.62 |
| PTVnd | 62.83 ± 7.83 | 71.60 ± 2.14 | 68.15 ± 1.40 |
| PTV1 | 37.12 ± 12.84 | 75.09 ± 2.99 | 65.56 ± 1.10 |
| PTV2 | 35.43 ± 19.79 | 62.25 ± 5.52 | 56.22 ± 1.99 |
| IB-L | 24.93 ± 7.23 | 69.06 ± 2.60 | 51.60 ± 5.25 (40.42–64.70) |
| IB-R | 23.81 ± 7.03 | 68.93 ± 2.45 | 52.20 ± 5.26 (37.52–66.33) |
| SMG-L | 36.50 ± 8.23 | 68.87 ± 2.49 | 53.04 ± 4.68 |
| SMG-R | 38.30 ± 8.74 | 68.79 ± 2.49 | 53.64 ± 4.87 |
Dmax, maximum dose; Dmean, mean dose; Dmin, minimum dose; IB-L, left Level IB; IB-R, right Level IB; PTV, planning target volume; PTV1, PTV of the clinical target volume at high risk for involvement; PTV2, PTV of the target volume at low risk for involvement; PTVnd, PTV of enlarged neck lymph nodes; PTVnx, PTV of nasopharyngeal gross tumour volume; SMG-L, left submandibular gland; SMG-R, right submandibular gland.
Values are given as median ± standard deviation.
Table 3.
Dose–volume histogram statistics for organs at risk (OARs)
| OARs | Variable | Median ± SD (Gy) |
|---|---|---|
| Brain stem | D1% | 48.51 ± 6.05 |
| Spinal cord | D1cm3 | 41.59 ± 5.37 |
| ON left | D1% | 19.88 ± 21.80 |
| ON right | D1% | 24.77 ± 22.04 |
| Optic chiasma | D1% | 25.32 ± 24.24 |
| Lens left | D1% | 5.14 ± 2.60 |
| Lens right | D1% | 5.14 ± 2.60 |
| Parotid left | Dmean | 38.24 ± 6.20 |
| Parotid right | Dmean | 37.66 ± 5.58 |
| Oral cavity | Dmean | 36.53 ± 4.01 |
| Larynx/larynx pharynx | Dmean | 36.54 ± 3.31 |
D1%, 1% of target volume received dose higher than (Gy); D1cm3, 1 cm3 of target volume received dose higher than (Gy); Dmean, mean dose; ON, optic nerve; SD, standard deviation.
Figure 1.
Dose distributions in the transverse planes at the level of the submandibular gland (SMG) in a T3N3M0 nasopharyngeal carcinoma case without Level IB node involvement. CTV-1, purple shadow; left SMG, green shadow; right SMG, blue shadow; 60-Gy isodose, brown line; 54-Gy isodose, sky blue line; 45-Gy isodose, blue line; 40-Gy isodose, pink line. 40-Gy isodose line covers the posterior one third to one half of the SMGs. For colour see online.
The Dmean for Level IB were ≥50 Gy in 61%/64.2% of the patient on the left/right side, and the average Dmean for the SMGs was approximately 53 Gy (Table 2). The Dmean of Level IB was <50 Gy in 48 cases on the left side (39%) and 44 cases on the right side (35.8%), with the lowest Dmean of SMGs was 48.36/48.77 Gy of the left/right side, respectively. Among the group with Dmean of unilateral/bilateral Level IB <50 Gy, the ipsilateral involved lymph nodes were either small or not located in Level IIA, making the PTVnd and high-dose curve of 66 Gy cover less volume of the ipsilateral IB and SMG, thus resulting in relatively lower dose to them.
Toxicities
During treatment, the worst acute mucositis was grade 1 or 2 in 84 (68.3%) patients and grade 3 in 39 (31.7%) patients, 10 of whom (8.1%) required the insertion of a nasogastric feeding tube for nutritional support. Other chemotherapy and acute radiation toxicities were summarized in the Table A1. Chronic xerostomia was recorded as grades 1 and 2 in 60 (48.8%) and 43 (35.0%) patients, respectively, at 1 year from the start of IMRT, although the percentage of combined grades 1 and 2 xerostomia fell to 20.3% at 3 years after treatment.
