Abstract
Ocular melanoma is the most common adult primary intraocular tumour. Although <1% of patients have metastatic disease at the time of initial diagnosis, most will develop metastasis at varying lengths of time. Metastasis surveillance is therefore critical in the follow-up of patients with ocular melanoma. Liver is the most common site of metastasis and prognosis is based on the treatment of liver metastasis. Hence, imaging of liver metastasis is vital. MRI is the most specific modality for imaging liver metastasis and is at least as sensitive as CT. Extrahepatic metastasis such as retroperitoneal nodules and bone metastases are also better evaluated on MRI. Gadolinium-based contrast agents are extremely helpful for detecting liver lesions. In particular, newer hepatobiliary contrast agents which offer an additional hepatobiliary phase of excretion help in the detection of even tiny liver metastases. Diffusion-weighted imaging is helpful when an i.v. contrast cannot be administered. Treated lesions are also better evaluated with MRI. CT is useful for evaluating lung nodules, large liver metastasis or in patients in whom MRI is medically contraindicated. The disadvantage lies in its inability to detect small liver metastasis and the radiation dose involved. The lesions treated with iodized oil as part of chemoembolization procedures can be followed on CT. Ultrasound can be used only for detecting hepatic metastases. However, it is heavily operator dependent, technically challenging and time consuming especially in patients who are large. Extrahepatic metastasis cannot be seen on ultrasound. Its utility is primarily for the biopsy of liver lesions. Positron emission tomography (PET)-CT can detect lung nodules and large liver lesions but is insensitive to small liver lesions. Moreover, the high radiation dose is a major disadvantage.
IMAGING OF OCULAR MELANOMA METASTASIS
Ocular melanoma is the most common adult primary intraocular tumour, with a stable incidence over the past 30 years of 5.1 per million. The overwhelming majority of those affected are Caucasian. In ocular melanoma, unlike most cancers, <1% of patients have metastatic disease at the time of initial diagnosis. However, unfortunately, many do go on to develop metastases. The Collaborative Ocular Melanoma Study, one of the largest prospective studies, with a longitudinal follow-up of 2320 patients, found a 10-year cumulative metastatic rate of 34%.1 The most frequent site of ocular melanoma metastasis is the liver (90%), followed by the lung (30%), bone (23%) and skin (17%). Metastatic disease is identified on an average about 3 years after the diagnosis of the primary tumour.2,3 The median survival time after diagnosis of metastasis in the largest series of patients with metastatic uveal melanoma was 3.6 months,4, although time to metastasis can be prolonged up to 42 years in some cases.5 As liver metastases are the most common cause of death in these patients, this review focused largely on imaging of the liver.
Given the prolonged time frame in which metastasis can occur, a rational surveillance programme is critical. The method of surveillance varies widely between institutions and in different parts of the world, and there is no universally accepted protocol. In Europe, ultrasound of the liver is typically performed every 6 months for 10 years, with CT or MRI being performed if a suspicious lesion is visualized.6 At tertiary-care centres in the USA, surveillance is usually carried out in a twofold manner, using MRI of the abdomen with i.v. contrast for optimal liver surveillance and CT chest, abdomen and pelvis for whole-body surveillance, with the timing based on the risk of metastasis indicated by the tumour histology and genetic profile.
ROLE OF GENETICS IN SURVEILLANCE OF METASTATIC OCULAR MELANOMA
Genetics plays a dominant role in the surveillance of ocular melanoma metastasis. Cytogenetic profiling is usually offered by the oncologists to patients undergoing treatment for ocular melanoma. Patients are characterized as Class 1 or Class 2 based on the specific genetic expression profiles. Class 1 tumours have a low risk of metastasis unlike Class 2 tumours, which are associated with an increased risk of metastasis and a much worse prognosis.7 The findings are summarized in Table 1.
Table 1.
Genetic expression profiles in ocular melanoma7
| Types | Chromosomal abnormalities |
|---|---|
| Class 1 Better prognosis and lower risk of metastasis |
6-p gain 8-q gain |
| Class 2 Increased risk of metastasis and a worse prognosis |
Loss of other copy of Chromosome 3 or Monosomy 3 Mutation of BAP1 (BRCA-1 associated protein 1) gene 1-p loss 8-p loss 8-q gain (seen in both) |
Surveillance protocols vary by institution. At our ocular melanoma clinic, Class 2 patients who have a higher risk of metastasis are followed with a CT of the chest and an MRI of the abdomen at 3-month intervals for the first 2 years, and then with an MRI abdomen every 6 months and a CT chest every 12 months. Patients with a lower risk of metastasis, namely Class 1 patients, are imaged with an abdomen MRI every 6–12 months and a chest radiograph every 12 months for 5 years.
LIVER FUNCTION TESTS
The COMS determined that liver function tests (LFTs) are a poor surveillance tool with a sensitivity of only 14.7%.1 Although other studies have showed abnormal LFTs, especially elevated transaminases and alkaline phosphatase, in a higher percentage of patients, this finding is seen only with large liver lesions or hepatomegaly. Given the association with large lesions, it is not surprising that abnormal LFTs have been associated with a poorer prognosis.8
IMAGING MODALITIES
CT
CT is by far the most commonly used modality for the evaluation of melanoma metastasis, at least in the USA. It has many advantages: it is universally available and well tolerated, can rapidly image the entire body, has consistent image quality from one examination to the next, even if performed on different equipment, and has robust and reproducible imaging features. CT remains the gold standard for the evaluation of the lungs. In addition, a standard contrast-enhanced CT of the chest, abdomen and pelvis study can evaluate the liver and other organs, muscles, subcutaneous tissues and bones. CT, however, does have some disadvantages. Its two major drawbacks are (1) it uses ionizing radiation; and (2) it is relatively insensitive to small liver lesions, especially those <1 cm. Currently, the typical radiation dose for an abdominal CT study is on the order of 10–15 mSv, but this will likely decrease substantially as recently developed dose reduction techniques are more widely implemented. Of these two drawbacks, the relative insensitivity to small liver metastases may be the more important reason to use MRI as the primary surveillance tool for the evaluation of liver metastasis. It is pertinent to note that liver metastases are the most common cause of death in these patients and that the theoretical risks of ionizing radiation are unlikely to be of any consequence in this group of patients.
