Abstract
Placental adhesion disorder (PAD) comprises placenta accreta, increta and percreta lesions; these are classified according to the depth of uterine invasion. Although PAD is considered a rare condition, its incidence has increased 10-fold in the last 50 years. Ultrasound is the primary imaging modality for the assessment of the placenta and in the majority of cases, it is sufficient for diagnosis; however, when ultrasound findings are suspicious or inconclusive, MRI is recommended as an adjunct imaging technique. Numerous MRI features of PAD have been described, including dark intraplacental bands, disorganized intraplacental vascularity and abnormal uterine bulging. This pictorial review describes and illustrates these characteristics and discusses their implications in planning delivery. In addition, we present a series of “pitfall” cases to aid the interpreting radiologist and discuss management of PAD. PAD is a clinical and diagnostic challenge that is encountered with increasing frequency, requiring a cohesive multidisciplinary approach to its management.
Placental adhesion disorder (PAD) comprises placenta accreta, increta and percreta lesions, which are classified according to the depth of uterine invasion by the trophoblastic tissue.
Although PAD is considered a rare condition, its incidence has increased 10-fold in the past 50 years.1 PAD is a condition associated with massive post-partum haemorrhage (PPH), high risk of multiple blood transfusions, emergency hysterectomy and maternal morbidity and mortality.2 It is the second highest reported cause of haemorrhage leading to peripartum hysterectomy in the UK.3
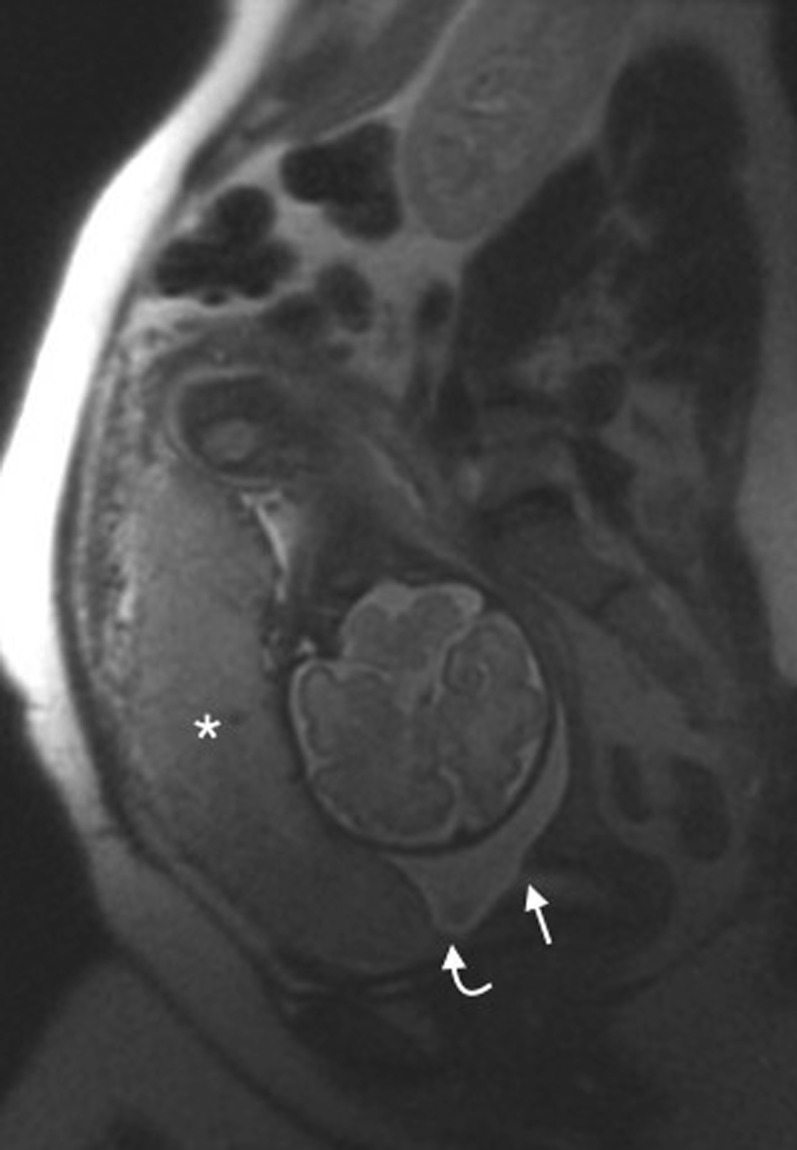
Ultrasound is the primary modality of placental imaging and in the majority of cases, it is sufficient for diagnosis; however, when ultrasound findings are suspicious or inconclusive, MRI is recommended as a supplementary imaging technique. In 2011, the UK Royal College of Obstetrics and Gynaecology and the National Institute of Clinical Excellence published guidelines advocating the use of MRI in cases of uncertainty4,5 and many radiology departments have therefore sought to increase their experience in performing and interpreting the images. The normal placenta is smooth and homogenous and returns intermediate T2 signal intensity (T2SI) (Figure 1).
