Abstract
Cancer of the pancreas remains one of the deadliest cancer types. Based on the GLOBOCAN 2012 estimates, pancreatic cancer causes more than 331000 deaths per year, ranking as the seventh leading cause of cancer death in both sexes together. Globally, about 338000 people had pancreatic cancer in 2012, making it the 11th most common cancer. The highest incidence and mortality rates of pancreatic cancer are found in developed countries. Trends for pancreatic cancer incidence and mortality varied considerably in the world. A known cause of pancreatic cancer is tobacco smoking. This risk factor is likely to explain some of the international variations and gender differences. The overall five-year survival rate is about 6% (ranges from 2% to 9%), but this vary very small between developed and developing countries. To date, the causes of pancreatic cancer are still insufficiently known, although certain risk factors have been identified, such as smoking, obesity, genetics, diabetes, diet, inactivity. There are no current screening recommendations for pancreatic cancer, so primary prevention is of utmost importance. A better understanding of the etiology and identifying the risk factors is essential for the primary prevention of this disease.
Keywords: Pancreatic cancer, Epidemiology, Incidence, Mortality, Trend, Risk factors
Core tip: Pancreatic cancer is the one of leading causes of cancer mortality and one of the most lethal malignant neoplasms across the world. The highest incidence and mortality rates of pancreatic cancer are found in developed countries. The estimated 5-year survival rate for pancreatic cancer is about 5%. The causes of pancreatic cancer are still insufficiently known, although certain risk factors have been identified, such as cigarette smoking, positive family history and genetics, diabetes mellitus, obesity, dietary factors, alcohol use, physical inactivity. There are no current screening recommendations for pancreatic cancer, so primary prevention is of utmost importance.
INTRODUCTION
Pancreatic cancer is the one of leading causes of cancer mortality in developed countries and one of the most lethal malignant neoplasms across the world[1-3]. The two main tumor types of pancreatic cancer are adenocarcinoma (that accounts for about 85% of cases), and pancreatic endocrine tumors (which are make up for less than 5% of all cases)[1,4,5].
Based on the GLOBOCAN 2012 estimates, pancreatic cancer causes more than 331000 deaths per year (accounts for 4.0% of all deaths), ranking as the seventh leading cause of cancer death in both sexes together[1]. About 338000 people had pancreatic cancer in 2012, making it the 11th most common cancer[1,2].The estimated 5-year survival rate for pancreatic cancer is less than 5%[2,4]. The incidence and mortality of pancreatic cancer worldwide correlated with increasing age and was slightly more common in men than in women[1-3]. In the past decades, pancreatic cancer mortality has been increasing in both genders (for example, in the United States, European countries, Japan, China)[1,3,6-8].
The causes of pancreatic cancer are still insufficiently known, although certain risk factors have been identified, such as cigarette smoking, positive family history and genetics, diabetes mellitus, obesity, dietary factors, alcohol use, physical inactivity[9-12].
Understanding the epidemiology of pancreatic cancer could be the key to elucidating the etiology of pancreatic cancer and thus the cornerstone of developing a prevention strategy.
INCIDENCE
The incidence of pancreatic cancer varies greatly across regions and populations. Incidence rates for pancreatic cancer in 2012 were highest in Northern America (7.4 per 100000 people) and Western Europe (7.3 per 100000 people), followed by other regions in Europe and Australia/New Zealand (equally about 6.5 per 100000 people) (Figure 1)[1]. The lowest rates (about 1.0 per 100000 people) were observed in Middle Africa and South-Central Asia. Differences in incidence rates were twenty fold between the populations with the highest rate (Czech Republic - 9.7), and the population with the lowest rate (Pakistan - 0.5). More than half of new cases (55.5%) were registered in more developed regions[1]. Slightly less than half (41.0%; 139363 of cases) of all new cases of pancreatic cancer in 2012 were recorded in the countries of Asia.
Figure 1.
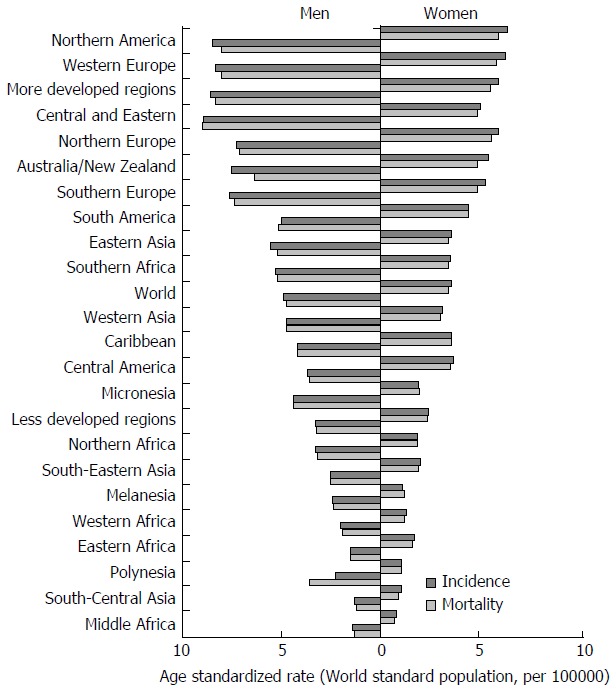
Pancreatic cancer incidence and mortality in men and women, by regions, GLOBOCAN 2012 estimates.
There are significant geographic variations in the incidence of pancreatic cancer by genders[1,2,6-8]. The incidence rate of pancreatic cancer among men in 2012 was 4.9 per 100000, and among women 3.6 per 100000 (Figure 2)[1]. In men, the risk of developing pancreatic cancer was high in Armenia (11.9) and Czech Republic (11.8), Slovakia and Hungary (equally - 11.5), then in Japan and Lithuania (equally - 10.6) (Figure 2A). In contrast, the risk of contracting pancreatic cancer in men was the lowest in Pakistan (0.5) and Guinea (0.4). The regions with the highest incidence rates of pancreatic cancer in women were Northern America (6.4 per 100000), Western Europe (6.3), and Northern Europe and Australia/New Zealand (5.9 and 5.4, respectively) (Figure 1)[1]. The lowest rates of pancreatic cancer incidence (less than 1.0 per 100000) were in Middle Africa and Polynesia. Women living in Hungary, Denmark, Finland and Armenia have the greatest risk (aproximatelly 7.5 per 100000) of dying from pancreatic cancer, while women in Tanzania, Guinea, Cameroon, Namibia and Pakistan have the lowest disease risk (less than 1.0 per 100000) (Figure 2B).