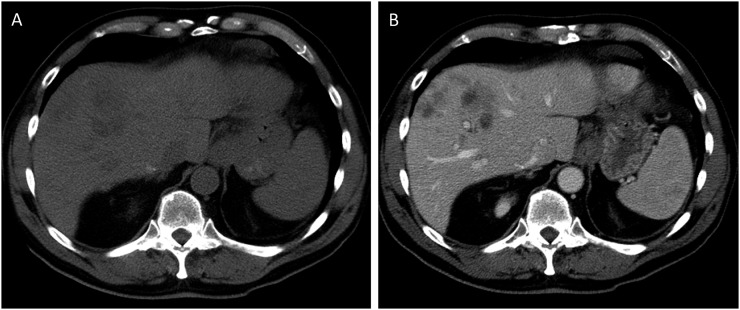
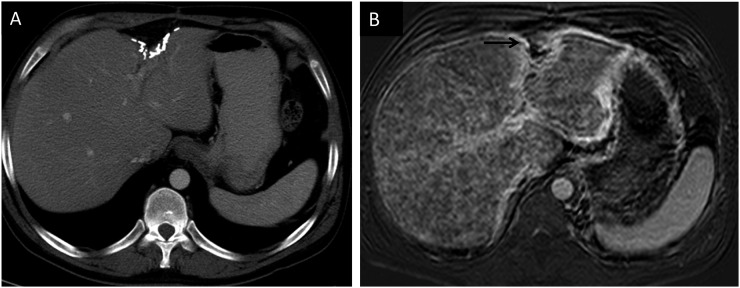
Surveillance CT studies should use an i.v. contrast, since the contrast increases both sensitivity and specificity for lesions in the liver and elsewhere (Figure 1a and b). When a liver lesion is large enough to be detected on CT scan, there are almost invariably more lesions elsewhere in the liver. One study described 90% of patients as having multiple lesions on CT.8 There are no CT features that are specific to melanoma: liver metastases appear the same as other hypervascular metastases and their appearance can overlap with that of benign lesions such as haemangiomas. Untreated metastases are hypodense and enhance with contrast, often intensely.8 Intratumoral haemorrhage is rare. Intrahepatic or extrahepatic biliary ductal dilatation is also uncommon. Other features that have been described include hepatomegaly and ascites, which are seen in up to one-quarter of patients and are associated with a poorer prognosis.8 In cases where there is a question as to whether a lesion is a metastasis, MRI will often provide additional information or a definitive answer.
Figure 1.
(a) Non-contrast CT abdomen demonstrating the ill-defined lesions in the right lobe of the liver. (b) Contrast-enhanced CT in the same patient. The lesions are better evaluated on the post-contrast images.
Treated lesions
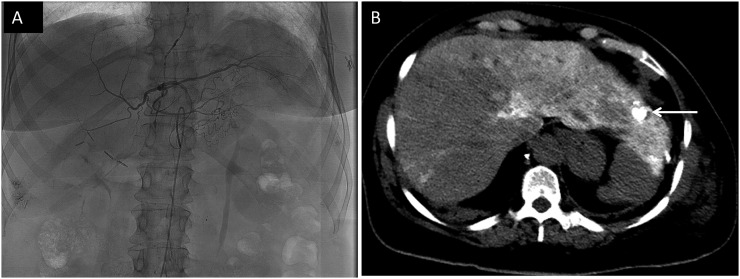
Given the multitude of treatment modalities for metastatic liver lesions, it is imperative to recognize the signs of disease progression in these patients and to distinguish between progression and effect of treatment. Hepatic arterial chemoembolization is one common form of treatment that entails injection of a chemoembolization material which includes iodized oil. The material is taken up in the tumour more than in the normal liver, and the iodine within it then appears as marked hyperdensity within the treated lesion (Figure 2a and b). This should not be mistaken for calcification; melanoma metastases do not calcify. Treated lesions are first assessed by the presence of iodized oil in the targeted segment of the liver, indicating areas where the tumour has taken up the agent. The agent is retained over time in the dead tumour but washes out from the normal liver and viable tumour. Thus, washout of dense material from treated tumours suggests residual viable tumour. This is similar to the assessment of locally treated hepatocellular carcinoma.9 The presence of new hypodense lesions or an increase in size of existing lesions suggests disease progression. CT surveillance for small areas of residual viable tumour following treatment with iodized oil agents can be challenging because the treated area is so dense that it cannot be assessed for contrast enhancement in the usual way. Once the iodized oil has dissipated, any new or increasing enhancing nodule suggests disease progression.
Figure 2.
(a) Angiogram image showing selective embolization of the left hepatic artery as part of transarterial chemoembolization (TACE). (b) Non-contrast CT abdomen image showing the presence of lipiodol–iodized oil which is used as part of TACE procedure (arrow). The hyperdense iodized oil should not be mistaken for calcification. Hyperdense opacities in the right lobe are from prior embolization.
