Abstract
Hyperlipidemia occurs in 95% of organ transplant recipients, however its effect on organ allograft rejection has not been investigated. We found that induction of hyperlipidemia in mice caused a significant acceleration of rejection of cardiac allografts. Accelerated rejection was associated with an aggressive T cell infiltrate that mediated significant tissue damage as well as increased serum levels of the proinflammatory cytokines IL-2, IL-6, and IL-17. Hyperlipidemic mice had an increased number of Th17 cells in their periphery and rejecting allografts from hyperlipidemic mice contained significant numbers of IL-17 producing T cells that were not detectable in transplants harvested from controls. Neutralization or genetic ablation of IL-17 prolonged survival of cardiac allografts transplanted into hyperlipidemic recipients, suggesting that IL-17 production promotes accelerated rejection. Analysis of alloreactive T cell frequencies directly ex vivo in naïve mice revealed that the frequency of donor reactive IL-17 producing cells in hyperlipidemic was increased prior to antigen exposure, suggesting that hyperlipidemia was sufficient to alter T cell alloreactivity and promote anti-donor Th17 responses on first exposure to antigen. Together, our data suggest that hyperlipidemia alters rejection by altering the types of T cell subsets that respond to donor antigen by promoting Th17 biased anti-donor reactivity.
Introduction
The canonical understanding of T cell mediated rejection of organ allografts holds that recognition of donor antigen together with costimulation leads to the activation of alloreactive T-helper type 1 (Th1) CD4 T cells and CD8 T cells that destroy the transplant (1–5). Based on this paradigm, a considerable amount of effort has been placed on inhibiting the activation of alloreactive T cells, particularly Th1 cells. However, most of our understanding of transplantation immunology has been gained from transplanting tissues or organs from one healthy animal into another of a different strain. There has been little consideration of how health conditions prevalent in the transplant patient population pre or posttransplant may affect responses to donor antigen or the regulation of such responses. Indeed, transplant patients typically suffer from preexisting health conditions that either result from or lead to systemic inflammation. How these conditions may affect rejection responses is not known.
Hyperlipidemia is a comorbidity that is highly relevant in transplantation. Hyperlipidemia, increased serum cholesterol and triglycerides, leads to development of atherosclerosis. Hyperlipidemia is known to cause atherosclerosis primarily as a result of increased serum cholesterol levels, but also causes systemic inflammation that can contribute to disease progression (6–14). Hyperlipidemic humans and mice exhibit increased levels of inflammatory cytokines in their serum, and exhibit increased inflammatory T cell responses (reviewed in [7]). A major recipient characteristic associated with cardiac allograft rejection after the first year is a history of ischemic heart disease resulting from coronary artery disease caused by hyperlipidemia (15). Ischemic cardiomyopathy resulting from hyperlipidemia is the cause of end-stage heart disease in approximately 40% of all patients requiring a heart transplant (15). Hyperlipidemia develops in 50% of heart transplant patients after the first year of transplant, and 95% of patients within 5 years (www.nhlbi.nih.gov). In spite of treatment, two-thirds of patients remain dyslipidemic and a significant number of heart transplant patients are statin intolerant (16,17). Thus alterations in responses to allografts as a result of hyperlipidemia are highly relevant in a clinical setting.
Limited reports have suggested that increased cholesterol contributes to neointimal proliferation in artery grafts (18), and atherosclerosis in cardiac allografts (19). However, little is known about how hyperlipidemia affects rejection and an effect of hyperlipidemia on anti-donor adaptive immune responses has not been described. We hypothesized that the ability of hyperlipidemia to promote inflammation may alter anti-donor responses or their regulation and thereby influence transplant outcome. To test our hypothesis, we analyzed heart transplant rejection in hyperlipidemic mice. Mice that are deficient in the cholesterol transport protein, apolipoprotein E, are one of the best-characterized models in which to study the effects of hyperlipidemia. ApoE deficient mice display lipid abnormalities similar those observed in transplant patients, including increased plasma cholesterol, increased LDL and VLDL, reduced HDL, and elevated triglycerides and are therefore a physiologically relevant model of hyperlipidemia (7,20–22). Importantly, plasma glucose levels in ApoE−/− mice are reduced (23), and ApoE−/− mice do not become obese as a result of high-fat diet (24) eliminating the confounding factors of diabetes and obesity, which are known to have independent effects on immunity.
Analysis of transplant rejection in ApoE−/− mice revealed that hyperlipidemia fundamentally alters transplant rejection through its ability to promote Th17 cell responses and anti-donor Th17 cell reactivity. In hyperlipidemic mice, IL-17 production plays a key role in the rejection response. These changes were also observed in C57BL/6 mice fed a high-fat diet. Our data therefore reveal a previously unappreciated effect of hyperlipidemia on T cell subsets that mediate transplant rejection.
Materials and Methods
Mice
Apoe<tm1Unc>/J (ApoE−/−) mice (20), B6.C-H2-Ab1bm12/KhEg (bm12), BALB/cJ, and C57BL/6 controls were obtained from Jackson Laboratories (Bar Harbor, ME). Mice in which the IL-17 gene is disrupted by the insertion of the cre gene were the kind gift of Dr. Brigitta Stockinger. These mice were crossed with ApoE−/− mice in our facility to generate mice in which IL-17 production is ablated. Mice were maintained on either high-fat diet, Western Diet, Teklad, 21% fat, or normal chow LabDiet, JL mouse breeder/auto, 12% fat obtained from Harlan Laboratories (Indianapolis, IN). Our Institutional Animal Care and Use Committee (IACUC) approved all procedures in accordance with NIH guidelines for the care and use of animals.
Serum lipid levels
Levels of serum cholesterol were measured using Cholesterol Rapid Liquid Reagent (Cliniqa, San Marcos, CA). Briefly, 10 μL serum was added into 1 mL reagent, and incubated at 37°C for 5 min. The absorbance of samples was read at 500 nm with iMark microplate absorbance reader (Bio-Rad, Hercules, CA). The cholesterol levels were calculated according to manufacturer’s instruction.
Serum cytokine determination
Serum was taken from heart transplant recipients at the time of rejection, or at 100 days in mice that did not reject. Serum from groups of 4–6 mice were pooled and assayed by Multiplex Elisa by Quansys (Logan, UT). Samples were diluted at ratios of 1 to 2 (sample to buffer) (50%), 1 to 20 (5%), and 1 to 200 (0.5%). Each dilution was loaded into three wells and measured in triplicate, a total of nine wells per sample. The optimal dilution was selected by finding the dilution where the pixel intensity values fall on the most linear portion of the standard curve. Measured pixel intensity values were regressed to interpolate concentrations in the standard curve.