Figure 2.
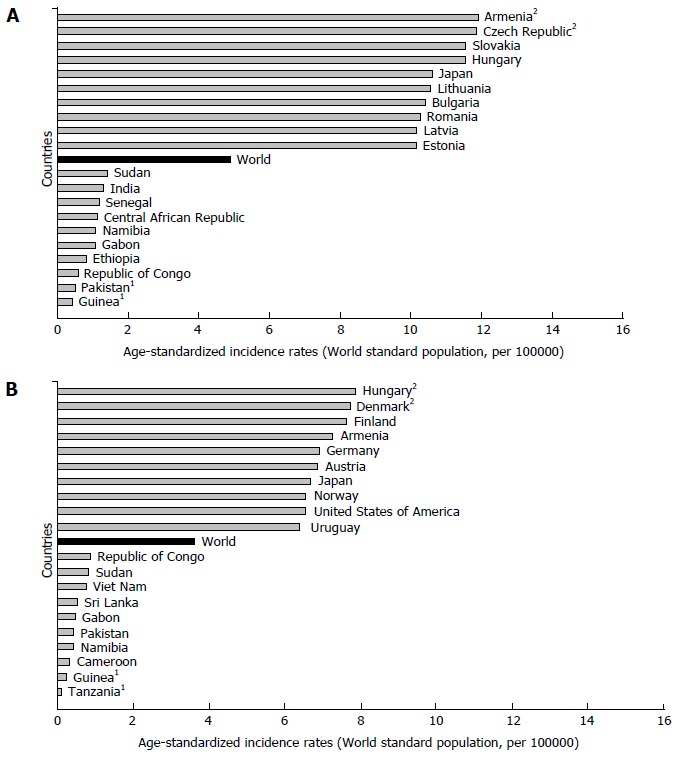
Pancreatic cancer incidence in men (A) and women (B), GLOBOCAN 2012 estimates. 1Country with the lowest mortality rates; 2Country with the highest mortality rates. GLOBOCAN 2012 estimates[1].
The incidence rates for both sexes increase with age, the highest in older than 70 years[3,4,6]. It is predominantly a disease of the elderly, and almost 90% of all cases are diagnosed after the age of 55 years[6,13].
Although it is not possible to fully explain the differences in the incidence of pancreatic cancer in different parts of the world, most of the international variation in the incidence of pancreatic cancer has been attributed to exposure to known or suspected risk factors related to lifestyle or the environment[9,10,13]. Tobacco smoking is likely to explain these international variations and gender differences[11]. Some findings may be indicating that obesity may have some effect on differences[14]. Additionally, some findings indicate the role of aging and hereditary and genetic factors. The reasons for higher incidence of pancreatic cancer in men are still insufficiently known: women are either less prone to these kinds of malignant tumors, or are less exposed to risk factors from the environment responsible for their occurrence[9,15]. Besides that, international differences probably reflect diagnostic capacity and the change in use of various diagnostic modalities[16]. In 2012, Europe carried one third of the overall incidence, which likely reflected the more accurate diagnosis of pancreatic cancer rather than etiology[17]. It should be noted that some differences in incidence of pancreatic cancer around the world may be attributed to the quality of registries, which coverage, completeness and accuracy varies by country[18].
MORTALITY
International mortality rates for pancreatic cancer vary significantly in different areas. Rates of mortality from pancreatic cancer in 2012 in both genders were highest in Northern America (6.9 per 100000 people) and Western Europe (6.8), followed by other European regions and Australia/New Zealand (approximately - 6.0, respectively) (Figure 1)[1]. The lowest mortality was recorded in the countries in Middle Africa and South-Central Asia (less than 1.0 per 1000000 people). The differences in mortality rates were nearly fifty fold between the populations with the highest and lowest rate (Armenia vs Tanzania: 8.9 vs 0.2). More than one third (111029 deaths) of all deceased from pancreatic cancer are residents of Europe. Slightly less than half (41.5%; 137251 deaths) of all deaths from pancreatic cancer were recorded in 2012 in Asian countries[1]. More than half (55.8%, 184429 deaths) of deceased of pancreatic cancer were registered in more developed regions. At least deaths were registered in Micronesia/Polynesia. The least number of deaths was registered in Micronesia/Polynesia.
Mortality of pancreatic cancer in both genders increases with age, and almost 90% of all deaths are registered after the age of 55 years[1,3]. The highest mortality rates in 2012 in males were recorded in Central and Eastern Europe (Latvia - 11.9, Hungary - 11.5) (Figure 3A)[1]. The mortality from pancreatic cancer was lowest (less than 1.0 per 100000 people) in Belize and Bahrain. The highest mortality rates in 2012 in females were recorded in Hungary (7.5) and Malta (7.2) (Figure 3B)[1]. The mortality from pancreatic cancer was lowest in women in Belize (0.8).
Figure 3.
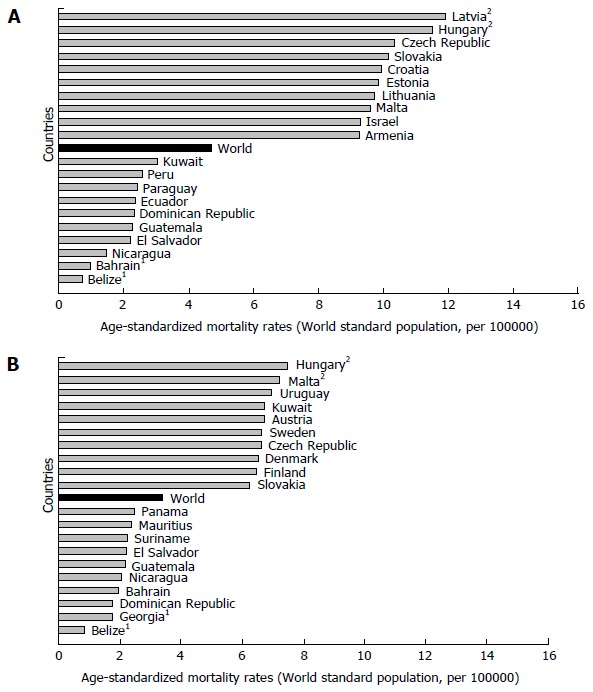
Pancreatic cancer mortality in men (A) and women (B), GLOBOCAN 2012 estimates. 1Country with the lowest mortality rates; 2Country with the highest mortality rates. GLOBOCAN 2012 estimates[1].
