Abstract
Objective:
Low-dose-rate brachytherapy (LDR-BT) in localized prostate cancer is available since 15 years in Italy. We realized the first national multicentre and multidisciplinary data collection to evaluate LDR-BT practice, given as monotherapy, and outcome in terms of biochemical failure.
Methods:
Between May 1998 and December 2011, 2237 patients with early-stage prostate cancer from 11 Italian community and academic hospitals were treated with iodine-125 (125I) or palladium-103 LDR-BT as monotherapy and followed up for at least 2 years. 125I seeds were implanted in 97.7% of the patients: the mean dose received by 90% of target volume was 145 Gy; the mean target volume receiving 100% of prescribed dose (V100) was 91.1%. Biochemical failure-free survival (BFFS), disease-specific survival (DSS) and overall survival (OS) were estimated using Kaplan–Meier method. Log-rank test and multivariable Cox regression were used to evaluate the relationship of covariates with outcomes.
Results:
Median follow-up time was 65 months. 5- and 7-year DSS, OS and BFFS were 99 and 98%, 94 and 89%, and 92 and 88%, respectively. At multivariate analysis, the National Comprehensive Cancer Network score (p < 0.0001) and V100 (p = 0.09) were correlated with BFFS, with V100 effect significantly different between patients at low risk and those at intermediate/high risk (p = 0.04). Short follow-up and lack of toxicity data represent the main limitations for a global evaluation of LDR-BT.
Conclusion:
This first multicentre Italian report confirms LDR-BT as an excellent curative modality for low-/intermediate-risk prostate cancer.
Advances in knowledge:
Multidisciplinary teams may help to select adequately patients to be treated with brachytherapy, with a direct impact on the implant quality and, possibly, on outcome.
INTRODUCTION
Males with localized prostate cancer and indication for curative treatment are candidates for radical prostatectomy (RP), external beam radiotherapy (EBRT) or brachytherapy (BT) depending on the disease features, patient age, health conditions and preferences. Few radiation oncology centres in Italy started low-dose-rate brachytherapy (LDR-BT) at the end of the 1990s and >4200 patients have been treated with this modality until 2014 in 13 institutes.
Long-term results have demonstrated the efficacy of this treatment modality and this approach is considered as an established option for low- and intermediate-risk disease.1–4
The aim of the present study was to realize the first Italian multicentre low-dose-rate prostate BT data collection, reporting the selection criteria, implant parameters and biochemical outcome of patients treated in Italy using this modality and comparing them with other multi-institutional reports.5–10
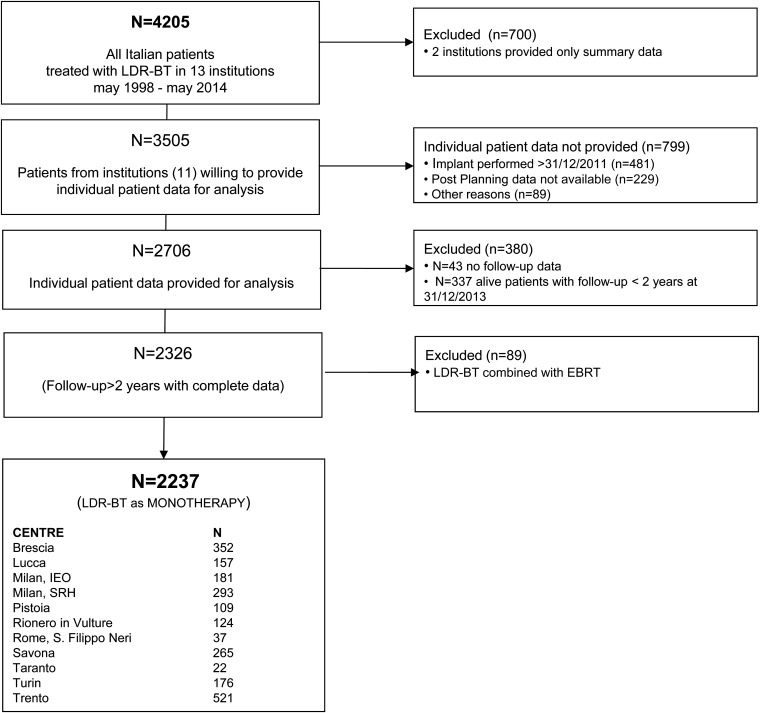
For this purpose, 11 Italian community and academic hospitals (Figure 1) pooled their data to generate a large patient cohort involving 2237 patients treated with LDR-BT over a period of 14 years, now with a minimum follow-up of 2 years.
Figure 1.
CONSORT flow diagram of the study. EBRT, external beam radiotherapy; LDR-BT, low-dose-rate brachytherapy.
METHODS AND MATERIALS
Study design and organization
All the 13 Italian centres performing LDR-BT for prostate cancer were contacted and invited to this study. It is a retrospective multicentre cohort study and consists in a centralized collection and analysis of the clinical and physical parameters of patients who underwent LDR-BT for prostate cancer. The inclusion criteria were as follows: (1) LDR-BT for early prostate cancer; (2) implant performed between May 1998 and December 2011; (3) LDR-BT given as monotherapy; (4) written informed consent and (5) follow-up of minimum of 2 years.
All participating centres were instructed to use the same database previously designed by expert personnel of one centre. Completely anonymized data collection was centrally coordinated by the epidemiology and biostatistics division of another centre.
Database structure, data collection instruments, manuals and processes, especially for the handling of missing data, were standardized and shared by all investigators. Data entry was performed locally by each local data management unit and sent to the coordinating centre for data cleaning and validation.
