Abstract
Objective:
To develop an alternative three-dimensional treatment plan with standardized fields class solution for whole-breast radiotherapy in patients with large/pendulous breast and/or high body mass index (BMI).
Methods:
Two treatment plans [tangential fields and standardized five-fields technique (S5F)] for a total dose of 50 Gy/25 fractions were generated for patients with large breasts [planning target volume (PTV) >1000 cm3 and/or BMI >25 kg m−2], supine positioned. S5F plans consist of two wedged tangential beams, anteroposterior: 20° for the right breast and 340° for the left breast, and posteroanterior: 181° for the right breast and 179° for the left breast. A field in field in medial–lateral beam and additional fields were added to reduce hot spot areas and extra–target-tissue irradiation and to improve dose distribution. The percentage of PTV receiving 95% of the prescribed dose (PTV V95%), percentage of PTV receiving 105% of the prescribed dose (PTV V105%), maximal dose to PTV (PTV Dmax), homogeneity index (HI) and conformity index were recorded. V10%, V20%, V105% and V107% of a “proper” normal tissue structure (body-PTV healthy tissue) were recorded. Statistical analyses were performed using SYSTAT v.12.0 (SPSS, Chicago, IL).
Results:
In 38 patients included, S5F improved HI (8.4 vs 10.1; p ≤ 0.001) and significantly reduced PTV Dmax and PTV V105%. The extra–target-tissue irradiation was significantly reduced using S5F for V105% (cm3) and V107% (cm3) with a very high difference in tissue irradiation (46.6 vs 3.0 cm3, p ≤ 0.001 for V105% and 12.2 vs 0.0 cm3, p ≤ 0.001 for V107% for tangential field and S5F plans, respectively). Only a slight increase in low-dose extra–target-tissue irradiation (V10%) was observed (2.2719 vs 1.8261 cm3, p = 0.002).
Conclusion:
The S5F technique in patients with large breast or high BMI increases HI and decreases hot spots in extra-target-tissues and can therefore be easily implemented in breast cancer radiotherapy.
Advances in knowledge:
The treatment planning strategy proposed in this study has several advantages: (a) it is extremely reliable as the standard supine positioning is used; (b) the standardized class solution allows for widespread use; (c) time and cost of treatment are not increased; and (d) it can be used for both large breasted and obese patients not compliant to different treatment positioning.
INTRODUCTION
The breast-conserving approach with the adjuvant use of radiotherapy (RT) has gained an important role in the treatment for early-stage breast cancer with excellent long-term local control and survival.1 During or shortly after the course of breast cancer RT, a large portion of patients will experience acute radiation dermatitis and breast oedema to some degree.2 There is accumulating clinical evidence that acute reactions are associated with the development of late toxicity which includes telangiectasia, breast induration and pain.3–5
The severity of RT-induced breast damage relates to the biological damage to the epidermis, dermis and connective tissue during treatment.6 Although all patients undergoing RT are at risk of these reactions, there are a number of intrinsic and extrinsic risk factors that can affect the severity.7 Intrinsic factors include age, current or previous history of smoking, malnourishment, concomitant disease/medication, obesity, the presence of skin folds and previously irradiated areas.8–10 Extrinsic factors relate to the type of treatment with regard to the energy of the radiation beam, the dose distribution with hot spots and/or the field size of the treated area.
Although the skin and subcutaneous tissues are not a dose-limiting tissue, skin toxicity, breast induration and pain are associated with impairment of the patients' quality of life, causing pain and discomfort and limiting activities.2,11
Furthermore, surgery, chemotherapy and RT can have a significant impact on health-related quality of life and can be worse in females with higher body mass index (BMI).12 The majority of existing studies on obesity and health-related quality of life in females with breast cancer have not specially focused on patients undergoing RT.12–14 Obesity has been associated with greater acute toxicity with adjuvant RT for breast cancer.12,13 Higher BMI has also previously been associated with breast cancer treatment-related lymphoedema and inferior disease-specific survival in both pre- and post-menopausal females.13–15 Overweight and obese females are at higher risk of developing treatment-related symptoms, with higher BMI found to be associated with development of long-term pain after breast cancer treatment.15,16
Moreover, the treatment of large or pendulous breasts has been associated with impaired cosmetic outcomes due to inhomogeneous dose distribution.17 This anatomic feature, characterized by the tendency of the breast to fall laterally and/or superiorly, leads to the inclusion of a larger portion of the lung and the heart in the treatment fields and increases inframammary folds yielding a bolus effect.18
In these patients, the challenge is to minimize these side effects without losing efficacy of the treatment.19,20
Prone position had been used in order to reduce the dose received by normal tissues and the size of hot spots.21,22 However, coexisting large or pendulous breasts and high BMI make this kind of approach difficult because of some positioning complications.