Mortality of pancreatic cancer is almost identical with its incidence, because it is one of the most fatal malignant tumors[19,20]. Reasons for the substantial differences in mortality rates of pancreatic cancer were not completely elucidated. Differences in rates of incidence can be apparent and specious. Specious differences may arise as a result of changes in the diagnosis of diseases and causes of death, as a result of a real shift in the incidence and/or fatality. Data on the incidence/mortality published by WHO are not of the same quality in all countries[18]. Although the quality (accuracy and completeness of cause of death registration, primarily) and the coverage of information in most developing countries can be considered limited, the registry often remains the only available source. Symptoms, signs and insufficiently defined conditions as the underlying cause of death are significantly more often mentioned in Serbia, the Russian Federation and Greece than in more developed countries such as the United states of America, United Kingdom, and Finland, which points to the need for a cautious interpretation of the data statistics of mortality in international comparisons[18]. Pancreatic cancer is difficult to diagnose. Malignant pancreatic neoplasm was among the most common cancers detected at autopsy studies[16,21]. It is known that for pancreatic cancer there is no workable modality of screening, early detection and effective treatment, which has the consequence of survival rates varying very little between developed and developing countries[22]. Current available treatment options for pancreatic cancer are limited. Due to the advanced stage at diagnosis, 80%-90% of patients have unresectable tumours and long-term survival after surgical resection is poor[13,19,23].
High smoking prevalence has been widely recognized as the main contributor to the high mortality rates of pancreatic cancer[11,24]. Numerous evidence support that diet (animal fat and meat consumption, etc.) plays a role in the development of pancreatic cancer[25,26]. In addition, the highest rates for pancreatic cancer mortality in eastern European countries suggested that other factors (including prevalence of diabetes, obesity, alcohol use) could influence the mortality of pancreatic cancer[27].
TEMPORAL TRENDS
Trends for pancreatic cancer mortality varied considerably in the world. Figure 4 represent pancreatic cancer mortality trends in 8 selected countries using official data for pancreatic cancer abstracted from the WHO database over the period 1955 to 2012[1]. For men in most developed countries (United States, the United Kingdom, Australia, Japan and Finland), a rise was observed between the early 1955 and the late 1980s, followed by a leveling off since 1990s (Figure 4A). Thus, rates for men in Eastern and Southern Europe (Hungary and Greece) rose continuously over the observed period. For women in the United Kingdom, Finland, Japan, United States and Australia, rates tended to rise up to the early 1990s, and to level off thereafter (Figure 4B). In Eastern and Southern European countries (such as Hungary and Greece), rates rose from the early 1970s to onwards.
Figure 4.
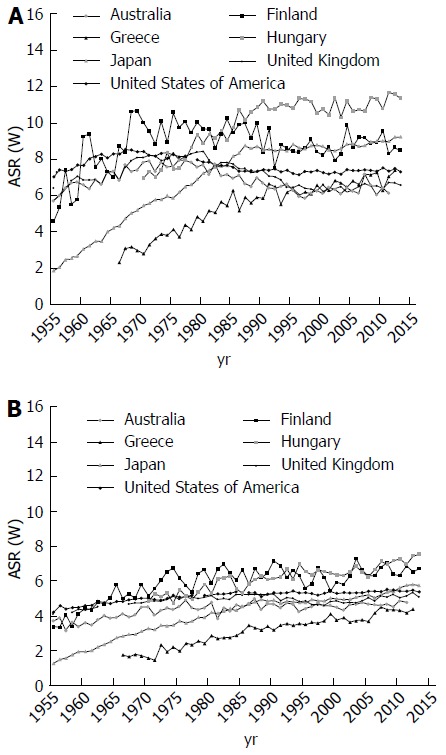
Pancreatic cancer mortality trends among men (A) and women (B) in selected countries. GLOBOCAN 2012 estimates[1].
In the United States, whites and blacks experienced opposite trends in pancreatic cancer death rates between 1975 and 2013[28]. In whites, pancreatic cancer death rates decreased by 0.2% per year from 1095 to 1996 and then increased by 0.4% per year through 2013. In contrast, the rates among blacks increased between 1975 and the late 1990s and then decreased thereafter. Also, in last decades a decreasing trend was found in both sexes in the United Kingdom, Sweden, Australia, the Netherlands, Mexico[13]. An increasing trend in both sexes was noticed in France, Japan, Brazil, Republic of Korea, and most countries of Central (Germany, Hungary), Eastern (Russian Federation, Poland, Romania, Serbia), and Southern Europe (Italy, Spain) [13,29-31].
To date, however, temporal trends in pancreatic cancer incidence and mortality have not been well understood. In both sexes, temporal trends in cigarette smoking prevalence were related to temporal trends in pancreatic cancer mortality with a roughly lag of several decades[9,11,24]. Decreased smoking, particularly in men, has been widely recognized as the main contributor to the decreases in mortality trends from pancreatic cancer in developed countries[24,32]. The mortality rate of pancreatic cancer began to decline earlier in the countries where the tobacco control started to be implemented earlier (such as United States, the United Kingdom, Australia). However, the recent increasing trends in some countries in the European Union suggest that other factors (including mainly obesity, physical inactivity, diabetes, and dietary factors), besides smoking, may have influenced the pancreatic cancer mortality[20,22,31,33]. In recent years, people in developing countries adopt lifestyles and behaviors that are typical for developed countries, such as cigarette smoking, higher consumption of saturated fat and calorie-rich foods, and reduced physical activity[25,27,34]. Besides, the trends might be due to disparities in socioeconomic circumstances between high- and low-income areas. Improved diagnostic and death certification of the disease might also partially explain the observed figures, at least part of the recent trends in some countries of Southern, Central and Eastern Europe[18,27,31]. It is unlikely, however, that progress in the treatment of pancreatic cancer could have a significant impact on mortality trends, also given the negligible changes in survival over the last decades[19,22,23,35]. Also, it is known that the changes in coding of pancreatic cancer had minimal influence on mortality trends of pancreatic cancer in the second half of the XX century[18,36].
SURVIVAL
Cancer of the pancreas remains one of the most deadly common cancer types: the Mortality/Incidence ratio is 98%[1]. The overall five-year survival rate is about 6% (ranges from 2% to 9%), but this partly reflects varying data quality worldwide[37,38]. For pancreatic cancer, survival rates vary very small between developed and developing countries[37].
Based on the United States National Cancer Institute’ data for pancreatic cancer in both sexes and all races, 9.4% are diagnosed at the local stage while the 5-year survival for localized disease was 29.3% during 2006-2012[3]. More than half (52%) of all cases were diagnosed at the distant stage with the 5-year survival rates of 2.6%.