For the purpose of this study, the following parameters have been collected: age, pre-treatment prostate-specific antigen (PSA) level, Gleason score, T-stage, National Comprehensive Cancer Network (NCCN) risk group classification,11 pre-implant prostate volume, pre-implant androgen deprivation (AD) therapy, implant date, radioactive isotope, prescription dose, post-implant dose received by 90% of target volume (D90), post-implant target volume that received 100% of the prescribed dose (V100), last follow-up date, last post-implant PSA dosage, biochemical failure (BF) and vital status data.
No formal ethics review committee was involved for this retrospective anonymized data collection; all patients gave their written informed consent for LDR-BT, and each step of patient care followed the basic principles outlined in the Declaration of Helsinki.
Statistical analysis
The analyses were performed with SAS statistical software for Windows 9.2 (SAS Institute Inc., Cary, NC).12 Continuous data were expressed as mean ± standard deviation if normally distributed and as median and range or interquartile range (IQR) otherwise; categorical variables were expressed as percentages.
Patients were stratified according to the NCCN risk group classification into low-, intermediate- and high-risk groups: “low risk” was defined as PSA level ≤10 ng ml−1, Gleason score ≤6 and Stage T1–T2a; “intermediate risk” was defined as one or more risk factors: PSA level 10–20 ng ml−1, Gleason score 7 and Stage T2b–T2c; and “high risk” was defined as one or more risk factors: PSA >20 ng ml−1, Gleason score >7 and Stage ≥T3a.11
BF was considered according to the Phoenix definition (PSA nadir plus 2 ng ml−1).13 Biochemical failure-free survival (BFFS) was calculated from the date of implantation to the date of event or latest follow-up.
Disease-specific survival and overall survival (OS) were calculated from date of implantation to date of death or latest follow-up. Survival experience was represented by the Kaplan–Meier approach, with differences between groups evaluated by the log-rank test. Multivariable Cox regression model including NCCN risk group classification, D90, post-implant V100, neoadjuvant AD therapy, patient age and prostatic volume at implant was used to evaluate the relationship of covariates with BFFS. All tests were two-sided.
RESULTS
11 Italian institutions provided clinical data of consecutive patients treated with LDR-BT for clinically localized prostate cancer. Between May 1998 and 31 December 2013, 2706 consecutive patients were treated. However, 380 patients did not reach a minimum follow-up of 2 years and an additional 89 patients were treated with a combination of LDR-BT and EBRT. All these patients were excluded. The last patient with at least 2 years of follow-up was treated on 27 December 2011 and the final number of included patients was 2237 (Figure 1).
Patient characteristics
The mean age of patients was 67 ± 7 years with a median pre-treatment PSA value of 6.5 ng ml−1 (PSA range: 0.64–96) and the mean pre-implant prostate volume (including the effect of an eventual neoadjuvant AD) of 35.7 ± 9.7 cm³.
According to the NCCN risk group classification, 66.4% (1485/2237) of the patients were classified as belonging to low-risk group, 26.0% (582/2237) patients as belonging to intermediate-risk group and 1.8% (41/2237) patients as belonging to high-risk group, while 5.8% (129/2237) patients could not be unequivocally categorized (Table 1).
Table 1.
Baseline characteristics
| Age, categorical (number of patients) (%) | |
| <50 years | 11 (0.5) |
| 50–59 years | 320 (14.3) |
| 60–69 years | 966 (43.2) |
| 70–79 years | 915 (40.9) |
| ≥80 years | 25 (1.1) |
| Age, continuous (years) | |
| Mean (SD) | 67 (7) |
| Median (range) | 68 (39–86) |
| NCCN risk group classification (number of patients) (%) | |
| Low risk | 1485 (66.4) |
| Intermediate risk | 582 (26.0) |
| High risk | 41 (1.8) |
| N/A | 129 (5.8) |
| Gleason score (number of patients) (%) | |
| ≤6 | 1861 (83.2) |
| 7 | 271 (12.1) |
| >7 | 28 (1.3) |
| N/A | 77 (3.4) |
| T stagea (number of patients) (%) | |
| T1 (a, b, c) | 1597 (71.4) |
| T2–T2a | 354 (15.8) |
| T2b–T2c | 141 (6.3) |
| T3 (a, b, c) | 1 (0.1) |
| N/A | 144 (6.4) |
| PSA category at entry (number of patients) (%) | |
| ≤10 ng ml−1 | 1937 (86.6) |
| 10–20 ng ml−1 | 260 (11.6) |
| >20 ng ml−1 | 14 (0.6) |
| N/A | 26 (1.2) |
| PSA at entry (ng ml−1) | |
| Median (range) | 6.5 (0.64–96) |
| Neoadjuvant AD therapy (number of patients) (%) | |
| No | 1099 (49.1) |
| Yes | 882 (39.4) |
| N/A | 256 (11.5) |
| Pre-implant prostate volume (cm3) | |
| N | 2115 |
| Mean (SD) | 35.7 (9.7) |
| Median (range) | 35.0 (11.5–83.5) |
AD, androgen deprivation; N/A, not available; NCCN, National Comprehensive Cancer Network; PSA, prostate-specific antigen; SD, standard deviation.
American Joint Committee on Cancer staging, 7th edn, 2009.
Table 2 reports the proportion of patients who had received AD before BT within each NCCN risk group. The higher the NCCN risk classification, the greater the proportion of patients with a history of AD before LDR-BT implantation.
Table 2.
Neoadjuvant androgen deprivation (AD) therapy and risk group classification
| Neoadjuvant AD therapy | NCCN risk group classification |
||||
|---|---|---|---|---|---|
| Low |
Intermediate |
High |
N/A |
Total |
|
| Number of patients (%) | Number of patients (%) | Number of patients (%) | Number of patients (%) | Total number of patients (%) | |
| No | 795 (58.0) | 248 (48.1) | 8 (36.4) | 48 (66.7) | 1099 (55.5) |
| Yes | 576 (42.0) | 268 (51.9) | 14 (63.6) | 24 (33.3) | 882 (44.5) |
| Total with AD therapy information | 1371 | 516 | 22 | 72 | 1981 |
| N/A | 114 | 66 | 19 | 57 | 256 |
| Total | 1485 | 582 | 41 | 129 | 2237 |
N/A, not available; NCCN, National Comprehensive Cancer Network.