Introducing improved radiation techniques, such as intensity-modulated RT (IMRT), has led to a reduction in acute skin toxicity and late fibrosis and improved cosmetic outcome.19,20 This type of treatment is, however, not available in all RT centres, whereas breast cancer RT is one of the most diffused treatments in radiation oncology.
The aim of our study was to develop an alternative simple and easily available three-dimensional (3D) treatment plan with a standardized fields class solution for patients with large/pendulous breast and/or high BMI in order to improve homogeneity and to reduce hot spots and extra–target-tissue dose irradiation.
METHODS AND MATERIALS
A retrospective cohort of patients who underwent conservative surgery and adjuvant breast irradiation with 3D conformal RT technique were selected for this study. Patients were selected based on their anatomy (large or pendulous breasts, CT simulation breast volume >1000 cm3 or when breast tissue tended to fall towards the mid axillary line, and/or a BMI of >25 kg m−2). Simulations were performed with patients positioned supine on a breast board with the ipsilateral arm raised above the head. The scan was extended from the jugular notch to 5 cm below the lower edge of the breast with a scan interval of 5 mm. The target volume, heart and lungs were manually contoured on each CT slice by a single radiation oncologist following the Radiation Therapy Oncology Group guidelines23 and heart atlas published by Feng et al.24 The clinical target volume was defined as the entire breast excluding the outer 4 mm from the skin surface. The planning target volume (PTV) was defined as clinical target volume + 5 mm in the direction of the chest wall. An apposite normal tissue structure (body-PTV healthy tissue) was generated subtracting the PTV from the body volume.
Treatment planning techniques
Eclipse™ v. 7.3.10 (Varian Medical Systems, Palo Alto, CA) treatment planning was used to generate two different treatment plans for each patient [a = “tangential fields” (TF); b = “standardized five-fields technique” (S5F)] described below. The pencil beam convolution 7.5.18.0 algorithm was used.
The total prescribed dose was 50 Gy at the isocentre in accordance with the International Commission on Radiation Units Measurement. All plans were optimized according to the following constraint for the PTV: V95% ≥ 95% (the volume receiving 95% of the prescription dose or more must be ≥95% of the PTV). This was considered as the primary constraint. As a secondary constraint, we considered the following: PTV V105% ≤ 5% of the prescribed dose.
Tangential fields treatment plans
Treatment plans consisted of a simple wedged tangential plan (with gantry angles optimized to match divergence of the posterior edges of the beam) to avoid contralateral breast irradiation and to minimize the ipsilateral lung and heart area in the beam's eye view or field-in-field technique, in which the dose on each of the two tangential beams was split into two different segments. The first segment was designed to encompass the entire breast. A second segment was then directed to this area of underdosing, in order to compensate for the drop in dose and to reduce hot spot areas. The weights of the two segments were determined through an iterative process repeated until optimal results were achieved. The weight of these segments is typically in the range of 10–15% of the total. The energy of the photon beams was 6 MV for TFs and 15 MV for field-in-field beams; in some cases, to increase dose coverage in depth, the energy of the tangential beams was also 15 MV.
Standardized five-fields technique treatment plans
Five-fields treatment plans were made with two wedged tangential beams such as above (fields 1–2), with an anteroposterior (AP) field with 20° rotation starting from the zero-position (linear accelerator's gantry 20° for right breast and 340° for left breast treatment) (field 3), and a posteroanterior (PA) field with 179° rotation starting from the zero position (linear accelerator's gantry 181° for right-breast treatment and 179° for left-breast treatment) in order to avoid collision between the linear accelerator and table (field 4). Moreover, a field in field with a weight of 10–15% and the same geometry of the medial–lateral beam (field 5) was added to reduce hot spot areas, to homogenize dose to the target and to improve dose distribution in the chest wall region. The fields AP and PA, with a weight of 10–15%, were optimized to compensate the dose fall localized at the centre of the breast, avoiding the extra-target-tissues at the level of the scapula and including not >1 cm of the lung in the field's beam's eye view. The energy of photon beams was 6 MV for TFs and 15 MV for field-in-field, AP and PA beams.
