Abstract
AIM
To conduct a prospective assessment of anti-hepatitis E virus (HEV) IgG seroprevalence in the Western Cape Province of South Africa in conjunction with evaluating risk factors for exposure.
METHODS
Consenting participants attending clinics and wards of Groote Schuur, Red Cross Children’s Hospital and their affiliated teaching hospitals in Cape Town, South Africa, were sampled. Healthy adults attending blood donor clinics were also recruited. Patients with known liver disease were excluded and all major ethnic/race groups were included to broadly represent local demographics. Relevant demographic data was captured at the time of sampling using an interviewer-administered confidential questionnaire. Human immunodeficiency virus (HIV) status was self-disclosed. HEV IgG testing was performed using the Wantai® assay.
RESULTS
HEV is endemic in the region with a seroprevalence of 27.9% (n = 324/1161) 95%CI: 25.3%-30.5% (21.9% when age-adjusted) with no significant differences between ethnic groups or HIV status. Seroprevalence in children is low but rapidly increases in early adulthood. With univariate analysis, age ≥ 30 years old, pork and bacon/ham consumption suggested risk. In the multivariate analysis, the highest risk factor for HEV IgG seropositivity (OR = 7.679, 95%CI: 5.38-10.96, P < 0.001) was being 30 years or older followed by pork consumption (OR = 2.052, 95%CI: 1.39-3.03, P < 0.001). A recent clinical case demonstrates that HEV genotype 3 may be currently circulating in the Western Cape.
CONCLUSION
Hepatitis E seroprevalence was considerably higher than previously thought suggesting that hepatitis E warrants consideration in any patient presenting with an unexplained hepatitis in the Western Cape, irrespective of travel history, age or ethnicity.
Keywords: Hepatitis E, Seroprevalence, South Africa, Pork consumption, Genotype
Core tip: This is a prospective seroprevalence study of 1161 participants assessing anti-hepatitis E virus (HEV) IgG seroprevalence in the Western Cape Province of South Africa. The only risk factors for seropositivity are pork consumption and individuals over 30 years of age. A recent clinical case suggests HEV genotype 3 may be circulating in South Africa.
INTRODUCTION
Globally, hepatitis E virus (HEV) is the most frequent aetiological cause of acute hepatitis[1]. It causes sporadic and epidemic infections, predominantly in young adults living in developing countries. In these regions, it is associated with HEV genotypes 1 and 2, which are obligate human pathogens and spread oro-faecally through infected water. Most patients experience a self-limiting hepatitis, except in pregnant women and patients with chronic liver disease, where mortality may reach 25% and 75% respectively[2].
In the developed world, hepatitis E is largely a porcine zoonosis caused by genotypes 3 and 4 and is a cause of self-limiting hepatitis in middle-aged and elderly men[2-4]. Chronic infection occurs in those who are immunosuppressed, including transplant recipients[5], patients with haematological malignancy and individuals with human immunodeficiency virus (HIV)[6]. An important route of infection is through consumption of infected pig meat products in the human food chain[2].
Hepatitis E, invariably genotypes 1 and 2, is seen in a number of African countries. There have been several outbreaks observed in sub-Saharan African refugee camps, including recently South Sudan and Uganda[7,8]. In the 1980’s a large outbreak was reported in Namibia where HEV genotype 2 is known to circulate[9]. In South Africa, very few data regarding HEV exists. Two seroprevalence studies from the 1990’s demonstrated low rates but these studies were potentially limited as the screening tests employed are now known to have had poor sensitivity[10]. In addition, very few cases of hepatitis E from South Africa have been reported in the literature but recently, two cases caused by HEV genotype 3 have been described[11,12].
Given the paucity of data, we elected to prospectively investigate the seroprevalence of anti-HEV IgG in a South African population in the Western Cape using a sensitive assay as well as assessing risk factors for anti-HEV IgG seropositivity. Unexpectedly, a case of acute hepatitis E infection was documented and is reported here.