Heterotopic heart transplantation
Mice in each group were maintained on a high-fat diet or normal chow throughout the experiment as indicated. Vascularized heterotopic cardiac transplants were performed into the abdomen as previously described (25). Graft function was assessed by daily palpation of the abdomen. Hearts were considered rejected when a heartbeat was no longer detectable by palpation. In all cases, rejection was verified by autopsy and visual inspection. Heart transplants that stopped beating within 48 h were assumed to be technical failures and eliminated from further analysis. All transplant recipients were 10–14 weeks of age.
Histology
Transplants were harvested at the time of rejection, or when indicated, and transverse sections prepared from formalin fixed or frozen tissue. Sections fixed in 10% buffered formalin were stained with hematoxylin and eosin (H&E) or Masson Trichrome Stain (Trichrome). Immunofluorescence staining was conducted as described (26) on frozen sections. Briefly, frozen graft sections were fixed in methanol, blocked with 1% bovine serum albumin in PBS-T for 1 h at room temperature. The sections were then stained with rat anti-mouse CD4, rat anti-mouse CD8a (BioLegend, San Diego, CA), goat anti-IL-17 (Santa Cruz Biotechnology, Dallas, TX) or isotype control antibodies overnight at 4°C. After washing with PBS, the sections were stained with DyLight 488 goat anti-rat IgG (BioLegend) or Cy3-conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), respectively at room temperature for 1 h. Lastly, the slides were stained with DAPI (Thermo Scientific, Waltham, MA). Images were captured using TE300 Nikon microscopy system. Three consecutive sections per graft were used for analyzing the infiltration of CD4+ and CD8+ cells. Sections were taken from similar areas of the graft for all groups. The positively stained cells in each randomly selected view of graft were counted under 20× magnification, and the average of cell count per mm2 from at least 15–25 nonoverlapping views was used to represent the number of CD4+ or CD8+ cells for each graft. ImageJ (version 1.46r, NIH) was used for area measurement.
Flow cytometry
Cell surface staining was performed using standard methods. The following antibodies were used: using anti-CD4 (GK1.5, Biolegend), anti-CD8 (53-6.7, BD Pharmingen San Jose, CA), anti-B220 (RA3-6B2, BD Pharmingen), anti-CD11b (M1/70, BD Pharmingen), and anti-CD11c HL3 (BD Pharmingen). Intracellular cytokine staining was performed on single cell suspensions prepared from lymph node and spleen. Cells were re-stimulated for 4 h in the presence of PMA (50 ng/mL)/ionomycin (1 mM, Sigma–Aldrich, St. Louis, MO), and monensin (eBioscience, San Diego, CA). Cells were then prepared using the intracellular fixation and permeabilization buffer set (eBioscience) according to the manufacturers instructions. Cells were stained with anti-IL17A (TC11-18H10.1 Biolegend, 17B7 eBioscience), or anti-IFN-γ (XMG1.2 Biolegend). All flow cytometry was performed on a FacsCanto II cytometer (BD Biosciences), and data were analyzed using FlowJo v X 10.0.7 software (Tree Star, Inc. Ashland, OR).
ELISpot
Age matched ApoE−/− and C57BL/6 mice were fed a high-fat diet or normal chow for 4 weeks. Animals were sacrificed and single cell suspensions prepared from spleens. Cells were stimulated with either irradiated allogeneic bm12 or syngeneic C57BL/6 splenocytes in ELISpot assays performed as described (27). Plates were developed after 48 h, and read on a CTL ImmunoSpot S5. Results were analyzed by subtraction of background response to syngeneic splenocytes from the total number of spots in wells stimulated with allogeneic splenocytes.
IL-17 neutralization
Neutralizing anti-IL-17 antibody (17F3 BioXcell, West Lebanon, NH) was administered intraperitoneally at a dose of 100 μg daily on days 0–3, followed by every other day until day 13 after transplantation. Neutralization of IL-17 was verified by serum ELISA assays.
Statistical analysis
All statistical calculations were preformed using GraphPad Prism 5.0b software (Graphpad Software, San Diego, CA). The Kaplan and Meier method with a 95% confidence interval was used for the calculation of survival curves. Survival curves were compared using the log rank test. p values <0.05 using Student’s t test were considered statistically significant.
Results
Hyperlipidemia leads to accelerated rejection of cardiac allografts
To test the hypothesis that hyperlipidemia alters transplant outcome, we examined survival of allogeneic hearts transplanted into hyperlipidemic recipients. To induce hyperlipidemia, groups of C57BL/6 or ApoE−/− mice (Apoe<tm1Unc>/J (ApoE−/−) C57BL/6 background) were placed on a high-fat diet for 4-weeks. Control C57BL/6 and ApoE−/− mice were fed normal chow. As expected, feeding ApoE−/− and C57BL/6 mice a high-fat diet led to an increase in serum cholesterol (Figure S1) and (20–22). The mice were then heterotopically transplanted with hearts from MHC class II disparate C57BL/6.C-H2bm12 mice (bm12). bm12 hearts transplanted into control C57BL/6 mice fed normal chow survived long-term (Figure 1A, MST>100 days) with histological evidence of chronic rejection as described previously (28). In contrast, bm12 hearts transplanted into ApoE−/− mice fed a high-fat diet were acutely rejected and stopped beating with a median survival time (MST) of 21.5 days (Figure 1A, p = 0.0014 vs. C57BL/6). Syngeneic hearts transplanted into ApoE−/− recipients fed a high-fat diet survived long-term (>100 days) demonstrating that loss of bm12 allografts resulted from alloreactivity (Figure 1B p = 0.008 vs. ApoE with high-fat diet).
Figure 1. Cardiac allograft rejection is accelerated in hyperlipidemic recipients.
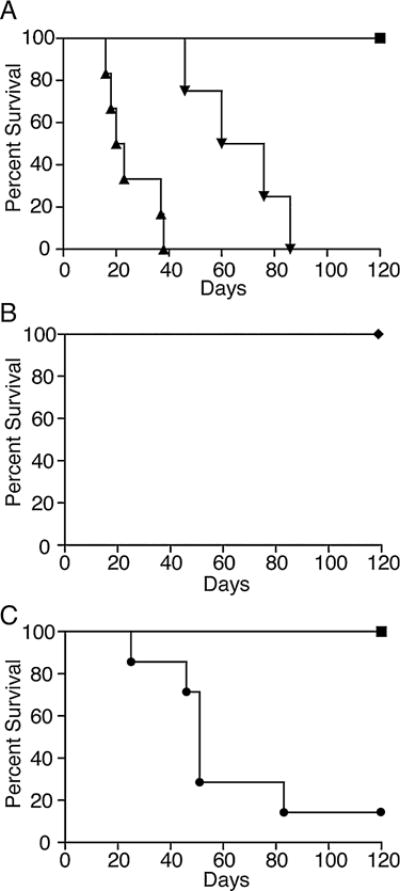
(A) ApoE−/− mice were fed high-fat diet (triangles, n = 6, p = 0.0014), or normal chow (inverted triangles, n = 4, p = 0.0027) for 4 weeks, after which they received a bm12 heart transplant. As a control, bm12 hearts were transplanted into C57BL/6 mice fed normal chow (n = 5 squares). (B) ApoE−/− mice were fed high-fat diet and received a syngeneic cardiac graft (diamonds, n = 3 p = 0.008 compared to mice fed a high-fat diet that received a bm12 heart). (C) C57BL/6 mice were fed high-fat diet (circles, n = 7 p = 0.006) or normal chow (n = 5 squares, shown as reference) for 4 weeks after which they received a bm12 cardiac graft. Shown is the percentage of hearts surviving in each group. Data are the combined results of multiple independent experiments. ApoE, apolipoproteinE.