Statistical analysis
Dose–volume histograms were generated for PTV and organs at risk for all plans. For PTV, the percentage of PTV receiving 95% of the prescribed dose (V95%), percentage of PTV receiving 105% of the prescribed dose (V105%), maximal dose to PTV (Dmax) and a homogeneity index (HI) defined as HI = 100 × (D2% − D98%)/Dp, where Dp is the prescribed dose, were chosen as parameters for comparison. Lower HI values indicate a more homogeneous target dose. V105% was chosen to specify the target volume receiving high doses. In addition, a conformity index (CI) was defined as CI = Vri/VPTV, where Vri is the volume encompassed by the reference isodose for the PTV (95% of the prescribed dose) and VPTV is the PTV volume expressed in cm3. Body-PTV healthy tissue V10%, V20%, V105% and V107% were defined as surrogates for extra–target-tissue irradiation. For each dosimetric variable, normality was tested using the Shapiro–Wilk test. For the ipsilateral lung, the two plans were compared in terms of mean lung dose, V2.5Gy, V5Gy, V20Gy, V40Gy and V50Gy and for the heart, mean heart dose, V2.5GyV5Gy, V15Gy and V25Gy. Comparisons were made by means of Mann–Whitney test.
The statistical analysis was performed using SYSTAT v. 12.0 (SPSS, Chicago, IL).
RESULTS
Patients' characteristics
38 patients were included in the analysis. Patients' characteristics are listed in Table 1. 17 (44.7%) patients were right sided, 21 left sided (55.3%). The mean BMI was 33.6 (standard deviation 7.1). 22 (57.9%) patients had a breast CT simulation volume >1000 cm3.
Table 1.
Patients' characteristics
| Patients' characteristics | n |
|---|---|
| Patients | 38 |
| Age, years | |
| Median | 65 |
| Range | 40–82 |
| Tumour side | |
| Right | 17 |
| Left | 21 |
| Breast separation (cm) | |
| Mean | 18.7 |
| SD | 2.6 |
| BMI | |
| Mean | 33.6 |
| SD | 7.1 |
| PTV (cm3) | |
| Mean | 1077.0 |
| SD | 362.4 |
BMI, body mass index; PTV, planning target volume; SD, standard deviation.
Target coverage
The analysis results for target coverage are reported in Table 2. The CI, as well as V95%, was not significantly different between the two groups (p = not significant). However, the constraint PTV V95% > 95% was achieved for all plans realized with S5F, but not for TF treatment plans. S5F improved dose homogeneity (8.4 vs 10.1; p ≤ 0.001). In particular, Dmax and PTV V105% were significantly reduced with this approach (106.9% vs 108.6%, 1.9% vs 4.46% respectively; p ≤ 0.001) (Figure 1).
Table 2.
Comparison of target coverage data (median values, range)
| Dosimetric data | Standard tangential | 5-fields technique | p-value |
|---|---|---|---|
| HI | |||
| Median | 10.1 | 8.4 | <0.001 |
| Range | 7.1–38.6 | 6.8–16.5 | |
| CI | |||
| Median | 1.0 | 1.0 | 0.795 |
| Range | 0.7–1.0 | 0.9–1.0 | |
| PTV V95% (%) | |||
| Median | 98.9 | 98.9 | 0.752 |
| Range | 71.9–99.9 | 95.1–99.9 | |
| PTV V105% (%) | |||
| Median | 4.5 | 1.9 | <0.001 |
| Range | 0.1–9.3 | 0.0–6.4 | |
| PTV Dmax% (%) | |||
| Median | 108.9 | 106.9 | <0.001 |
| Range | 105.1–117.0 | 105.2–111.0 | |
| PTV Dmax (Gy) | |||
| Median | 54.1 | 53.4 | <0.001 |
| Range | 52.7–57.5 | 52.5–55.3 | |
CI, conformity index; HI, homogeneity index; PTV, planning target volume.
HI = 100 × (D2% − D98%)/prescribed dose.
CI = volume of PTV encompassed by 95% of the prescribed dose/PTV volume.
