Abstract
AIM
To determine the effects of ω-3 fatty acids (ω-3FA) on the toll-like receptor 4 (TLR4)/nuclear factor κB p56 (NF-κBp56) signal pathway in the lungs of rats with severe acute pancreatitis (SAP).
METHODS
A total of 56 Sprague-Dawley rats were randomly divided into 4 groups: control group, SAP-saline group, SAP-soybean oil group and SAP-ω-3FA group. SAP was induced by the retrograde infusion of sodium taurocholate into the pancreatic duct. The expression of TLR4 and NF-κBp56 in the lungs was evaluated by immunohistochemistry and Western blot analysis. The levels of inflammatory cytokines interleukin-6 and tumor necrosis factor-alpha in the lungs were measured by enzyme-linked immunosorbent assay.
RESULTS
The expression of TLR4 and NF-κBp56 in lungs and of inflammatory cytokines in serum significantly increased in the SAP group compared with the control group (P < 0.05), but was significantly decreased in the ω-3FA group compared with the soybean oil group at 12 and 24 h (P < 0.05).
CONCLUSION
During the initial stage of SAP, ω-3FA can efficiently lower the inflammatory response and reduce lung injury by triggering the TLR4/NF-κBp56 signal pathway.
Keywords: Severe acute pancreatitis, ω-3 fatty acids, Lung injury, Toll-like receptor 4, Nuclear factor-κB p56, Cytokine
Core tip: There is no report about the correlation between ω-3 fatty acids and toll-like receptor 4 (TLR4) expression in lungs of animals with severe acute pancreatitis (SAP). In this study, we investigated the effects of ω-3 fatty acids (ω-3FA) on TLR4 and nuclear factor κB p56 (NF-κBp56) in lungs of rats with SAP and the levels of cytokines in serum to examine the effects of ω-3FA on TLR4 and NF-κBp56 of lungs in rats with SAP.
INTRODUCTION
Severe acute pancreatitis (SAP) is a critical illness associated with long-term treatment and high mortality. Mortality can approach 50% due to induction of systemic inflammatory response syndrome (SIRS) during the early stages of the disease, subsequently leading to multiple organ dysfunction syndrome (MODS)[1]. Acute lung injury is common, with approximately 20% of patients developing acute respiratory distress syndrome (ARDS). ARDS is a primary cause of death during the early stages of SAP[2,3].
Recent reports have highlighted the role of activation of inflammatory cytokines and signal pathways in pancreatic tissues during the process of SAP[4,5]. Activation of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), and infiltration of inflammatory cells in early SAP can lead to pathological injury not only in pancreatic tissue but also in extra-pancreatic organs, through activation of downstream inflammatory mediators by the amplification of a series of cascade reactions[6]. These injuries can then induce SIRS or even MODS[7,8]. It has been shown that toll-like receptor 4 (TLR4) plays a critical role in the initiation of SAP. TLR4 can regulate the transcription of inflammatory cytokines, which leads to local inflammation in multiple systems and/or organs[9-12].
Toll-like receptors (TLRs) are key modulators of the innate immune response. Members of the TLR family recognize and bind to their corresponding ligand to activate signal transduction pathways and thus produce different biological functions in response to various stimuli[13]. TLR4 is the first reported TLR by which the mediated signal pathway can non-specifically bind in pathogen-associated molecular patterns[14]. Nuclear factor-κB p56 (NF-κBp56), a nuclear transcription factor present in a wide variety of cells, shares an upstream/downstream relationship with TLR4[15]. NF-κBp56 mainly functions at the level of regulating inflammation, cell survival, and apoptosis[16].
Under normal circumstances, members of NF-κBp56 form homomeric or heteromeric dimers in cytoplasm, which exert their function on the activation/inhibition of transcription[17]. Following activation of the TLR4-mediated signal transduction pathway, NF-κBp56 activation and transcription of related inflammatory cytokines are then stimulated[18]. Several studies have confirmed that the expression and activation of TLR4 and NF-κBp56 were up-regulated, and a large number of inflammatory cytokines were detected in the SAP rat model induced in a variety of ways[19-21].
Thus, it is highly likely that the TLR4/NF-κBp56 signal pathway is closely related to the occurrence and development of SAP. The global inflammation in SAP is an important step in the initiation of MODS, in which TLR4 functions as a “messenger”[22]. During the onset stage of SAP, through TLR4, local inflammation mediates the activation of various inflammatory cytokines, which in turn spread to other organs and lead to inflammation of multiple organs[23].
Parenteral nutrition support with ω-3 fatty acids (ω-3FA) alters cytokine production and reduces the rate of complications, for example, the duration of mechanical ventilation and the prevalence of nosocomial infections[24-26]. Thus, ω-3FA, which are major components of fish oil-supplemented parenteral nutrition, offers a potential positive effect on the anti-inflammatory response. Recent animal studies have also shown that ω-3FA can have an anti-inflammatory role in pancreatitis[27-29]. However, there are no data on the correlation between ω-3FA and the TLR4/NF-κBp56 signal pathway in the lungs of subjects with SAP. In this study, we investigated the effects of ω-3FA on the expression of TLR4 and NF-κBp56 in the lungs of rats with SAP, as well as the levels of cytokines in lungs.