Some intercountry survival differences for pancreatic cancers exist over Europe: 5-year survival rate was less than 3% in both sexes in England and Wales[39], Denmark and Sweden - 3.8%[22], in Italy - 1.2%[40]. EUROCARE Working Group analyzed the survival of cancer patients diagnosed from 1990 to 1994 in 22 European countries and showed that 5-year survival rates were highest in men in Estonia (7.0%) and in women in Czech Republic (7.5%), while lowest survival rates were recorded in men in Malta (0.0%) and in women in Slovenia (1.3%)[37]. Survival from pancreatic cancer in Germany in the early 21st century was 9.0%[35].
Survival rates of pancreatic cancer in population are affected by many factors, such as the type of cancer, the staging at the time of diagnosis, serum albumin level, and tumor size, treatment modality, availability and differences in health care systems, and other factors including age, sex, overall health, lifestyle[23,37,41-50]. Besides, pancreatic cancer survival rates could be influenced by factors such as the validity of cancer registry, exhaustiveness and quality of registration data, completeness of follow-up[22,51].
ETIOLOGY AND RISK FACTORS
To date, the causes of pancreatic cancer are still insufficiently known, although certain risk factors have been identified. People are at higher risk to develop pancreatic cancer with any of the risk factors such as smoking, obesity, genetics, diabetes, diet, inactivity[9,12,13,52].
In the study in the United Kingdom in 2011 it was estimated that around 26.2% of pancreatic cancers in men and 31.0% in women was linked to tobacco smoking[9]. The International Agency for Research on Cancer confirmed that smoking is causally associated with pancreatic cancer[11,33]. A recent meta-analysis that included 82 studies found that the risk of pancreatic cancer was RR = 1.7 for current and RR = 1.2 for former smokers[53,54]. Cigarette smoking causes an increase in the risk of pancreatic cancer by 75 percent compared to non-smokers, and the risk persists for at least 10 years after smoking cessation[34,54]. The European (EPIC) study showed in 2012 that risk increased for every five cigarettes smoked per day[55]. Also, the same EPIC study noticed that passive smoking can increase the risk of pancreatic cancer by 50%[54].
The risk of developing pancreatic cancer increases with age[13,56]. Over 80% of pancreatic cancers develop between the ages of 60 and 80 years. Pancreatic cancer rarely occurs before the age of 40, and more than half of cases of pancreatic adenocarcinoma occur in those over 70. Pancreatic cancer affects men and women equally[1,56]. Studies in the United States have shown that pancreatic cancer is more common in the African American population than it is in the white population[57,58]. Some of this increased risk may be due to socioeconomic factors and to cigarette smoking[3,24,53,56].
According to an American Cancer Society study, obesity has been associated with increased mortality from cancer of the pancreas: risk of pancreatic cancer among obese (body mass index BMI ≥ 30) men and women compared with men and women of normal body mass index (< 25) was RR = 2.08[59]. A burden study published in 2011 estimated that, in the United Kingdom, around 12.8% of pancreatic cancers in men and 11.5% in women can attributed to overweight/obesity[9]. The recent meta-analysis has confirmed the hypothesis that both general and abdominal obesity are associated with increased pancreatic cancer risk[60]. Besides, physical inactivity has been linked with increased risk of cancer of the pancreas.
There is some evidence that intake of red or processed meat and high-temperature cooking may increase the risk of pancreatic cancer. In a large United Kingdom cohort study in 2016, low meat eaters (about 30%-45% lower mortality), as well as vegetarians and vegans (about 50% lower mortality) had lower mortality for pancreatic cancer compared with regular meat eaters[61]. The EPIC study found no association between intakes of red and processed meat and pancreatic cancer risk, while poultry consumption was associated with an increased risk[62]. A recent meta-analysis of 11 prospective studies found a positive association between pancreatic cancer incidence and processed meat consumption[63]. But, some studies have not supported these findings[64], or have provided support for the association among men only[65]. On the other hand, frequent nut consumption is inversely associated with risk of pancreatic cancer in women[66,67]. A recent large nested case-control study in 2010 showed increased risk even at consumption of 60 g/d or more of liquor (spirits), and found no association with beer or wine[68]. Findings from the latest meta-analysis support that fruit and vegetable intake is associated inversely with the risk of pancreatic cancer[69]. Additionally, a summary review of meta-analytical studies showed that the major protective factor is increasing fruit or folate intake, with respective population preventable fractions of 0-12%[70]. In the Italian population, 11.9% of pancreatic cancers were attributable to a low adherence to Mediterranean diet[71].
Diabetes mellitus is linked with increased risk of pancreatic tumors[70,72]. Both type I and type II diabetes have doubled the risk of pancreatic cancer[72-74]. The pancreatic cancer burden study in the Italian population estimated that 9.7% of pancreatic cancers were attributable to diabetes[71]. The United States National Cancer Institute estimates that diabetes is associated with a 1.8-fold increased risk of pancreatic cancer, particularly in Hispanic men and Asians in comparison with whites and blacks[75]. Pancreatic cancer risk decreased with duration of diabetes, but a 30% excess risk persists for more than two decades after diabetes diagnosis[76]. Oral antidiabetics or insulin use were associated with a reduced risk of pancreatic cancer[75,76].
Some studies showed that Helicobacter pylori (H. pylori) infection is the major risk factors associated with pancreatic cancer, with estimated population attributable fraction of 4%-25%[70]. But, other studies did not observe an association of H. pylori infection with pancreatic cancer[77].
Patients with pancreatitis, especially the chronic or recurrent forms, had a moderate excess of pancreatic cancer risk[78]. About 4% of chronic pancreatitis patients developed pancreatic cancer[79]. It is estimated that 1.34% of pancreatic cancers are atributable to chronic pancreatitis, but for those who were under the age of 65 that risk was two times higher[80]. Patients with hereditary pancreatitis (rare, autosomal-dominant disease, usually occurs at a young age) have a risk that is 50-60 times greater than expected[81].
It is estimated that 5%-10% of pancreatic cancers are hereditary[9,52]. A family history of pancreatic cancer in a parent, sibling or child was associated with increased risk of pancreatic cancer[82]. People with at least two first-degree relatives (mother, father, brother, sister) with pancreatic cancer have almost double the risk of people without pancreatic cancer in their family[83].