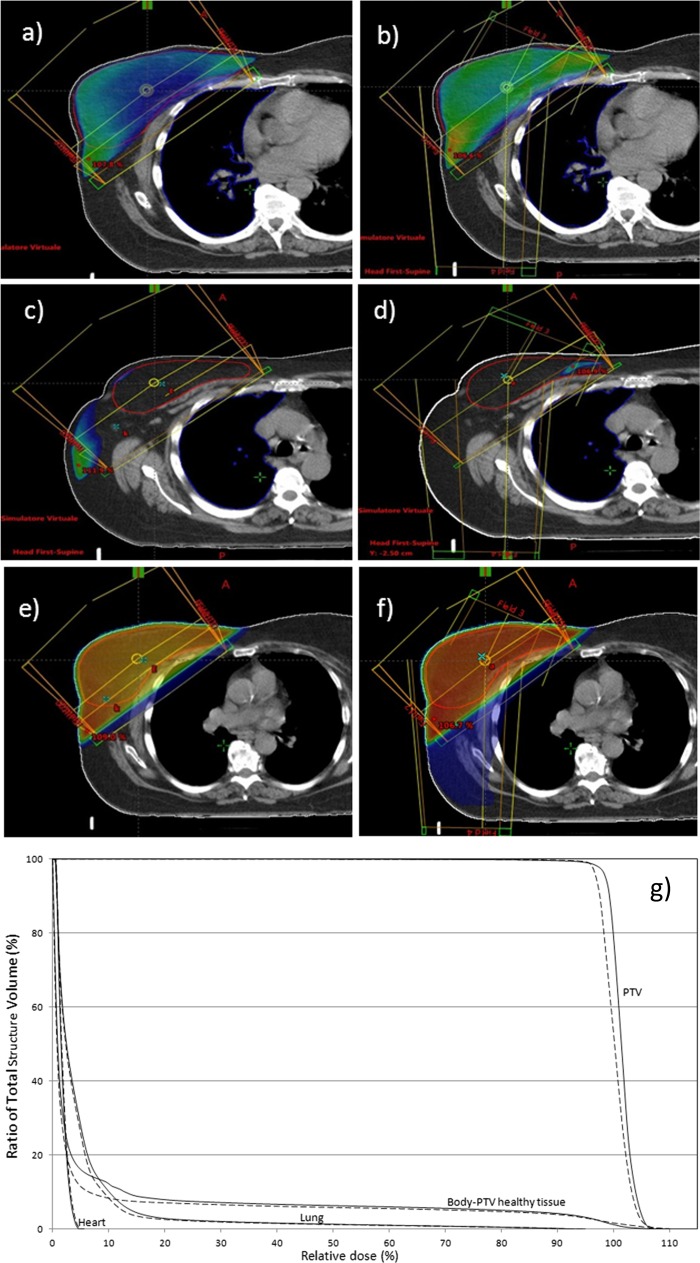
Figure 1.
(a, b) 95% Isodose distribution: (a) tangential fields (TF) technique; (b) standardized five-fields technique (S5F). (c, d) Body-planning target volume (PTV) healthy tissue 105% isodose distribution (extra–target-tissue irradiation): (c) TF technique; (d) S5F. (e, f) Body-PTV healthy tissue 10% isodose distribution (extra–target-tissue irradiation): (e) TF technique; (f) S5F. (g) Dose–volume histogram (DVH): PTV, ipsilateral lung, heart and body-PTV healthy tissue DVH for TF (dashed line) and Standardized five-fields technique (solid line).
Normal tissue irradiation
Normal tissue dosimetric data are summarized in Table 3 (median value and range).
Table 3.