MATERIALS AND METHODS
Experimental animal model and grouping
All experiments were approved by the Ethics Committee for Animal Research at Jinling Hospital, Nanjing, China. Healthy adult male Sprague-Dawley rats, weighing 230-250 g, were provided by the Experimental Animal Center of Jinling Hospital. All 56 animals were randomly divided into 4 groups: healthy controls (n = 8); SAP-saline group (n = 16); SAP-soybean oil group (n = 16); and SAP-ω-3FA group (n = 16). Under pentobarbital anesthesia (50 mg/kg body weight), a laparotomy was performed and 5% sodium taurocholate in distilled water (1 mL/kg body weight) was injected into the bilio-pancreatic duct at the rate of 0.2 mL/min using a micro-infusion pump. Controls received an intraductal infusion of saline (0.2 mL/min)[30]. After SAP induction, the SAP-ω-3FA group received an intravenous injection of a combination of the soybean-based compound and 0.2 g/kg fish oil (Omegaven; Fresenius, Bad Homburg, Germany). The SAP-soybean oil group received a soybean-based fat solution without additional fish oil [Intralipid medium-chain triglyceride/long-chain triglyceride 20%; Braun, Melsungen, Germany], and the SAP-saline group received the same volume of saline.
Eight animals from each group were sequentially killed after 12 and 24 h (SAP-saline group, SAP-soybean oil group and SAP-ω-3FA group) by a lethal dose of pentobarbital (200 mg/kg intravenous). The entire lung was removed, and a sample was immediately frozen at -80 °C for biochemical analysis. The lung was then fixed in 10% formalin in anatomic orientation for histological analysis. The lung tissues and whole blood were obtained for subsequent analysis.
Measurement of amylase levels in serum
Serum measurement of measurement of amylase (AMY) concentration was conducted by an automated HITACHI-7150 analyzer to ensure successful SAP models.
Measurement of TNF-α and IL-6 levels in lungs
TNF-α and IL-6 levels in lungs were measured using a commercial ELISA according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO, United States). The levels of TNF-α and IL-6 were found to be a minimum of 7 pg/mL and 60 pg/mL, respectively.
Pathological examination of lungs
The lung tissue was fixed by 40 g/L formaldehyde, embedded in paraffin, and stained with hematoxylin-eosin. All microscopic sections were analyzed blindly. One slide for each rat per group was studied, and 10 random fields per slide were evaluated. Lung histopathological changes were graded on a scale of 0 to 3 (normal 0, mild 1, moderate 2, severe 3) for alveolar and interstitial edema, inflammatory cell infiltration, and alveolar and interstitial hemorrhage[31].
Immunohistochemistry of TLR4 and NF-κBp56 in lung tissues
The S-P method was used to detect the expression of TLR4 and NF-κBp56 proteins in lung tissues. Fixed sections were incubated with the appropriate primary and secondary antibodies. Color reactions were developed using diaminobenzidine solution according to the manufacturer’s instructions. Phosphate buffered saline was used as a negative control, in lieu of antibody. For semi-quantitative analyses, areas of positive staining were defined by two independent investigators using Image-Pro 6.0 Plus (MediaCybernetics). Five fields of view for each section were randomly selected, images acquired, and integrated optical density (IOD) determined [density (mean) = IOD/area].
Western blot of TLR4 and NF-κBp56 in lung tissues
Lung tissue samples were homogenized with lysate buffer (10 mmol/L Tris at pH 7.5, 10 mmol/L NaCl, 0.1 mmol/L EDTA, 0.5% Triton-X 100, 0.02 mmol/L NaN3, and 0.2 mmol/L phenylmethanesulphonylfluoride), treated with a sodium dodecyl sulfate-polyacrylamide loading buffer at 95 °C for 5 min, and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated protein was transblotted from the gel to the polyvinylidine difluoride membrane (Bio-Rad, Hercules, CA, United States) at 300 mA for 1.5 h at 4 °C. The membrane was blocked with 5% non-fat dried milk in Tris-buffered saline with 0.05% Tween20 (TBST) for 1 h at room temperature, washed 3 times for 10 min each time in TBST, and incubated with a primary antibody in a 1:500 dilution of goat anti-rat TLR4 and anti-rat NF-κBp56 monoclonal antibody (Upstate, Charlottesville, VA, United States) in TBST containing 5% non-fat dried milk for 2 h at room temperature. After washing 3 times with TBST for 10 min each time, the membranes were incubated with a 1:5000 dilution of peroxidase-conjugated goat anti-rat immunoglobulin G (Sigma-Aldrich) for 1 h at room temperature. After washing, the membranes were analyzed by the enhanced fluorescence system (Pierce Biotechnology, Rockford, IL, United States).
Statistical analysis
The results for AMY, cytokine and histopathology score of the lungs represent the mean of 8 experiments and are expressed as mean ± SE. Experimental data from different groups were compared using the non-paired Student’s t-test and one-way analysis of variance. The western blot data were from 8 experiments and are expressed as mean optical density, which was analyzed using the Mann-Whitney nonparametric U test. A P value less than 0.05 (2-tailed) was considered statistically significant. All tests were performed using the statistical package SPSS software 11.0 (SPSS, Chicago, IL, United States) in Windows 7.