MATERIALS AND METHODS
HEV seroprevalence and risk factors
The study was designed to cover all age groups in addition to reflecting the population of the Western Cape in terms of ethnic distribution. Participants were asked to self-identify their ethnicity and were randomly recruited and sampled from the three major ethnic groups aged 0 to > 60 years old from both a hospital and non-hospital setting. Participants were recruited from the general medical and Emergency Unit inpatients and outpatients of Groote Schuur, Red Cross Childrens, New Somerset and UCT Private Academic Hospitals between 28/02/14-12/02/2015. Furthermore, healthy blood donors, prior to screening, from two blood donation centres in Cape Town, South Africa were included. Participants with known or reported liver disease were excluded. Following informed consent, blood samples were drawn and each participant completed a structured questionnaire for demographic and known risk factors for hepatitis E acquisition, these included, consumption of pork, sausage, bacon/ham, fish and shellfish; type of dwelling (formal dwelling or informal dwelling/shack in back yard, informal dwelling/shack not in back yard, other); access to piped water (piped water inside dwelling, piped water inside yard, piped water outside yard, no access to piped water); proximity to coast (coastal, < 5 km, 5-10 km, > 10 km) and refuse disposal (removed by local authority/private company, communal refuse dump, own refuse dump, no rubbish disposal/other). Given the massive upscaling of HIV testing in South Africa, participants were asked to self-disclose their HIV status, if known. Blood samples were stored at -70 °C and tested in batches for HEV anti-IgG using the Wantai® assay (Beijing, China) according to the manufacturer’s instructions.
Statistical analysis
Age-standardized seroprevalence was calculated using the 2014 South Africa Mid-Year population estimates as the reference population[13]. Age-specific percentages were applied to the South African population, and then summed to determine the age-standardized seroprevalence for anti-HEV IgG in the population as a whole as well as between different ethnic groups. Continuous variables are described using counts and percentages and independent risk factors were explored by uni-variate binary logistic regression. Factors, which were found to be statistically significant on uni-variate analysis, were then modelled with multi-variate binary logistic regression using maximum-likelihood estimation with parameters (0, 1), analysed with IBM SPSS V22.0. Statistical significance was accepted if P < 0.05.
Clinical case
A documented case of acute hepatitis E presenting to New Somerset hospital is described; the clinical course and laboratory findings are recorded.
Ethics
Ethics approval was granted by the Faculty of Health Sciences Human Research Ethics Committee of the University of Cape Town. Patients ≥ 18 years provided consent whilst parents/guardians provided permission/consent for minors. Adolescents provided assent.
RESULTS
About 1161 participants were included in the study with the 3 major ethnic groups in the Western Cape viz. Black Africans (n = 392, 33.5%), Mixed Ancestry (n = 455, 38.9%) and Whites (n = 322, 27.5%), proportionally sampled. The mean age of the population sampled was 36.4 years (SD 22.3) and 53.3% were male. Notably, Whites < 16 years and Black Africans > 60 years of age were relatively under-represented due to low attendance at sampling locations (Table 1 and Figure 1).
Table 1.