Comparison of organ at risk (median values, ranges)
| Organs at risk | Standard tangential | Five-fields technique | p-value |
|---|---|---|---|
| Lung (ipsilateral) | |||
| MLD (Gy) | |||
| Median | 3.8 | 4.1 | 0.585 |
| Range | 1.8–12.1 | 2.2–12.2 | |
| V2.5Gy (%) | |||
| Median | 25.7 | 28.8 | 0.010 |
| Range | 13.4–45.8 | 20.8–45.7 | |
| V5Gy (%) | |||
| Median | 13.0 | 14.8 | 0.112 |
| Range | 4.9–37.3 | 7.2–38.9 | |
| V20Gy (%) | |||
| Median | 5.0 | 5.2 | 0.986 |
| Range | 1.0–23.3 | 0.6–23.3 | |
| V40Gy (%) | |||
| Median | 2.8 | 2.8 | 0.901 |
| Range | 0.1–16.9 | 0.0–14.9 | |
| V50Gy (%) | |||
| Median | 0.0 | 0.0 | 0.124 |
| Range | 0.0–1.8 | 0.0–0.9 | |
| Heart (left sided) | |||
| MHD (Gy) | |||
| Median | 2.0 | 1.9 | 0.562 |
| Range | 1.1–2.6 | 1.3–2.5 | |
| V2.5Gy (%) | |||
| Median | 17.1 | 16.5 | 0.656 |
| Range | 3.5–28.8 | 6.5–27.9 | |
| V5Gy (%) | |||
| Median | 4.3 | 4.1 | 0.519 |
| Range | 0.0–8.2 | 0.0–7.5 | |
| V15Gy (%) | |||
| Median | 0.5 | 0.4 | 0.769 |
| Range | 0.0–1.7 | 0.0–1.6 | |
| V25Gy (%) | |||
| Median | 0.2 | 0.1 | 0.934 |
| Range | 0.0–0.8 | 0.0–0.8 | |
| Heart (right sided) | |||
| MHD (Gy) | |||
| Median | 0.8 | 0.9 | 0.534 |
| Range | 0.4–1.2 | 0.5–1.2 | |
| V2.5Gy (%) | |||
| Median | 0.0 | 0.0 | 0.820 |
| Range | 0–8.5 | 0.0–4.2 | |
| V5Gy (%) | |||
| Median | 0.0 | 0.0 | 0.264 |
| Range | 0.0–0.0 | 0.0–0.0 | |
| V15Gy (%) | |||
| Median | 0.0 | 0.0 | 1000 |
| Range | 0.0–0.0 | 0.0–0.0 | |
| V25Gy (%) | |||
| Median | 0.0 | 0.0 | 1000 |
| Range | 0.0–0.0 | 0.0–0.0 | |
| Body-PTV healthy tissue | |||
| V10% (cm3) | |||
| Median | 1826.1 | 2271.9 | 0.002 |
| Range | 942.6–3472.8 | 1117.2–3601.0 | |
| V20% (cm3) | |||
| Median | 1561.0 | 1587.0 | 0.720 |
| Range | 810.2–2974.4 | 824.2–2577.5 | |
| V105% (cm3) | |||
| Median | 46.6 | 3.0 | <0.001 |
| Range | 0.0–394.6 | 0.0–41.0 | |
| V107% (cm3) | |||
| Median | 12.2 | 0.0 | <0.001 |
| Range | 0.0–298.8 | 0.0–5.4 | |
MLD, mean lung dose; MHD, mean heart dose; PTV, planning target volume.
Lung and heart dosimetric parameters analysed showed no differences between the two techniques (Table 3), with the exception of lung V2.5Gy which was increased in S5F treatment plans (28.8% vs 25.7%, p = 0.010). Particularly, heart dosimetry (mean heart dose, V2.5Gy, V5Gy, V15Gy, V25Gy) was not different regardless of the breast cancer side. On the contrary, high-dose extra–target-tissue irradiation (body-PTV healthy tissue) was significantly reduced using S5F; in particular, absolute V105 values are reduced by 15 times (46.6 vs 3.0 cm3, p ≤ 0.001) and V107% from 12.2 vs 0 cm3 (p ≤ 0.001) (Figure 1). However, a slight increase in low-dose extra–target-tissue irradiation (body-PTV healthy tissue V10%) was observed using S5F (2.2719 vs 1.8261 cm3, p = 0.002) (Figure 1).
DISCUSSION
The aim of this study was to develop a simple and easily available alternative to the tangential 3D treatment plan with a standardized fields class solution for patients with large/pendulous breast and/or high BMI in order to reduce hot spots particularly at the inframammary fold and extra–target-tissues irradiation. In our series, the S5F showed improved dose homogeneity (8.4 vs 10.1; p ≤ 0.001), lower PTV V105% (4.5 vs 1.9%; p ≤ 0.001) and extra–target-tissue irradiation (body-PTV healthy tissue) (46.6 vs 3.0 cm3, p ≤ 0.001).
Several attempts had been carried out in order to achieve a better dose distribution and thus reduce radiation-induced toxicity, including the use of novel techniques (inverse/forward IMRT), different patient positioning (lateral/prone decubitus) and using immobilization devices that displace the breast from the chest wall.