Clinical outcomes and patterns of treatment failure
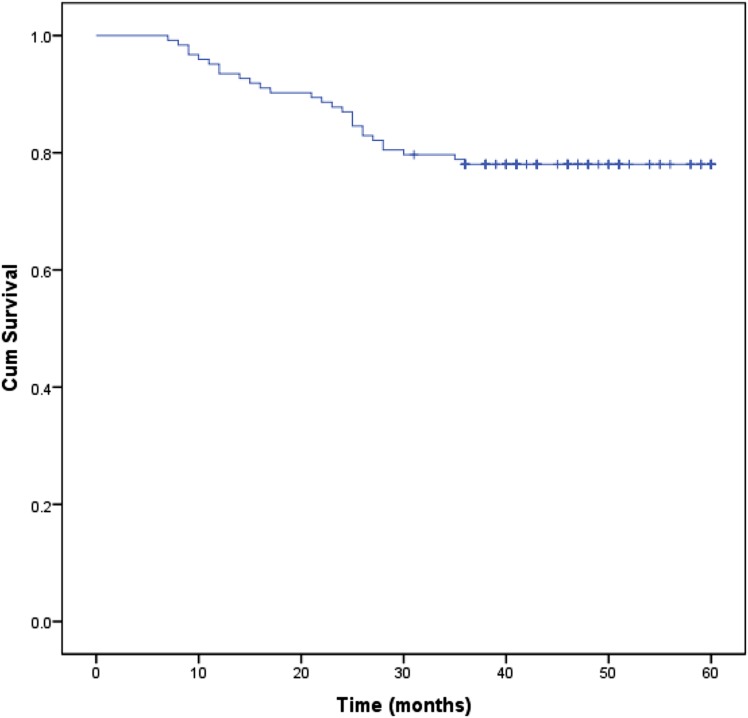
After a median follow-up of 41 months (range, 7–60 months; 31 October 2013), two patients had developed regional failure at Level IB (1.6%) (Table 4). Other sites of failure included local failure in 6 (4.9%) patients, regional nodal failure other than Level IB in 4 (3.2%) patients, simultaneous local and regional nodal failure in 1 (0.8%) patient, simultaneous regional nodal and distant failure in 1 (0.8%) patient and distant failure alone in 24 (19.5%) patients. All the failure cases received salvage surgery, reirradiation and/or chemotherapy. By 31 October 2013, 27 patients had died, 96 patients were still alive including the 2 patients who experienced Level IB recurrence and none were lost to follow-up. Among the patients who died, 1 (3.7%) patient died in a traffic accident, 1 (3.7%) patient died of intercurrent illness and 25 (92.6%) patients died of local/regional recurrence and/or DM. The 3y-LC and 3y-RC were both 93.5%. The 1-year OS, 2-year OS and 3y-OS rates were 93.5%, 87.0% and 78.0%, respectively (Figure 2).
Table 4.
Pattern of Level IB nodal failure in two patients with intensity-modulated radiotherapy
| Items | Case 1 | Case 2 |
|---|---|---|
| Criterion (1): L/R | +/+ | +/+ |
| Criterion (2): L/R | +/+ | +/+ |
| FV-TNM stage | T3N2M0 | T2N2M0 |
| Recurrent stage | T1N2M0 | T0N1M0 |
| Relapsed Level IB | Bilateral | Right |
| Other relapsed regions | NP/bilateral parotid LN | – |
| IB-L, Dmean (Dmin–Dmax), Gy | 46.92 (23.25–65.08) | 46.69 (8.81–64.39) |
| IB-R, Dmean (Dmin–Dmax), Gy | 47.12 (22.01–70.66) | 47.68 (10.4–70.00) |
| Time to failure (months) | 60 | 43 |
| Salvage treatment | RT + chemo | Surgery |
| Treatment response | CR | CR |
chemo, chemotherapy; CR, complete response; Dmax, maximum dose; Dmean, mean dose; Dmin, minimum dose; FV, first visit; IB-L, left level IB; IB-R, right Level IB; LN, lymph node; L/R, left/right side of the neck; NP, nasopharynx; RT, radiotherapy.