RESULTS
Serum levels of AMY in rats
Serum AMY was determined and showed a consistent change. Hyperamylasemia was found in all SAP groups, and AMY levels were significantly higher than those in the control group (Table 1).
Table 1.
Serum amylase levels in the different groups
| Group | Time | Cases | Mean ± SE (IU/L) |
| Control | 8 | 1316.34 ± 115.21 | |
| SAP-saline | 12 h | 8 | 4637.11 ± 621.57a |
| 24 h | 8 | 4352.54 ± 436.41d | |
| SAP-soybean oil | 12 h | 8 | 4978.25 ± 918.48b |
| 24 h | 8 | 4245.89 ± 646.27e | |
| SAP-ω-3FA | 12 h | 8 | 4638.63 ± 586.14c |
| 24 h | 8 | 4163.47 ± 653.64f |
P < 0.05;
P < 0.05;
P < 0.01;
P < 0.05;
P < 0.05;
P < 0.05 vs control group. SAP: Severe acute pancreatitis; ω-3FA: ω-3 fatty acids.
Measurement of TNF-α and IL-6 levels in lungs
Compared with the control group, TNF-α and IL-6 levels in the lungs of the SAP-saline group, SAP-soybean oil group and SAP-ω-3FA group were significantly higher at each time point (P < 0.05). Moreover, lung TNF-α and IL-6 levels in the SAP-ω-3FA group were lower than those in the SAP-soybean oil group at each time point (P < 0.05) (Table 2).
Table 2.
Changes in tumor necrosis factor-alpha and interleukin-6 in the lungs of the groups at each time point (pg/mL, mean ± SE, n = 8)
| Control | SAP-saline | SAP-soybean oil | SAP-ω-3FA | |
| TNF-α (12 h) | 24.18 ± 1.05 | 43.24 ± 4.26a | 46.17 ± 13.63ad | 33.42 ± 6.37abc |
| IL-6 (12 h) | 625.11 ± 124.1 | 826.31 ± 76.34a | 827 ± 44.36ad | 739.67 ± 65.23abc |
| TNF-α (24 h) | 24.22 ± 1.03 | 38.67 ± 4.87a | 42.45 ± 11.14ad | 30.37 ± 10.23abc |
| IL-6 (24 h) | 623.21 ± 124.3 | 909.54 ± 26.23a | 925.18 ± 34.52ad | 801.27 ± 51.29abc |
P < 0.05 vs control group at the same time point;
P > 0.05,
P < 0.05 vs SAP-saline group;
P < 0.05 vs SAP-soybean oil group. SAP: Severe acute pancreatitis; ω-3FA: ω-3 fatty acids.
Histopathology of lung tissues
Significantly greater pulmonary interstitial alveolar edema, and a larger number of alveolar bleeding inflammatory cells were detected in the lungs of the SAP-saline, SAP-soybean oil and SAP-ω-3FA groups, but the progression of inflammation was different among the groups: lung inflammation was significantly decreased in the SAP-ω-3FA group compared with the SAP-soybean oil group at the same time point (P < 0.05); no significant differences in histopathological changes were detected between the SAP-saline group and the SAP-soybean oil group at each time point; and no histopathological changes were detected in the control group (Figure 1, Table 3).
Figure 1.
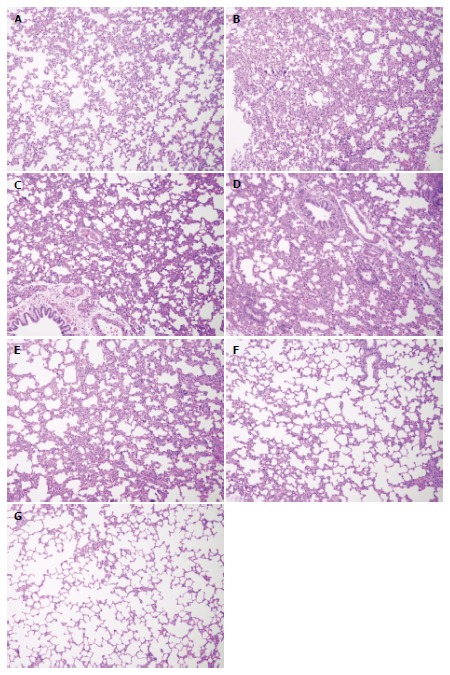
Representative photographs of lung histology in rats from each group (magnification × 200). A: HC group; B: SAP-saline 12 h group; C: SAP-saline 24 h group; D: SAP-soybean oil 12 h group; E: SAP-soybean oil 24 h group; F: SAP-ω-3FA 12 h group; G: SAP-ω-3FA 24 h group. HC: Healthy control; SAP: Severe acute pancreatitis; ω-3FA: ω-3 fatty acids.
Table 3.