Treatment procedures
A similar BT protocol was carried out by all 11 institutions. In all the institutions, a multidisciplinary uro-oncologic team, caring also for patients with prostate cancer, was active.
Seed implantation was performed using a transperineal approach with transrectal ultrasound guidance. The radioactive isotope implanted was iodine-125 (125I) in most of the cases and palladium-103 (103Pd) in some of the most dated cases. The intended prescribed dose was changed depending on the isotope used (145 Gy and 135 Gy using 125I and 103Pd, respectively). Dose was prescribed to the prostate volume as defined at ultrasound images and a choice of a margin around prostate was operator dependent, usually ranging between 3 and 5 mm to account for possible extraprostatic extension and for seed release uncertainties.
Neoadjuvant AD therapy with an antiandrogen and/or a luteinizing hormone-releasing hormone analogue was prescribed mainly for volume reduction in patients with large prostate for a short period (3–6 months; median 4 months).14
Post-implant CT dosimetry was performed within 1 month (mainly on Day 30) of implantation.15
Patients were followed up every 3–6 months with PSA assays for the first 2 years and every 6–12 months thereafter.
Treatment data
125I and 103Pd seeds were implanted in 2185 (97.7%) patients and 52 (2.3%) patients, respectively. The mean D90 was 146 Gy (±28 Gy) for patients with 125I seeds and 130 Gy (±24 Gy) for patients with 103Pd seeds; the V100 was 91.2% (±7.4%) for patients with 125I seeds and 87.9% (±8.1%) for patients with 103Pd implants (Table 3).
Table 3.
Treatment modality and dosimetry
| Dosimetry | Radioactive isotope |
Total | |
|---|---|---|---|
|
125I |
103Pd |
|
|
| N = 2185 | N = 52 | N = 2237 | |
|
D90 (Gy) | |||
| N | 2173 | 50 | 2223 |
| Mean (SD) | 146 (28) | 130 (24) | 145 (28) |
| Median (IQR) | 149 (124–167) | 134 (123–146) | 149 (124–166) |
|
D90 (% of the prescribed dose) | |||
| N | 2173 | 50 | 2223 |
| Mean (SD) | 1.01 (0.2) | 0.97 (0.17) | 1.01 (0.2) |
| Median (IQR) | 1.03 (0.86–1.15) | 0.99 (0.91–1.08) | 1.03 (0.86–1.15) |
|
V100 (%) | |||
| N | 2172 | 52 | 2224 |
| Mean (SD) | 91.2 (7.4) | 87.9 (8.1) | 91.1 (7.4) |
| Median (IQR) | 93 (89–96) | 90 (87–93) | 93 (89–96) |
125I, iodine-125; 103Pd, palladium-103; D90, dose received by 90% of target volume; IQR, interquartile range; SD, standard deviation; V100, target volume receiving 100% of the prescribed dose.
Outcome data
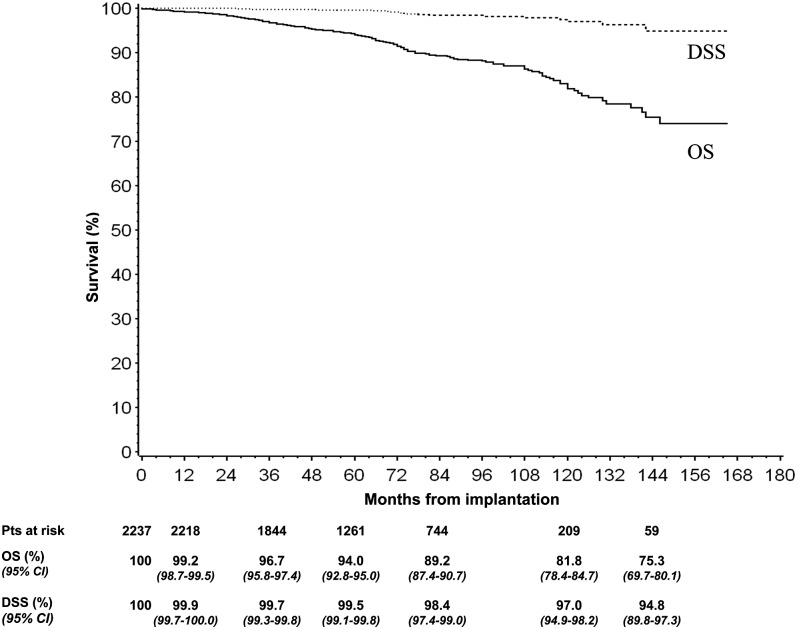
The overall median follow-up time was 65 months (IQR: 42–93 months). 204 deaths were recorded. 172 patients died without any BF, while 32 males died after a BF. For 26 of them, the fatal event was related to metastatic disease progression (Table 4). The 3-, 5- and 7-year OS rates were 96.7, 94.0 and 89.2%, respectively, and the 3-, 5- and 7-year disease-specific survival rates were 99.7, 99.5 and 98.4%, respectively (Figure 2).
Table 4.
Events
| Outcomes | N (%) |
|---|---|
| Follow-up duration (months)a (%) | |
| Mean (SD) | 69 (34) |
| Median (IQR) | 65 (42–93) |
| Vital status (number of patients) (%) | |
| Alive | 2033 (90.9) |
| Dead | 204 (9.4) |
| Death without BF | 172 |
| ≤24 months | 33 |
| >24 months | 139 |
| Death after BF | 32 |
| ≤24 months | 1 |
| >24 months | 31 |
| Of whom owing to prostatic cancer | 26 |
| BF (number of patients) (%) | |
| No | 2030 (90.7) |
| Yes | 207 (9.3) |
| Alive at the last follow-up | 175 |
| Death after BF | 32 |
| Time to BF (months)a | |
| N | 207 |
| Mean (SD) | 48 (29) |
| Median (IQR) | 42 (24–64) |
BF, biochemical failure; IQR, interquartile range; SD, standard deviation.