Liver metastases can vary in number and extent of liver involved. In widespread metastases, it is helpful to report the estimated percentage of liver involvement and progression of disease in the right and left lobes separately, as these are important for guiding treatment decisions.
The most common complications of liver metastases include portal vein thrombosis, involvement of the hepatic veins and inferior vena cava. Uncommon complications of treatment include biliary necrosis and superinfection of the necrotic tumour.
Common extrahepatic sites of metastasis are the lungs, bones, muscles and subcutaneous adipose tissue. Lymph nodes in the retroperitoneum and mesentery are also frequently involved. Lymphatic metastasis should be suspected when lymph nodes are >1.5 cm in short axis, with surrounding infiltration and a round contour.10 Melanoma can also metastasize to the gallbladder, pancreas, kidneys, adrenal glands, heart and pericardium. Unlike other metastatic cancers, the presence of extrahepatic metastases in ocular melanoma does not worsen the survival prognosis, since prognosis is determined by liver involvement,8 although extrahepatic metastatic disease does affect the treatment strategy. It is of utmost importance to evaluate the liver accurately, since the presence or absence of liver metastases is the main determinant of mortality rates.
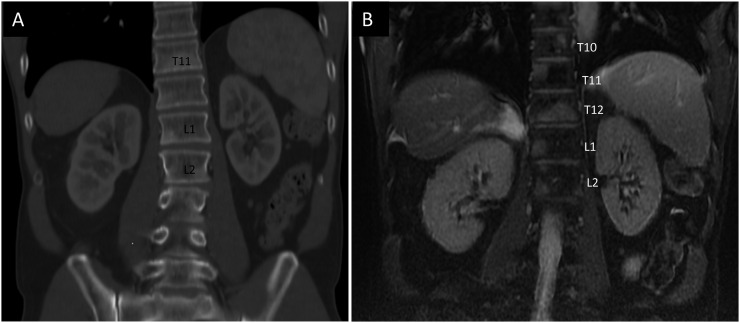
Bone metastases frequently involve the thoracic and lumbar spine. Melanoma metastases can be faintly sclerotic or lytic but are frequently subtle or invisible on CT and require a high degree of suspicion for detection. Bone metastases are seen much better on MRI (Figure 3a and b).
Figure 3.
(a) Coronal CT image demonstrating bony metastases seen as sclerotic densities in T11, L1 and L2 vertebral bodies. (b) Coronal MRI gradient echo, non-contrast, T2 weighted fat-suppressed sequence on the same day demonstrating several other bony metastases in vertebral bodies T10, T11, T12, L1 and L2.
Positron emission tomography CT
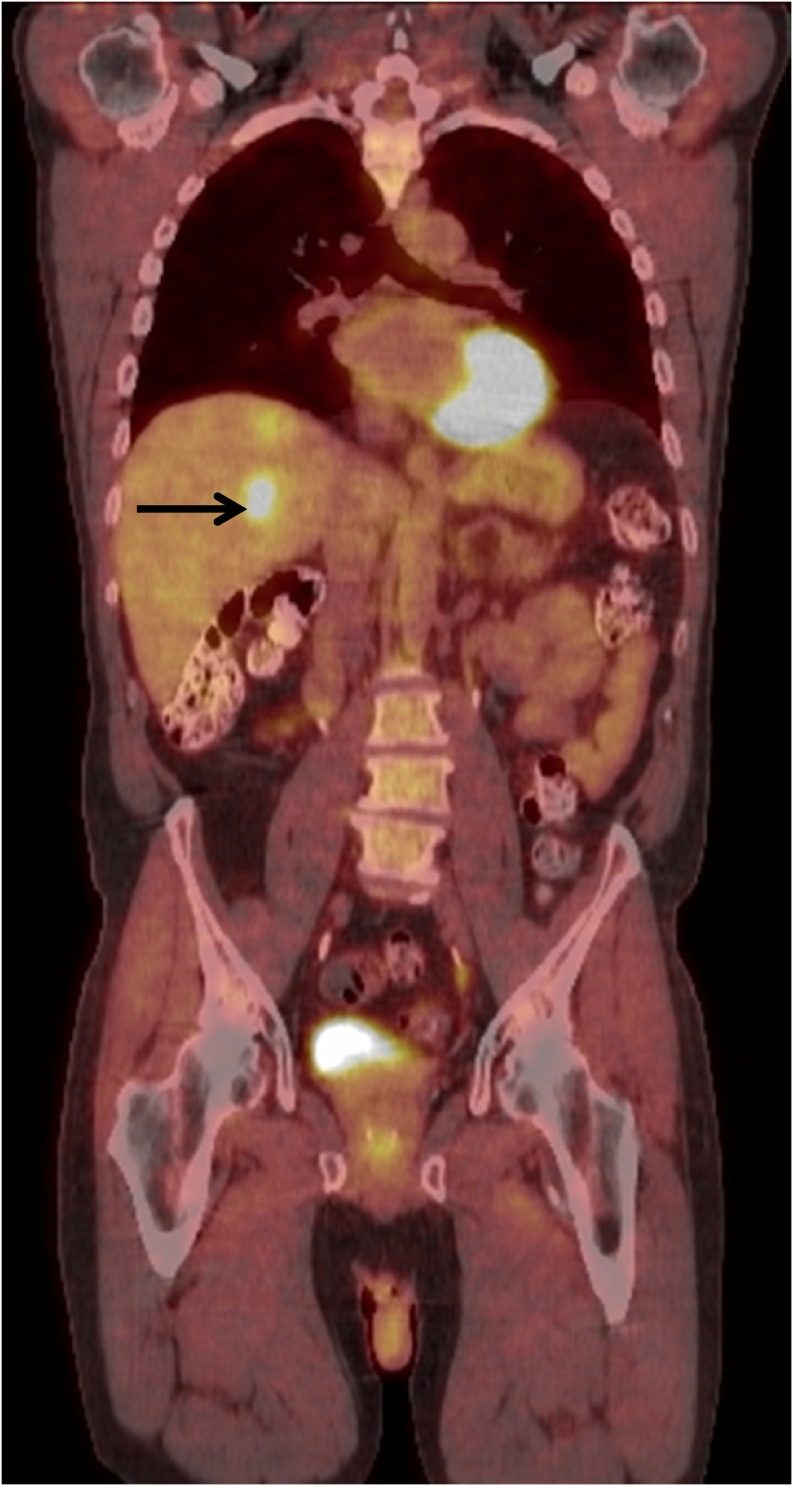
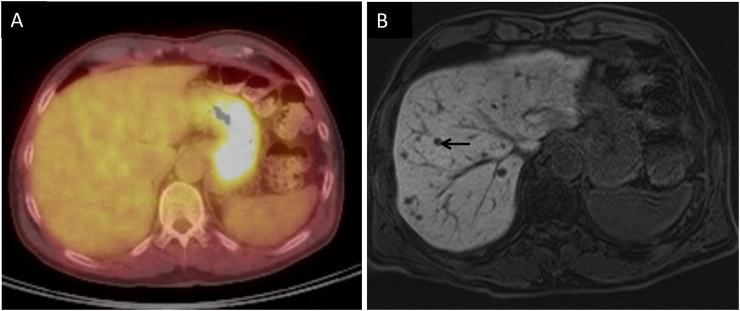
PET-CT is also commonly used to evaluate melanoma metastasis. Uveal melanoma metastases are fludeoxyglucose (FDG) avid, similar to metastases from cutaneous melanoma. Metastatic lung nodules are FDG avid and easily seen if they are larger than about 12 mm.11 Metastatic lymph nodes can easily be seen in the mediastinum and abdomen. Bone metastases are also easily identified on PET-CT. Large liver metastasis can be seen on PET-CT (Figure 4). However, PET-CT is much less sensitive than MRI for evaluating liver metastases, because the normal mottled hepatic uptake of FDG obscures small FDG-avid lesions owing to a poor target-to-background ratio (Figure 5a and b). Localization of small metastases is also limited by respiratory motion artefacts.6 In a study by Orcurto et al,12 only 4% of 108 hepatic lesions were detected by PET alone as compared with 65% by MRI alone.