There are many of inherited genetic disorders which are known to increase the risk for pancreatic cancer, including Lynch syndrome, Peutz-Jeghers syndrome, the Familial atypical multiple mole melanoma syndrome, Hereditary breast and ovarian cancer syndrome, Li-Fraumeni syndrome, Familial adenomatous polyposis, etc[83]. Individuals with mutations or deletion in such genes as PRSS1, K-ras, p16, p53, and BRCA2 have an increased risk of developing pancreatic cancer[84].
Some findings show a link between pancreatic cancer and previous cancers (cancer of the gallbladder, lung, stomach, uterus, breast, colon, etc.) and other conditions (Crohn’s disease, gastric ulcer)[84-86].
Other potential risk factors include aspirin use, occupational exposure to certain pesticides, and dietary factors such as carbohydrate or sugar intake[70,86]. Most of pancreatic cancer risk factors are only weakly associated with the disease. Additionally, many people with pancreatic cancer do not have any one specific risk factor for it.
PREVENTION
There are no current screening recommendations for pancreatic cancer, so primary prevention is of utmost importance. A better understanding of the etiology and identifying the risk factors is essential for the primary prevention of this disease. Potentially modifiable risk factors include tobacco smoking, obesity, and diabetes mellitus, diet, alcohol consumption. So far, the best preventive strategy against pancreatic cancer is risk reduction, including lifestyle modification (smoking cessation, healthy weight, diet high in fruits and vegetables, regular exercises), and regular control of health issues[9,13,52,53].
Lifestyle modifications
Control of smoking offers the best available strategy for reducing the incidence of pancreatic cancer. It has been estimated that about 30% of pancreatic cancers could be prevented by prevention of smoking[9]. A European-wide prospective study in 2009 showed that risk is reduced to the levels of a non-smoker after just five years of cessation[54].
Epidemiological data show that dietary factors which are associated with increased risk of pancreatic cancer are meat, red meat in particular, and energy: preventive measures include recommendations for reducing the intake. Protection is mainly provided by means of a “prudent”, well balanced diet, diet containing ample fruits and vegetables, nut consumption, vitamins (β-carotene, vitamin D and E), and more dietary compounds[26,87,88]. Also, it is necessary to limit the alcohol use, which is known to increase the pancreatic cancer risk through development of chronic pancreatitis and cirrhosis[89].
Chemoprevention, with agents such as COX inhibitors and aspirin, could mean benefit for persons at high risk for pancreatic cancer and needs to be verified in future[90,91].
Chronic pancreatitis caused by heavy alcohol consumption or by an underlying inherited disorder is associated with a high risk of pancreatic cancer. Reduced alcohol use has been shown to reduce the risk of pancreatic cancer. Screening PRSS1 (Cationic trypsinogen gene) mutation as the mutation that causes hereditary pancreatitis is being performed for the development of a screening programme aimed at detecting pancreatic cancer at an early stage[92].
Patients with cystic neoplasms of the pancreas develop pancreatic cancer in about 60% to 70% of patients[93]. The complete extirpation of cystic neoplasms is now performed as a cancer preventive strategy[94].
Some risk factors such as age, gender, race, and family history cannot be modified. But, there is secondary prevention that might lower pancreatic cancer risk.
Screening and early detection
There is no reliable screening test currently available to screen the general population and detect pancreatic cancer early[95,96]. The research into the area of screening for pancreatic cancer has increased significantly in the past decade. Several screening tests were investigated to aid in the identification or earlier diagnosis of pancreatic cancer for the general population, including the blood markers for pancreatic cancer CA19-9, SPAN-1, CA-50, DUPAN-2, cell surface associated mucins (MUC), carcinoembryonic antigen, and heat shock proteins[10,97]. But these tests have not been well studied yet[98].
Although there are no screening tests currently available to screen the general population, researchers are working on developing effective screening tests that may be applied to groups of persons which have an increased risk of developing pancreatic cancer (such as persons with family history of pancreatic cancer, with abnormalities in certain genes - such as BRCA2, p16, HNPCC, and with histories of familial pancreatitis and Peutz-Jeghers syndrome, etc.)[98,99]. Also, question is which access points (stool, pancreatic juice, saliva and blood) could be appropriate to study these potential screening markers. For persons with high risk of pancreatic cancer (including patients with hereditary pancreatitis or for persons with family members with pancreatic cancer), some screening techniques (i.e., endoscopic ultrasound and spiral computerized tomography) are promising but have not been fully evaluated[100]. In patients with hereditary pancreatitis (in PRSS1 mutation carriers) who have a higher risk of early onset of pancreatic cancer, screening can begin at age 40[92]. There is no consensus about when is screening initiating recommended for other persons. However, it remains an area of great interest[101].
CONCLUSION
Around the world significant efforts are being made in order to better understand pancreatic cancer. Detailed epidemiological analyses of pancreatic cancer trends and further analytical epidemiological researches will help guide future cancer control strategies.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Serbia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest.