From date of low-dose-rate brachytherapy implantation.
Figure 2.
Kaplan–Meier analysis of overall survival (OS) and disease-specific survival (DSS) with 95% confidence interval (CI). Pts, patients.
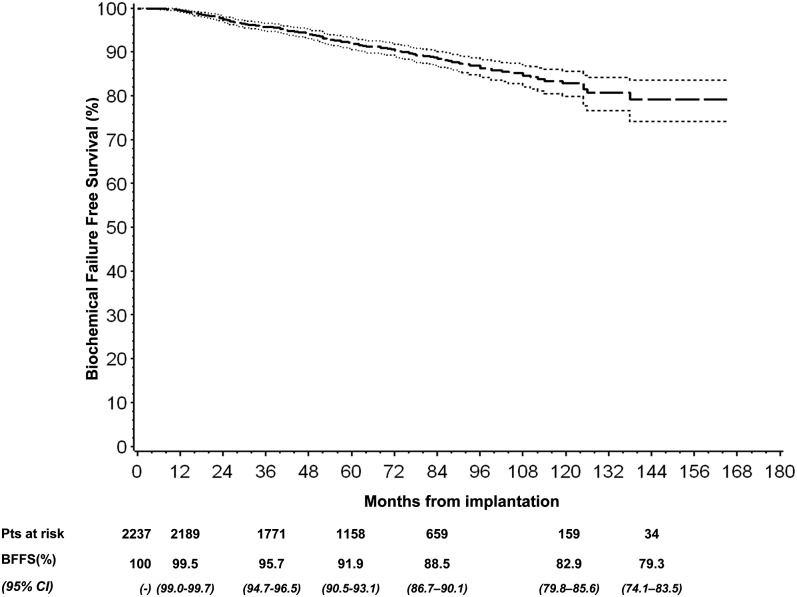
207 patients experienced a BF and 175 of them were alive at the last follow-up. The median time elapsed between LDR-BT implantation and occurrence of BF was approximately 42 months (IQR: 24–64 months) (Table 4). The 3-, 5- and 7-year BFFS was 95.7, 91.9 and 88.5%, respectively (Figure 3 and Table 5).
Figure 3.
Kaplan–Meier analysis of biochemical failure-free survival (BFFS) with 95% confidence interval (CI). Pts, patients.
Table 5.
Biochemical failure-free survival (BFFS) in subgroups
| Subgroups | Total | Events | Univariate analysis |
Multivariate analysisc |
||||
|---|---|---|---|---|---|---|---|---|
| 3-year BFFS (%) | 5-year BFFS (%) | 7-year BFFS (%) | p-valuea | RR (95% CI) | p-valueb | |||
| All patientsb | 2237 | 207 | 95.7 | 91.9 | 88.5 | |||
| NCCN risk group classification | ||||||||
| Low risk | 1485 | 93 | 97.0 | 94.8 | 92.8 | <0.001 | 1 | |
| Intermediate risk | 582 | 91 | 93.8 | 86.0 | 78.4 | 2.60 (1.93–3.51) | <0.0001 | |
| High risk | 41 | 10 | 78.9 | 73.0 | 73.0 | 3.02 (1.38–6.60) | 0.006 | |
|
V100 (%) | ||||||||
| <90% | 642 | 77 | 95.3 | 90.1 | 86.4 | 0.03 | 1 | |
| ≥90% | 1582 | 128 | 96.0 | 92.8 | 89.5 | 0.76 (0.55–1.04) | 0.09 | |
|
D90 (% of the prescribed dose) | ||||||||
| <90% | 668 | 62 | 95.8 | 91.1 | 89.1 | 0.49 | 1 | |
| ≥90% | 1555 | 143 | 95.8 | 92.3 | 88.5 | 1.01 (0.72–1.42) | 0.93 | |
| Neoadjuvant AD therapy | ||||||||
| No | 1099 | 96 | 96.3 | 92.6 | 89.2 | 0.59 | 1 | |
| Yes | 882 | 80 | 95.8 | 92.0 | 89.1 | 0.79 (0.58–1.07) | 0.13 | |
| Radioactive isotope | ||||||||
| 125I | 2185 | 202 | 95.7 | 91.9 | 85.5 | 0.45 | 1 | |
| 103Pd | 52 | 5 | 96.1 | 91.7 | 89.1 | 0.92 (0.37–2.25) | 0.85 | |
| Age (years) | ||||||||
| <55 | 102 | 7 | 96.9 | 95.7 | 90.9 | 0.42 | 1 | |
| 55–64 | 637 | 56 | 94.6 | 93.4 | 90.8 | 1.31 (0.59–2.88) | 0.51 | |
| 65–74 | 1187 | 112 | 96.3 | 91.5 | 88.0 | 1.38 (0.64–2.97) | 0.42 | |
| ≥75 | 311 | 32 | 95.2 | 88.7 | 84.7 | 1.50 (0.65–3.46) | 0.34 | |
| Interactionsd | ||||||||
| NCCN risk group–V100 | ||||||||
| ≥90% vs <90% (low-risk group) | 0.53 (0.33–0.84) | 0.04 | ||||||
| ≥90% vs <90% (intermediate-/high-risk group) | 1.09 (0.69–1.74) | |||||||
| NCCN risk group–neoadjuvant AD therapy | ||||||||
| AD therapy vs no AD therapy (low-risk group) | 0.56 (0.35–0.89) | 0.07 | ||||||
| AD therapy vs no AD therapy (intermediate-/high-risk group) | 1.09 (0.69–1.71) | |||||||
| NCCN risk group–radioactive isotope | ||||||||
| 103Pd vs125I (low-risk group) | 0.43 (0.10–1.76) | 0.10 | ||||||
| 103Pd vs125I (intermediate-/high-risk group) | 2.34 (0.72–7.61) | |||||||
125I, iodine-125; 103Pd, palladium-103; AD, androgen deprivation; CI, confidence interval; D90, dose received by 90% of target volume; NCCN, National Comprehensive Cancer Network; RR, relative risk; V100; target volume receiving 100% of prescribed dose.