Figure 1.
Normal placenta: sagittal T2 single-shot fast spin-echo image of a normal anterior placenta (asterisk). The leading edge of the placenta (curved arrow) is clearly anterior and superior to the internal cervical os (arrow).
Numerous MRI features of PAD have been described; this pictorial review aims to describe and review these characteristics and discuss their implications in planning delivery.
ACCRETA, INCRETA AND PERCRETA
In normal placentation, extravillous trophoblast invades the decidua and converts the spiral arterioles of the endometrium to uteroplacental vessels (decidualization); the trophoblastic proliferation leads to the formation of chorionic villi. If the underlying endometrium is deficient, decidualization fails and the trophoblast or chorionic villi invade and penetrate the myometrium. In placenta accreta, the chorionic villi are implanted on the myometrium with no intervening decidua. In increta, the myometrium is invaded by the placental villous tissue. In percreta, the chorionic villi penetrate the serosal layer of the uterus or even beyond into adjacent organs.6
The radiological definitions of accreta, increta and percreta are less specific. For the purposes of this article, accreta is defined as partial myometrial invasion, increta is total myometrial invasion and percreta is invasion involving the complete myometrium, serosal layer and beyond. Imaging appearances of placenta percreta are rarely equivocal, but the appearances of placenta accreta and increta are subtle and identification relies on the ancillary signs of PAD described below.
Risk factors
Patients at risk of PAD are those with a scarred uterus. Prior caesarean section and placenta praevia are the two most important risk factors for PAD (thought to be due to the deficiency of the decidua at the site of the scar). Other proposed risk factors include conservative myomectomy, uterine artery embolization, curettage and previous uterine rupture.7
Rationale of MRI
The clinical consequence of PAD is massive PPH at the time of placental separation. Accurate prenatal identification of PAD is crucial to optimize the management of patient delivery, including timing and site, availability of blood products and recruitment of a skilled anaesthetic, surgical and interventional radiology (IR) team. Caesarean section is usually planned at 36 weeks of gestation to minimize the risk of spontaneous labour, and surgical planning can be individualized according to the imaging findings and patient risk factors.
MRI should complement and not substitute ultrasound and is useful in cases where ultrasound is inconclusive or evaluation of the placenta is limited.8,9 The reported sensitivity and specificity of ultrasound for the diagnosis of PAD with ultrasound is 77–93% and 71–95%, respectively.10 MRI has an overall sensitivity of 75–100% and specificity of 65–100%. Its negative-predictive value is 79–92% and its positive-predictive value is 67–84.4%.11 Clearly, both imaging modalities perform better in non-equivocal cases. Principals of MRI scanning in pregnancy and suggested MRI protocol are described in Tables 1 and 2.
Table 1.
Principles of MRI imaging the placenta and suggested imaging protocol
| Principle | Comments |
|---|---|
| Flexibility | Use a body coil for comfort |
| Minimize scan time | Artefact from foetal movement and comfort of the mother are significant considerations Tailor the scan to the individual patient |
| Consideration of the patient needs | If preferred, scan in the lateral decubitus position for comfort |
| i.v. contrast should be avoided | Owing to lack of human clinical data and potential toxicity12 |
Table 2.
Suggested imaging protocol
| Sequence | Imaging planea | Rationale |
|---|---|---|
| T2 SSFSE | Coronal, sagittal, axial (LFOV ± SFOV) | Anatomical assessment, assess dark bands Assess position of placenta in relation to the cervix |
| T2 BGE | Coronal, sagittal, axial (LFOV ± SFOV) | Anatomical assessment and assessment of vascularity |
| T1 | Sagittalb | Assess for retroplacental haemorrhage |
| DWI (50, 100, 800) | Sagittalb | Assess for invasion |
BGE, balanced gradient echo; DWI, diffusion-weighted imaging; LFOV, large field of view; SFOV, small field of view; SSFSE, single-shot fast spin echo.
At our institution, we plan the scan according to the plane of the mother.
Choice of imaging plane should be tailored to the individual patient to minimize scan time.
Positive MRI findings in placental adhesion disorder
The following criteria are considered useful in the MRI assessment of PAD.