Lung histopathology score in each group of rats (mean ± SE, n = 8)
| Group | Pulmonary interstitial edema | Exudation of inflammatory cells | Alveolar bleeding | Alveolar edema |
| Control | 0.32 ± 0.06 | 0.21 ± 0.01 | 1.14 ± 0.02 | 0.14 ± 0.02 |
| SAP-saline | ||||
| 12 h | 1.24 ± 0.11a | 1.37 ± 0.14a | 0.87 ± 0.09a | 1.13 ± 0.08a |
| 24 h | 2.53 ± 0.21a | 2.67 ± 0.26a | 1.12 ± 0.12a | 2.14 ± 0.11a |
| SAP-soybean oil | ||||
| 12 h | 1.24 ± 0.21ad | 1.35 ± 0.24ad | 0.90 ± 0.25ad | 1.14 ± 0.12ad |
| 24 h | 2.15 ± 0.28abc | 2.38 ± 0.30abc | 0.85 ± 0.15abc | 1.95 ± 0.21abc |
| SAP-ω-3FA | ||||
| 12 h | 0.94 ± 0.16abc | 1.12 ± 0.15abc | 0.72 ± 0.38abc | 0.94 ± 0.36abc |
| 24 h | 2.15 ± 0.28abc | 2.38 ± 0.30abc | 0.85 ± 0.15abc | 1.95 ± 0.21abc |
P < 0.05 vs the control group;
P > 0.05,
P < 0.05 vs SAP-saline group at the same time point;
P < 0.05 vs SAP-soybean oil group at the same time point. SAP: Severe acute pancreatitis; ω-3FA: ω-3 fatty acids.
Immunohistochemistry of TLR4 and NF-κBp56 in lung tissues
The immunohistochemistry results indicated that TLR4 and NF-κBp56 expression in the lungs was mainly in neutrophils, macrophages, bronchial epithelial cells, alveolar epithelial cells and microvascular endothelium cells. The TLR4 and NF-κBp56 positive rate was significantly higher in the SAP-soybean oil group than in the SAP-ω-3FA group (P < 0.05) (Figures 2 and 3).
Figure 2.
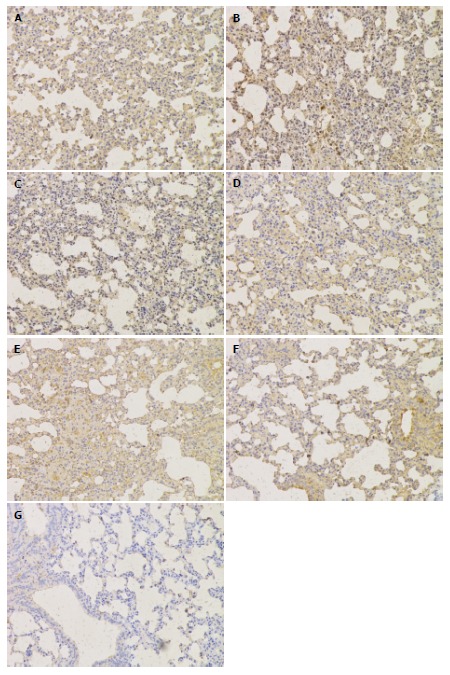
Immunohistochemical analysis of the toll-like receptor 4 protein in lungs from each group (magnification × 200). A: HC group; B: SAP-saline 12 h group; C: SAP-saline 24 h group; D: SAP-soybean oil 12 h group; E: SAP-soybean oil 24 h group; F: SAP-ω-3FA 12 h group; G: SAP-ω-3FA 24 h group. HC: Healthy control; SAP: Severe acute pancreatitis; ω-3FA: ω-3 fatty acids.
Figure 3.
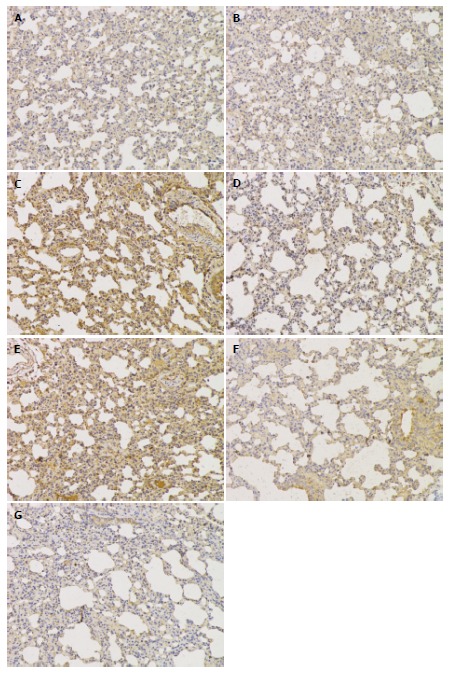
Immunohistochemical analysis of the nuclear factor-κB p56 protein in lungs from each group (magnification × 200). A: HC group; B: SAP-saline 12 h group; C: SAP-saline 24 h group; D: SAP-soybean oil 12 h group; E: SAP-soybean oil 24 h group; F: SAP-ω-3FA 12 h group; G: SAP-ω-3FA 24 h group. HC: Healthy control; SAP: Severe acute pancreatitis; ω-3FA: ω-3 fatty acids.