Figure 4.
Coronal fused positron emission tomography CT image demonstrating a hypermetabolic lesion within the liver (arrow) consistent with metastasis.
Figure 5.
(a) Fused positron emission tomography CT images through the liver showing no focal fludeoxyglucose uptake to suggest metastasis. (b) T1 weighted hepatobiliary-phase post-contrast image performed on the same day demonstrating multiple, tiny, rounded hypointense lesions consistent with metastases. An arrow points to one of these lesions.
Although PET-CT is a powerful tool, it has several disadvantages: it is relatively expensive and is not universally available; it is insensitive to subcentimetre lesions and entails a substantial amount of radiation (approximately 30 mSv), which can be a consideration when it is used as a surveillance tool, especially in patients who are young. We do not routinely use PET-CT as a surveillance tool, but rather as a problem-solving modality.
MRI
MRI is the study of choice for evaluating liver metastases. The superiority of MRI for liver metastases is already clearly established for other tumours and its superiority may be even greater for melanoma, given the unique MRI features of melanoma metastases, as described below. MRI is more specific than and at least as sensitive as CT for detecting liver metastasis.13 In our experience, lesions <1 cm are much better evaluated on MRI. Given the continuing improvements in the speed and resolution of MRI, and the numerous and varied ways in which tissues can be characterized, there is little doubt that MRI should soon replace CT as the standard modality for liver imaging in melanoma.
MRI protocol
Most imaging protocols include a dual-echo T1 weighted gradient-recalled echo sequence, including both opposed-phase and in-phase (water relative to fat) components, a T2 weighted fast spin-echo sequence and dynamic multiphasic contrast-enhanced fat-suppressed T1 weighted gradient-recalled echo sequences through the liver prior to and following i.v. administration of gadolinium-based hepatobiliary or extracellular contrast agents. Additional sequences that have proven extremely useful are diffusion-weighted sequences and delayed post-contrast sequences following the use of a hepatocyte-avid contrast agent that is taken up and retained by the normal liver but not by the tumour. Table 2. In our experience, image quality is adequate on appropriately performed studies on 1.5-T systems and 3.0-T systems; however, some open magnet systems have significant disadvantages. In particular, low-field 0.3-T systems have a relatively poor spatial and temporal resolution and image quality and should be avoided if possible. Fortunately, many new MRI systems at 1.5 and 3.0 T have wide bores that increase patient tolerance.
Table 2.