Because ApoE deficient mice fed a high-fat diet have very high serum lipid levels, we also analyzed transplant survival in ApoE−/− mice fed normal chow that are known to have lipid levels similar to humans with hyperlipidemia (23). Hearts from bm12 mice transplanted into ApoE−/− mice fed normal chow were also acutely rejected (Figure 1A, MST = 61 days, p = 0.0027 compared with C57BL/6 controls), although rejection was slower than in ApoE−/− mice fed a high-fat diet (p = 0.004). To assess whether acceleration of rejection was related to the ApoE mutation, we also examined whether raising lipid levels in wild-type mice would also affect rejection. bm12 hearts transplanted into C57BL/6 mice fed a high-fat diet also exhibited accelerated rejection (MST = 51 days) when compared to bm12 hearts transplanted into C57BL/6 controls fed normal chow (Figure 1C, p = 0.006 between groups). Thus, accelerated rejection occurs independently of ApoE deficiency and in mice with clinically relevant lipid levels.
Hyperlipidemia promotes an aggressive rejection response
To assess differences in rejection in mice fed a high-fat diet, when compared with mice fed normal chow, we examined damage to rejected hearts in each group histologically. bm12 hearts transplanted into C57BL/6 controls exhibited signs of chronic rejection such as vasculopathy and fibrosis but contained few infiltrating lymphocytes or damage to the myocardium, consistent with other reports (Figures 2A and S2). Syngeneic hearts transplanted into ApoE−/− mice fed a high-fat diet did not contain a lymphocytic infiltration, and no tissue damage was apparent (Figures 2B and S2). In contrast, bm12 hearts rejected by ApoE−/− mice fed a high-fat diet contained a significant lymphocytic infiltrate, exhibited extensive damage to vessels and myocardium, and contained areas of necrosis and fibrosis throughout the tissue consistent with acute rejection (Figures 2C and S2). Similar findings were observed in bm12 hearts rejected by C57BL/6 mice fed a high-fat diet, although damage was less severe than that observed ApoE−/− mice with higher lipid levels (Figure 2D). We were also able to detect significantly higher numbers of CD4+ and CD8+ cells infiltrating bm12 grafts recovered from ApoE−/− recipients fed a high-fat diet when compared with bm12 grafts from C57BL/6 controls fed a normal diet (Figures 2E and F p < 0.005). Together, these data suggest that hyperlipidemia promotes aggressive rejection of allogeneic heart transplants and that the effect on rejection is dependent on the degree of recipient hyperlipidemia.
Figure 2. Hyperlipidemia promotes an aggressive rejection response.
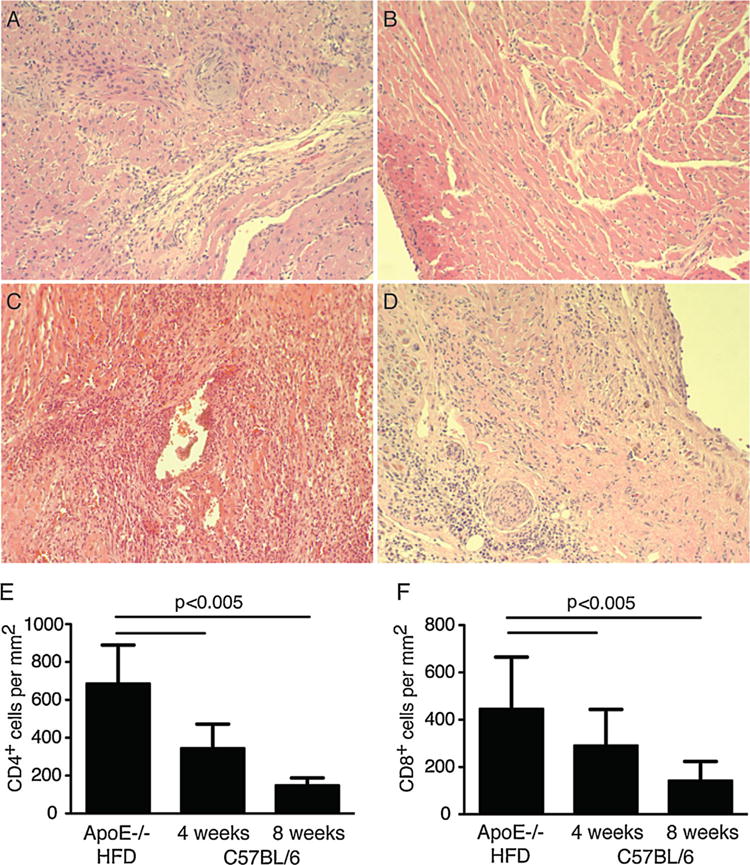
(A) H&E staining of tissue sections from a bm12 heart harvested after 100 days from C57B/6 control mice fed normal chow; (B) a syngeneic heart harvested after 100 days from an ApoE−/− mouse fed a high-fat diet; (C) a bm12 heart harvested at the time of rejection from an ApoE−/− mouse fed a high-fat diet (day 21); (D) a bm12 heart harvested at the time of rejection from a C57BL/6 mouse fed a high-fat diet (day 60). 20× magnification is shown. (E) Quantification of CD4+ graft infiltrating cells in bm12 heart transplants from ApoE−/− mice fed a high-fat diet (HFD) at the time of rejection, or C57BL/6 mice fed normal chow at 4 or 8 weeks following transplant. (F) Quantification of CD8+ graft infiltrating cells in bm12 heart transplants from ApoE−/−mice fed a high-fat diet (HFD) at the time of rejection, or C57BL/6 mice fed normal chow at 4 or 8 weeks following transplant. All data shown are representative of at least three experiments containing groups of at least 3 mice. ApoE, apolipoproteinE; H&E, hematoxylin and eosin.