Dark intraplacental bands
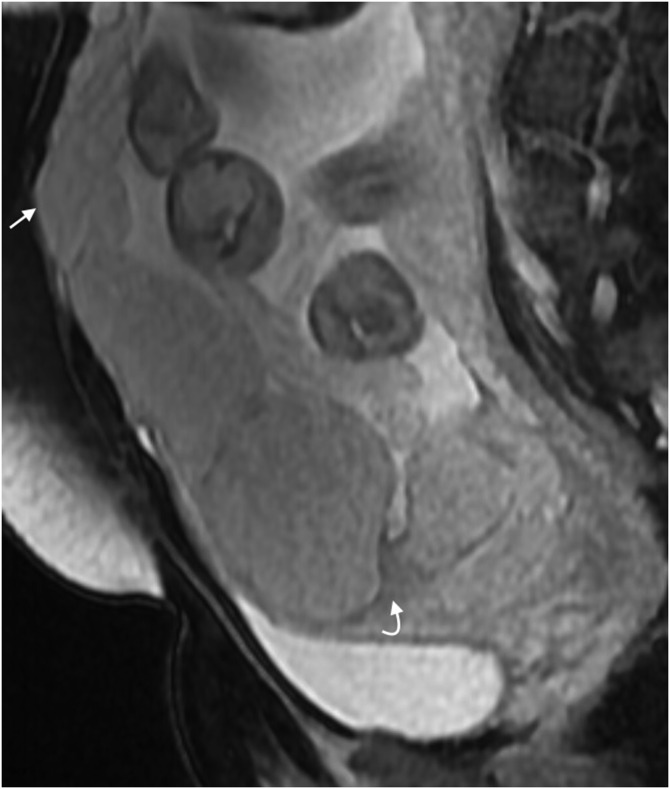
Placental dark bands are thick lines that arise from the maternal surface and are thought to represent fibrin, possibly due to frequent haemorrhage and infarction.11 Dark intraplacental bands are frequently observed in normal patients, but in the presence of risk factors, these should raise suspicion of PAD. They are nodular or linear areas of low signal intensity on T2 weighted images; they have a varying thickness and random distribution. They must be differentiated from placental septa, which tend to be thin and smooth; if the placenta is homogeneous and smooth without placental bands, it is unlikely that there is underlying PAD.13
Dark intraplacental bands return low T2SI on single-shot fast spin-echo (SSFSE) and balanced gradient-echo (BGE) images (Figure 2a,b). It should be noted that a high T2SI on BGE sequences indicates flow in a vessel and excludes the dark band. T1 sequences should be scrutinized to exclude recent haemorrhage (Figure 3a–d).
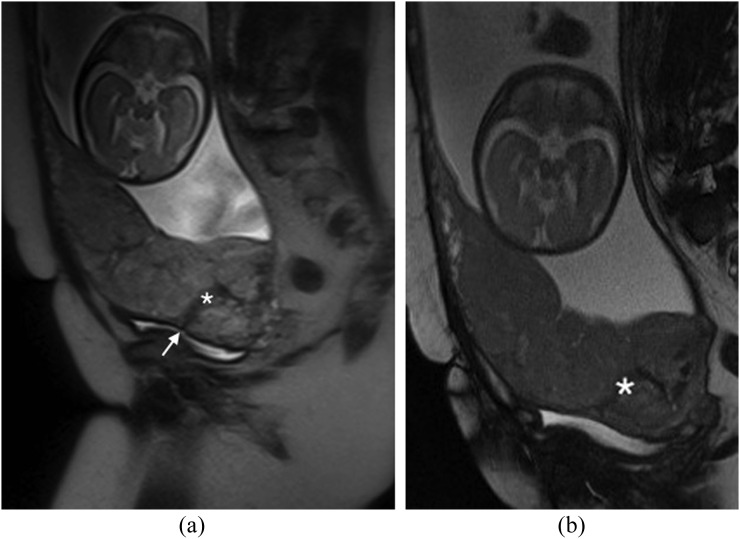
Figure 2.
Dark intraplacental bands in a placenta percreta: (a) sagittal T2 single-shot fast spin-echo image demonstrates the typical appearance of a dark intraplacental band (asterisk) in a patient with a complete placenta praevia and a prior caesarean section. The caesarian section scar is visible (arrow). (b) Sagittal T2 balanced gradient-echo image demonstrates persistent low T2 signal intensity of the dark intraplacental band (asterisk), this allows differentiation from an abnormal vessel. Histology confirmed a placenta percreta.
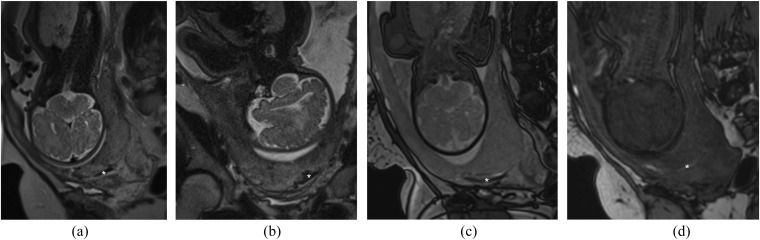
Figure 3.