Summary of MRI sequences on 1.5-T magnet for imaging ocular melanoma metastasis in the abdomen
| Sequence | Comments |
|---|---|
| Multiplanar survey, using balanced steady-state free precession or other techniques | |
| T2 weighted fast spin echo axial and coronal planes | Two sequences of axial images with TE of 80 and 200 ms are helpful. The higher TE value differentiates cysts from solid masses whereas the lower TE sequence demonstrates metastasis better |
| Dual-echo fat-water in-phase and opposed-phase gradient echo images | On 1.5 T: TE of 2.2 and 4.4 ms (On 3 T: TE of 1.1 and 2.2 ms) If possible, this can be obtained using 3D Dixon fat-water separation technique to yield fat-suppressed images (examples of such sequences include LAVA-flex, mDixon or VIBE-Dixon based on the vendor) |
| Multiphasic 3D fat-suppressed gradient echo images following contrast administration | Shortest possible TR and TE, and overlapping thinnest possible slices, within MRI unit capabilities. Hepatobiliary phase (10–20 min) obtained with hepatocyte-avid agent is extremely useful |
| Diffusion-weighted sequence | B-values of 800 and 20–50 These are highly useful if contrast cannot be administered |
3D, three-dimensional; LAVA-flex, liver acquisition with volume acquisition with Dixon method of water–fat separation; mDixon, multipoint Dixon; TE, echo time; TR, repetition time; VIBE-Dixon, volume interpolated body examination with Dixon method of water–fat separation.
Imaging characteristics
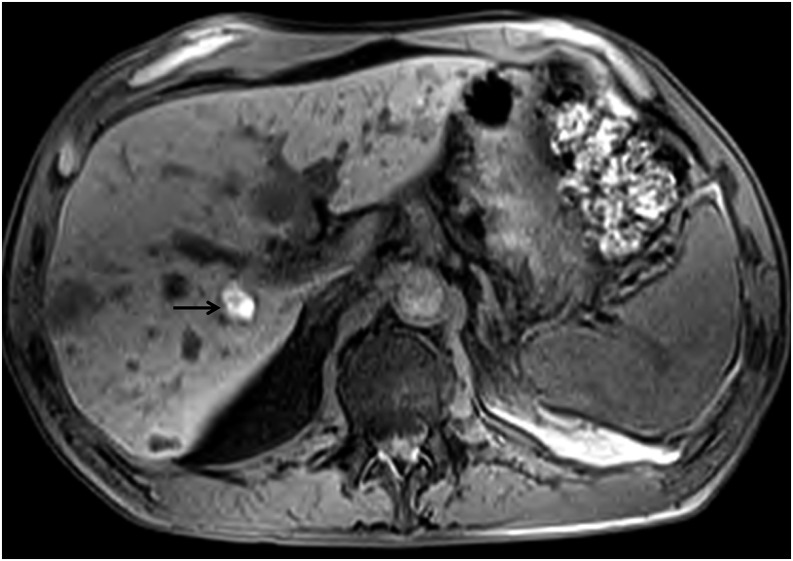
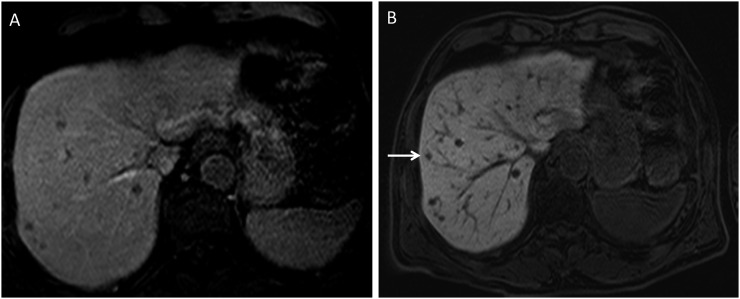
Many types of melanin cause shortening of T1 and T2 relaxation times, yielding an almost pathognomonic high signal on T1 weighted images. Even very small melanotic deposits of this type can be visualized as bright foci on unenhanced T1 weighted images, especially when the dynamic range is increased through the suppression of the fat signal. Although some studies report T1 bright melanotic lesions as the predominant type, involving approximately 73% of lesions,14 our experience indicates otherwise. Melanoma metastases which are bright on T1 weighted sequences vary between 20% and 50% (Figure 6). There is an overwhelming predominance of T1 hypointense metastatic lesions in the liver. Moreover, T1 hyperintense lesions suggest the presence of well-differentiated tumour metastases which are often more indolent and less aggressive. T1 hypointense lesions usually grow more rapidly and are more aggressive. In such cases, gadolinium-based contrast agents are extremely useful. The phase during which the lesions are best visualized varies: many are hypervascular and are seen best on arterial phase imaging; others are better visualized on the portal venous phase. Thus, a multiphasic dynamic imaging protocol is essential for all contrast-enhanced studies. When a hepatocyte-avid contrast agent such as gadoxetate disodium (Eovist and Primovist) is used, an additional imaging phase of great utility can be obtained. These agents are retained in normal liver parenchyma and on a delayed, hepatobiliary phase, even tiny lesions will stand out as markedly hypointense spots against the strongly enhanced normal liver parenchyma (Figure 7a and b).
Figure 6.
T1 weighted pre-contrast image showing multiple metastatic lesions, very few of which are T1 bright. The T1 bright lesion as indicated by an arrow is in the right lobe. Most melanoma metastases are not bright on T1 weighted sequences.
Figure 7.
(a) Pre-contrast T1 MRI fat-suppressed image demonstrating multiple hypodense lesions concerning metastases in a patient with uveal melanoma. (b) Post-contrast T1 MRI hepatobiliary-phase image demonstrating several more lesions in addition to the previously seen lesions. One of the smaller lesions, seen only on the hepatobiliary phase, is indicated by an arrow.