Peer-review started: July 4, 2016
First decision: August 19, 2016
Article in press: September 28, 2016
P- Reviewer: Neninger E, Peng SY S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. Accessed 2016-03-04. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975-2013, National Cancer Institute. Bethesda, MD, 2016. Available from: http://seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 4.Hidalgo M, Cascinu S, Kleeff J, Labianca R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL, Heinemann V. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. 2013;15:8–18. doi: 10.1016/j.pan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malvezzi M, Carioli G, Bertuccio P, Rosso T, Boffetta P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann Oncol. 2016;27:725–731. doi: 10.1093/annonc/mdw022. [DOI] [PubMed] [Google Scholar]
- 7.Qiu D, Katanoda K, Marugame T, Sobue T. A Joinpoint regression analysis of long-term trends in cancer mortality in Japan (1958-2004) Int J Cancer. 2009;124:443–448. doi: 10.1002/ijc.23911. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Yang GH, Lu XH, Huang ZJ, Li H. Pancreatic cancer mortality in China (1991-2000) World J Gastroenterol. 2003;9:1819–1823. doi: 10.3748/wjg.v9.i8.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105 Suppl 2:S77–S81. doi: 10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 11.Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116:963–971. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
- 12.Willett WC. Diet and cancer. Oncologist. 2000;5:393–404. doi: 10.1634/theoncologist.5-5-393. [DOI] [PubMed] [Google Scholar]
- 13.Bosetti C, Bertuccio P, Negri E, La Vecchia C, Zeegers MP, Boffetta P. Pancreatic cancer: overview of descriptive epidemiology. Mol Carcinog. 2012;51:3–13. doi: 10.1002/mc.20785. [DOI] [PubMed] [Google Scholar]
- 14.Genkinger JM, Spiegelman D, Anderson KE, Bernstein L, van den Brandt PA, Calle EE, English DR, Folsom AR, Freudenheim JL, Fuchs CS, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer. 2011;129:1708–1717. doi: 10.1002/ijc.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 16.Avgerinos DV, Björnsson J. Malignant neoplasms: discordance between clinical diagnoses and autopsy findings in 3,118 cases. APMIS. 2001;109:774–780. doi: 10.1034/j.1600-0463.2001.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 18.Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171–177. [PMC free article] [PubMed] [Google Scholar]
- 19.Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 2013;6:321–337. doi: 10.1177/1756283X13478680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levi F, Lucchini F, Negri E, La Vecchia C. Pancreatic cancer mortality in Europe: the leveling of an epidemic. Pancreas. 2003;27:139–142. doi: 10.1097/00006676-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Sens MA, Zhou X, Weiland T, Cooley AM. Unexpected neoplasia in autopsies: potential implications for tissue and organ safety. Arch Pathol Lab Med. 2009;133:1923–1931. doi: 10.5858/133.12.1923. [DOI] [PubMed] [Google Scholar]
- 22.Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JW. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345–1389. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Lambe M, Eloranta S, Wigertz A, Blomqvist P. Pancreatic cancer; reporting and long-term survival in Sweden. Acta Oncol. 2011;50:1220–1227. doi: 10.3109/0284186X.2011.599338. [DOI] [PubMed] [Google Scholar]
- 24.Weiss W, Benarde MA. The temporal relation between cigarette smoking and pancreatic cancer. Am J Public Health. 1983;73:1403–1404. doi: 10.2105/ajph.73.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Zhao Z, Berkel HJ. Animal fat consumption and pancreatic cancer incidence: evidence of interaction with cigarette smoking. Ann Epidemiol. 2005;15:500–508. doi: 10.1016/j.annepidem.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Anderson KE, Mongin SJ, Sinha R, Stolzenberg-Solomon R, Gross MD, Ziegler RG, Mabie JE, Risch A, Kazin SS, Church TR. Pancreatic cancer risk: associations with meat-derived carcinogen intake in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort. Mol Carcinog. 2012;51:128–137. doi: 10.1002/mc.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarosz M, Sekuła W, Rychlik E. Influence of diet and tobacco smoking on pancreatic cancer incidence in poland in 1960-2008. Gastroenterol Res Pract. 2012;2012:682156. doi: 10.1155/2012/682156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J, Siegel R, Jemal A. Pancreatic cancer death rates by race among US men and women, 1970-2009. J Natl Cancer Inst. 2013;105:1694–1700. doi: 10.1093/jnci/djt292. [DOI] [PubMed] [Google Scholar]
- 29.Ilić M, Vlajinac H, Marinković J, Kocev N. Pancreatic cancer mortality in Serbia from 1991-2010 - a joinpoint analysis. Croat Med J. 2013;54:369–375. doi: 10.3325/cmj.2013.54.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wingo PA, Cardinez CJ, Landis SH, Greenlee RT, Ries LA, Anderson RN, Thun MJ. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer. 2003;97:3133–3275. doi: 10.1002/cncr.11380. [DOI] [PubMed] [Google Scholar]
- 31.La Vecchia C, Bosetti C, Lucchini F, Bertuccio P, Negri E, Boyle P, Levi F. Cancer mortality in Europe, 2000-2004, and an overview of trends since 1975. Ann Oncol. 2010;21:1323–1360. doi: 10.1093/annonc/mdp530. [DOI] [PubMed] [Google Scholar]
- 32.Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking - IARC Monographs on the Evaluation of Carcinogenic Risks to Humans,Vol. 83 Lyon: International Agency for research on Cancer WHO mortality database, WHO/Geneva, 2004) Available from: http://www3.who.int. Accessed on 04/03/2016. [PMC free article] [PubMed]
- 34.Rastogi T, Devesa S, Mangtani P, Mathew A, Cooper N, Kao R, Sinha R. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol. 2008;37:147–160. doi: 10.1093/ije/dym219. [DOI] [PubMed] [Google Scholar]
- 35.Hiripi E, Gondos A, Emrich K, Holleczek B, Katalinic A, Luttmann S, Sirri E, Brenner H. Survival from common and rare cancers in Germany in the early 21st century. Ann Oncol. 2012;23:472–479. doi: 10.1093/annonc/mdr131. [DOI] [PubMed] [Google Scholar]
- 36.Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49:1–32. [PubMed] [Google Scholar]
- 37.Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, Grosclaude P, Hédelin G, Matsuda T, Møller H, et al. EUROCARE-3: survival of cancer patients diagnosed 1990-94--results and commentary. Ann Oncol. 2003;14 Suppl 5:v61–118. doi: 10.1093/annonc/mdg754. [DOI] [PubMed] [Google Scholar]
- 38.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitry E, Rachet B, Quinn MJ, Cooper N, Coleman MP. Survival from cancer of the pancreas in England and Wales up to 2001. Br J Cancer. 2008;99 Suppl 1:S21–S23. doi: 10.1038/sj.bjc.6604576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasquali C, Sperti C, Filipponi C, Pedrazzoli S. Epidemiology of pancreatic cancer in Northeastern Italy: incidence, resectability rate, hospital stay, costs and survival (1990-1992) Dig Liver Dis. 2002;34:723–731. doi: 10.1016/s1590-8658(02)80024-x. [DOI] [PubMed] [Google Scholar]