Data (%) for total population overall and age group seroprevalence and 95%CI in 3 racial groups in Western Cape, South Africa
| Age group (yr) |
Total population (n = 1161) |
Black African population (n = 389) |
MA/Coloured population (n = 452) |
White population (n = 320) |
||||
| IgG (n) | HEV Seroprevalence % (95%CI) | IgG (n) | HEV Seroprevalence % (95%CI) | IgG (n) | HEV Seroprevalence % (95%CI) | IgG (n) | HEV Seroprevalence % (95%CI) | |
| 0-4 | 6/72 | 8.3 (3.9-17.0) | 2/36 | 5.6 (1.5-18.1) | 4/31 | 12.9 (5.1-28.9) | 0/51 | 0 (0-43.4) |
| 5-9 | 6/78 | 7.7 (3.6-15.8) | 1/26 | 3.8 (0.7-18.9) | 5/45 | 11.1 (4.8-23.5) | 0/71 | 0 (0-35.4) |
| 10-14 | 7/72 | 9.7 (4.8-18.7) | 3/27 | 11.1 (3.9-28.1) | 4/42 | 9.5 (3.8-22.1) | 0/31 | 0 (0-56.2) |
| 15-19 | 8/95 | 8.4 (4.3-15.7) | 4/23 | 17.4 (7.0-37.1) | 4/42 | 9.5 (3.8-22.1) | 0/301 | 0 (0-11.4) |
| 20-29 | 17/179 | 9.5 (6.0-14.7) | 2/62 | 3.2 (0.9-11.0) | 9/54 | 16.7 (9.0-28.7) | 6/63 | 9.5 (4.4-19.3) |
| 30-39 | 42/142 | 29.6 (22.7-37.5) | 14/52 | 26.9 (16.8-40.3) | 17/45 | 37.8 (25.1-52.4) | 11/45 | 24.4 (14.2-38.7) |
| 40-49 | 67/153 | 43.8 (36.2-51.7) | 22/62 | 35.5 (24.7-47.9) | 22/49 | 44.9 (31.9-58.7) | 23/42 | 54.8 (39.9-68.8) |
| 50-59 | 62/126 | 49.2 (40.6-57.8) | 15/36 | 41.7 (27.1-57.8) | 26/47 | 55.3 (41.2-68.6) | 21/43 | 48.8 (34.6-63.2) |
| 60-69 | 67/152 | 44.1 (36.4-52.0) | 24/43 | 55.8 (41.1-69.6) | 19/58 | 32.8 (22.1-45.6) | 24/51 | 47.1 (34.1-60.5) |
| 70-79 | 34/68 | 50.0 (38.4-61.6) | 6/17 | 35.3 (17.3-58.7) | 16/29 | 55.2 (37.5-71.6) | 12/22 | 54.5 (34.7-73.1) |
| 80+ | 8/24 | 33.3 (18.0-53.3) | 1/5 | 20.0 (3.6-62.4) | 2/10 | 20.0 (5.7-51.0) | 5/9 | 55.6 (26.7-81.1) |
| Total | 324/1161 | 27.9 (25.3-30.5) | 94/389 | 24.2 (20.2-28.7) | 128/452 | 28.3 (24.4-32.6) | 102/320 | 31.9 (27.0-37.2) |
Overall seroprevalence was 27.9% and age adjusted seroprevalence was 21.9%.
Numbers too small to calculate a meaningful confidence interval. HEV: Hepatitis E virus.
Figure 1.
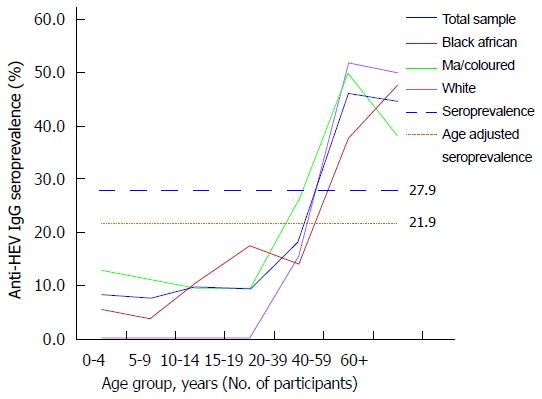
Overall seroprevalence curve and individual seroprevalence curves in 3 racial groups in Western Cape, South Africa. Anti-HEV IgG seroprevalence by age in Western Cape, South Africa.
The overall anti-HEV IgG seroprevalence was 27.9% (n = 324/1161, 95%CI: 25.3-30.5) and the age adjusted seroprevalence was 21.9%. Seroprevalence in children (< 19 years old) was approximately 10% with a rapid increase in seroprevalence in individuals older than 20 years of age (Figure 1). Various demographic and environmental factors were explored with univariate analysis, age, ethnicity, gender, HIV status, consumption of pork, sausages, bacon/ham, fish, shellfish, type of dwelling, access to water, proximity to the coast and method of refuse disposal (Table 2). Age group ≥ 30 years (P < 0.001), pork consumption (P < 0.001) and bacon/ham consumption (P = 0.004) were strongly associated with a positive HEV IgG. However as the pork and bacon/ham are not independent from one another, only pork consumption was used in the multivariate analysis along with age group (< 30 years, ≥ 30 years).
Table 2.