Pitfall—clot mimicking a dark intraplacental band: (a) sagittal T2 single-shot fast spin-echo (SSFSE) image demonstrates a linear low T2 signal intensity (T2SI) region (asterisk) in the expected region of the previous caesarean section scar. (b) Coronal T2 SSFSE image of the same area, the linear region of low T2SI (asterisk) is again visualized. (c) Sagittal T2 balanced gradient-echo image demonstrates persistent low T2SI (asterisk), suggestive but not typical of a dark intraplacental band. (d) Sagittal T1 weighted image demonstrates this area returns high T1 signal intensity (asterisk) in keeping with haemorrhage. Overall appearances are not in keeping with a dark intraplacental band. At caesarean section, the placenta was removed normally.
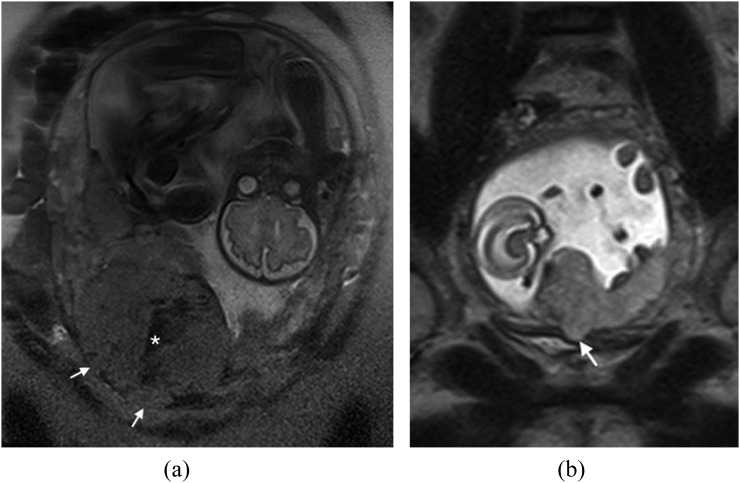
Disorganized abnormal intraplacental vascularity
This feature was described by Derman et al9 in 2011 and is related to the tortuous dilated (>6 mm) disorganized vessels within the placenta that are located in some areas of dark intraplacental bands. It is suggested that the extent of abnormal vessels is related to the degree of invasion, with the most bizarre vasculature existing in cases of percreta. Identification of abnormal vascularity relies on the comparison between T2 SSFSE and BGE sequences, with vessels returning high T2SI on BGE (absence of flow void indicates slow-moving blood as seen in “lacunes” described on ultrasound) (Figure 4a–d). These appearances are absent in non-invaded cases.
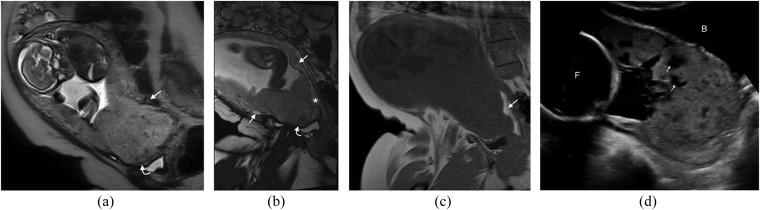
Figure 4.
Disorganized vascularity: (a) sagittal T2 single-shot fast spin-echo demonstrates a complete placenta praevia. The low T2 signal intensity serpiginous vessels along the posterior myometrium are noted (arrow). In addition, the placenta is heterogeneous with a low uterine bulge (curved arrow). (b) Coronal oblique balanced gradient-echo image in the same patient demonstrates extensive disorganized vascularity in the anterior and posterior myometrium (arrows). A uterine bulge (curved arrow) is again noted. (c) Coronal oblique T1 weighted image demonstrates a high T1 signal intensity collection (arrow) in keeping with retroplacental haemorrhage. This case was proven to be a placenta percreta at histology. The appearences of the abnormal vessels should be reported as the information will facilitate surgical planning. (d) Ultrasound image in a different patient demonstrating multiple tortuous hypoechoic structures within the placenta (arrows) in keeping with lacunae. The bladder (B) and the foetus (F) can be noted.