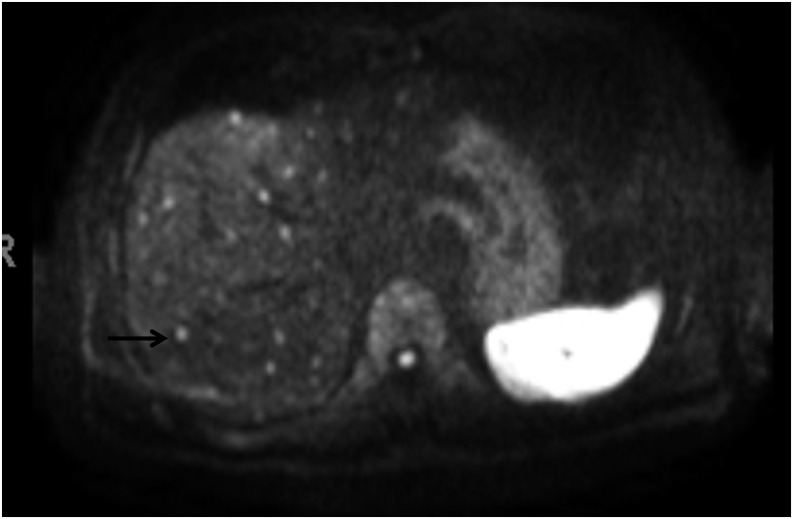
Diffusion-weighted sequences are sensitive to microenvironments that restrict the motion of water molecules and are remarkably sensitive in depicting melanoma metastases, within and outside the liver. These sequences also show promise in assessing tumour response to treatment, and changes in diffusion characteristics have been shown to precede changes in the tumour volume.15 Although diffusion-weighted images do not completely obviate the benefit of gadolinium contrast agents, in patients with severe renal failure which may preclude the use of gadolinium contrast, the diffusion-weighted sequences are particularly beneficial for evaluating metastasis (Figure 8). Moreover, this sequence is based on echoplanar imaging with a short acquisition time, making it one of the most effective sequences in evaluation.
Figure 8.
Diffusion-weighted sequence of the same patient in Figure 5 showing the multiple tiny metastatic lesions (one of which is indicated by an arrow) not visualized on positron emission tomography CT.
As they increase in number, metastases can coalesce. In addition, ocular melanoma metastases can occasionally develop a diffuse infiltrating pattern that is rare in other tumours: these infiltrative metastases can engulf pre-existing metastases, spreading through hepatic sinusoids while preserving the vascular architecture and can be mistaken for segmental oedema (Figure 9a and b). The finding is prognostically important: in our experience, this pattern is associated with an especially poor prognosis.
Figure 9.
(a) Post-contrast CT image showing a normal-appearing liver. Surgical clips are outlining the tumour resection site in the left lobe. (b) T1 weighted post-contrast MRI image in the same patient showing innumerable tiny metastases throughout the liver. Surgical clips seen on CT are seen as susceptibility artefacts (arrow). In this repeatedly imaged patient, the liver metastases were evident on MRI studies 1 year before they could be seen on CT.
Treated lesions
Imaging evaluation following treatment is complicated. Most patients are imaged 4–6 weeks after chemoembolization. Traditionally used methods of assessing treatment response such as response evaluation criteria in solid tumours criteria are not useful for evaluating treatment response in patients with melanoma for several reasons. Firstly, response evaluation criteria in solid tumours criteria use complete disappearance of lesions as complete response and a decrease in size of <30% as partial response. When metastatic uveal melanoma liver lesions are treated with local chemotherapy, size rarely decreases substantially. Hence, size is not an accurate indicator of response. The European Association for the Study of the Liver recommends as an alternative that any enhancement in a lesion should be considered evidence of residual or viable tumour. However, if there is iodized oil in the treated segment, enhancement frequently cannot be assessed on contrast-enhanced CT. In these cases, contrast-enhanced MRI is particularly useful, since iodized oil is invisible on MRI. There is, however, a confounding factor for MRI. Because some melanoma metastases are bright on T1 weighted pre-contrast sequences, it can be difficult to establish whether they enhance on the post-contrast sequences. Subtraction sequences may be helpful in these cases, although if T1 is extremely short in a melanotic metastasis, even subtraction techniques may fail to depict enhancement. This is one of the few disadvantages of MRI vs CT for hepatic imaging in patients with melanoma, but it is outweighed by its substantial advantages already discussed. As mentioned previously, T1 bright lesions tend to be less aggressive, and this can be determined by stable size on serial MRI.
Alternatively, change in diffusion, as assessed using apparent diffusion coefficient (ADC) mapping, has also been reported to correlate with response to therapy.15 In viable tumours with an intact cell membrane, there is a decrease in the Brownian movement of water, which leads to a low ADC value. With treatment, there is necrosis and disruption of the cell membrane; hence, the ADC value increases. Although absolute ADC values can be obtained, a visual gestalt of the lesion is practical and useful. While diffusion-weighted images are highly effective for showing most melanoma metastases, those with extremely short T1 and T2 signal, seen as hyperintense on T1 and hypointense on T2 weighted sequences, have little signal on the T2 weighted echoplanar images used for diffusion weighting, rendering this technique less useful for this subset of metastases.
The percentage of the mass showing either arterial or venous enhancement is also known to decrease after treatment.15
In our experience, following locoregional therapy, most treated lesions show characteristic perilesional enhancement. This should not be mistaken for tumour viability and is similar to findings in locoregionally treated hepatocellular carcinoma.16 The presence of an enhancing nodule within a hypovascular treated metastasis is more concerning for the residual or recurrent viable tissue. The presence of haemorrhage from treatment is an extra confounding factor which should not be mistaken for residual tumour. Subtraction images can be helpful, although spatial misregistration between enhanced and unenhanced images can produce pitfalls on subtraction images. Sometimes, the presence of oedema and haemorrhage in the lesion can increase the size of a treated metastasis, giving a false-positive result of increase in tumour burden. This occurs especially when imaged before 4 weeks of treatment. This pitfall can be resolved by imaging the patient again in 4–6 weeks to confirm the change in size. Once the acute signs have subsided, an increase in the size of the lesion is most concerning for progression of disease.