- 41.Carrato A, Falcone A, Ducreux M, Valle JW, Parnaby A, Djazouli K, Alnwick-Allu K, Hutchings A, Palaska C, Parthenaki I. A Systematic Review of the Burden of Pancreatic Cancer in Europe: Real-World Impact on Survival, Quality of Life and Costs. J Gastrointest Cancer. 2015;46:201–211. doi: 10.1007/s12029-015-9724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fesinmeyer MD, Austin MA, Li CI, De Roos AJ, Bowen DJ. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1766–1773. doi: 10.1158/1055-9965.EPI-05-0120. [DOI] [PubMed] [Google Scholar]
- 43.Sharma C, Eltawil KM, Renfrew PD, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J Gastroenterol. 2011;17:867–897. doi: 10.3748/wjg.v17.i7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klint A, Engholm G, Storm HH, Tryggvadóttir L, Gislum M, Hakulinen T, Bray F. Trends in survival of patients diagnosed with cancer of the digestive organs in the Nordic countries 1964-2003 followed up to the end of 2006. Acta Oncol. 2010;49:578–607. doi: 10.3109/02841861003739330. [DOI] [PubMed] [Google Scholar]
- 45.Saif MW. Pancreatic neoplasm in 2011: an update. JOP. 2011;12:316–321. [PubMed] [Google Scholar]
- 46.Oh SY, Edwards A, Mandelson MT, Lin B, Dorer R, Helton WS, Kozarek RA, Picozzi VJ. Rare long-term survivors of pancreatic adenocarcinoma without curative resection. World J Gastroenterol. 2015;21:13574–13581. doi: 10.3748/wjg.v21.i48.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beger HG, Rau B, Gansauge F, Leder G, Schwarz M, Poch B. Pancreatic cancer--low survival rates. Dtsch Arztebl Int. 2008;105:255–262. doi: 10.3238/arztebl.2008.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dimastromatteo J, Houghton JL, Lewis JS, Kelly KA. Challenges of Pancreatic Cancer. Cancer J. 2015;21:188–193. doi: 10.1097/PPO.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linder S, Boström L, Nilsson B. Pancreatic carcinoma incidence and survival in Sweden in 1980-2000: a population-based study of 16,758 hospitalized patients with special reference to different therapies. Eur J Surg Oncol. 2007;33:616–622. doi: 10.1016/j.ejso.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T, Yagi S, Kinoshita H, Sakamoto Y, Okada K, Uryuhara K, Morimoto T, Kaihara S, Hosotani R. Long-term survival after resection of pancreatic cancer: a single-center retrospective analysis. World J Gastroenterol. 2015;21:262–268. doi: 10.3748/wjg.v21.i1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenner H, Hakulinen T. Population-based monitoring of cancer patient survival in situations with imperfect completeness of cancer registration. Br J Cancer. 2005;92:576–579. doi: 10.1038/sj.bjc.6602323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393:535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 54.Vrieling A, Bueno-de-Mesquita HB, Boshuizen HC, Michaud DS, Severinsen MT, Overvad K, Olsen A, Tjønneland A, Clavel-Chapelon F, Boutron-Ruault MC, et al. Cigarette smoking, environmental tobacco smoke exposure and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;126:2394–2403. doi: 10.1002/ijc.24907. [DOI] [PubMed] [Google Scholar]
- 55.Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, Canzian F, Steplowski E, Arslan AA, Gross M, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170:403–413. doi: 10.1093/aje/kwp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.American Cancer Society. Cancer Facts and Figures 2013. Atlanta, Ga: American Cancer Society; 2013. Available from: http://www.cancer.org. [Google Scholar]
- 57.Silverman DT, Hoover RN, Brown LM, Swanson GM, Schiffman M, Greenberg RS, Hayes RB, Lillemoe KD, Schoenberg JB, Schwartz AG, et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology. 2003;14:45–54. doi: 10.1097/00001648-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 58.Jemal A, Simard EP, Xu J, Ma J, Anderson RN. Selected cancers with increasing mortality rates by educational attainment in 26 states in the United States, 1993-2007. Cancer Causes Control. 2013;24:559–565. doi: 10.1007/s10552-012-9993-y. [DOI] [PubMed] [Google Scholar]
- 59.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 60.Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ, Norat T. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 61.Appleby PN, Crowe FL, Bradbury KE, Travis RC, Key TJ. Mortality in vegetarians and comparable nonvegetarians in the United Kingdom. Am J Clin Nutr. 2016;103:218–230. doi: 10.3945/ajcn.115.119461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rohrmann S, Linseisen J, Nöthlings U, Overvad K, Egeberg R, Tjønneland A, Boutron-Ruault MC, Clavel-Chapelon F, Cottet V, Pala V, et al. Meat and fish consumption and risk of pancreatic cancer: results from the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2013;132:617–624. doi: 10.1002/ijc.27637. [DOI] [PubMed] [Google Scholar]
- 63.Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer. 2012;106:603–607. doi: 10.1038/bjc.2011.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arem H, Mayne ST, Sampson J, Risch H, Stolzenberg-Solomon RZ. Dietary fat intake and risk of pancreatic cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Ann Epidemiol. 2013;23:571–575. doi: 10.1016/j.annepidem.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aschebrook-Kilfoy B, Cross AJ, Stolzenberg-Solomon RZ, Schatzkin A, Hollenbeck AR, Sinha R, Ward MH. Pancreatic cancer and exposure to dietary nitrate and nitrite in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2011;174:305–315. doi: 10.1093/aje/kwr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bao Y, Hu FB, Giovannucci EL, Wolpin BM, Stampfer MJ, Willett WC, Fuchs CS. Nut consumption and risk of pancreatic cancer in women. Br J Cancer. 2013;109:2911–2916. doi: 10.1038/bjc.2013.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu L, Wang Z, Zhu J, Murad AL, Prokop LJ, Murad MH. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. 2015;73:409–425. doi: 10.1093/nutrit/nuv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michaud DS, Vrieling A, Jiao L, Mendelsohn JB, Steplowski E, Lynch SM, Wactawski-Wende J, Arslan AA, Bas Bueno-de-Mesquita H, Fuchs CS, et al. Alcohol intake and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium (PanScan) Cancer Causes Control. 2010;21:1213–1225. doi: 10.1007/s10552-010-9548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu QJ, Wu L, Zheng LQ, Xu X, Ji C, Gong TT. Consumption of fruit and vegetables reduces risk of pancreatic cancer: evidence from epidemiological studies. Eur J Cancer Prev. 2016;25:196–205. doi: 10.1097/CEJ.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 70.Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol. 2015;44:186–198. doi: 10.1093/ije/dyu240. [DOI] [PubMed] [Google Scholar]