Risk factor analysis
| Potential risk factors (n = 131) |
Univariate analyses |
|||
| Groups | n (IgG%) | P value | OR (95%CI) | |
| Race (n = 161) | Black African (n = 389) | 94 (24.2%) | 0.073 | - |
| MA/Coloured (n = 452) | 128 (28.3%) | 1.24 (0.91-1.69) | ||
| White (n = 320) | 102 (31.9%) | 1.47 (1.06-2.04) | ||
| Gender (n = 161) | Male (n = 541) | 161 (29.8%) | 0.189 | - |
| Female (n = 620) | 163 (26.3%) | 0.842 (0.65-1.09) | ||
| Pork (n = 131) | Yes (n = 690) | 228 (33.0%) | < 0.001 | 1.93 (1.45-2.55) |
| No (n = 441) | 90 (20.4%) | - | ||
| Sausage (n = 131) | Yes (n = 853) | 248 (29.1%) | 0.210 | 1.22 (0.90-1.66) |
| No (n = 278) | 70 (25.2%) | - | ||
| Bacon/Ham (n = 131) | Yes (n = 707) | 220 (31.1%) | 0.004 | 1.50 (1.14-1.98) |
| No (n = 424) | 98 (23.1%) | - | ||
| Fish (n = 131) | Yes (n = 1055) | 299 (28.3%) | 0.532 | 1.19 (0.69-2.03) |
| No (n = 76) | 19 (25.0%) | |||
| Shellfish (n = 131) | Yes (n = 548) | 163 (29.7%) | 0.238 | 1.17 (0.90-1.52) |
| No (n = 583) | 155 (26.6%) | - | ||
| Type of dwelling (n = 161) | Formal Dwelling (n = 1050) | 296 (28.2%) | 0.642 | - |
| Shack in Yard (n = 88) | 21 (24.0%) | 0.80 (0.48-1.40) | ||
| Shack not in Yard (n = 19) | 5 (26.3%) | 0.91 (0.33-2.55) | ||
| Other (n = 4) | 2 (50.0%) | 2.55 (0.36-18.17) | ||
| Water access (n = 1161) | Piped dwelling (n = 1041) | 292 (28.0%) | 0.516 | - |
| Piped yard (n = 68) | 17 (25.0%) | 0.855 (0.49-1.51) | ||
| Community tap (n = 44) | 11 (25.0%) | 0.855 (0.43-1.71) | ||
| No water access (n = 8) | 4 (50.0%) | 2.565 (0.64-10.32) | ||
| Proximity to coast (n = 1160) | Coastal (n = 114) | 32 (28.0%) | 0.935 | - |
| Under 5 km (n = 209) | 62 (29.7%) | 1.081 (0.65-1.79) | ||
| 5-10 km (n = 313) | 85 (27.2%) | 0.955 (0.59-1.54) | ||
| Over 10 km (n = 524) | 145 (27.7%) | 0.980 (0.62-1.54) | ||
| Refuse disposal (n = 1160) | Local authority (n = 1124) | 315 (28.0%) | 0.725 | - |
| Communal dump (n = 20) | 5 (25.0%) | 0.856 (0.31-2.40) | ||
| Own dump (n = 8) | 3 (37.5%) | 1.541 (0.37-6.50) | ||
| No rubbish disposal (n = 8) | 1 (12.5%) | 0.367 (0.05-3.00) | ||
| HIV status (n = 891) | Positive (n = 60) | 14 (23.3%) | 0.340 | 1.35 (0.73-2.50) |
| Negative (n = 831) | 242 (29.1%) | - | ||
| Age group (n = 1156) | < 30 yr (n = 496) | 44 (8.9%) | < 0.001 | - |
| ≥ 30 yr (n = 660) | 280 (42.4%) | 7.569 (5.36-10.70) | ||
| Multivariate analysis (n = 1126) | ||||
| Pork | Yes (n = 686) | 228 (33.2%) | < 0.001 | 7.679 (5.38-10.96) |
| Age group | ≥ 30 yr (n = 660) | 276 (42.3%) | < 0.001 | 2.021 (1.50-2.73) |
The model was statistically significant (P < 0.001) and therefore was able to determine the risk factors of people presenting with HEV IgG positive compared to those that were not and explained between 15%-22% of the variance . With pork in their diet, an individual had an increased risk (OR = 2.02, 95%CI: 1.5-2.73) of being HEV IgG positive compared to those who did not have pork in their diet. Individuals 30 years old or over had an increased risk (OR = 7.679, 95%CI: 5.38-10.96, P < 0.001) of being HEV IgG positive compared to those under 30 years old. No other risk factors were associated with HEV seropositivity (Table 2) and therefore excluded from the multivariate analysis.