Western blot of TLR4 and NF-κBp56 in lung tissues
The Western blotting results showed the expression level of TLR4 and NF-κBp56 in the lungs of the SAP-saline, SAP-soybean oil and SAP-ω-3FA groups compared with the control group at 12 and 24 h. The expression levels of both proteins in the groups are shown in Figure 4 and Table 4. Compared with the control group, the SAP-saline group and SAP-soybean oil group showed a significantly higher expression level of TLR4 in the lungs at each time point (P < 0.05). TLR4 expression was much lower in the SAP-ω-3FA group. Furthermore, NF-κBp56 expression level was also significantly increased in the SAP groups compared with the control group, while NF-κBp56 expression in the SAP-ω-3FA group was a little lower than that in the SAP-soybean oil group (P < 0.05). These results suggest that while the expression of TLR4 and NF-κBp56 significantly increased in the lungs of rats with SAP, ω-3FA down-regulated TLR4 expression and inhibited NF-κBp56 expression in the lungs of rats with SAP.
Figure 4.
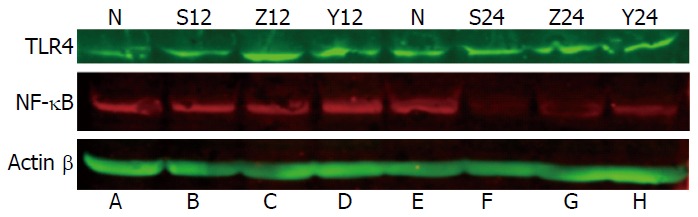
Western blot analysis of the toll-like receptor 4 and nuclear factor-κB protein levels in lungs from each group. A: HC group; B: SAP-saline 12 h group; C: SAP-soybean oil 12 h group; D: SAP-ω-3FA 12 h group; E: HC group; F: SAP-saline 24 h group; G: SAP-soybean oil 24 h group; H: SAP-ω-3FA 24 h group. HC: Healthy control; SAP: Severe acute pancreatitis; ω-3FA: ω-3 fatty acids.
Table 4.
Densitometrically quantified toll-like receptor 4 and nuclear factor-κB p56 bands in the lungs of each group of rats, optical density (mean ± SE)
| Group | Time | Cases | TLR4 | NF-κBp56 |
| Control | 8 | 2.3 ± 0.3 | 2.5 ± 0.2 | |
| SAP-saline | 12 h | 8 | 3.9 ± 0.4a | 4.0 ± 0.3a |
| 24 h | 8 | 4.0 ± 0.2a | 4.1 ± 0.4a | |
| SAP-soybean oil | 12 h | 8 | 3.8 ± 0.3ad | 3.9 ± 0.3ad |
| 24 h | 8 | 3.9 ± 0.4ad | 3.8 ± 0.2ad | |
| SAP-ω-3FA | 12 h | 8 | 2.7 ± 0.3abc | 3.0 ± 0.2abc |
| 24 h | 8 | 3.1 ± 0.4abc | 2.9 ± 0.4abc |
P < 0.05 vs the control group;
P > 0.05,
P < 0.05 vs SAP-saline group at the same time point;
P < 0.05 vs SAP-soybean oil group at the same time point. NF-κBp56: Nuclear factor-κB p56; SAP: Severe acute pancreatitis; TLR4: Toll-like receptor 4; ω-3FA: ω-3 fatty acids.
DISCUSSION
The first sign of multiple organ failure in SAP is often impaired lung function due to ARDS[32]. As a consequence of overactive SIRS, TNF-α and IL-6 are activated within the circulation and attack the pulmonary vascular endothelium[33,34]. Inflammatory cytokines play an important role in lung injury in SAP and in the translation of local pancreatic damage to a systemic inflammatory response[35,36]. The increase in lung pathological damage and lung edema is evidence of SAP-associated lung injury. TLRs are proteins that can trigger the inflammatory cascade reaction. Therefore, an investigation into the tissue-specific expression of TLRs (mainly TLR4) in lungs and determining their roles is of great significance in understanding the pathogenesis of SAP.
It is currently thought that TLRs may play a central role in the recognition of endogenous or exogenous antigens in the immune system and in the initiation of signal transduction in the inflammatory reaction during SAP. Some researchers believe that TLR4 may play an important role in the synthesis and release of inflammatory cytokines, and up-regulation of the TLR4 gene may be related to the development and progression of organ injury during SAP[37,38]. Some studies have indicated that when SAP is stimulated, the expression of cytokines and cell adhesion molecules is significantly up-regulated in the pancreas, thereby promoting the accumulation of excessive neutrophils in the area of inflammation leading to injury of the pancreas and other organs[39,40]. Our study showed that, during the early stages of SAP, there was obvious expression of TLR4 and NF-κBp56 in lung tissues. This expression increased as SAP developed; peak expression was seen at 24 h. Changes in the concentration of TNF-α and IL-6 in lungs were consistent with pathological changes in lung tissues.