As mentioned earlier, it is important to document both the percentage of liver involvement and, especially in patients who are being treated with catheter-based techniques, the progression separately in both treated and untreated liver lobes.
Whole-body diffusion-weighted imaging
Whole-body diffusion-weighted imaging is a promising modality for screening, given that it eliminates the radiation associated with a conventional CT and PET. Studies have shown that in cutaneous melanoma, whole-body diffusion-weighted imaging is as sensitive as conventional MRI sequences in the detection of extracranial metastases. It has enormous potential, but at this stage, it is more of a research option than a clinical reality.17
Ultrasound
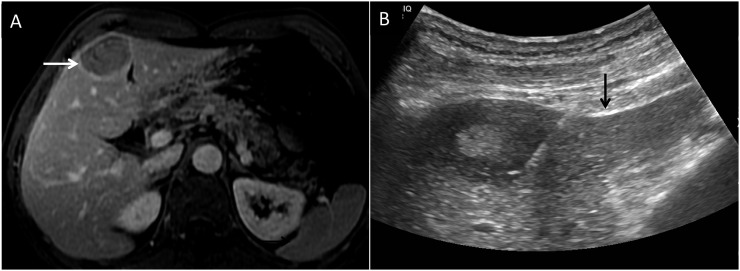
Ultrasound can be used for evaluating melanoma metastases, and many centres especially in Europe routinely use ultrasound as a screening modality.18 In the right hands, ultrasound can be both sensitive and specific: liver metastases usually show up as hypoechoic nodules, and a hypoechoic liver nodule in a patient with melanoma should be considered to be a metastasis until proven otherwise. However, ultrasound is highly operator dependent and a thorough evaluation of the liver is both time consuming and technically challenging, particularly in patients who are large. Furthermore, ultrasound is of almost no utility in surveillance for tumour outside the liver. On the other hand, ultrasound is indispensable as a modality for obtaining tissue diagnosis by fine-needle aspiration cytology or core biopsy (Figure 10a and b).
Figure 10.
(a) Post-contrast T1 weighted fat-suppressed image showing metastatic lesion (arrow) in the medial segment of the left lobe of the liver. (b) Ultrasound-guided core biopsy of the same lesion was performed which confirmed metastases. The arrow is seen pointing to the needle.
Contrast-enhanced ultrasound
Contrast-enhanced ultrasound has a clear advantage over conventional ultrasound, but is not widely used in the USA. With contrast imaging, which utilizes microbubbles, metastatic lesions show rapid enhancement and washout.19 Contrast-enhanced ultrasound can be used in patients with renal failure and is considered as diagnostically reliable as contrast-enhanced MRI and more than contrast-enhanced CT in the evaluation of metastatic lesions to the liver.19 Accordingly, it is a cost-effective and radiation-free alternative to follow up patients.
CONCLUSION
Surveillance of metastasis in ocular melanoma is vital. Liver is the most common site of metastasis and surveillance with CT or MRI is preferred. The CT and MRI features of these metastases are important in recognizing initial metastasis and in the follow-up of the lesions after treatment. Ultrasound is preferred for interventional procedures. PET-CT, although preferred for bone metastasis, has a low sensitivity for evaluating liver metastasis (Table 3).
Table 3.
Ocular melanoma metastasis imaging
| Key points to remember |
|---|
| Liver is the most common site of metastasis and prognosis is based on the surveillance and treatment of liver metastasis |
| MRI |
| The most specific modality for imaging liver metastasis and is at least as sensitive as CT |
| Majority of melanoma metastases are not T1 bright. T1 hypointense lesions are aggressive and grow rapidly |
| Newer hepatobiliary contrast agents help in detection of very small liver metastases |
| Diffusion-weighted imaging is helpful when i.v. contrast cannot be administered |
| Treated lesions are also better evaluated with MRI. Decrease in size is not a criterion for response to treatment. Perilesional enhancement does not indicate progression in treated lesions, rather nodular enhancement does |
| CT |
| Useful for lung metastasis and when MRI is medically contraindicated |
| Locoregional treated lesions can be also assessed with CT |
| Lesions treated with chemoembolization are assessed by presence of iodized oil in the treated segment. Washout out of the dense material suggests viable tumour |
| Ultrasound is mainly useful for biopsy of liver lesions |
| PET-CT |
| Detects lung nodules and large liver lesions but is insensitive to small liver lesions |
| High radiation dose is a major disadvantage |
PET, positron emission tomography.
Contributor Information
Rashmi Balasubramanya, Email: rasbal75@yahoo.com.
Santosh Kumar Selvarajan, Email: santosh.rad@gmail.com.
Mougnyan Cox, Email: mougnyan.cox@gmail.com.
Ganesh Joshi, Email: drganeshj@gmail.com.
Sandeep Deshmukh, Email: sandeep.deshmukh@jefferson.edu.
Donald G Mitchell, Email: donald.mitchell@jefferson.edu.
Patrick O'Kane, Email: patrick.okane@jefferson.edu.