Clinical case of hepatitis E
In January 2014, a 54-year-old white male presented to New Somerset Hospital, Cape Town, with acute liver failure secondary to a severe hepatitis. Initial clinical examination revealed marked icterus, mild abdominal ascites and smooth non-tender hepatomegaly, as well as signs of chronic liver disease including palmar erythema and spider telangiectasia on the chest wall. The patient worked as a truck driver but had no history of recent travel (in the last 3 mo prior to presentation) outside of the Western Cape. He was not known to have any medical co-morbidities but reportedly consumed significant alcohol. At presentation, blood tests demonstrated (normal range in parentheses): Bilirubin 407 μmol/L (0-18 μmol/L), albumin 28 g/L (35-52 g/L), alanine aminotransferase (ALT) 3054 IU/L (0-40 IU/L), prothrombin time-International normalized ratio (PT-INR) 2.74. Serology for hepatitis A, B, C and HIV were negative. Despite maximal supportive care he demised on day 3 following admission as a result of fulminant hepatic failure. On the day of his death, hepatitis E IgM test returned positive and subsequently HEV RNA genotype 3e was confirmed by polymerase chain reaction (PCR) (see Figure 2).
Figure 2.
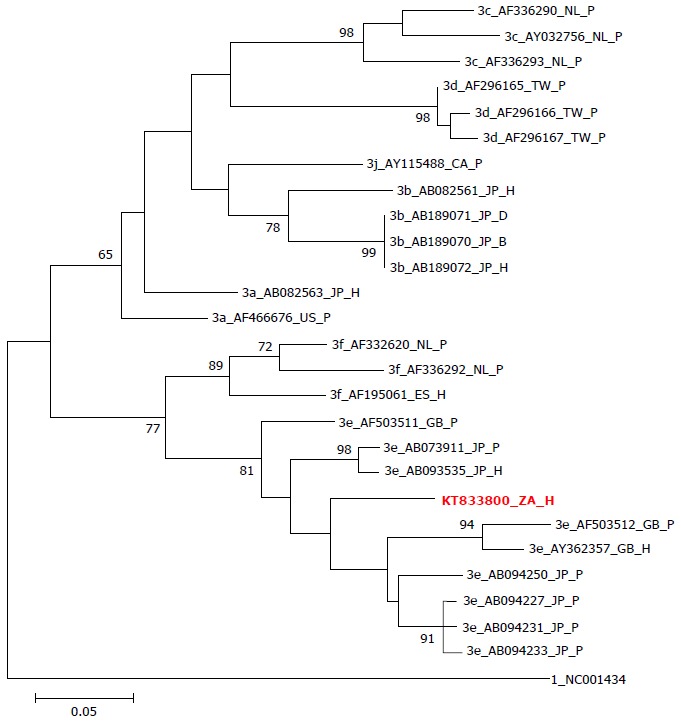
A maximum likelihood tree constructed in MEGA6 from an alignment of a 301nt fragment of ORF2. Bootstrap support above 60% is shown. Our patient’s viral sequence, GenBank accession KT833800, is highlighted in red. The tree is rooted on the HEV reference sequence (genotype 1). Sequences for comparison have names starting with genotype, followed by Genbank accession number, followed by country ISO 3166-1 abbreviation (CA: Canada; ES: Spain; GB: United Kingdom; JP: Japan; NL: The Netherlands; TW: Taiwan; US: United States of America; ZA: South Africa), and ending in source (D: Deer; H: Human; P: Pig). The patient’s viral sequence clusters within genotype 3 (with subgenotype 3e), in keeping with other viruses recently described in South Africa[11,12,24].