Hyperlipidemia promotes a Th17-biased rejection response
We next measured the levels of proinflammatory cytokines in the serum of mice that had received cardiac grafts. ApoE−/− and C57BL/6 mice fed a high-fat diet that rejected a bm12 heart transplant had higher levels of the proinflammatory cytokines IL-2, IL-6, and IL-17 in their serum when compared with control C57BL/6 mice fed a normal diet that received a bm12 heart transplant but did not reject their grafts for over 100 days (Figure 3A). Levels of IL-1α, IFN-γ, TNF-α or IL-12p70, and IL-4 were unchanged between groups indicating that this was a specific effect rather than a general increase in cytokine production (Figure S3). There were no significant differences in the frequencies of CD4 T cells, CD8 T cells, B220+ B cells or CD11b/c+ antigen presenting cells in peripheral lymphoid tissue of mice fed a high-fat diet when compared to controls (Figure S4). Increased IL-17 production led us to examine whether CD4 T cells from mice that had rejected bm12 heart grafts produced IL-17. In lymph nodes from ApoE−/−mice fed a high-fat diet that had received a bm12 heart graft, the absolute number of CD4 T cells producing IL-17 was greater than observed in C57BL/6 controls fed normal chow that were sacrificed 100 days after transplantation (Figure 3B). Similarly the number of IFN-γ producing cells was increased (Figure 3B). We also observed an increase in the frequency of IL-17 producing CD4 cells in the spleens of recipients fed high-fat diet (Figure 3C).
Figure 3. Hyperlipidemia promotes an IL-17 mediated rejection response.
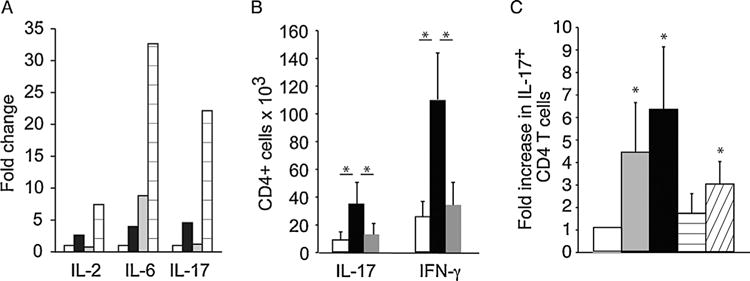
(A) Fold change in serum levels of proinflammatory cytokines were determined in C57BL/6 mice fed normal chow (white bars) or high-fat diet (black bars), ApoE−/− fed normal chow (gray bars) or a high-fat diet (lined bars) for 4 weeks that received a bm12 heart transplant. Serum was collected at the time of rejection, or at 100 days. Pooled serum from 4 to 6 mice was analyzed by Multiplex Elisa. (B) Absolute number of CD4 cells producing IL-17 or IFN-γ in the lymph nodes of C57BL/6 fed normal chow (white bars) or ApoE−/− mice fed a high-fat diet (black bars) that received a bm12 heart transplant as determined by flow cytometry. Untransplanted ApoE−/− mice on a high-fat diet (gray bars) were used as controls. n = 3–6 mice per group. Asterisk indicates p < 0.05. (C) Fold change in the frequency of CD4 T cells expressing IL-17 in the spleen was determined in ApoE−/− mice fed normal chow (gray bars) or a high-fat diet (black bars) and C57BL/6 mice fed normal chow (horizontal lines) or high-fat diet (diagonal lines) at the time of rejection of bm12 cardiac grafts, or at 100 days. Splenocytes were restimulated with PMA/ionomycin, and assayed for expression of IL-17 by intracellular staining and flow cytometry. Frequencies in naïve C57BL/6 mice (white bar) were used as an inter-experimental control. Asterisk indicates p value <0.05 relative to control. ApoE, apolipoproteinE; IFN-g, interferon gamma; IL, interleukin; PMA, phorbol myristate acetate.
Rejecting cardiac allografts from hyperlipidemic mice contain IL-17 producing T cells that are not observed in mice with normal lipid levels
To examine a possible role for IL-17 producing T cells in the rejection process, we examined intra-graft expression of IL-17. We did not detect IL-17 producing cells in bm12 hearts transplanted into control C57BL/6 mice fed a normal diet (Figure 4A). In contrast, analysis of tissue sections from bm12 hearts harvested at the time of rejection from ApoE−/− mice fed a high-fat diet revealed a significant number of graft infiltrating T cells producing IL-17 (Figure 4B). Similarly, while we were unable to detect IL-17 producing cells in fully allogeneic BALB/c hearts that were rejected by C57BL/6 controls (Figure 4C), BALB/c hearts rejected by ApoE−/− mice fed a high-fat diet contained a significant number of IL-17 producing graft infiltrating T cells (Figure 4D). Thus, hyperlipidemia promotes an anti-donor response that involves significant production of IL-17 in both MHC class II as well as fully mismatched cardiac allografts.
Figure 4. Detection of IL-17 production in heart transplants by immunofluorescence.
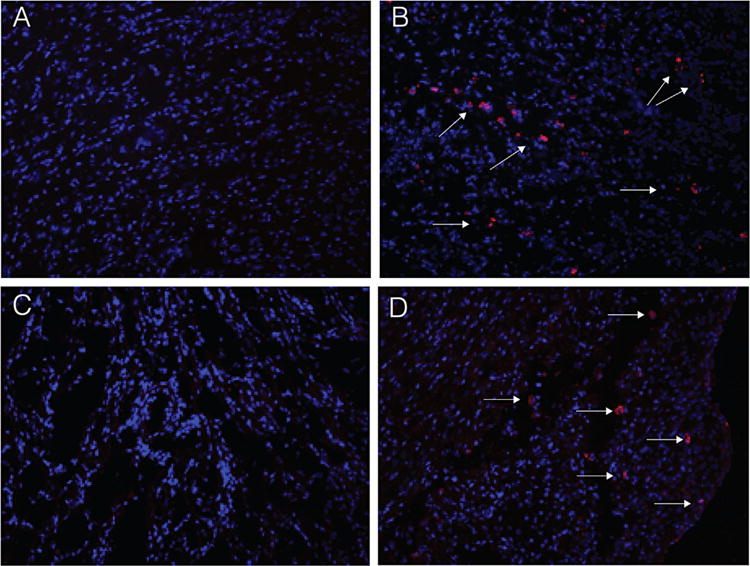
Shown is staining for IL-17 (red) in bm12 hearts transplanted into C57BL/6 controls (A); bm12 hearts transplanted into ApoE−/− mice fed a high-fat diet (B); or BALB/c hearts transplanted into C57BL/6 controls (C); and BALB/c hearts transplanted into ApoE−/− mice fed a high-fat diet (D). Representative data are shown. Nuclei are counterstained with DAPI shown in blue. Magnification 20×. ApoE, apolipoproteinE; DAPI, 4′,6-diamidino-2-phenylindole; IL, interleukin.