- 71.Rosato V, Polesel J, Bosetti C, Serraino D, Negri E, La Vecchia C. Population attributable risk for pancreatic cancer in Northern Italy. Pancreas. 2015;44:216–220. doi: 10.1097/MPA.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 72.Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol. 2014;21:2453–2462. doi: 10.1245/s10434-014-3625-6. [DOI] [PubMed] [Google Scholar]
- 73.Haugvik SP, Hedenström P, Korsæth E, Valente R, Hayes A, Siuka D, Maisonneuve P, Gladhaug IP, Lindkvist B, Capurso G. Diabetes, smoking, alcohol use, and family history of cancer as risk factors for pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Neuroendocrinology. 2015;101:133–142. doi: 10.1159/000375164. [DOI] [PubMed] [Google Scholar]
- 74.Stevens RJ, Roddam AW, Beral V. Pancreatic cancer in type 1 and young-onset diabetes: systematic review and meta-analysis. Br J Cancer. 2007;96:507–509. doi: 10.1038/sj.bjc.6603571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li D, Tang H, Hassan MM, Holly EA, Bracci PM, Silverman DT. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control. 2011;22:189–197. doi: 10.1007/s10552-010-9686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bosetti C, Rosato V, Li D, Silverman D, Petersen GM, Bracci PM, Neale RE, Muscat J, Anderson K, Gallinger S, et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the International Pancreatic Cancer Case-Control Consortium. Ann Oncol. 2014;25:2065–2072. doi: 10.1093/annonc/mdu276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen XZ, Schöttker B, Castro FA, Chen H, Zhang Y, Holleczek B, Brenner H. Association of helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: A ten-year follow-up of the ESTHER cohort study. Oncotarget. 2016;7:17182–17193. doi: 10.18632/oncotarget.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ekbom A, McLaughlin JK, Karlsson BM, Nyrén O, Gridley G, Adami HO, Fraumeni JF. Pancreatitis and pancreatic cancer: a population-based study. J Natl Cancer Inst. 1994;86:625–627. doi: 10.1093/jnci/86.8.625. [DOI] [PubMed] [Google Scholar]
- 79.Kudo Y, Kamisawa T, Anjiki H, Takuma K, Egawa N. Incidence of and risk factors for developing pancreatic cancer in patients with chronic pancreatitis. Hepatogastroenterology. 2011;58:609–611. [PubMed] [Google Scholar]
- 80.Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA, Silverman DT, Ji BT, Gallinger S, Holly EA, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012;23:2964–2970. doi: 10.1093/annonc/mds140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lowenfels AB, Maisonneuve P, Whitcomb DC. Risk factors for cancer in hereditary pancreatitis. International Hereditary Pancreatitis Study Group. Med Clin North Am. 2000;84:565–573. doi: 10.1016/s0025-7125(05)70240-6. [DOI] [PubMed] [Google Scholar]
- 82.Jacobs EJ, Chanock SJ, Fuchs CS, Lacroix A, McWilliams RR, Steplowski E, Stolzenberg-Solomon RZ, Arslan AA, Bueno-de-Mesquita HB, Gross M, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Int J Cancer. 2010;127:1421–1428. doi: 10.1002/ijc.25148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greer JB, Whitcomb DC, Brand RE. Genetic predisposition to pancreatic cancer: a brief review. Am J Gastroenterol. 2007;102:2564–2569. doi: 10.1111/j.1572-0241.2007.01475.x. [DOI] [PubMed] [Google Scholar]
- 84.Slebos RJ, Hoppin JA, Tolbert PE, Holly EA, Brock JW, Zhang RH, Bracci PM, Foley J, Stockton P, McGregor LM, et al. K-ras and p53 in pancreatic cancer: association with medical history, histopathology, and environmental exposures in a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9:1223–1232. [PubMed] [Google Scholar]
- 85.Shen M, Boffetta P, Olsen JH, Andersen A, Hemminki K, Pukkala E, Tracey E, Brewster DH, McBride ML, Pompe-Kirn V, et al. A pooled analysis of second primary pancreatic cancer. Am J Epidemiol. 2006;163:502–511. doi: 10.1093/aje/kwj073. [DOI] [PubMed] [Google Scholar]
- 86.Hemminki K, Li X, Sundquist J, Sundquist K. Cancer risks in Crohn disease patients. Ann Oncol. 2009;20:574–580. doi: 10.1093/annonc/mdn595. [DOI] [PubMed] [Google Scholar]
- 87.Pericleous M, Rossi RE, Mandair D, Whyand T, Caplin ME. Nutrition and pancreatic cancer. Anticancer Res. 2014;34:9–21. [PubMed] [Google Scholar]
- 88.Koushik A, Spiegelman D, Albanes D, Anderson KE, Bernstein L, van den Brandt PA, Bergkvist L, English DR, Freudenheim JL, Fuchs CS, et al. Intake of fruits and vegetables and risk of pancreatic cancer in a pooled analysis of 14 cohort studies. Am J Epidemiol. 2012;176:373–386. doi: 10.1093/aje/kws027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lucenteforte E, La Vecchia C, Silverman D, Petersen GM, Bracci PM, Ji BT, Bosetti C, Li D, Gallinger S, Miller AB, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012;23:374–382. doi: 10.1093/annonc/mdr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, Chen JQ, Xie L, Wang J, Li T, He Y, Gao Y, Qin X, Li S. Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: a systematic review and meta-analysis. BMC Med. 2014;12:55. doi: 10.1186/1741-7015-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stan SD, Singh SV, Brand RE. Chemoprevention strategies for pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2010;7:347–356. doi: 10.1038/nrgastro.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vitone LJ, Greenhalf W, Howes NR, Neoptolemos JP. Hereditary pancreatitis and secondary screening for early pancreatic cancer. Rocz Akad Med Bialymst. 2005;50:73–84. [PubMed] [Google Scholar]
- 93.Walsh RM, Vogt DP, Henderson JM, Hirose K, Mason T, Bencsath K, Hammel J, Brown N. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery. 2008;144:677–684; discussion 684-685. doi: 10.1016/j.surg.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 95.Greenhalf W, Grocock C, Harcus M, Neoptolemos J. Screening of high-risk families for pancreatic cancer. Pancreatology. 2009;9:215–222. doi: 10.1159/000210262. [DOI] [PubMed] [Google Scholar]
- 96.Shin EJ, Canto MI. Pancreatic cancer screening. Gastroenterol Clin North Am. 2012;41:143–157. doi: 10.1016/j.gtc.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cappelli G, Paladini S, D’Agata A. [Tumor markers in the diagnosis of pancreatic cancer] Tumori. 1999;85:S19–S21. [PubMed] [Google Scholar]
- 98.Gemmel C, Eickhoff A, Helmstädter L, Riemann JF. Pancreatic cancer screening: state of the art. Expert Rev Gastroenterol Hepatol. 2009;3:89–96. doi: 10.1586/17474124.3.1.89. [DOI] [PubMed] [Google Scholar]
- 99.Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–781; quiz 665. doi: 10.1016/j.cgh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, Ali SZ, Jagannath S, Petersen GM, Fishman EK, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–621. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 101.James TA, Gibbs JF. Pancreatic cancer screening: identifying premalignant disease. Future Oncol. 2005;1:191–195. doi: 10.1517/14796694.1.2.191. [DOI] [PubMed] [Google Scholar]