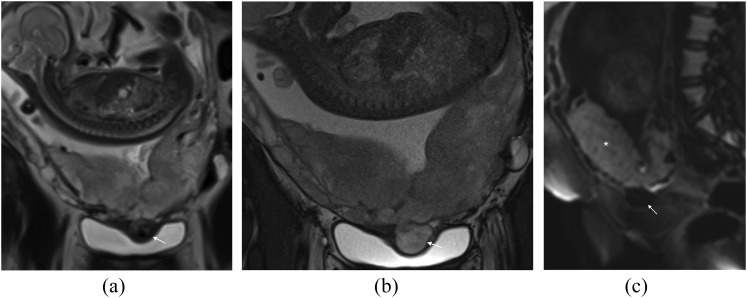
Abnormal uterine bulging (including invasion of adjacent organ and tenting of the bladder)
Focal bulging raises the suspicion of invasion; however, this sign is specific but not sensitive, as it exists only in cases of percreta.11 Bulging is seen as an interruption in the myometrial wall at the site of placental invasion; in patients with prior caesarean section, the placenta may be seen invading or tenting the bladder (Figure 5a,b), clinical history is useful in these cases as if invasion is severe the patient may complain of haematuria.
Figure 5.
Abnormal uterine bulge: (a) coronal T2 single-shot fast spin-echo (SSFSE) image in a patient with placenta percreta. An abnormal uterine bulge can be noted in two areas (arrows). Note also the typical appearance of the dark intraplacental band (asterisk). Placenta percenta was confirmed at histology. (b) Coronal T2 SSFSE image demonstrating an abnormal uterine bulge (arrow) through the previous caesarean section scar in a different patient this scan was acquired at 18/40. The placenta is normal in appearence and this is a case of scar dehiscence not placenta percreta.
Diffusion-weighted imaging is particularly a useful sequence when assessing for an abnormal uterine bulge, as the placental tissue will demonstrate persistent high SI on the late B-value images and reveal its true external contour (Figure 6a–c); in difficult cases, correlation with ultrasound will help clarify.
Figure 6.
Pitfall—abnormal uterine bulge: (a) coronal single-shot fast spin-echo image demonstrates a heterogeneous placenta with low T2 signal intensity bulge indenting the bladder (arrow). (b) Coronal T2 balanced gradient-echo image demonstrates the abnormal bulge (arrow) indenting the bladder; it has the same signal intensity as the placenta on these sequences. (c) Sagittal B-800 diffusion-weighted image clearly demonstrates normal restricted diffusion in the placenta (asterisk) but not in the abnormal bulge (arrow). These appearances are consistent with a bladder varix; this proved to be vital information for the obstetrics team to facilitate planning of delivery.
Heterogeneous placenta
The normal placenta is smooth and homogeneous and returns intermediate T2SI (Figure 1); PAD is unlikely in a homogeneous placenta.
As pregnancy progresses, the placenta normally becomes heterogeneous, the so-called “heterogeneity of ageing”. A subjective assessment of the degree of heterogeneity may be attempted; a mild-to-moderate degree of heterogeneity is deemed a non-useful sign of invasion and marked heterogeneity (as a result of dark intraplacental bands) is considered a strong indicator of invasion.11
Pitfalls in interpretation
In our practice, we have noted a number of signs and appearances that have complicated the interpretation of placental MRI. We present the following “pitfalls” based on our experience. In most cases, correlation with ultrasound is helpful.
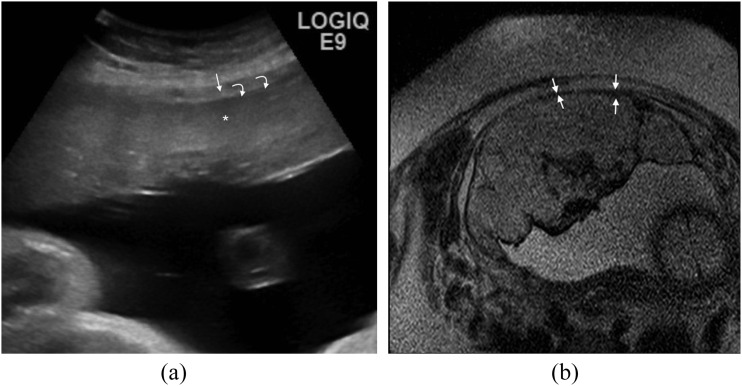
Thinning or loss of retroplacental T2 dark zone
This is related to the ultrasound “review area” of the retroplacental clear space. On ultrasound, a retroplacental hypoechoic line (Figure 7a) is usually seen in the normal placenta, and the absence of this has been described in cases of PAD (Figure 7b). However, it is often absent in normal pregnancies and therefore not considered sensitive. On MRI, as pregnancy progresses, the myometrium becomes thin and difficult to visualize (even when the scan is performed perpendicular to the myometrium/placenta interface), and relying on this sign alone can lead to false-positive interpretation.13
Figure 7.
