Abstract
BACKGROUND
Differentiating between partial adhesive small bowel obstruction (aSBO) likely to resolve with medical management and complete obstruction requiring operative intervention remains elusive. We implemented a standardized protocol for the management of aSBO and reviewed our experience retrospectively.
METHODS
Patients with symptoms of aSBO were admitted for intravenous fluid resuscitation, bowel rest, nasogastric tube decompression, and abdominal examinations every 4 hours. Laboratory values and a computed tomography scan of the abdomen and pelvis with intravenous contrast were obtained. Patients with peritonitis or computed tomography scan findings suggesting bowel compromise were taken to the operating room for exploration following resuscitation. All other patients received 80 mL of Gastroview (GV) and 40 mL of sterile water via nasogastric tube. Abdominal plain films were obtained at 4, 8, 12, and 24 hours. If contrast did not reach the colon within 24 hours, then operative intervention was performed.
RESULTS
Over 1 year, 91 patients were admitted with aSBO. Sixty-three patients received GV, of whom 51% underwent surgery. Twenty-four patients went directly to the operating room because of clinical or imaging findings suggesting bowel ischemia. Average time to surgery was within 1 day for the no-GV group and 2 days for the GV group. Patients passing GV to the colon within 5 hours of administration had a 90% rate of resolution of obstruction. There was a direct relationship between the duration of time before passing GV to the colon and hospital length of stay (HLOS) (r2 = 0.459). Patients who received GV and did not require surgery had lower HLOS (3 days vs. 11 days, p < 0.0001).
CONCLUSION
The GV protocol facilitated early recognition of complete obstruction. Administration of GV had diagnostic and therapeutic value and did not increase HLOS, morbidity, or mortality.
LEVEL OF EVIDENCE
Therapeutic study, level V. Epidemiologic study, level V.
Keywords: Adhesive small bowel obstruction, mechanical small bowel obstruction, Gastroview, Gastrografin, adhesiolysis
Adhesive disease is the most frequently encountered disorder of the small intestine. In one review of 87 studies including 110,076 patients, the incidence of adhesive small bowel obstruction (aSBO) following all types of abdominal operations was 2.4%.1 In North America, there are more than 300,000 annual hospital admissions for aSBO accounting for 850,000 days of inpatient care, costing more than $1.3 billion in medical expenditures and contributing to more than 2,000 deaths annually.2
A Nationwide Inpatient Sample study performed in 2009 enrolled 27,046 patients with aSBO and demonstrated that delay in surgery was associated with increased hospital length of stay (HLOS) and mortality.3 Surgery was performed on 18% of all patients enrolled.3 For this subgroup, 32% stayed in the hospital more than 1 week, 25% required bowel resection, 19% experienced a complication, and 3% died.3 A delay in four or more days was associated with a 64% increase in mortality.3 Finding nonviable bowel at the time of exploration was associated with a fourfold increase in mortality.3 The imperative nature of early surgical intervention for aSBO was substantiated by a review of 9,297 patients from the National Surgical Quality Improvement Program from 2005 to 2011.4 Keenan et al.4 observed that patients who underwent surgery after a preoperative HLOS of 3 days had increased overall 30-day morbidity and that patients who received their operation after 4 days had increased total HLOS.4 Consistent with these findings, a 2013 update of World Society of Emergency Surgery guidelines for the management of aSBO recommends that nonoperative management should not exceed 3 days.5
However, operative intervention is associated with significant risks including enterotomy, bowel resection with anastamotic complications, short bowel syndrome, prolonged ileus, hernia formation, and recurrent symptoms.1,3,4 Because of the substantial operative risks associated with adhesiolysis, operative exploration is traditionally reserved for those afflicted with complete obstruction and those with evidence of bowel ischemia. Despite medical advances, differentiating between partial and complete obstruction remains difficult. The necessity for a standardized protocol-driven approach to managing aSBO with attention to early recognition of strangulation has been well articulated and part of the rationale for the development of such a protocol at our institution.6
Before adoption of water-soluble contrast administration strategies, some authors made the decision to operate based on a history of obstipation, the presence of mesenteric edema, and a lack of small bowel fecalization on computed tomography (CT) scan.7–10 The presence of all three signs was found to have a positive predictive value of 90% for the necessity of surgical exploration.7–9 One author found that clinical judgment had sensitivity of 48% in detecting strangulation in the preoperative setting,10 whereas multiple reviews have concluded that CTis 84% to 100% sensitive for detecting ischemia and strangulation.11–14
MD-Gastroview (GV; Mallinckrodt, Inc., St. Louis, MO) is a water-soluble contrast agent that creates an osmotic gradient in the gastrointestinal lumen, which may transmit pressure across an obstruction.15 The use of contrast agents to encourage resolution of partial aSBO has been effective in some studies but remains controversial.15,16 Odds of resolution are improved if the contrast progresses to the colon within 24 hours.7 Goussous et al.7 compared the use of GV to historic controls and demonstrated an improvement in the resolution of partial obstruction while decreasing HLOS. With the implementation of such a protocol, our objectives were to identify complete obstruction early, resolve partial obstruction, operate within 3 days of admission when necessary, and decrease HLOS. We hypothesized that administration of GV would allow for early identification of complete bowel obstruction.