Pignol et al11 in a multicentre randomized trial with a total of 358 patients showed how IMRT technique improved radiation treatment quality by reducing the clinical significant maximum dose gradient (105% vs 110%; p ≤ 0.001), PTV V105% (7.5% vs 16.9%), and reported how these dosimetric benefits translated into a lower proportion of patients experiencing acute moist desquamation (31.2% vs 47%; p = 0.002). A British study with median follow-up of 5 years, which included 1145 treated patients, showed that the use of forward planned IMRT reduces the rates of telangiectasia (odds ratio 0.58, 95% confidence interval: 0.36–0.92, p = 0.021).25
Similarly, a study carried out after 4.7 years of median follow-up showed a reduction in chronic breast oedema (3% vs 30%; p = 0.007) and hyperpigmentation (3 vs 41%; p = 0.001), especially in patients with larger breasts (volume >1600 cm3).26
Even though breast cancer RT is one of the most diffused applications of radiation oncology, this technique is not, however, always available in all RT centres, as the use of IMRT is not always justified due to the cost of technology, particularly when less expensive alternatives that yield similar target dose coverage and normal organ sparing are readily available.
Lateral decubitus position decreases separation and improves dose homogeneity in the target by changing breast shape. Recently, Kirova et al27 reported in a study carried out on 56 patients that a maximum dose to the breast of 53.48 Gy on average, a low mean lung and heart dose of 0.96 and 1.35 Gy, respectively, and an incidence of acute grade 3 dermatitis of 1.8% was acceptable.
The greatest challenge of this positioning lies in the complexity of daily set-up. Prone set-up yields the same advantages of lateral decubitus RT with a simpler set-up and better accuracy. Moreover, several studies have investigated prone RT where dosimetric studies22,28 showed increased homogeneity and reduced lung doses with prone positioning in comparison with supine positioning, whereas in a large single-centre study by Stegman et al,29 that reviewed the data of 245 patients treated over a 12-year period, planning was with opposed coplanar beams and the median hot spot percentage was 106% (interquartile range 104–108%) in the majority of cases.
Grade 3 acute skin toxicity reported with prone positioning ranges from 2% to 14.5%.29,30 Even though prone set-up is considered a reasonable option for large breasted patients, it, however, requires patients' compliance and some older patients may not be able to maintain the position. Additionally, in our series 63.0% of patients had a BMI >30% and 94.3% had a BMI >25. In these overweight/obese patients, if a large bore CT scan is not available, prone set-up may not be feasible.
Finally, approaches were proposed which included the construction of a thermoplastic mould or reinforced polyvinyl chloride ring and Styrofoam™ (The Dow Chemical Company, Midland, MI)17,31,32 to pull the lateral breast tissue anteriorly and upright and to decrease breast separation.
Recently Arenas et al18 reported on the use of breast cups on patients with large or pendulous breasts.
The use of breast cups resulted in a significant reduction of the PTV volume (from 1640 to 1283 cm3) of the irradiated volume (from 2154 to 1477 cm3) and of the CI (from 1383 to 1213). Furthermore, the use of breast cups also led to significant dose reductions in V20 for the lung (from 13.7% to 1.7%) and V5 for the heart (from 9.8% to 2.7%).
Despite these encouraging results, however, these devices may be limited in standard application due to decreased reproducibility, patient discomfort and costs.
On the contrary, the treatment planning strategy proposed in this study has several advantages: (a) it is extremely reliable as a standard supine positioning is used; (b) the standardized class solution allows for widespread use; (c) time and cost of treatment are not increased; and (d) it can be used for both large breasted and obese patients not compliant to different treatment positioning.
There are some drawbacks in our study. First, treatment plans were performed by an “expert” operator. Second, low doses (lung V2.5Gy and body-PTV healthy tissue V10%) were slightly increased using S5F due to the contribution derived both from the AP and PA field. However, we believe this is limited as the two fields had low weight (10–15%). Furthermore, we have no clinical data on the efficacy of this treatment in reducing skin toxicity. For this reason, we are currently evaluating this 3D treatment strategy in a clinical setting.
In conclusion, S5F in patients with large breast or high BMI increases HI and decreases Dmax hot spots in extra-target-tissues by a factor of 15 and can therefore be easily implemented in post-operative breast cancer RT.
Contributor Information
Gerardina Stimato, Email: g.stimato@unicampus.it.
Edy Ippolito, Email: e.ippolito@unicampus.it.
Sonia Silipigni, Email: s.silipigni@unicampus.it.
Cristina Di Venanzio, Email: c.divenanzio@unicampus.it.
Carla Germana Rinaldi, Email: carlagermana@hotmail.it.
Diego Gaudino, Email: d.gaudino@unicampus.it.
Michele Fiore, Email: m.fiore@unicampus.it.