Criteria (1) and (2) refer to the inclusion criteria indicated in patient eligibility.
Figure 2.
Survival curve for the entire cohort of patients with nasopharyngeal carcinoma. Cum, cumulative.
DISCUSSION
Several previous studies have demonstrated an incidence of Level IB metastasis to be <5%. Studies have demonstrated that Levels II, III, IV and V are most commonly involved (in order), whereas Level IB is less often involved.14,15,17 Tan et al16 found an involvement rate of Level IB of only 0.9% and skip metastasis in only 2.4%. They demonstrated that IB involvement in patients with NPC was rare, irrespective of the presence of Level II lymph node metastases and suggested that routine prophylactic irradiation to Level IB could be omitted. In a retrospective study, Chen et al19 spared Level IB lymph nodes during IMRT in 120 patients with NPC without IB involvement and found no Level IB recurrence during a median follow-up of 54 months. However, the extent of cervical nodal involvement, especially the Level II status of the enrolled cases, was not mentioned. To the best of our knowledge, few institutions have routinely omitted ENI to Level IB in IB-negative patients with NPC, and no prospective studies have been reported.
Different treatment centres have developed their own guidelines regarding Level IB delineation and dose definition when applying IMRT to NPC cases.1–3,12,13 According to the RTOG 0225 protocol, CTV59.4 should include Levels I–V nodal regions.1 The treatment guidelines at the Prince of Wales Hospital in Hong Kong define bilateral neck Levels Ib–V electively irradiated with a total dose of 54–60 Gy for node-negative NPC cases,2 whereas guidelines at the National Cancer Centre of Singapore delineated neck Level IB for elective irradiation and include it in the CTV-60 Gy only if there was nodal disease in the ipsilateral neck.3 The Cancer Institute and Hospital of Chinese Academy of Medical Sciences includes Level IB in the high-risk CTV and prescribes 60 Gy in cases with ipsilateral Level IIA lymphadenopathy or a maximum dimension of ipsilateral Level II lymph node ≥2 cm.12 The 2010 expert consensus guidelines for IMRT target delineation and dose delivery for NPC published by the Chinese NPC Clinical Stage Working Committee indicated that Level IB should be included in the high-risk CTV and delivered 60 Gy in cases meeting the following conditions: (1) maximum dimension of Level IIA lymph node ≥3 cm or ES and (2) number of ipsilateral node-positive regions ≥4.13
In our prospective study, all IB-negative NPC cases met at least one of the following criteria: (1) unilateral or bilateral Level II involvement with one of the followings: LN-IIA (+), or DLN-II ≥2 cm ES−1 and (2) ≥2 unilateral node-positive regions. Bilateral Level IB nodes were not contoured as part of the treatment target and treated electively. This cohort thus met all of the criteria for Ib ENI based on any of the above guidelines or protocols, and the results demonstrated a low Level IB nodal failure rate (1.6%) when ENI to Level IB was omitted after a median follow-up of 41 months. Both patients who developed Level IB recurrence were successfully salvaged and remained alive at the last follow-up.
Several institutions have reported excellent tumour control rates after treating NPC by IMRT.1–4 Kam et al2 from Hong Kong and Tham et al3 from Singapore reported a similar result of 3y-OS and 3-year LC of 94% and 90%, respectively, in patients with NPC treated by IMRT (AJCC Stage III/IV cases accounted for about 60% of the patients enrolled), and DM accounted for most treatment failure. In our study, despite the excellent 3-year LC and RC rates, OS was lower than in the above studies. This apparent discrepancy may be due to the higher percentage of patients with AJCC Stage III/IV cancer (91.1%) in our study. Thus, DM accounted for most treatment failures (19.5%), and omitting ENI to Level IB did not compromise LC or RC in these patients.