IL-17 mediates cardiac graft rejection in hyperlipidemic recipients
We next examined whether production of IL-17 is involved in accelerated rejection of cardiac allografts in hyperlipidemic recipients. Survival of bm12 hearts transplanted into IL-17 deficient ApoE−/− mice (C57BL6 background) fed a high-fat diet was prolonged when compared to IL-17 sufficient controls (Figure 5A, p = 0.009). To confirm that these results were not due to the congenital lack of IL-17 in these mice, we also performed antibody neutralization experiments. ApoE−/− mice that had been fed a high-fat diet for 4 weeks were treated with neutralizing anti-IL-17 antibody for a period of 13 days. Survival of bm12 grafts in these mice was prolonged when compared to controls (Figure 5B, p = 0.02). These data demonstrate that IL-17 production in hyperlipidemic recipients plays a significant role in promoting accelerated rejection of cardiac allografts.
Figure 5. Graft survival is prolonged in the absence of IL-17.
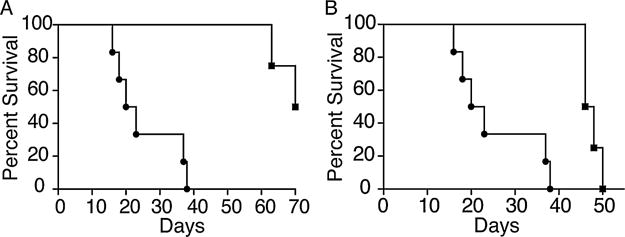
(A) IL-17−/− ApoE−/− mice (n = 4, p = 0.009) were fed a high-fat diet for 4 weeks, and then received a bm12 heart transplant (squares). (B) ApoE−/− mice were fed a high-fat diet for 4 weeks and then received a bm12 heart transplant along with IL-17 neutralizing antibody for 2 weeks (squares) as described (n = 4, p = 0.02). Survival of bm12 hearts transplanted into ApoE−/− mice fed a high-fat diet (circles) from Figure 1 are shown for reference in panels A and B. ApoE, apolipoproteinE; IL, interleukin.
Hyperlipidemia increases the frequency of alloreactive T cells and promotes Th17 responses prior to antigen challenge
Analysis of alloreactive T cell frequencies directly ex vivo revealed that the frequency of bm12 reactive IL-17, IL-2, and IFN-γ producing cells in ApoE−/− mice fed a high-fat diet was significantly higher than in ApoE−/− mice maintained on normal chow prior to exposure to alloantigen (Figure 6A). C57BL/6 mice fed a high-fat diet also consistently contained a higher frequency of bm12 reactive IL-17, and IL-2 producing cells when compared to C57BL/6 mice fed normal chow although this difference did not reach statistical significance (Figure 6A). The frequency of BALB/c reactive IL-17, IL-2, and IFN-γ producing cells was also higher in the spleens of ApoE−/− mice fed high-fat diet when compared to controls (Figure 6B), suggesting that an increase in alloreactive T cell frequencies is not restricted to an anti-bm12 response. These data suggest that that induction of hyperlipidemia is sufficient to lead to an increase in the frequency of T cells capable of responding to alloantigen in the absence of prior to antigen exposure, and that hyperlipidemia promotes Th17 alloreactivity.
Figure 6. Hyperlipidemia increases the frequency of alloreactive cells.
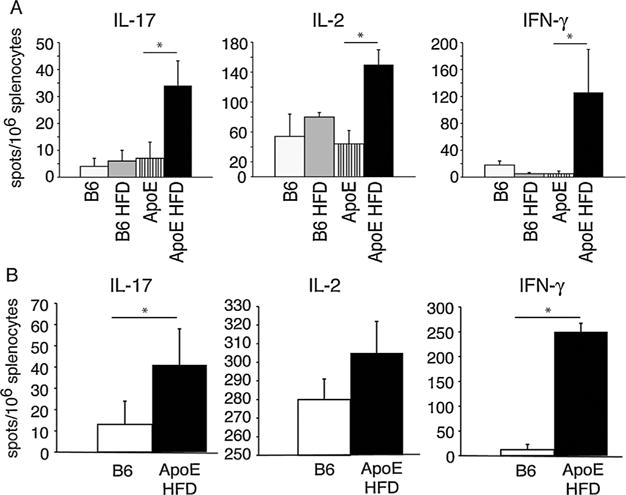
(A) ApoE−/− mice and C57BL/6 mice were fed high-fat diet (HFD), or normal chow for 4 weeks. Splenocytes were then harvested and stimulated with irradiated bm12 or syngeneic splenocytes and assayed for production of IL-17, IL-2, and IFN-γ by ELISpot. (B) ApoE−/− mice were fed a high-fat diet and C57BL/6 controls were fed normal chow for 4 weeks. Splenocytes were then harvested and stimulated with irradiated BALB/c or syngeneic splenocytes and assayed for production of IL-17, IL-2, and IFN-γ by ELISpot. n ≥ 5 in all groups. Asterisk indicates p < 0.05. ApoE, apolipoproteinE; IFN-g, interferon gamma; IL, interleukin.
Discussion
Modern immunosuppressive regimens have significantly improved cardiac transplant survival in the first year after transplant, yet the mortality rate beyond the first year of heart transplantation has not significantly improved in the last two decades (15). This startling fact prompted us to examine how concomitant health conditions prevalent in the transplant population may affect transplant outcome. Our data suggest that the common condition of hyperlipidemia can powerfully accelerate the rejection of cardiac allografts. Mechanistically, hyperlipidemia promotes a rejection response involving Th17 cells and requires production of IL-17. An increased frequency of alloreactive T cells was observed in hyperlipidemic mice upon antigen exposure, suggesting that hyperlipidemia is sufficient to drive these alterations. Our data therefore reveal a previously unappreciated effect of hyperlipidemia on transplant rejection and demonstrate that hyperlipidemia fundamentally alters the T cell subsets that mediate rejection.
Th17 cells represent a unique CD4 T cell lineage derived from naïve T cells stimulated in the presence of TGF-β plus IL-6 or IL-21. There has been great interest in the potential role of IL-17 producing cells in rejection, the majority of studies which have suggested that Th17 cells play a role in rejection have relied on models with questionable physiological relevance (29–43). Except perhaps for the case of Obliterative bronchiolitis in lung transplants (44), it has been difficult to detect a role for IL-17 producing cells in wild-type mice and IL-17 is not typically detected within rejecting grafts. In contrast, rejection in hyperlipidemic mice was associated with an increase the frequency of CD4 T cells producing IL-17. While we were unable to detect IL-17 producing T cells infiltrating hearts rejected by normal mice, transplants rejected by hyperlipidemic mice contained a significant number of IL-17 producing cells. To our knowledge, IL-17 is has not been observed in rejecting cardiac transplants except in the case of mice not able to generate a Th1 response (29,30). Both genetic ablation and neutralization of IL-17 significantly prolonged graft survival in hyperlipidemic recipients demonstrating that hyperlipidemia promotes a rejection response that involves a significant Th17 component in which IL-17 plays a mechanistic role in mediating rejection. Together our data demonstrate that Th17 cells play a critical role in rejection when examined in the context of physiologic conditions present in the transplant patient population. This shifts the current paradigm by demonstrating that rejection must be studied in the context of conditions such as hyperlipidemia that play a previously undescribed role in altering rejection.