Lucio Trodella, Email: l.trodella@unicampus.it.
Rolando Maria D'Angelillo, Email: r.dangelillo@unicampus.it.
Sara Ramella, Email: s.ramella@unicampus.it.
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG): Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011; 378: 1707–16. doi: 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feight D, Baney T, Bruce S, McQuestion M. Putting evidence into practice. Clin J Oncol Nurs 2011; 15: 481–92. doi: 10.1188/11.CJON.481-492 [DOI] [PubMed] [Google Scholar]
- 3.Tanteles GA, Whitworth J, Mills J, Peat I, Osman A, McCann GP, et al. Can cutaneous telangiectasiae as late normal-tissue injury predict cardiovascular disease in women receiving radiotherapy for breast cancer? Br J Cancer 2009; 101: 403–9. doi: 10.1038/sj.bjc.6605182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lilla C, Ambrosone CB, Kropp S, Helmbold I, Schmezer P, von Fournier D, et al. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Res Treat 2007; 106: 143–50. doi: 10.1007/s10549-006-9480-9 [DOI] [PubMed] [Google Scholar]
- 5.Keller LM, Sopka DM, Li T, Klayton T, Li J, Anderson PR, et al. Five-year results of whole breast intensity modulated radiation therapy for the treatment of early stage breast cancer: the Fox Chase Cancer Center experience. Int J Radiat Oncol Biol Phys 2012; 84: 881–7. [DOI] [PubMed] [Google Scholar]
- 6.Princess Royal Radiotherapy Review Team. Managing radiotherapy induced skin reactions, a toolkit for healthcare professionals. Leeds: Leeds Teaching Hospitals NHS Trust; 2011. [Google Scholar]
- 7.Porock D. Factors influencing the severity of radiation skin and oral mucosal reactions: development of a conceptual framework. Eur J Cancer Care (Engl) 2002; 11: 33–43. [PubMed] [Google Scholar]
- 8.Wells M, MacBride S. Radiation skin reactions. In: Faithfull S, Wells M, eds. Supportive care in radiotherapy. London, UK: Churchill Livingstone; 2004. [Google Scholar]
- 9. NHS Quality Improvement Scotland. 2010 Best practice statement. Skincare of patients receiving radiotherapy. Available from: http://tinyurl.com/l2hl9jh.
- 10.Rutter CE, Qin L, Higgins SA, Moran MS, Evans SB. Dosimetric and clinical predictors of the development of moist desquamation in breast cancer irradiation. J Radiat Oncol 2014; 3: 147–52. [Google Scholar]
- 11.Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 2008; 26: 2085–92. doi: 10.1200/JCO.2007.15.2488 [DOI] [PubMed] [Google Scholar]
- 12.Morcos B, Ahmad FA, Anabtawi I, Sba' AM, Shabani H, Yaseen R. Development of breast cancer-related lymphedema: is it dependent on the patient, the tumor or the treating physicians? Surg Today 2014; 44: 100–6. doi: 10.1007/s00595-013-0494-8 [DOI] [PubMed] [Google Scholar]
- 13.Paxton RJ, Phillips KL, Jones LA, Chang S, Taylor WC, Courneya KS, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer 2012; 118: 4024–31. doi: 10.1002/cncr.27389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010; 123: 627–35. doi: 10.1007/s10549-010-0990-0 [DOI] [PubMed] [Google Scholar]
- 15.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol 2002; 20: 1128–43. doi: 10.1200/JCO.20.4.1128 [DOI] [PubMed] [Google Scholar]
- 16.Forsythe LP, Alfano CM, George SM, McTiernan A, Baumgartner KB, Bernstein L, et al. Pain in long-term breast cancer survivors: the role of body mass index, physical activity, and sedentary behavior. Breast Cancer Res Treat 2013; 137: 617–30. doi: 10.1007/s10549-012-2335-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zierhut D, Flentje M, Frank C, Oetzel D, Wannenmacher M. Conservative treatment of breast cancer: modified irradiation technique for women with large breasts. Radiother Oncol 1994; 31: 256–61. [DOI] [PubMed] [Google Scholar]
- 18.Arenas M, Hernández V, Farrús B, Müller K, Gascón M, Pardo A, et al. Do breast cups improve breast cancer dosimetry? A comparative study for patients with large or pendulous breasts. Acta Oncol 2014; 53: 795–801. doi: 10.3109/0284186X.2014.893062 [DOI] [PubMed] [Google Scholar]