Level IB and SMGs, which are anterior to the IIA region, were not contoured as part of the CTV and were not a constraint for optimizing the current radiation protocol. Despite our attempt to minimize the dose to Level IB and the SMGs, further analysis demonstrated that bilateral Level IB nodes still received unplanned irradiation of around 51 Gy. Simultaneously, the Dmean to the left and right SMGs were as high as 53 Gy. It is known that when we prescribe a Dmean of 60–66 Gy to Level IIA, which lies adjacent to Level IB and SMGs, and constrain the dose to other structures such as the spinal cord, oral cavity, larynx, constricture muscles and PGs, the isodose curve will extend to Level IB and SMGs if they are not part of the avoidance structures (Figure 1). Chen et al19 reported that ≥40-Gy isodose lines covered the posterior part of the SMGs, although they did not provide the dosimetric details of the SMGs and IB in their patients. Both Dmean to Levels IB and SMGs were much lower in the current study than at 60 and 62 Gy, respectively, when we prescribed ENI to Levels IB of 54–60 Gy in IB-negative NPC in a previous retrospective study.20 It is interesting that both patients in the current study who experienced IB relapses had IB Dmean <50 Gy, equivalent to a relatively low IB relapse rate [2.1% for the left side (1/48) and 4.5% (2/44) for the right side] in patients who received Level IB Dmean <50 Gy. Since many of our patients received a mean dose ≥50 Gy to Level IB, addition studies will be needed to determine whether a similar low rate of failure is observed when the dose of Level IB is reduced <50 Gy.
Chronic xerostomia recorded in the present study was mild, with 48.8% of the patients developing grade 1, 35.0% grade 2 and no cases of grade 3 or worse at 1 year from the start of IMRT. These levels fell to 20.3% of grade 1 or 2 xerostomia at 3 years after treatment, and they were comparable to 16.2% rates reported for RTOG 02-25 Trial (16.2% grade 2–3 at 1 year) and a Hong Kong trial (23% of grade ≥2 at 2 years).1,2 The comparable incidence of xerostomia despite the omission of ENI to Level IB may be attributable to the high percentage of Stage III/IV cases (>90%), comparable dose to the PGs and the unavoidable irradiation dose to the SMGs. Chen et al19 reported a comparable low rate of 16.7% of grade 2 or worse xerostomia; however, they did not provide information on the time points of the xerostomia evaluation or the Dmean of the PGs and SMGs.19 Murdoch-Kinch et al7 reported that SMG salivary flow rates depended on the Dmean, with a threshold of 39 Gy. However, it may not be feasible to limit the Dmean of SMGs to 39 Gy in patients with extensive nodal disease as defined in our study. Nevertheless, the relatively low Level IB failure rate in the present study suggests that further studies should be conducted to investigate whether constraining the SMG Dmean to 39 Gy is feasible, in an attempt to preserve SMG function in selective early-staged and node-negative patients.21
To the best of our knowledge, this is the first prospective study to address the value of not contouring Level IB as part of the CTV and prescribed ENI dose in patients with NPC with Level II lymph node involvement. Our results suggest that the rate of Level IB nodal failure is low when ENI to Level IB is omitted in patients with IB-negative NPC. Although the lack of a randomized control arm and the unplanned dose shed to IB were major limitations of the present study, the relatively large sample size, relatively lower dose to the IB and low rate of Level IB failure (1.6%) still support the use of the current protocol. The consistent and long-term follow-up, as well as the 100% follow-up rate, ensured that the vast majority of nodal failures were recorded. Another limitation was that patients with III–IVB disease received different chemotherapy regimens, which may have introduced bias to the results. This issue should be addressed and improved in a future Phase 3 trial.