Thinning of the myometrium: (a) ultrasound image demonstrating the normal hyperechoic placenta (asterisk) surrounded by the hypoechoic myometrium (arrow). The thin hypoechoic line at the inner aspect of the myometrium (curved arrows) represents the retroplacental clear space. On colour Doppler, a normal organized pattern of subplacental flow that parallels the myometrium is expected. (b) Axial T2 single-shot fast spin-echo image demonstrates thinning of the myometrium (arrows) in a case of placenta percreta. This assessment must be made in three planes and if there is doubt, correlation with ultrasound is often useful.
Bladder varices
Bladder varices are common and can mimic a uterine bulge (Figure 6a–c). DWI sequences and correlation with ultrasound will help avoid this pitfall; however, the obstetrician should be made aware of their presence, as it will alter the surgical approach (bleeding bladder varices are difficult to control). Increased vascularity around the uterine wall especially above the bladder and around the cervix is common.
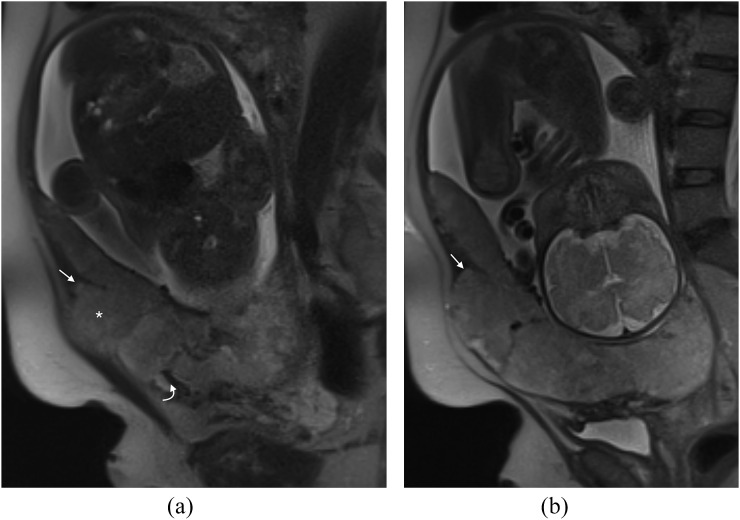
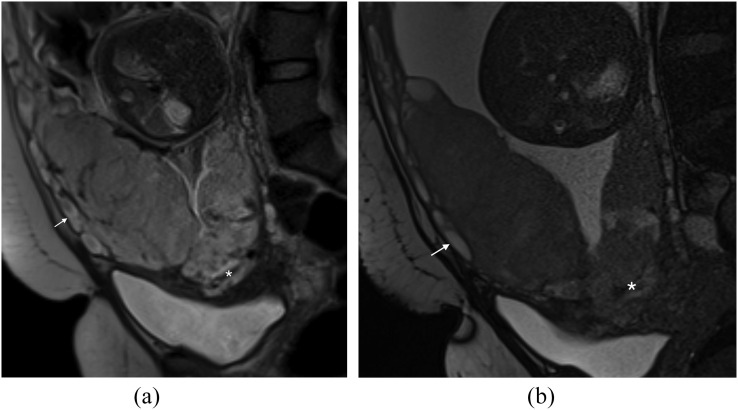
Uterine dehiscence
In our practice, we have seen one case of uterine dehiscence mimicking a placenta percreta (Figure 8a,b). There is abnormal uterine bulging and abnormal appearance of the placenta with dark bands and increased heterogeneity. In fact, the abnormal bulge is as a result of the placenta filling the breach in the myometrium.
Figure 8.
Pitfall—uterine dehiscence: (a) sagittal T2 single-shot fast spin-echo (SSFSE) image demonstrates a dark band in the expected site of the previous caesarean section (curved arrow) and a bulge superiorly (asterisk). This has the appearance of a percreta but at surgery, massive uterine dehiscence was found; the placenta had been “plugging” the breach in the myometrium. Note the myometrium is ending in a “V” shape (arrow), in retrospect in keeping with uterine retraction and dehiscence. (b) Sagittal T2 SSFSE image in the same patient again showing the “V”-shaped uterine retraction (arrow) in keeping with dehiscence.
Focal bulge in the region of the maternal umbilicus
This is an anecdotal pitfall sign that we commonly notice in our practice. As pregnancy progresses, the rectus sheath separates and a focal bulge is noted in the anterior myometrium in the region of the maternal umbilicus (Figure 9). As the underlying myometrium is usually normal, it is unlikely that these cases represent percreta. The case illustrated (Figure 9) was a percreta in the low uterine segment but not in the anterior abdominal wall.
Figure 9.