In recent years, a new ω-3 fish oil emulsion was found to not only supply energy but also to exert some important biological effects, such as immune regulation and organ protection[41-43]. The main mechanisms of action are as follows. On the one hand, ω-3FA can enter the phospholipid pool of the cell membrane to replace ω-6FA, and reach an appropriate ω-3/ω-6 ratio (1:2 to 1:4). They can competitively combine with cyclooxygenase and lipoxygenase to inhibit production of inflammatory mediators from ω-6FA which have a strong pro-inflammatory action and immune regulation. On the other hand, by reducing the expression of surface receptors and molecules in immune cell membranes, ω-3FA can participate in the immune response, reduce the activity of immune cells and the production of cytokines, and inhibit the inflammatory response[44-46]. In our study, ω-3FA reduced the release of the inflammatory cytokines TNF-α and IL-6, and reduced lung injury.
However, it is unclear whether ω-3FA inhibits the inflammatory response by reducing the expression of TLR4 in lungs. In our study, we found that the expression of TLR4 and NF-κBp56 was decreased in the lungs of rats treated with ω-3FA. The inflammatory response and pathological changes in the lungs improved after the application of ω-3FA. This suggested that ω-3FA indirectly inhibits the expression of TLR4 by inhibiting activation of NF-κBp56.
In conclusion, TLR4 may play an important role during SAP-induced lung injury. TLR4 activation results in a variety of inflammatory reactions, and ω-3FA can inhibit the inflammatory response and reduce lung injury by inhibiting the TLR4/NF-κBp56 signal pathway. Development of these therapeutic strategies should help reduce the complications and mortality associated with SAP.
COMMENTS
Background
Acute lung injury (ALI) is one of the most common complications of severe acute pancreatitis (SAP) and often occurs in the early stage of the disease, and may progress to adult respiratory distress syndrome. Toll-like receptor 4 (TLR4) can regulate the transcription of inflammatory cytokines, which leads to ALI.
Research frontiers
It has been found that ω-3 fatty acids (ω-3FA) reduces the release of the inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), and reduces lung injury. It is unclear whether ω-3FA can inhibit the inflammatory response by reducing expression of TLR4 in the lungs.
Innovations and breakthroughs
In this study, the authors found that ω-3FA inhibited the inflammatory response and reduced lung injury by inhibiting the TLR4/nuclear factor-κB p56 (NF-κBp56) signal pathway.
Applications
The findings of this study may help to further understand the role of TLR4 during the course of SAP-induced lung injury.
Peer-review
This is a well-intentioned basic study that has evaluated the effects of ω-3FA on expression of TLR4 and NF-κBp56 of lungs with SAP and the levels of cytokines in rat.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study has been approved by the Institutional Review Board of Jinling Hospital, Nanjing, China.
Institutional animal care and use committee statement: The study was approved by the Institutional Animal Care and Use Committee of Jinling Hospital. All operations were performed according to international guidelines concerning the care and treatment of experimental animals.
Conflict-of-interest statement: The authors declare that there is no conflict of interest related to this study.
Data sharing statement: No additional unpublished data are available.
Peer-review started: July 4, 2016
First decision: August 29, 2016
Article in press: October 10, 2016
P- Reviewer: Pai CG S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang FF
References
- 1.Gasparović V, Daković K, Gornik I, Radonić R. Severe acute pancreatitis as a part of multiple dysfunction syndrome. Coll Antropol. 2014;38:125–128. [PubMed] [Google Scholar]
- 2.Surbatović M, Jovanović K, Radaković S, Filipović N. [Pathophysiological aspects of severe acute pancreatitis-associated lung injury] Srp Arh Celok Lek. 2005;133:76–81. doi: 10.2298/sarh0502076s. [DOI] [PubMed] [Google Scholar]
- 3.Hoque R, Malik AF, Gorelick F, Mehal WZ. Sterile inflammatory response in acute pancreatitis. Pancreas. 2012;41:353–357. doi: 10.1097/MPA.0b013e3182321500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong JA, Cash N, Soares PM, Souza MH, Sutton R, Criddle DN. Oxidative stress in acute pancreatitis: lost in translation? Free Radic Res. 2013;47:917–933. doi: 10.3109/10715762.2013.835046. [DOI] [PubMed] [Google Scholar]
- 5.Escobar J, Pereda J, Arduini A, Sandoval J, Sabater L, Aparisi L, López-Rodas G, Sastre J. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: a key role for protein phosphatases. Curr Pharm Des. 2009;15:3027–3042. doi: 10.2174/138161209789058075. [DOI] [PubMed] [Google Scholar]