CONCLUSIONS
Based on the results of this single-institution, Phase 2 study, ENI to Level IB may not be necessary in IB-negative patients with NPC treated with IMRT. Omitting ENI to IB did not compromise Level IB recurrence or treatment end points. However, Level IB received unplanned but unavoidable high-dose irradiation. The current treatment plan optimization protocol does not effectively minimize the mean dose to the SMGs, and a further, well-designed, Phase 3 study is warranted to investigate this approach.
Acknowledgments
ACKNOWLEDGMENTS
The authors thank Drs Quynh-Thu Le, MD; Zhijian Chen, MD; and Derui Li, MD, for their valuable support.
APPENDIX A
Table A1.
Type and frequency of acute adverse effects in 123 patients
| Number of patients by toxicity grade |
|||||
|---|---|---|---|---|---|
| Adverse effect | 1 | 2 | 3 | 4 | 5 |
| Allergy/immunology | 5 | 0 | 0 | 0 | 0 |
| Auditory/hearing | 12 | 36 | 20 | 0 | 0 |
| Blood/bone marrow | 9 | 25 | 58 | 8 | 0 |
| Constitutional symptom | 30 | 43 | 30 | 3 | 0 |
| Dermatologic/skin | 29 | 54 | 20 | 0 | 0 |
| Gastrointestinal | 6 | 40 | 73 | 4 | 0 |
| Hepatic | 38 | 10 | 3 | 0 | 0 |
| Infection febrile neutropaenia | 7 | 11 | 10 | 2 | 0 |
| Metabolic/laboratory | 33 | 20 | 21 | 7 | 0 |
| Neurology | 36 | 9 | 5 | 3 | 0 |
| Pain | 12 | 39 | 19 | 3 | 0 |
| Pulmonary | 19 | 15 | 1 | 0 | 0 |
| Renal/genitourinary | 21 | 15 | 5 | 0 | 0 |
Contributor Information
Mei Li, Email: 25088443@qq.com.
Xiao-Guang Huang, Email: xghuang2012@163.com.
Zhi-Ning Yang, Email: tteerry@gmail.com.
Jia-Yang Lu, Email: 328796180@qq.com.
Yi-Zhou Zhan, Email: 6780540@qq.com.
Wen-Jia Xie, Email: xwj7203@163.com.
Dong-Jie Zhou, Email: 773531642@qq.com.
Li Wang, Email: 570683335@qq.com.
Di-Xia Zhu, Email: gonnawalk@163.com.
Zhi-Xiong Lin, Email: limei00182@139.com.
FUNDING
Supported by the Young Investigator grants of Cancer Hospital, Shantou University Medical College 2013A002 (ML). No additional external funding was received for this study.
REFERENCES
- 1.Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 2009; 27: 3684–90. doi: 10.1200/JCO.2008.19.9109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kam MK, Teo PM, Chau RM, Cheung KY, Choi PH, Kwan WH, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2004; 60: 1440–50. doi: 10.1016/j.ijrobp.2004.05.022 [DOI] [PubMed] [Google Scholar]
- 3.Tham IW, Hee SW, Yeo RM, Salleh PB, Lee J, Tan TW, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy-the national cancer centre singapore experience. Int J Radiat Oncol Biol Phys 2009; 75: 1481–6. doi: 10.1016/j.ijrobp.2009.01.018 [DOI] [PubMed] [Google Scholar]
- 4.Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 2011; 80: 661–8. doi: 10.1016/j.ijrobp.2010.03.024 [DOI] [PubMed] [Google Scholar]
- 5.Milne RW, Dawes C. The relative contributions of different salivary glands to the blood group activity of whole saliva in humans. Vox Sang 1973; 25: 298–307. doi: 10.1111/j.1423-0410.1973.tb04377.x [DOI] [PubMed] [Google Scholar]
- 6.Tabak LA. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol 1995; 57: 547–64. doi: 10.1146/annurev.ph.57.030195.002555 [DOI] [PubMed] [Google Scholar]