REFERENCES
- 1.Singh AD, Kivelä T. The collaborative ocular melanoma study. Ophthalmol Clin North Am 2005; 18: 129–42, ix. doi: 10.1016/j.ohc.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 2.Desjardins L, Dorval T, Levy C, Cojean I, Schlienger P, Salmon RJ, et al. Randomised study on adjuvant therapy by DTIC in choroidal melanoma. [In French.] Ophtalmologie 1998; 12: 168–73. [Google Scholar]
- 3.Marshall E, Romaniuk C, Ghaneh P, Wong H, McKay M, Chopra M, et al. MRI in the detection of hepatic metastases from high-risk uveal melanoma: a prospective study in 188 patients. Br J Ophthalmol 2013; 97: 159–63. doi: 10.1136/bjophthalmol-2012-302323 [DOI] [PubMed] [Google Scholar]
- 4.Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol 2005; 123: 1639–43. doi: 10.1001/archopht.123.12.1639 [DOI] [PubMed] [Google Scholar]
- 5.Shields JA, Augsburger JJ, Donoso LA, Bernardino VB, Jr, Portenar M. Hepatic metastasis and orbital recurrence of uveal melanoma after 42 years. Am J Ophthalmol 1985; 100: 666–8. doi: 10.1016/0002-9394(85)90621-X [DOI] [PubMed] [Google Scholar]
- 6.Servois V, Mariani P, Malhaire C, Petras S, Piperno-Neumann S, Plancher C, et al. Preoperative staging of liver metastases from uveal melanoma by magnetic resonance imaging (MRI) and fluorodeoxyglucose-positron emission tomography (FDG-PET). Eur J Surg Oncol 2010; 36: 189–94. doi: 10.1016/j.ejso.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 7.Harbour JW. The genetics of uveal melanoma: an emerging framework for targeted therapy. Pigment Cell Melanoma Res 2012; 25: 171–81. doi: 10.1111/j.1755-148X.2012.00979.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel M, Winston CB, Marr BP, Carvajal RD, Schwartz GK, Wolchok J, et al. Characterization of computed tomography scan abnormalities in patients with biopsy-proven hepatic metastases from uveal melanoma. Arch Ophthalmol 2011; 129: 1576–82. doi: 10.1001/archophthalmol.2011.263 [DOI] [PubMed] [Google Scholar]
- 9.Lim HS, Jeong YY, Kang HK, Kim JK, Park JG, et al. Imaging features of hepatocellular carcinoma after transcatheter arterial chemoembolization and radiofrequency ablation. AJR Am J Roentgenol 2006; 187: W341–9. [DOI] [PubMed] [Google Scholar]
- 10.Tojo D, Wenig BL, Resnick KI. Incidence of cervical metastasis from uveal melanoma: implications for treatment. Head Neck 1995; 17: 137–9. [DOI] [PubMed] [Google Scholar]
- 11.Mayerhoefer ME, Prosch H, Herold CJ, Weber M, Karanikas G. Assessment of pulmonary melanoma metastases with 18F-FDG PET/CT: which PET-negative patients require additional tests for definitive staging? Eur Radiol 2012; 22: 2451–7. doi: 10.1007/s00330-012-2499-x [DOI] [PubMed] [Google Scholar]
- 12.Orcurto V, Denys A, Voelter V, Schalenbourg A, Schnyder P, Zografos L, et al. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography and magnetic resonance imaging in patients with liver metastases from uveal melanoma: results from a pilot study. Melanoma Res 2012; 22: 63–9. doi: 10.1097/CMR.0b013e32834d3dcb [DOI] [PubMed] [Google Scholar]
- 13.Semelka RC, Martin DR, Balci C, Lance T. Focal liver lesions: comparison of dual-phase CT and multisequence multiplanar MR imaging including dynamic gadolinium enhancement. J Magn Reson Imaging 2001; 13: 397–401. doi: 10.1002/jmri.1057 [DOI] [PubMed] [Google Scholar]
- 14.Maeda T, Tateishi U, Suzuki S, Arai Y, Kim EE, Sugimura K. Magnetic resonance screening trial for hepatic metastasis in patients with locally controlled choroidal melanoma. Jpn J Clin Oncol 2007; 37: 282–6. doi: 10.1093/jjco/hym018 [DOI] [PubMed] [Google Scholar]
- 15.Buijs M, Vossen JA, Hong K, Georgiades CS, Geschwind JF, Kamel IR. Chemoembolization of hepatic metastases from ocular melanoma: assessment of response with contrast-enhanced and diffusion-weighted MRI. AJR Am J Roentgenol 2008; 191: 285–9. doi: 10.2214/AJR.07.2467 [DOI] [PubMed] [Google Scholar]
- 16.Gervais DA, Kalva S, Thabet A. Percutaneous image-guided therapy of intra-abdominal malignancy: imaging evaluation of treatment response. Abdom Imaging 2009; 34: 593–609. doi: 10.1007/s00261-008-9476-5 [DOI] [PubMed] [Google Scholar]
- 17.Petralia G, Padhani A, Summers P, Alessi S, Raimondi S, Testori A, et al. Whole-body diffusion-weighted imaging: is it all we need for detecting metastases in melanoma patients? Eur Radiol 2013; 23: 3466–76. doi: 10.1007/s00330-013-2968-x [DOI] [PubMed] [Google Scholar]
- 18.Rivoire M, Kodjikian L, Baldo S, Kaemmerlen P, Négrier S, Grange JD, et al. Treatment of liver metastases from uveal melanoma. Ann Surg Oncol 2005; 12: 422–8. [DOI] [PubMed] [Google Scholar]
- 19.De Toni EN, Gallmeier E, Auernhammer CJ, Clevert DA. Contrast-enhanced ultrasound for surveillance of choroidal carcinoma patients: features of liver metastasis arising several years after treatment of the primary tumor. Case Rep Oncol 2011; 4: 336–42. doi: 10.1159/000329453 [DOI] [PMC free article] [PubMed] [Google Scholar]