- 6.Fisic E, Poropat G, Bilic-Zulle L, Licul V, Milic S, Stimac D. The Role of IL-6, 8, and 10, sTNFr, CRP, and Pancreatic Elastase in the Prediction of Systemic Complications in Patients with Acute Pancreatitis. Gastroenterol Res Pract. 2013;2013:282645. doi: 10.1155/2013/282645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, Dixit VK. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surg. 2013;2013:367581. doi: 10.1155/2013/367581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirota M, Sugita H, Maeda K, Ichibara A, Ogawa M. [Concept of SIRS and severe acute pancreatitis] Nihon Rinsho. 2004;62:2128–2136. [PubMed] [Google Scholar]
- 9.Barak B, Feldman N, Okun E. Toll-like receptors as developmental tools that regulate neurogenesis during development: an update. Front Neurosci. 2014;8:272. doi: 10.3389/fnins.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao HK, Zhou ZG, Li Y, Chen YQ. Toll-like receptor 4 Asp299Gly polymorphism is associated with an increased risk of pancreatic necrotic infection in acute pancreatitis: a study in the Chinese population. Pancreas. 2007;34:295–298. doi: 10.1097/mpa.0b013e318032674a. [DOI] [PubMed] [Google Scholar]
- 11.Bogaerts M, Deggoujf N, Huart C, Hupin C, Laureyns G, Lemkens P, Rombaux P, Ten Bosch Jv, Gordts F. Physiology of the mouth and pharynx, Waldeyer’s ring, taste and smell. B-ENT. 2012;8 Suppl 19:13–20. [PubMed] [Google Scholar]
- 12.Li G, Wu X, Yang L, He Y, Liu Y, Jin X, Yuan H. TLR4-mediated NF-κB signaling pathway mediates HMGB1-induced pancreatic injury in mice with severe acute pancreatitis. Int J Mol Med. 2016;37:99–107. doi: 10.3892/ijmm.2015.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Xu J, Hu CD, Pan ZL, Zhang YC. The relationship between SNPs in the genes of TLR signal transduction pathway downstream elements and rheumatoid arthritis susceptibility. Tsitol Genet. 2014;48:24–29. [PubMed] [Google Scholar]
- 14.Murad S. Toll-like receptor 4 in inflammation and angiogenesis: a double-edged sword. Front Immunol. 2014;5:313. doi: 10.3389/fimmu.2014.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Kong X, Zhou H, Zhang X, Liu J, Yan J, Xie H, Xie Y. oxLDL/β2GPI/anti-β2GPI complex induced macrophage differentiation to foam cell involving TLR4/NF-kappa B signal transduction pathway. Thromb Res. 2014;134:384–392. doi: 10.1016/j.thromres.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh G, Wang VY, Huang DB, Fusco A. NF-κB regulation: lessons from structures. Immunol Rev. 2012;246:36–58. doi: 10.1111/j.1600-065X.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Lu Y, Ma L, Cao X, Xiao J, Chen J, Jiao S, Gao Y, Liu C, Duan Z, et al. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-κB signaling and protects against endotoxin shock. Immunity. 2014;40:501–514. doi: 10.1016/j.immuni.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Xiping Z, Dijiong W, Jianfeng L, Qihui C, Jing Y, Penghui J, Meijuan Y, Ninni Z. Effects of Salvia miltiorrhizae on ICAM-1, TLR4, NF-kappaB and Bax proteins expression in multiple organs of rats with severe acute pancreatitis or obstructive jaundice. Inflammation. 2009;32:218–232. doi: 10.1007/s10753-009-9124-4. [DOI] [PubMed] [Google Scholar]
- 20.Souza-Fonseca-Guimaraes F, Parlato M, Philippart F, Misset B, Cavaillon JM, Adib-Conquy M. Toll-like receptors expression and interferon-γ production by NK cells in human sepsis. Crit Care. 2012;16:R206. doi: 10.1186/cc11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immunol. 2004;172:20–24. doi: 10.4049/jimmunol.172.1.20. [DOI] [PubMed] [Google Scholar]
- 22.Giakoustidis A, Mudan SS, Giakoustidis D. Dissecting the stress activating signaling pathways in acute pancreatitis. Hepatogastroenterology. 2010;57:653–656. [PubMed] [Google Scholar]
- 23.Sharif R, Dawra R, Wasiluk K, Phillips P, Dudeja V, Kurt-Jones E, Finberg R, Saluja A. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813–819. doi: 10.1136/gut.2008.170423. [DOI] [PubMed] [Google Scholar]
- 24.Koopmann MC, Baumler MD, Boehler CJ, Chang FL, Ney DM, Groblewski GE. Total parenteral nutrition attenuates cerulein-induced pancreatitis in rats. Pancreas. 2010;39:377–384. doi: 10.1097/MPA.0b013e3181bb908e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei QC, Wang XY, Xia XF, Zheng HZ, Bi JC, Tian F, Li N. The role of omega-3 fatty acids in acute pancreatitis: a meta-analysis of randomized controlled trials. Nutrients. 2015;7:2261–2273. doi: 10.3390/nu7042261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss G, Meyer F, Matthies B, Pross M, Koenig W, Lippert H. Immunomodulation by perioperative administration of n-3 fatty acids. Br J Nutr. 2002;87 Suppl 1:S89–S94. doi: 10.1079/bjn2001461. [DOI] [PubMed] [Google Scholar]