DISCUSSION
The seroprevalence in Western Cape is much higher than has been previously described in South African populations[10,14]. We found that the overall seroprevalence was 27.9% (21.9% when age-adjusted) and was similar between genders, ethnic groups and HIV status. The age-related seroprevalence curve shows that HEV IgG seropositivity increases with age, but in a non-linear fashion. Seroprevalence is very low in children and young adults, rapidly increasing between the ages of 20 and 30 years and then plateaus off. Multivariate risk factor analysis demonstarted that the only other significant association with anti-HEV IgG seropositivity was the consumption of pork.
Two previous studies from South Africa in the 1990’s described seroprevalence rates of 10.7% and 1.8%-2.6%, respectively. The overall seroprevalence in the current study was an order of magnitude higher. The reason for this difference could relate to geographical location, as both the above previous studies were performed in different areas of South Africa and it is known that HEV seroprevalence can vary enormously between regional areas within countries[15]. Another possible explanation is that there has been an increase in the exposure rates of hepatitis E in South Africa over the last 20 years, which may include changes in migration, diet and enzootic disease. However, the likely dominant explanation relates to the sensitivity of the IgG assays employed in the previous studies. The first generation of anti-HEV IgG assays used in studies in the 1990’s lacked sensitivity and generally reported spuriously low seroprevalence rates[16]. In contrast, the assay employed in our study has a sensitivity of 98% at detecting previous infection and produces much higher estimates of seroprevalence[17,18]. However, in common with all currently available anti-HEV IgG assays, the specificity of the Wantai® assay for detecting previous infection has not been fully established although indirect evidence suggests that it has an acceptable specificity. This includes very low seroprevalence rates in young children (in the current study and others from Nepal, Bangladesh and France)[19] and adult populations (Fiji 2%[20], New Zealand 4%[21] and Scotland 4.6%[22])[19-21]. Hence, the high seroprevalence rate reported in our study is probably due to the high sensitivity of the assay used.
The shape of the anti-HEV seroprevalence curve in Western Cape (low rates in childhood, with a rapid increase in early adulthood) is similar to that seen in hyperendemic developing countries such as Nepal and Bangladesh, where HEV genotype 1 predominates[18]. However, the risk factor analysis showed that only pork consumption was significantly associated with HEV seropositivity which is typical of European populations with high incidences of locally-acquired porcine HEV genotype 3[3,15,23]. This finding contrasts with previous data from a different area of South Africa, which showed that rural mud hut dwellers with no access to clean water had higher seropositivity[13]. Moreover, there have been no outbreaks of hepatitis E reported in South Africa which would also argue against genotype 1 possibly being the dominant circulating genotype. Our clinical case, in addition to others, suggests that HEV genotype 3 may be currently circulating in Western Cape. The other cases include two of HEV genotype 3 infection in immunosuppressed patients documented in Cape Town[11,12].
The strengths of the current study are that a highly sensitive, partially validated assay was employed to estimate seroprevalence and that the study population comprised of large numbers of individuals, including adults and children, from all racial groups. The main limitation of the study is that the population was a heterogeneous “convenience population” comprising hospital in-patients, outpatients and healthy blood donors and may not be representative of the population of Western Cape as a whole. The population studied may have over or under-represented certain population subgroups. This includes white children and elderly black South Africans, very few of whom were included in the study.
In conclusion, HEV is endemic in the Western Cape, South Africa with seroprevalence of 27.9%. Seroprevalence in children is very low with a rapid increase in early adulthood. The only risk factors for seropositivity are pork consumption and individuals over 30 years of age. Recent cases suggest HEV genotype 3 may be currently circulating in South Africa. The diagnosis of hepatitis E should be considered in any patient presenting with hepatitis in Western Cape, irrespective of travel history, age or ethnicity.