Induction of hyperlipidemia was sufficient to lead to an increase in the frequency of alloreactive T cells in naïve hyperlipidemic mice, a significant proportion of which produced IL-17. While recent studies suggesting that proatherogenic conditions promote Th17 responses (26) and other studies have suggested that hyperlipidemia promotes production of proinflammatory cytokines (reviewed in [7]), to our knowledge, there have not been previous reports describing an effect of alloreactive T cells. Since hyperlipidemia is a common health condition in patients both prior to and following cardiac transplant these data demonstrate a need to fully understand how this condition may alter anti-donor responses in order to improve outcomes.
As discussed above, ApoE deficient mice are a useful model to study the effects of hyperlipidemia. Several studies have suggested that ApoE deficient mice have changes in immune responses (45). However, it is not clear whether these changes are due to the effect of lipid levels on cells of the immune system, or some undefined alternate role for ApoE. We demonstrate that ApoE−/− mice exhibit accelerated rejection of bm12 grafts when compared with ApoE−/− controls fed normal diet. The effect observed is increased in mice fed a high-fat diet. Furthermore, we observed that in wild-type C57BL/6 mice, raising lipid levels through feeding a high-fat diet results in accelerated rejection of bm12 allografts. Within the group of C57BL/6 mice fed a high-fat diet, there was a trend towards mice with higher lipid levels rejecting more quickly than mice with lower lipid levels. These data suggest that the observed effect on rejection is therefore a direct result of increased lipid levels and is dependent on the degree of recipient hyperlipidemia.
Transplant patients suffer from a variety of concomitant health conditions yet surprisingly little is known about whether these conditions have an effect on rejection or the ability to induce tolerance. Indeed, much of the current paradigm of transplant rejection is based on work performed in animal models that do not capture conditions prevalent in the transplant patient population. Our data indicate that hyperlipidemia promotes an aggressive rejection response that results in accelerated rejection of allogeneic hearts transplants. The majority of transplant patients develop hyperlipidemia, and two-thirds remain dyslipidemic despite treatment. Our data suggest in mice that hyperlipidemia induced as a result of diet profoundly changes the host’s immune response to donor antigens. An important therapeutic issue is determining whether the effects of hyperlipidemia in the immune system can be reversed, by interventions that lower plasma lipid levels. This will depend in part on whether hyperlipidemia induces a transient inflammatory state, or if exposure to high lipid levels permanently alters T cell phenotype.
These studies provide the proof-of-principle for examining whether similar changes are observed in humans with hyperlipidemia. If hyperlipidemia leads to similar alterations in humans it may be possible to improve transplant outcomes by targeting T cell subsets that contribute to rejection in hyperlipidemic hosts. While the effects of hyperlipidemia on immune status in humans are poorly understood, it is becoming clear that hyperlipidemia can promote IL-17 responses and inflammation (7,46). Improving control of hyperlipidemia or developing new approaches that target novel effectors and promote regulation may allow for the establishment of transplant tolerance.
Supplementary Material
Figure S1: Serum cholesterol levels in mice fed a high-fat diet. Shown are levels of cholesterol in C57BL/6 mice (top panel) or ApoE−/− mice (bottom panel) prior to starting them on a high-fat diet, after being fed a high fed for 4-weeks on the day of transplant (HFD), or at the time the mice were sacrificed and hearts harvested (Harvest). n = 5–6 mice per group. Asterisk indicates p < 0.05. A full discussion of the plasma cholesterol levels in ApoE−/− and C57BL/6 mice fed normal chow or high fat diet can be found in (47). For reference, transplant patients are considered hyperlipidemic when they have plasma cholesterol levels greater than 200 mg/dL. A retrospective study of lipid control in transplant patients in a single center identified a range of from 91 mg/dl to 1665 mg/dL among patients tested (48). It is important to note that wild-type mice have a different plasma cholesterol composition, with high high density lipoprotein (HDL), and low low density lipoprotein (LDL) levels compared with humans. While the goal for human transplant patients is less than 100 mg/dL LDL, C57BL/6 mice fed normal diet have an LDL of 7 mg/dL. In ApoE−/− mice, in contrast to wild-type mice, low density LDL and VLDL particles are enriched, similar to what is observed in hyperlipidemic humans.
Figure S2: Trichrome staining of tissue sections from a transplanted bm12 heart harvested after 100 days from C57B/6 control mice fed normal chow (A); a syngeneic heart harvested after 100 days from an ApoE−/− mouse fed a high-fat diet (B); a bm12 heart harvested at the time of rejection from a C57BL/6 mouse fed a high-fat diet (C, day 60); a bm12 heart harvested at the time of rejection from an ApoE−/− mouse fed a high-fat diet (D, day 21); a bm12 heart harvested at the time of rejection from an ApoE−/− mouse fed a normal diet (E, day 60). Representative tissue sections are shown. 20× magnification is shown.
Figure S3: Serum cytokine concentrations. ApoE−/− and C57BL/6 mice were fed high-fat diet or normal chow for 4 weeks before receiving a bm12 heart transplant. Mice were sacrificed at the time of rejection, and pooled serum for each group was assayed for expression of IL-1α, IFN-γ, TNF-α, IL-12p70, IL-2 IL-4, IL-6, and IL-17 by multiplex ELISA. (A) Serum cytokine level of IL-2, IL-6, and IL-17 expressed in pg/ml in C57BL/6 mice fed normal chow (white bars), C57BL/6 mice fed a high-fat diet (light gray bars), ApoE−/− mice fed normal chow (dark gray bars), and ApoE−/− mice fed a high-fat diet (black bars). n = 6 mice per group. (B) Serum cytokine level of IL-1α, IFN-γ, TNF-α, or IL-12p70, and IL-4 expressed in pg/ml in in C57BL/6 mice fed normal chow (white bars), C57BL/6 mice fed a high-fat diet (light gray bars), ApoE−/− mice fed normal chow (dark gray bars), and ApoE−/− mice fed a high-fat diet (black bars). n = 4–6 mice per group. Asterisk represents p < 0.05.