- 19.Freedman GM, Anderson PR, Li J, Eisenberg DF, Hanlon AL, Wang L, et al. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am J Clin Oncol 2006; 29: 66–70. doi: 10.1097/01.coc.0000197661.09628.03 [DOI] [PubMed] [Google Scholar]
- 20.Moody AM, Mayles WP, Bliss JM, A'Hern RP, Owen JR, Regan J, et al. The influence of breast size on late radiation effects and association with radiotherapy dose inhomogeneity. Radiother Oncol 1994; 33: 106–12. [DOI] [PubMed] [Google Scholar]
- 21.Formenti SC, Gidea-Addeo D, Goldberg JD, Roses DF, Guth A, Rosenstein BS, et al. Phase I–II trial of prone accelerated intensity modulated radiation therapy to the breast to optimally spare normal tissue. J Clin Oncol 2007; 25: 2236–42. [DOI] [PubMed] [Google Scholar]
- 22.Ramella S, Trodella L, Ippolito E, Fiore M, Cellini F, Stimato G, et al. Whole-breast irradiation: a subgroup analysis of criteria to stratify for prone position treatment. Med Dosim 2012; 37: 186–91. doi: 10.1016/j.meddos.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 23.Li XA, Tai A, Arthur DW, Buchholz TA, Macdonald S, Marks LB, et al. ; Radiation Therapy Oncology Group Multi-Institutional and Multiobserver Study. Variability of target and normal structure delineation for breast cancer radiotherapy: an RTOG Multi-Institutional and Multiobserver Study. Int J Radiat Oncol Biol Phys 2009; 73: 944–51. doi: 10.1016/j.ijrobp.2008.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011; 79: 10–18. doi: 10.1016/j.ijrobp.2009.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukesh MB, Barnett GC, Wilkinson JS, Moody AM, Wilson C, Dorling L, et al. Randomized controlled trial of intensity-modulated radiotherapy for early breast cancer: 5-year results confirm superior overall cosmesis. J Clin Oncol 2013; 31: 4488–95. doi: 10.1200/JCO.2013.49.7842 [DOI] [PubMed] [Google Scholar]
- 26.Harsolia A, Kestin L, Grills I, Wallace M, Jolly S, Jones C, et al. Intensity-modulated radiotherapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int J Radiat Oncol Biol Phys 2007; 68: 1375–80. doi: 10.1016/j.ijrobp.2007.02.044 [DOI] [PubMed] [Google Scholar]
- 27.Kirova YM, Hijal T, Campana F, Fournier-Bidoz N, Stilhart A, Dendale R, et al. Whole breast radiotherapy in the lateral decubitus position: a dosimetric and clinical solution to decrease the doses to the organs at risk (OAR). Radiother Oncol 2014; 110; 477–81. doi: 10.1016/j.radonc.2013.10.038 [DOI] [PubMed] [Google Scholar]
- 28.Varga Z, Hideghéty K, Mezo T, Nikolényi A, Thurzó L, Kahán Z. Individual positioning: a comparative study of adjuvant breast radiotherapy in the prone versus supine position. Int J Radiat Oncol Biol Phys 2009; 75: 94–100. doi: 10.1016/j.ijrobp.2008.10.045 [DOI] [PubMed] [Google Scholar]
- 29.Stegman LD, Beal KP, Hunt MA, Fornier MN, McCormick B. Long-term clinical outcomes of whole-breast irradiation delivered in the prone position. Int J Radiat Oncol Biol Phys 2007; 68: 73–81. [DOI] [PubMed] [Google Scholar]
- 30.Bergom C, Kelly T, Morrow N, Wilson JF, Walker A, Xiang Q, et al. Prone whole-breast irradiation using three-dimensional conformal radiotherapy in women undergoing breast conservation for early disease yields high rates of excellent to good cosmetic outcomes in patients with large and/or pendulous breasts. Int J Radiat Oncol Biol Phys 2012; 83: 821–8. doi: 10.1016/j.ijrobp.2011.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentel GC, Marks LB. A simple device to position large/flaccid breasts during tangential breast irradiation. Int J Radiat Oncol Biol Phys 1994; 29: 879–82. [DOI] [PubMed] [Google Scholar]
- 32.Huppert N, Jozsef G, Dewyngaert K, Formenti SC. The role of a prone setup in breast radiation therapy. Front Oncol 2011; 1: 31. doi: 10.3389/fonc.2011.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]