The sum of the number of patients among levels of a variable could be not equal to the total number of patients (N = 2237).
The “not available (N/A)” subgroups, although included both in univariate and multivariate models, have not been shown in this table.
Log-rank test for univariate analysis.
Wald test from Cox regression multivariate model.
Includes NCCN risk group, V100, D90, neoadjuvant AD therapy, radioactive isotope, age and prostatic volume at implantation.
Includes the same variables of c plus the interaction term.
Multivariate analysis showed that BFFS was significantly higher among patients in the low-risk group (p < 0.0001) and close to be significantly higher among those with V100 ≥ 90% (p = 0.09). In particular, after inclusion of the interaction term of the two factors in the model, we found that V100 ≥ 90% increased BFFS only in the subset of patients in the low-risk group [relative risk = 0.53 (95% confidence interval: 0.33–0.84], while no effect was found among the patients in the intermediate-/high-risk group [relative risk = 1.09 (95% confidence interval: 0.69–1.74); p = 0.04] for the interaction term. No other factor exhibited significant influence on BFFS (Table 5).
To check for potential prognostic factors on OS, we performed univariate and multivariate analyses. The intermediate-/high-risk group showed the worst OS compared with the low-risk group (p = 0.04 and p = 0.07, respectively); OS was also worst in elderly patients (p < 0.0001) (Table 6).
Table 6.
Overall survival (OS) in subgroups
| Subgroups | Total | Events | Univariate analysis |
Multivariate analysisc |
||||
|---|---|---|---|---|---|---|---|---|
| 3-year OSc (%) | 5-year OS (%) | 7-year OS (%) | p-valuea | RR (95% CI) | p-valueb | |||
| All patientsb | 2237 | 204 | 96.7 | 94.0 | 89.0 | |||
| NCCN risk group classification | ||||||||
| Low risk | 1485 | 117 | 97.2 | 94.7 | 91.1 | 0.001 | 1 | |
| Intermediate risk | 582 | 69 | 95.3 | 91.9 | 85.7 | 1.37 (1.01–1.87) | 0.04 | |
| High risk | 41 | 8 | 92.9 | 89.6 | 77.1 | 2.12 (0.94–4.76) | 0.07 | |
|
V100 (%) | ||||||||
| <90% | 642 | 54 | 97.6 | 94.7 | 90.1 | 0.16 | 1 | |
| ≥90% | 1582 | 146 | 96.4 | 93.8 | 89.1 | 1.30 (0.92–1.85) | 0.14 | |
|
D90 (% of the prescribed dose) | ||||||||
| <90% | 668 | 50 | 96.6 | 93.9 | 89.9 | 0.43 | 1 | |
| ≥90% | 1555 | 150 | 96.9 | 94.2 | 89.2 | 1.22 (0.85–1.76) | 0.28 | |
| Neoadjuvant AD therapy | ||||||||
| No | 1099 | 89 | 97.2 | 94.0 | 89.7 | 0.30 | 1 | |
| Yes | 882 | 99 | 95.9 | 93.3 | 88.6 | 0.98 (0.73–1.32) | 0.90 | |
| Radioactive isotope | ||||||||
| 125I | 2185 | 197 | 96.6 | 94.1 | 89.3 | 0.96 | 1 | |
| 103Pd | 52 | 7 | 98.0 | 91.6 | 86.6 | 1.29 (0.60–2.78) | 0.51 | |
| Age (years) | ||||||||
| <55 | 102 | 1 | 99.0 | 99.0 | 99.0 | <0.0001 | 0.05 (0.01–0.39) | 0.001 |
| 55–64 | 637 | 38 | 97.9 | 96.5 | 92.7 | 0.33 (0.21–0.52) | <0.0001 | |
| 65–74 | 1187 | 117 | 96.0 | 92.9 | 88.4 | 0.55 (0.39–0.77) | <0.001 | |
| ≥75 | 311 | 48 | 95.9 | 91.2 | 81.5 | 1 | – | |
125I, iodine-125; 103Pd, palladium-103; AD, androgen deprivation; CI, confidence interval; D90, dose received by 90% of target volume; NCCN, National Comprehensive Cancer Network; RR, relative risk; V100, target volume receiving 100% of prescribed dose.
The sum of the number of patients among levels of a variable could be not equal to the total number of patients (N = 2237).
The “not available (N/A)” subgroups, although included both in univariate and multivariate models, have not been shown in this table.
Log-rank test for univariate analysis.
Wald test from Cox regression multivariate model.
Includes NCCN risk group, V100, D90, neoadjuvant AD therapy, radioactive isotope, age and prostatic volume at implantation.
DISCUSSION
This early report is a retrospective multicentre cohort study and gives an efficient picture of the practice of BT in Italy, including 11/13 centres practising it and most of the patients treated in Italy. To the best of our knowledge, this is the largest LDR-BT European series ever reported.