COMMENTS
Background
Globally, hepatitis E virus (HEV) is the most frequent aetiological cause of acute hepatitis. It causes sporadic and epidemic infections, predominantly in young adults living in developing countries. In these regions, it is associated with HEV genotypes 1 and 2, which are obligate human pathogens and spread oro-faecally through infected water. Most patients experience a self-limiting hepatitis, except in pregnant women and patients with chronic liver disease, where mortality may reach 25% and 75% respectively.
Research frontiers
Many previous seroprevalanece studies in HEV employed poor assays and have in due course underestimated the extent of the problem in these geographical settings. The authors used a highly sensitive and specific assay; Wantai® demonstrating a seroprevalence of 27.9%. It also demonstrated a potential circulating gentotype previously thought not to be circulating in this particular area: HEV3.
Innovations and breakthroughs
Only pork consumption was significantly associated with HEV seropositivity which is typical of European populations with high incidences of locally-acquired porcine HEV genotype 3. This finding contrasts with previous data from a different area of South Africa, which showed that rural mud hut dwellers with no access to clean water had higher seropositivity. Moreover, there have been no outbreaks of hepatitis E reported in South Africa which would also argue against genotype 1 possibly being the dominant circulating genotype.
Applications
The diagnosis of hepatitis E should be considered in any patient presenting with hepatitis in Western Cape, irrespective of travel history, age or ethnicity.
Peer-review
This study describes the prevalence of anti-HEV IgG in hospital patients and blood donors in Western Cape, South Africa, and identified consumption of pork as its risk factor. The study also reported a case of fulminant hepatic failure following acute HEV infection with genotype 3 in a patient who had chronic alcoholic liver disease. The study provides useful information for better understanding of HEV epidemiology in South Africa.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: Ethics approval was granted by the Faculty of Health Sciences Human Research Ethics Committee of the University of Cape Town, reference HREC 018/2014.
Informed consent statement: All participants, or their legal guardian, provided written consent prior to study enrollment.
Conflict-of-interest statement: Dalton HR has had travel and accommodation costs and consultancy fees from GlaxoSmithKline, Wantai and Gilead; and travel, accommodation and lecture fees from Merck, GFfe Blut GmBh and the Gates foundation. Sonderup M has received travel awards and consultancy fees from AbbVie, Gilead and Roche.
Data sharing statement: There is no additional data to share.
Peer-review started: July 12, 2016
First decision: July 29, 2016
Article in press: October 19, 2016
P- Reviewer: Nakano T, Shimakawa Y, Vento S S- Editor: Gong ZM L- Editor: A E- Editor: Liu WX
References
- 1.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 2.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 3.Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698–709. doi: 10.1016/S1473-3099(08)70255-X. [DOI] [PubMed] [Google Scholar]
- 4.Dalton HR, Hunter JG, Bendall R. Autochthonous hepatitis E in developed countries and HEV/HIV coinfection. Semin Liver Dis. 2013;33:50–61. doi: 10.1055/s-0033-1338114. [DOI] [PubMed] [Google Scholar]
- 5.Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 6.Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med. 2009;361:1025–1027. doi: 10.1056/NEJMc0903778. [DOI] [PubMed] [Google Scholar]
- 7.Browne LB, Menkir Z, Kahi V, Maina G, Asnakew S, Tubman M, Elyas HZ, Nigatu A, Dak D, Maung UA, et al. Notes from the field: hepatitis E outbreak among refugees from South Sudan - Gambella, Ethiopia, April 2014-January 2015. MMWR Morb Mortal Wkly Rep. 2015;64:537. [PMC free article] [PubMed] [Google Scholar]
- 8.Gerbi GB, Williams R, Bakamutumaho B, Liu S, Downing R, Drobeniuc J, Kamili S, Xu F, Holmberg SD, Teshale EH. Hepatitis E as a cause of acute jaundice syndrome in northern Uganda, 2010-2012. Am J Trop Med Hyg. 2015;92:411–414. doi: 10.4269/ajtmh.14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaäcson M, Frean J, He J, Seriwatana J, Innis BL. An outbreak of hepatitis E in Northern Namibia, 1983. Am J Trop Med Hyg. 2000;62:619–625. doi: 10.4269/ajtmh.2000.62.619. [DOI] [PubMed] [Google Scholar]
- 10.Grabow WO, Favorov MO, Khudyakova NS, Taylor MB, Fields HA. Hepatitis E seroprevalence in selected individuals in South Africa. J Med Virol. 1994;44:384–388. doi: 10.1002/jmv.1890440412. [DOI] [PubMed] [Google Scholar]
- 11.Andersson MI, Preiser W, Maponga TG, Heys I, Taljaard JJ, van Rensburg C, Tedder RS, Ijaz S. Immune reconstitution hepatitis E: a neglected complication of antiretroviral therapy in Africa? AIDS. 2013;27:487–489. doi: 10.1097/QAD.0b013e32835b1074. [DOI] [PubMed] [Google Scholar]
- 12.Andersson MI, Stead PA, Maponga T, van der Plas H, Preiser W. Hepatitis E virus infection: An underdiagnosed infection in transplant patients in Southern Africa? J Clin Virol. 2015;70:23–25. doi: 10.1016/j.jcv.2015.06.081. [DOI] [PubMed] [Google Scholar]
- 13.Mid Year Population Estimates (2014), South Africa Statistics Accessed: 2015-03-09. Available from: http://www.statssa.gov.za/publications/P0302/P03022014.pdf.