Figure S4: Lymphocyte populations not affected by hyperlipidemia. ApoE−/− and C57BL/6 mice were fed high-fat diet or normal chow for 4 weeks before receiving a bm12 heart transplant. Mice were sacrificed at the time of rejection, and (A) the frequencies of CD4+, CD8+, B220+, and CD11b/c+ cells were assayed by cell surface staining and flow cytometry. Naïve C57BL/6 mice fed normal chow (white bars) were used as a control. n > 5 in each group. (B) The frequency of CD44hi cells was determined by flow cytometry. Data is shown gated on CD4+FoxP3− n > 4 in each group.
Acknowledgments
The authors thank Philip W. Hinds (Department of Developmental, Molecular and Chemical Biology, Tufts University School of Medicine) for critical review of the manuscript. We also thank Wayne Hancock (The Children’s Hospital of Philadelphia and University of Pennsylvania) for providing helpful insight and critical review of the manuscript. This work was supported in part by a grant from a Grant in Aid from the American Heart Association to J.I., an American Heart Association Scientist Development Grant to J.B., and an American Society of Transplantation/Genentech Basic Science Fellowship Grant to J.Y.
Abbreviations
- ApoE
apolipoproteinE
- DAPI
4′,6-diamidino-2-phenylindole
- H&E
hematoxylin and eosin
- HDL
high density lipoprotein
- IFN-γ
interferon gamma
- IL
interleukin
- LDL
low density lipoprotein
- MHC
major histocompatibility complex
- MST
median survival time
- PMA
phorbol myristate acetate
- PBS
phosphate buffered saline
- Th1
T-helper type 1
- Th17
T-helper type 17
- TGF-β
transforming growth factor beta
- TNF-α
tumor necrosis factor alpha
- Trichrome
Masson Trichrome Stain
- VLDL
very low density lipoprotein
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Strom TB, Roy-Chaudhury P, Manfro R, et al. The Th1/Th2 paradigm and the allograft response. Curr Opin Immunol. 1996;8:688–693. doi: 10.1016/s0952-7915(96)80087-2. [DOI] [PubMed] [Google Scholar]
- 2.Le Moine A, Goldman M, Abramowicz D. Multiple pathways to allograft rejection. Transplantation. 2002;73:1373–1381. doi: 10.1097/00007890-200205150-00001. [DOI] [PubMed] [Google Scholar]
- 3.Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest. 1997;100:550–557. doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation. 2012;93:1–10. doi: 10.1097/TP.0b013e31823cab44. [DOI] [PubMed] [Google Scholar]
- 5.Rocha PN, Plumb TJ, Crowley SD, Coffman TM. Effector mechanisms in transplant rejection. Immunol Rev. 2003;196:51–64. doi: 10.1046/j.1600-065x.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 6.Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Atherosclerosis as an inflammatory disease. Curr Pharm Des. 2012;18:4266–4288. doi: 10.2174/138161212802481237. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 8.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 10.Tedgui A, Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 11.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: Lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 12.Smith E, Prasad K-MR, Butcher M, et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng HP, Burris RL, Nagarajan S. Attenuated atherosclerotic lesions in apoE-Fcgamma-chain-deficient hyperlipidemic mouse model is associated with inhibition of Th17 cells and promotion of regulatory T cells. J Immunol. 2011;187:6082–6093. doi: 10.4049/jimmunol.1004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820–5827. doi: 10.4049/jimmunol.1000116. [DOI] [PubMed] [Google Scholar]
- 15.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report-2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Pflugfelder PW, Huff M, Oskalns R, Rudas L, Kostuk WJ. Cholesterol-lowering therapy after heart transplantation: A 12-month randomized trial. J Heart Lung Transplant. 1995;14:613–622. [PubMed] [Google Scholar]
- 17.Zakliczynski M, Boguslawska J, Wojniak E, et al. In the era of the universal use of statins dyslipidemia’s are still common in heart transplant recipients: A cross-sectional study. Transplant Proc. 2011;43:3071–3073. doi: 10.1016/j.transproceed.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 18.Shi C, Lee W-S, Russell ME, et al. Hypercholesterolemia exacerbates transplant arteriosclerosis via increased neointimal smooth muscle cell accumulation. Circulation. 1997;96:2722–2728. doi: 10.1161/01.cir.96.8.2722. [DOI] [PubMed] [Google Scholar]
- 19.Russell PS, Chase CM, Colvin RB. Accelerated atheromatous lesions in mouse hearts transplanted to apolipoprotein-E-deficient recipients. Am J Pathol. 1996;149:91–99. [PMC free article] [PubMed] [Google Scholar]
- 20.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitman SC. A practical approach to using mice in atherosclerosis research. Clin Biochem Rev. 2004;25:81–93. [PMC free article] [PubMed] [Google Scholar]
- 22.Plump AS, Breslow JL. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu Rev Nutr. 1995;15:495–518. doi: 10.1146/annurev.nu.15.070195.002431. [DOI] [PubMed] [Google Scholar]
- 23.Moghadasian MH, McManus BM, Nguyen LB, et al. Pathophysiology of apolipoprotein E deficiency in mice: Relevance to apo E-related disorders in humans. FASEB J. 2001;15:2623–2630. doi: 10.1096/fj.01-0463com. [DOI] [PubMed] [Google Scholar]
- 24.Davis HR, Jr, Hoos LM, Tetzloff G, et al. Deficiency of Niemann-Pick C1 Like 1 prevents atherosclerosis in ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:841–849. doi: 10.1161/01.ATV.0000257627.40486.46. [DOI] [PubMed] [Google Scholar]
- 25.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Lim H, Kim YU, Sun H, et al. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity. 2014;40:153–165. doi: 10.1016/j.immuni.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagley J, Sawada T, Wu Y, Iacomini J. A critical role for interleukin 4 in activating alloreactive CD4 T cells. Nat Immunol. 2000;1:257–261. doi: 10.1038/79811. [DOI] [PubMed] [Google Scholar]
- 28.Sayegh MH, Wu Z, Hancock WW, et al. Allograft rejection in a new allospecific CD4+ TCR transgenic mouse. Am J Transplant. 2003;3:381–389. doi: 10.1034/j.1600-6143.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 29.Yuan X, Ansari MJ, D’Addio F, et al. Targeting Tim-1 to overcome resistance to transplantation tolerance mediated by CD8 T17 cells. Proc Natl Acad Sci USA. 2009;106:10734–10739. doi: 10.1073/pnas.0812538106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanaudenaerde BM, Dupont LJ, Wuyts WA, et al. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J. 2006;27:779–787. doi: 10.1183/09031936.06.00019405. [DOI] [PubMed] [Google Scholar]
- 32.Van Kooten C, Boonstra JG, Paape ME, et al. Interleukin-17 activates human renal epithelial cells in vitro and is expressed during renal allograft rejection. J Am Soc Nephrol. 1998;9:1526–1534. doi: 10.1681/ASN.V981526. [DOI] [PubMed] [Google Scholar]