Pitfall—focal bulge in the region of the maternal umbilicus: sagittal T2 balanced gradient-echo image demonstrates a dark band in the low uterine segment at the expected site of the caesarean-section scar (curved arrow); in addition, there is placenta percreta in the region of the cervix. The bulge in the anterior abdominal wall (arrow) could be mistaken for an area of invasion, but it is in fact a common normal finding due to separation of the rectus muscles as pregnancy progresses.
Localized areas of abnormality
These are often accreta and will require care at delivery, although hysterectomy is often avoided. The placenta looks abnormal in just one area with an odd dark band and heterogeneity (Figure 10a,b). The surgical team should be informed of these focal areas of abnormality, as they should arrange delivery in the appropriate setting with an experienced team and ensure availability of extra blood products. IR involvement is not routinely required in these cases.
Figure 10.
Localized area of abnormality: (a) sagittal T2 single-shot fast spin-echo image demonstrates a complete placenta praevia with a solitary low T2 signal intensity linear area in keeping with a dark intraplacental band (asterisk). Note the increased vascularity (arrow) along the anterior myometrium. (b) Sagittal T2 balanced gradient-echo image is confirms the presence of the dark intraplacental band (asterisk) and increased vascularity (arrow). The MRI provided essential pre-delivery information. This placenta came away in a piecemeal fashion with increased blood loss.
Management of placental adhesion disorder
The traditional management of PAD was caesarean and hysterectomy with associated morbidity and haemorrhage. During the past few decades, other therapeutic options have been proposed such as non-separation placental hysterectomy, caesarean section with avoidance of placental removal combined with methotrexate, compression sutures, B-Lynch suture or balloon tamponade with the placenta remaining in situ.14,15 Despite these measures, delayed hysterectomy was often required, with associated infertility, adhesions and related complications.16
Interventional radiologists are increasingly involved in cases of PAD for elective or emergency pelvic devascularization procedures. Two techniques are proposed: (1) prophylactic placement of percutaneous balloon catheters in both the internal iliac arteries17–19 or (2) uterine artery embolization.20,21 Although the sample size in the literature is small, these techniques offer a successful clinical result with prevention of hysterectomy and improvement of blood loss during the procedure compared with caesarean section associated with haemostatic sutures or arterial ligation.22 Direct IR involvement should be considered in patients suspected of placenta percreta. In cases of suspected accreta, the delivery may be planned in an environment where a skilled IR team is available to intervene in the event of emergency PPH.
SUMMARY
The incidence rate of PAD is increasing and an accurate antenatal diagnosis is now expected. Delivery in these cases should be planned in the multidisciplinary setting. MRI is a useful addition to ultrasound in patients with high risk of PAD and can be utilized to help plan safe delivery in the appropriate setting with the appropriate team available. Skilled interpreters are few in number and diagnosis remains challenging. As our experience and cohort increases, we hope the currently established MRI criteria of PAD will continue to be validated.
Contributor Information
Faye Cuthbert, Email: faye.cuthbert@bsuh.nhs.uk.
Mireia Teixidor Vinas, Email: mireia.teixidorvinas@bsuh.nhs.uk.
Elspeth Whitby, Email: e.whitby@sheffield.ac.uk.
REFERENCES
- 1.Chantraine F, Blacher S, Berndt S, Palacios-Jaraquemada J, Sarioglu N, Nisolle M, et al. Abnormal vascular architecture at the placental-maternal interface in placenta increta. Am J Obstet Gynecol 2012; 207: 188.e1–9. doi: 10.1016/j.ajog.2012.06.083 [DOI] [PubMed] [Google Scholar]
- 2.Eshkoli T, Weintraub AY, Sergienko R, Sheiner E. Placenta accreta: risk factors, perinatal outcomes, and consequences for subsequent births. Am J Obstet Gynecol 2013; 208: 219.e1–7. doi: 10.1016/j.ajog.2012.12.037 [DOI] [PubMed] [Google Scholar]
- 3.Knight M; UKOSS. Peripartum hysterectomy in the UK: management and outcomes of the associated haemorrhage. BJOG 2007; 114: 1380–7. doi: 10.1111/j.1471-0528.2007.01507.x [DOI] [PubMed] [Google Scholar]
- 4.Placenta Praevia, Placenta Praevia Accreta and Vasa Praevia: Diagnosis and Management. RCOG (Green-top guideline No. 27). Internet; 2011; 4.6.
- 5.NICE, Caesarean Section, in CG132; 2011.