- 27.Mayer K, Meyer S, Reinholz-Muhly M, Maus U, Merfels M, Lohmeyer J, Grimminger F, Seeger W. Short-time infusion of fish oil-based lipid emulsions, approved for parenteral nutrition, reduces monocyte proinflammatory cytokine generation and adhesive interaction with endothelium in humans. J Immunol. 2003;171:4837–4843. doi: 10.4049/jimmunol.171.9.4837. [DOI] [PubMed] [Google Scholar]
- 28.Alhan E, Türkyilmaz S, Erçin C, Kaklikkaya N, Kural BV. Effects of omega-3 fatty acids on acute necrotizing pancreatitis in rats. Eur Surg Res. 2006;38:314–321. doi: 10.1159/000094019. [DOI] [PubMed] [Google Scholar]
- 29.Foitzik T, Eibl G, Schneider P, Wenger FA, Jacobi CA, Buhr HJ. Omega-3 fatty acid supplementation increases anti-inflammatory cytokines and attenuates systemic disease sequelae in experimental pancreatitis. JPEN J Parenter Enteral Nutr. 2002;26:351–356. doi: 10.1177/0148607102026006351. [DOI] [PubMed] [Google Scholar]
- 30.Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411–416. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenstein A, Milani R, Fernezlian SM, Leme AS, Capelozzi VL, Martins MA. Acute lung injury in two experimental models of acute pancreatitis: infusion of saline or sodium taurocholate into the pancreatic duct. Crit Care Med. 2000;28:1497–1502. doi: 10.1097/00003246-200005000-00040. [DOI] [PubMed] [Google Scholar]
- 32.Lei H, Minghao W, Xiaonan Y, Ping X, Ziqi L, Qing X. Acute lung injury in patients with severe acute pancreatitis. Turk J Gastroenterol. 2013;24:502–507. doi: 10.4318/tjg.2013.0544. [DOI] [PubMed] [Google Scholar]
- 33.Pooran N, Indaram A, Singh P, Bank S. Cytokines (IL-6, IL-8, TNF): early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol. 2003;37:263–266. doi: 10.1097/00004836-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Stimac D, Fisić E, Milić S, Bilić-Zulle L, Perić R. Prognostic values of IL-6, IL-8, and IL-10 in acute pancreatitis. J Clin Gastroenterol. 2006;40:209–212. doi: 10.1097/00004836-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Bhatia M. Acute pancreatitis as a model of SIRS. Front Biosci (Landmark Ed) 2009;14:2042–2050. doi: 10.2741/3362. [DOI] [PubMed] [Google Scholar]
- 36.Gunjaca I, Zunic J, Gunjaca M, Kovac Z. Circulating cytokine levels in acute pancreatitis-model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation. 2012;35:758–763. doi: 10.1007/s10753-011-9371-z. [DOI] [PubMed] [Google Scholar]
- 37.Wu HS, Zhang L, Chen Y, Guo XJ, Wang L, Xu JB, Wang CY, Zhang JH. Effect of nitric oxide on toll-like receptor 2 and 4 gene expression in rats with acute lung injury complicated by acute hemorrhage necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int. 2005;4:609–613. [PubMed] [Google Scholar]
- 38.Pastor CM, Pugin J, Kwak B, Chanson M, Mach F, Hadengue A, Frossard JL. Role of Toll-like receptor 4 on pancreatic and pulmonary injury in a mice model of acute pancreatitis associated with endotoxemia. Crit Care Med. 2004;32:1759–1763. doi: 10.1097/01.ccm.0000133020.47243.8e. [DOI] [PubMed] [Google Scholar]
- 39.Ueki M, Taie S, Chujo K, Asaga T, Iwanaga Y, Ono J, Maekawa N. Urinary trypsin inhibitor reduces inflammatory response in kidney induced by lipopolysaccharide. J Biosci Bioeng. 2007;104:315–320. doi: 10.1263/jbb.104.315. [DOI] [PubMed] [Google Scholar]
- 40.Vonlaufen A, Xu Z, Daniel B, Kumar RK, Pirola R, Wilson J, Apte MV. Bacterial endotoxin: a trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology. 2007;133:1293–1303. doi: 10.1053/j.gastro.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 41.Mayer K, Schaefer MB, Seeger W. Fish oil in the critically ill: from experimental to clinical data. Curr Opin Clin Nutr Metab Care. 2006;9:140–148. doi: 10.1097/01.mco.0000214573.75062.0a. [DOI] [PubMed] [Google Scholar]
- 42.Wu GH, Gao J, Ji CY, Pradelli L, Xi QL, Zhuang QL. Cost and effectiveness of omega-3 fatty acid supplementation in Chinese ICU patients receiving parenteral nutrition. Clinicoecon Outcomes Res. 2015;7:369–375. doi: 10.2147/CEOR.S81277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura K, Kariyazono H, Komokata T, Hamada N, Sakata R, Yamada K. Influence of preoperative administration of omega-3 fatty acid-enriched supplement on inflammatory and immune responses in patients undergoing major surgery for cancer. Nutrition. 2005;21:639–649. doi: 10.1016/j.nut.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Heller AR, Rössler S, Litz RJ, Stehr SN, Heller SC, Koch R, Koch T. Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit Care Med. 2006;34:972–979. doi: 10.1097/01.CCM.0000206309.83570.45. [DOI] [PubMed] [Google Scholar]
- 45.Stehr SN, Heller AR. Omega-3 fatty acid effects on biochemical indices following cancer surgery. Clin Chim Acta. 2006;373:1–8. doi: 10.1016/j.cca.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]