- 7.Murdoch-Kinch CA, Kim HM, Vineberg KA, Ship JA, Eisbruch A. Dose-effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys 2008; 72: 373–82. doi: 10.1016/j.ijrobp.2007.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saibishkumar EP, Jha N, Scrimger RA, MacKenzie MA, Daly H, Field C, et al. Sparing the parotid glands and surgically transferred submandibular gland with helical tomotherapy in post-operative radiation of head and neck cancer: a planning study. Radiother Oncol 2007; 85: 98–104. doi: 10.1016/j.radonc.2007.09.014 [DOI] [PubMed] [Google Scholar]
- 9.Liu ZY. Lymphology. Beijing, China: Medicine Science and Technology Press of China; 1995. Chinese. [Google Scholar]
- 10.Li ZQ, Pan QC, Chen JJ. Clinical and experimental study on nasopharyngeal carcinoma. Guangzhou, China: Guangdong Science and Technology Press; 1983. Chinese. [Google Scholar]
- 11.Ng SH, Chang JT, Chan SC, Ko SF, Wang HM, Liao CT, et al. Nodal metastases of nasopharyngeal carcinoma: patterns of disease on MRI and FDG PET. Eur J Nucl Med Mol Imaging 2004; 31: 1073–80. doi: 10.1007/s00259-004-1498-9 [DOI] [PubMed] [Google Scholar]
- 12.Lin DM. Nasopharyngeal carcinoma. In: Yin YZ, Xu GZ, eds. Beijing, China: Beijing Union Medical University Press; 2008. pp. 443–84. Chinese. [Google Scholar]
- 13.Committee. CNCSW. Intensity-modulated radiotherapy target delineation and dose delivery for nasopharyngeal carcinoma—2010 expert consensus guidelines [In Chinese.] Chin J Radiat Oncol. 2011; 20: 267–79. [Google Scholar]
- 14.Pan WR, Suami H, Corlett RJ, Ashton MW. Lymphatic drainage of the nasal fossae and nasopharynx: preliminary anatomical and radiological study with clinical implications. Head Neck 2009; 31: 52–7. doi: 10.1002/hed.20926 [DOI] [PubMed] [Google Scholar]
- 15.Ho FC, Tham IW, Earnest A, Lee KM, Lu JJ. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. BMC Cancer 2012; 12: 98. doi: 10.1186/1471-2407-12-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan JM, Liang Y, Zhao C, Li MY, Liu JJ. Prophylactic irradiation at the level Ib for nasopharyngeal carcinoma: should that be a routine? [In Chinese.] Nan Fang Yi Ke Da Xue Xue Bao 2011; 31: 1989–92. [PubMed] [Google Scholar]
- 17.Grégoire V, Levendag P, Ang KK, Bernier J, Braaksma M, Budach V, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol 2003; 69: 227–36. doi: 10.1016/j.radonc.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 18.van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 1990; 177: 379–84. doi: 10.1148/radiology.177.2.2217772 [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Ou D, He X, Hu C. Sparing level Ib lymph nodes by intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma. Int J Clin Oncol 2014; 19: 998–1004. doi: 10.1007/s10147-013-0650-6 [DOI] [PubMed] [Google Scholar]
- 20.Li M, Huang XG, Yang ZN, Zhan YZ, Lin ZX. Study of level Ib delineation, dose analysis and regional recurrence in level Ib lymph node-negative nasopharyngeal carcinoma patients treated by intensity-modulated radiotherapy. [In Chinese.] Cancer Res Clinic 2015; 27: 27–31. [Google Scholar]
- 21.Huang L, Zhang W, Zhuang T, Wu F, Li D, Zheng M, et al. Submandibular gland sparing in intensity-modulated radiotherapy for N0-stage nasopharyngeal carcinoma. Br J Radiol 2014; 87: 20130651. doi: 10.1259/bjr.20130651 [DOI] [PMC free article] [PubMed] [Google Scholar]