- 14.Tucker TJ, Kirsch RE, Louw SJ, Isaacs S, Kannemeyer J, Robson SC. Hepatitis E in South Africa: evidence for sporadic spread and increased seroprevalence in rural areas. J Med Virol. 1996;50:117–119. doi: 10.1002/(SICI)1096-9071(199610)50:2<117::AID-JMV3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Mansuy JM, Saune K, Rech H, Abravanel F, Mengelle C, L Homme S, Destruel F, Kamar N, Izopet J. Seroprevalence in blood donors reveals widespread, multi-source exposure to hepatitis E virus, southern France, October 2011. Euro Surveill. 2015;20:27–34. [PubMed] [Google Scholar]
- 16.Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116–138. doi: 10.1128/CMR.00057-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 18.Kmush BL, Labrique AB, Dalton HR, Ahmed ZB, Ticehurst JR, Heaney CD, Nelson KE, Zaman K. Two Generations of “Gold Standards”: The Impact of a Decade in Hepatitis E Virus Testing Innovation on Population Seroprevalence. Am J Trop Med Hyg. 2015;93:714–717. doi: 10.4269/ajtmh.15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izopet J, Labrique AB, Basnyat B, Dalton HR, Kmush B, Heaney CD, Nelson KE, Ahmed ZB, Zaman K, Mansuy JM, et al. Hepatitis E virus seroprevalence in three hyperendemic areas: Nepal, Bangladesh and southwest France. J Clin Virol. 2015;70:39–42. doi: 10.1016/j.jcv.2015.06.103. [DOI] [PubMed] [Google Scholar]
- 20.Halliday JS, Harrison GL, Brown A, Hunter JG, Bendall R, Penny D, Toatu T, Abdad MY, Klenerman P, Barnes E, et al. Hepatitis E virus infection, Papua New Guinea, Fiji, and Kiribati, 2003-2005. Emerg Infect Dis. 2014;20:1057–1058. doi: 10.3201/eid2006.130562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalton HR, Fellows HJ, Gane EJ, Wong P, Gerred S, Schroeder B, Croxson MC, Garkavenko O. Hepatitis E in new zealand. J Gastroenterol Hepatol. 2007;22:1236–1240. doi: 10.1111/j.1440-1746.2007.04894.x. [DOI] [PubMed] [Google Scholar]
- 22.Cleland A, Smith L, Crossan C, Blatchford O, Dalton HR, Scobie L, Petrik J. Hepatitis E virus in Scottish blood donors. Vox Sang. 2013;105:283–289. doi: 10.1111/vox.12056. [DOI] [PubMed] [Google Scholar]
- 23.Dalton HR, Pas SD, Madden RG, van der Eijk AA. Hepatitis e virus: current concepts and future perspectives. Curr Infect Dis Rep. 2014;16:399. doi: 10.1007/s11908-014-0399-8. [DOI] [PubMed] [Google Scholar]
- 24.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]