Our results indicate that an implant of good quality, both for case selection and post-implant dosimetric parameters, has been obtained in most patients. The selection criteria show adherence to accepted guidelines based on the European Society for Radiotherapy and Oncology–European Association of Urology–European Organization for Research and Treatment of Cancer recommendations,16,17 with implant being used for 66.4% of cases in the low-risk group and 26.0% of cases in the intermediate-risk group, with a prescribed dose of 145 Gy for I125; a short AD therapy, was given to 39.4% of patients (mainly for downsizing). Post-implant data show a mean D90 of 146 Gy and a mean V100 value of 91.2% for patients with I125 implants.
The BFFS rates at 5 and 7 years were estimated to be 91.9% and 88.5% for the whole group, respectively, with an event rate of 6.3% (93/1485) in the low-risk group, 15.6% (91/582) in the intermediate-risk group and 24.4% (10/41) in the high-risk group, respectively (p < 0.0001). Our results, in agreement with other multi-institutional reports5–8,10 and with two recently published monoinstitutional series,18,19 indicate that LDR-BT with permanent implant as monotherapy is an adequate modality for the radical treatment of low- and intermediate-risk prostate cancer. Only 89 patients with unfavourable factors were treated combining BT and EBRT and were excluded in the present analysis. Durable cancer control was reported with the BT and EBRT combination in these patients,20 but there may be an increased toxicity,21 and LDR-BT alone can produce excellent biochemical control for low- as well as intermediate-risk disease.4
Approximately 40% of patients in the present series had previously undergone neoadjuvant AD therapy for 3–6 months. This short period of AD did not impact on BFFS. This is in agreement with other reports.10,22
The impact of post-implant dosimetric parameters on BFFS in multi-institutional studies is not univocal. Zelefsky et al5 noted a significant impact of D90 on BFFS after a median follow-up of 63 months. These data are reinforced by Stone et al.9 Contrarily, no post-implant dosimetric factors predicted for biochemical control in a UK multi-institutional series with a median follow-up of 21 months.7 Morris et al8 reported results of the British Columbia Cancer Agency BT database after a median follow-up of 7.5 years: D90 values of <130 Gy were predictive of an increased risk of recurrence but only for the subset of males who did not receive AD therapy.10 In our cohort, post-implant V100, but not D90, impacted significantly on biochemical control, in spite of a strict correlation between them. The 5- and 7-year BFFS was 90.1 vs 92.8% and 86.4 vs 89.5% for V100 <90% vs ≥90% (p = 0.03), whereas the difference was not statistically significant for D90 <130 Gy or ≥130 Gy (p = 0.49). These results were confirmed after multivariate analysis (p = 0.09 and p = 0.93, respectively). We think that this may be a consequence of the preponderance of good-quality implants in the present series: only 30% (668/2237) of patients had a D90 <90% of the prescribed dose and median D90 was 149 Gy for patients with I125 seeds. Furthermore, the evaluation of post-implant dosimetric parameters and their impact on BFFS may be less robust owing to differences in post-planning CT timing and interpretation among different institutions;23 the average D90 in our cohort had a standard deviation of 28 Gy. A more standard approach to volume delineation should be an important aspect of quality assurance in prostate BT.17 As stated by Morris et al10 in their study, “dose metrics are not equivalent to oncologic end points and must be calibrated against disease-free survival using biochemical and clinical end points for each institution”.
Prostate BT seems comparable with both EBRT and RP.4,24 No randomized trials are available and many comparative outcome studies are largely single-centre studies with limited generalizability, and a population-based study provides the best outcome data. This report details PSA and OS outcomes after LDR-BT in a large and consecutive population-based cohort of patients and together with other multi-institutional studies,5–8,10 it might give more generalizable data.
There are several limitations of our study. Firstly, data were collected retrospectively. Not all the institutions provided sufficient data and not all the information we had planned to get were actually available in the local databases. The lack of important covariates (comorbidity, smoking history, centre-specific policy regarding frequency of PSA testing during all over the follow-up and site of clinical failure) results in a substantial loss of strength of our multivariate and subgroup analyses. Secondly, the median follow-up was only of 65 months; this time is too short for a substantial evaluation of the real biochemical outcome after a radical treatment. This relatively short follow-up duration and the high rate of patients lost to long-term follow-up produce a loss of power for the 10-year survival estimates. For this reason, in our analysis, we showed estimates at earlier time points.
Thirdly, although these LDR-BT results are encouraging, our series may include the selection bias, especially in the intermediate-risk group (selection of more favourable cases). Therefore, direct comparison of our findings in the intermediate-risk patients with those obtained with other treatment modalities such as EBRT and RP should be performed with caution.
Finally, our study does not provide any data on BT side effects. Evaluation of treatment sequelae in a retrospective multi-institutional study is difficult and uncertain. Still, BT complications and their impact on quality of life in the series of a participating centre have been reported.25
The retrospective character of our report carries the well-known risks of missing data and selection bias. However, our report provides information on BT results in nearly all patients who underwent LDR-BT in Italy. Reporting these data is now particularly crucial for a realistic comparison with other modalities of radical treatment, such as new surgery and radiotherapy techniques or radiotherapy fractionation schemes, which are rapidly spreading in the community,26,27 despite a less mature evidence of efficacy and perhaps a higher cost.
CONCLUSION
In conclusion, the findings in this largest European series are in agreement with those previously reported in literature and confirm that LDR-BT is an excellent curative modality for low- and intermediate-risk prostate cancer. The work of the multidisciplinary team involved in the treatment of patients with prostate cancer may help to select adequately patients to be treated with BT and this may impact on the implant quality and possibly on outcome.
Acknowledgments
ACKNOWLEDGMENTS
The contributions of Prof. Sergio Cosciani and Prof. Claudio Simeone to the Brescia Uro-oncology multidisciplinary team are gratefully acknowledged. We thank Cristiana Fodor, MSc, for her valuable help in data management in the final part of this work.
Contributor Information
Giovanni Fellin, Email: giovanni.fellin@apss.tn.it.