- 33.Tang JL, Subbotin VM, Antonysamy MA, Troutt AB, Rao AS, Thomson AW. Interleukin-17 antagonism inhibits acute but not chronic vascular rejection. Transplantation. 2001;72:348–350. doi: 10.1097/00007890-200107270-00035. [DOI] [PubMed] [Google Scholar]
- 34.Itoh S, Nakae S, Axtell RC, et al. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol. 2010;30:235–240. doi: 10.1007/s10875-009-9366-9. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida S, Haque A, Mizobuchi T, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 36.Antonysamy MA, Fanslow WC, Fu F, et al. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- 37.Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY. Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol. 2002;197:322–332. doi: 10.1002/path.1117. [DOI] [PubMed] [Google Scholar]
- 38.Vokaer B, Van Rompaey N, Lemaitre PH, et al. Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. J Immunol. 2010;185:3417–3425. doi: 10.4049/jimmunol.0903961. [DOI] [PubMed] [Google Scholar]
- 39.Litjens NH, van de Wetering J, van Besouw NM, Betjes MG. The human alloreactive CD4+ T-cell repertoire is biased to a Th17 response and the frequency is inversely related to the number of HLA class II mismatches. Blood. 2009;114:3947–3955. doi: 10.1182/blood-2009-03-211896. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Simeoni E, Fleury S, et al. Gene transfer of soluble interleukin-17 receptor prolongs cardiac allograft survival in a rat model. Eur J Cardiothorac Surg. 2006;29:779–783. doi: 10.1016/j.ejcts.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 41.Agorogiannis EI, Regateiro FS, Howie D, Waldmann H, Cobbold SP. Th17 cells induce a distinct graft rejection response that does not require IL-17A. Am J Transplant. 2012;12:835–845. doi: 10.1111/j.1600-6143.2011.03971.x. [DOI] [PubMed] [Google Scholar]
- 42.Tiriveedhi V, Takenaka M, Ramachandran S, et al. T regulatory cells play a significant role in modulating MHC class I antibody-induced obliterative airway disease. Am J Transplant. 2012;12:2663–2674. doi: 10.1111/j.1600-6143.2012.04191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan JA, Adams AB, Burlingham WJ. The emerging role of TH17 cells in organ transplantation. Transplantation. 2014;97:483–489. doi: 10.1097/TP.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 45.Zhang HL, Wu J, Zhu J. The immune-modulatory role of apolipoprotein E with emphasis on multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Dev Immunol. 2010;2010:186813. doi: 10.1155/2010/186813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cimato TR, Palka BA, Lang JK, Young RF. LDL cholesterol modulates human CD34+ HSPCs through effects on proliferation and the IL-17 G-CSF axis. PLoS ONE. 2013;8:e73861. doi: 10.1371/journal.pone.0073861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plump AS, Smith JD, Hayek T, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 48.Schonder KS, McKaveney TP, Lynch KJ. Retrospective analysis of hyperlipidemia management in a transplant population. Pharmacotherapy. 2005;25:918–923. doi: 10.1592/phco.2005.25.7.918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Serum cholesterol levels in mice fed a high-fat diet. Shown are levels of cholesterol in C57BL/6 mice (top panel) or ApoE−/− mice (bottom panel) prior to starting them on a high-fat diet, after being fed a high fed for 4-weeks on the day of transplant (HFD), or at the time the mice were sacrificed and hearts harvested (Harvest). n = 5–6 mice per group. Asterisk indicates p < 0.05. A full discussion of the plasma cholesterol levels in ApoE−/− and C57BL/6 mice fed normal chow or high fat diet can be found in (47). For reference, transplant patients are considered hyperlipidemic when they have plasma cholesterol levels greater than 200 mg/dL. A retrospective study of lipid control in transplant patients in a single center identified a range of from 91 mg/dl to 1665 mg/dL among patients tested (48). It is important to note that wild-type mice have a different plasma cholesterol composition, with high high density lipoprotein (HDL), and low low density lipoprotein (LDL) levels compared with humans. While the goal for human transplant patients is less than 100 mg/dL LDL, C57BL/6 mice fed normal diet have an LDL of 7 mg/dL. In ApoE−/− mice, in contrast to wild-type mice, low density LDL and VLDL particles are enriched, similar to what is observed in hyperlipidemic humans.
Figure S2: Trichrome staining of tissue sections from a transplanted bm12 heart harvested after 100 days from C57B/6 control mice fed normal chow (A); a syngeneic heart harvested after 100 days from an ApoE−/− mouse fed a high-fat diet (B); a bm12 heart harvested at the time of rejection from a C57BL/6 mouse fed a high-fat diet (C, day 60); a bm12 heart harvested at the time of rejection from an ApoE−/− mouse fed a high-fat diet (D, day 21); a bm12 heart harvested at the time of rejection from an ApoE−/− mouse fed a normal diet (E, day 60). Representative tissue sections are shown. 20× magnification is shown.
Figure S3: Serum cytokine concentrations. ApoE−/− and C57BL/6 mice were fed high-fat diet or normal chow for 4 weeks before receiving a bm12 heart transplant. Mice were sacrificed at the time of rejection, and pooled serum for each group was assayed for expression of IL-1α, IFN-γ, TNF-α, IL-12p70, IL-2 IL-4, IL-6, and IL-17 by multiplex ELISA. (A) Serum cytokine level of IL-2, IL-6, and IL-17 expressed in pg/ml in C57BL/6 mice fed normal chow (white bars), C57BL/6 mice fed a high-fat diet (light gray bars), ApoE−/− mice fed normal chow (dark gray bars), and ApoE−/− mice fed a high-fat diet (black bars). n = 6 mice per group. (B) Serum cytokine level of IL-1α, IFN-γ, TNF-α, or IL-12p70, and IL-4 expressed in pg/ml in in C57BL/6 mice fed normal chow (white bars), C57BL/6 mice fed a high-fat diet (light gray bars), ApoE−/− mice fed normal chow (dark gray bars), and ApoE−/− mice fed a high-fat diet (black bars). n = 4–6 mice per group. Asterisk represents p < 0.05.
Figure S4: Lymphocyte populations not affected by hyperlipidemia. ApoE−/− and C57BL/6 mice were fed high-fat diet or normal chow for 4 weeks before receiving a bm12 heart transplant. Mice were sacrificed at the time of rejection, and (A) the frequencies of CD4+, CD8+, B220+, and CD11b/c+ cells were assayed by cell surface staining and flow cytometry. Naïve C57BL/6 mice fed normal chow (white bars) were used as a control. n > 5 in each group. (B) The frequency of CD44hi cells was determined by flow cytometry. Data is shown gated on CD4+FoxP3− n > 4 in each group.