- 6.Dekan S, Linduska N. Normal and pathological placental development: MRI and pathology. In: Prayer D, ed. Fetal MRI. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. pp. 403–42. [Google Scholar]
- 7.Josephs SC, Obstetric and gynecologic emergencies: a review of indications and interventional techniques. Semin Intervent Radiol 2008; 25: 337–46. doi: 10.1055/s-0028-1102992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhawary TM, Dabees NL, Youssef MA, Diagnostic value of ultrasonography and magnetic resonance imaging in pregnant women at risk for placenta accreta. J Matern Fetal Neonatal Med 2013; 26: 1443–9. doi: 10.3109/14767058.2013.784740 [DOI] [PubMed] [Google Scholar]
- 9.Derman AY, Nikac V, Haberman S, Zelenko N, Opsha O, Flyer M. MRI of placenta accreta: a new imaging perspective. AJR Am J Roentgenol 2011; 197: 1514–21. doi: 10.2214/AJR.10.5443 [DOI] [PubMed] [Google Scholar]
- 10.Elsayes KM, Trout AT, Friedkin AM, Liu PS, Bude RO, Platt JF, et al. Imaging of the placenta: a multimodality pictorial review. Radiographics 2009; 29: 1371–91. doi: 10.1148/rg.295085242 [DOI] [PubMed] [Google Scholar]
- 11.Rahaim NS, Whitby EH, The MRI features of placental adhesion disorder and their diagnostic significance: systematic review. Clin Radiol 2015; 70: 917–25. doi: 10.1016/j.crad.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 12.Tremblay E, Thérasse E, Thomassin-Naggara I, Trop I. Quality initiatives: guidelines for use of medical imaging during pregnancy and lactation. Radiographics 2012; 32: 897–911. doi: 10.1148/rg.323115120 [DOI] [PubMed] [Google Scholar]
- 13.Baughman WC, Corteville JE, Shah RR, Placenta accreta: spectrum of US and MR imaging findings. Radiographics 2008; 28: 1905–16. doi: 10.1148/rg.287085060 [DOI] [PubMed] [Google Scholar]
- 14.Kayem G, Davy C, Goffinet F, Thomas C, Clément D, Cabrol D. Conservative versus extirpative management in cases of placenta accreta. Obstet Gynecol 2004; 104: 531–6. doi: 10.1097/01.AOG.0000136086.78099.0f [DOI] [PubMed] [Google Scholar]
- 15.Eller AG, Porter TF, Soisson P, Silver RM. Optimal management strategies for placenta accreta. BJOG 2009; 116: 648–54. doi: 10.1111/j.1471-0528.2008.02037.x [DOI] [PubMed] [Google Scholar]
- 16.Carlson KJ, Outcomes of hysterectomy. Clin Obstet Gynecol 1997; 40: 939–46. doi: 10.1097/00003081-199712000-00029 [DOI] [PubMed] [Google Scholar]
- 17.Clausen C, Stensballe J, Albrechtsen CK, Hansen MA, Lönn L, Langhoff-Roos J. Balloon occlusion of the internal iliac arteries in the multidisciplinary management of placenta percreta. Acta Obstet Gynecol Scand 2013; 92: 386–91. doi: 10.1111/j.1600-0412.2012.01451.x [DOI] [PubMed] [Google Scholar]
- 18.Panici PB, Anceschi M, Borgia ML, Bresadola L, Masselli G, Parasassi T, et al. Intraoperative aorta balloon occlusion: fertility preservation in patients with placenta previa accreta/increta. J Matern Fetal Neonatal Med 2012; 25: 2512–6. doi: 10.3109/14767058.2012.712566 [DOI] [PubMed] [Google Scholar]
- 19.Teixidor Viñas M, Chandraharan E, Moneta MV, Belli AM. The role of interventional radiology in reducing haemorrhage and hysterectomy following caesarean section for morbidly adherent placenta. Clin Radiol 2014; 69: e345–51. doi: 10.1016/j.crad.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 20.Hansch E, Chitkara U, McAlpine J, El-Sayed Y, Dake MD, Razavi MK. Pelvic arterial embolization for control of obstetric hemorrhage: a five-year experience. Am J Obstet Gynecol 1999; 180(6 Pt 1): 1454–60. doi: 10.1016/S0002-9378(99)70036-0 [DOI] [PubMed] [Google Scholar]
- 21.Sivan E, Spira M, Achiron R, Rimon U, Golan G, Mazaki-Tovi S, et al. Prophylactic pelvic artery catheterization and embolization in women with placenta accreta: can it prevent cesarean hysterectomy? Am J Perinatol 2010; 27: 455–61. doi: 10.1055/s-0030-1247599 [DOI] [PubMed] [Google Scholar]
- 22.Mei J, Wang Y, Zou B, Hou Y, Ma T, Chen M, et al. Systematic review of uterus-preserving treatment modalities for abnormally invasive placenta. J Obstet Gynaecol 2015; 35: 777–82. doi: 10.3109/01443615.2015.1011106 [DOI] [PubMed] [Google Scholar]