Maria A Mirri, Email: iaia.mirri@gmail.com.
Luigi Santoro, Email: luigi.santoro@ieo.it.
Barbara A Jereczek-Fossa, Email: barbara.jereczek@ieo.it.
Claudio Divan, Email: claudio.divan@apss.tn.it.
Salvatore Mussari, Email: salvatore.mussari@apss.tn.it.
Francesco Ziglio, Email: francesco.ziglio@apss.tn.it.
Beniamino La Face, Email: brachiterapia@spedalicivili.brescia.it.
Fernando Barbera, Email: barbera.f@tin.it.
Michela Buglione, Email: buglione@med.unibs.it.
Laura Bandera, Email: laurabandera81@gmail.com.
Barbara Ghedi, Email: bghedi@libero.it.
Nadia G Di Muzio, Email: dimuzio.nadia@hsr.it.
Andrea Losa, Email: losa.andrea@hsr.it.
Paola Mangili, Email: mangili.paola@hsr.it.
Luciano Nava, Email: lucianonava@alice.it.
Renato Chiarlone, Email: renatochiarlone@gmail.com.
Nunzia Ciscognetti, Email: nunziaciscognetti@yahoo.it.
Emilio Gastaldi, Email: emilio.gastaldi@tiscali.it.
Federica Cattani, Email: federica.cattani@ieo.it.
Ruggero Spoto, Email: ruggero.spoto@ieo.it.
Andrea Vavassori, Email: andrea.vavassori@ieo.it.
Francesca R Giglioli, Email: francescaromanagiglioli@gmail.com.
Alessia Guarneri, Email: alessia.guarneri.ag@gmail.com.
Valentina Cerboneschi, Email: vale.cerboneschi@gmail.it.
Marcello Mignogna, Email: m.mignogna@usl2.toscana.it.
Mauro Paoluzzi, Email: m.paoluzzi@usl2.toscana.it.
Valentina Ravaglia, Email: v.ravaglia@usl2.toscana.it.
Costanza Chiumento, Email: chiumento.costanza@gmail.com.
Stefania Clemente, Email: stefaniaclementesc@gmail.com.
Vincenzo Fusco, Email: fuscovincenzo@hotmail.com.
Roberto Santini, Email: r.santini@usl3.toscana.it.
Marco Stefanacci, Email: m.stefanacci@usl3.toscana.it.
Francesco P Mangiacotti, Email: f.mangiacotti@sanfilipponeri.roma.it.
Marco Martini, Email: martinimarco69@gmail.com.
Tiziana Palloni, Email: t.palloni@gmail.com.
Giuseppe Schinaia, Email: giuseppe.schinaia@uniroma1.it.
Grazia Lazzari, Email: lazzarigrazia@gmail.com.
Giovanni Silvano, Email: gisilva@tin.it.
Stefano Magrini, Email: magrini@med.unibs.it.
Umberto Ricardi, Email: umberto.ricardi@unito.it.
Riccardo Santoni, Email: riccardo.santoni@uniroma2.it.
Roberto Orecchia, Email: roberto.orecchia@ieo.it.
REFERENCES
- 1.Taira AV, Merrick GS, Butler WM, Galbreath RW, Lief J, Adamovich E, et al. Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys 2011; 79: 1336–42. doi: 10.1016/j.ijrobp.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 2.Crook J, Borg J, Evans A, Toi A, Saibishkumar EP, Fung S, et al. 10-year experience with I-125 prostate brachytherapy at the Princess Margaret Hospital: results for 1,100 patients. Int J Radiat Oncol Biol Phys 2011; 80: 1323–9. doi: 10.1016/j.ijrobp.2010.04.038 [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Chou JF, Pei X, Yamada Y, Kollmeier M, Cox B, et al. Predicting biochemical tumor control after brachytherapy for clinically localized prostate cancer: the Memorial Sloan-Kettering Cancer Center experience. Brachytherapy 2012; 11: 245–9. doi: 10.1016/j.brachy.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 4.Grimm P, Billiet I, Bostwick D, Dicker AP, Frank S, Immerzeel J, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int 2012; 109(Suppl. 1): 22–9. doi: 10.1111/j.1464-410X.2011.10827.x [DOI] [PubMed] [Google Scholar]
- 5.Zelefsky MJ, Kuban DA, Levy LB, Potters L, Beyer DC, Blasko JC, et al. Multi-institutional analysis of long-term outcome for stages T1–T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys 2007; 67: 327–33. doi: 10.1016/j.ijrobp.2006.08.056 [DOI] [PubMed] [Google Scholar]
- 6.Guedea F, Aguilo F, Polo A, Langley S, Laing R, Henderson A, et al. Early biochemical outcomes following permanent interstitial brachytherapy as monotherapy in 1050 patients with clinical T1–T2 prostate cancer. Radiother Oncol 2006; 80: 57–61. doi: 10.1016/j.radonc.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 7.Mitchell DM, Mandall P, Bottomley D, Hoskin PJ, Logue JP, Ash D, et al. Report on the early efficacy and tolerability of I(125) permanent prostate brachytherapy from a UK multi-institutional database. Clin Oncol (R Coll Radiol) 2008; 20: 738–44. doi: 10.1016/j.clon.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 8.Morris WJ, Keyes M, Spadinger I, Kwan W, Liu M, McKenzie M, et al. Population-based 10-year oncologic outcomes after low-dose-rate brachytherapy for low-risk and intermediate-risk prostate cancer. Cancer 2013; 119: 1537–46. doi: 10.1002/cncr.27911 [DOI] [PubMed] [Google Scholar]
- 9.Stone NN, Potters L, Davis BJ, Ciezki JP, Zelefsky MJ, Roach M, et al. Customized dose prescription for permanent prostate brachytherapy: insights from a multicenter analysis of dosimetry outcomes. Int J Radiat Oncol Biol Phys 2007; 69: 1472–77. doi: 10.1016/j.ijrobp.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 10.Morris WJ, Spadinger I, Keyes M, Hamm J, McKenzie M, Pickles T. Whole prostate D90 and V100: a dose-response analysis of 2000 consecutive (125)I monotherapy patients. Brachytherapy 2014; 13: 32–41. doi: 10.1016/j.brachy.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Reese AC, Pierorazio PM, Han M, Partin AW. Contemporary evaluation of the National Comprehensive Cancer Network prostate cancer risk classification system. Urology 2012; 80: 1075–9. doi: 10.1016/j.urology.2012.07.040 [DOI] [PubMed] [Google Scholar]
- 12.SAS Institute Inc. Corporation, Statistical analysis software 9.2. Cary, NC: SAS Institute; 2008. [Google Scholar]
- 13.Abramowitz MC, Li T, Buyyounouski MK, Ross E, Uzzo RG, Pollack A, et al. The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer 2008; 112: 55–60. doi: 10.1002/cncr.23139 [DOI] [PubMed] [Google Scholar]
- 14.Lee LN, Stock RG, Stone NN. Role of hormonal therapy in the management of intermediate—to high-risk prostate cancer treated with permanent radioactive seed implantation. Int J Radiat Oncol Biol Phys 2002; 52: 444–52. doi: 10.1016/S0360-3016(01)02598-6 [DOI] [PubMed] [Google Scholar]
- 15.Rivard MJ, Coursey BM, DeWerd LA, Hanson WF, Huq MS, Ibbott GS, et al. Update of AAPM Task Group No. 43 Report: a revised AAPM protocol for brachytherapy dose calculations. Med Phys 2004; 31: 633–74. doi: 10.1118/1.1646040 [DOI] [PubMed] [Google Scholar]
- 16.Ash D, Flynn A, Battermann J, de Reijke T, Lavagnini P, Blank L; ESTRA/EAU Urological Brachytherapy Group; EORTC Radiotherapy Group. ESTRO/EAU/EORTC recommendations on permanent seed implantation for localized prostate cancer. Radiother Oncol 2000; 57: 315–21. doi: 10.1016/S0167-8140(00)00306-6 [DOI] [PubMed] [Google Scholar]
- 17.Salembier C, Lavagnini P, Nickers P, Mangili P, Rijnders A, Polo A, et al. Tumour and target volumes in permanent prostate brachytherapy: a supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapy. Radiother Oncol 2007; 83: 3–10. doi: 10.1016/j.radonc.2007.01.014 [DOI] [PubMed] [Google Scholar]
- 18.Guarneri A, Botticella A, Filippi AR, Munoz F, Beltramo G, Casetta G, et al. 125I brachytherapy for localized prostate cancer: a single institution experience. Tumori 2013; 99: 83–7. doi: 10.1700/1248.13793 [DOI] [PubMed] [Google Scholar]
- 19.Giberti C, Chiono L, Gallo F, Schenone M, Gastaldi E. Radical retropubic prostatectomy versus brachytherapy for low-risk prostatic cancer: a prospective study. World J Urol 2009; 27: 607–12. doi: 10.1007/s00345-009-0418-9 [DOI] [PubMed] [Google Scholar]
- 20.Critz FA, Benton JB, Shrake P, Merlin ML. 25-year disease-free survival rate after irradiation for prostate cancer calculated with the prostate specific antigen definition of recurrence used for radical prostatectomy. J Urol 2013; 189: 878–83. doi: 10.1016/j.juro.2012.10.061 [DOI] [PubMed] [Google Scholar]
- 21.Lawton CA, Yan Y, Lee WR, Gillin M, Firat S, Baikadi M, et al. Long-term results of an RTOG Phase II Trial (00-19) of external-beam radiation therapy combined with permanent source brachytherapy for intermediate-risk clinically localized adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 2012; 82: e795–801. doi: 10.1016/j.ijrobp.2011.11.040 [DOI] [PubMed] [Google Scholar]
- 22.Merrick GS, Butler WM, Wallner KE, Galbreath RW, Allen ZA, Adamovich E. Androgen-deprivation therapy does not impact cause-specific or overall survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 2006; 65: 669–77. doi: 10.1016/j.ijrobp.2006.01.030 [DOI] [PubMed] [Google Scholar]
- 23.Crook J, Milosevic M, Catton P, Yeung I, Haycocks T, Tran T, et al. Interobserver variation in postimplant computed tomography contouring affects quality assessment of prostate brachytherapy. Brachytherapy 2002; 1: 66–73. doi: 10.1016/S1538-4721(02)00014-4 [DOI] [PubMed] [Google Scholar]
- 24.Kupelian PA, Potters L, Khuntia D, Ciezki JP, Reddy CA, Reuther AM, et al. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or = 72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1–T2 prostate cancer. Int J Radiat Oncol Biol Phys 2004; 58: 25–33. [DOI] [PubMed] [Google Scholar]
- 25.Caffo O, Fellin G, Bolner A, Coccarelli F, Divan C, Frisinghelli M, et al. Prospective evaluation of quality of life after interstitial brachytherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2006; 66: 31–7. doi: 10.1016/j.ijrobp.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 26.Mahmood U, Pugh T, Frank S, Levy L, Walker G, Haque W, et al. Declining use of brachytherapy for the treatment of prostate cancer. Brachytherapy 2014; 13: 157–62. doi: 10.1016/j.brachy.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 27.Martin JM, Handorf EA, Kutikov A, Uzzo RG, Bekelman JE, Horwitz EM, et al. The rise and fall of prostate brachytherapy: use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer 2014; 120: 2114–21. doi: 10.1002/cncr.28697 [DOI] [PMC free article] [PubMed] [Google Scholar]