PATIENTS AND METHODS
Institutional review board approval was obtained for this case series. We reviewed patients admitted to our acute care surgery service from January 2013 to December 2013 with symptoms of obstruction and suspicion for aSBO. Patients without a history of abdominal surgery were excluded. We implemented a standard-of-care protocol based on previously published literature.3–5,7 Upon presentation to the emergency department, initial management included volume resuscitation with intravenous (IV) isotonic fluid administration and nasogastric tube (NGT) decompression. Laboratory data were obtained including complete blood cell count, basic metabolic panel, and lactate levels. Foley catheters were placed to monitor the resuscitation in patients not previously anuric due to end-stage renal disease. Patients presenting with symptoms of peritonitis or symptoms of ischemic bowel such as localized abdominal tenderness associated with fever, tachycardia, and leukocytosis underwent operative management as soon as they were adequately resuscitated. CT scan with IV contrast was obtained. If there were CT findings of mesenteric edema, pneumatosis, perforation, closed-loop obstruction, or swirl sign with free fluid, then the patient underwent operative exploration. In the absence of these signs, the patient was monitored with serial abdominal examinations. Complete blood cell count, basic metabolic panel, and lactate levels were assessed every 6 hours to closely follow the efficacy of the resuscitation as well as identify early progression of ischemia. Following gastric decompression overnight or for a minimum of 6 hours, the patient was evaluated for aspiration risk factors including paraesophageal hernia, hiatal hernia, chronic obstructive pulmonary disease, or other cause of pulmonary insufficiency requiring home oxygen therapy, age greater than 65 years, or advanced frailty as determined by the clinical judgment of the admitting team. Patients who presented bed bound, with limited independent functional status, or required assistance for primary activities of daily living were examples of those deemed frail. Patients who were not at increased risk for aspiration received 80-mL GV followed by 40-mL sterile water via NGT. The NGT was clamped following the administration of GV and remained clamped unless the patient developed nausea or increasing abdominal pain, at which time the NGTwas returned to suction. Patients with increased aspiration risk underwent GV administration under fluoroscopy. Abdominal plain films were then taken 4, 8, 12, and 24 hours following contrast administration. The intervals for plain films were selected based on a physiologic small bowel transit time of 1 hour to 2 hours for emptying of 50% of small bowel contents17 and were similar to those used for a radiology-driven protocol for a small bowel follow-through series, which was previously established at our institution. We collaborated with our radiology colleagues to ensure that a majority of the studies would be performed on the ward to prevent significant delays in contrast administration. The study was concluded when contrast reached the colon and the patient had a bowel movement. Increased abdominal pain, development of peritonitis, progressive nausea, worsening fever and leukocytosis, or failure to pass contrast to the colon after 24 hours were considered indications for surgery. If the patient’s symptoms resolved before GV administration, the NGT was removed and a feeding challenge was performed.
This data were evaluated as a case series with Level V evidence. Categorical variables were reported as frequencies and percentages. Two-way analysis of variance and Bonferroni’s multiple comparisons test were preformed when appropriate with a 0.05 α level to comparevariables for significance among cohorts. Statistical analyses were performed using GraphPad Prism 6.00. Quantitative variables between two discrete groups were compared with Student’s unpaired t tests. Data are presented as averages or medians with interquartile range (IQR) or percent as indicated.
RESULTS
Ninety-one patients presented with symptoms of aSBO for 102 total admissions. Sixty-two percent of all patients were female, and the average patient age was 60 years. Eighteen percent of all patients were found to have a ventral hernia, and 63% of these hernias were involved in the obstructive process. Patients with a ventral hernia that was involved in the obstructive process were noted to have a significantly higher bodymass index than that of all other patients (35.7 vs. 27.4, p < 0.05). One patient was excluded from the data analysis. This patient was admitted for lung transplantation, underwent multiple operations over a 5-month hospital stay, and eventually developed multiple organ system failure. The family elected to withdraw care.
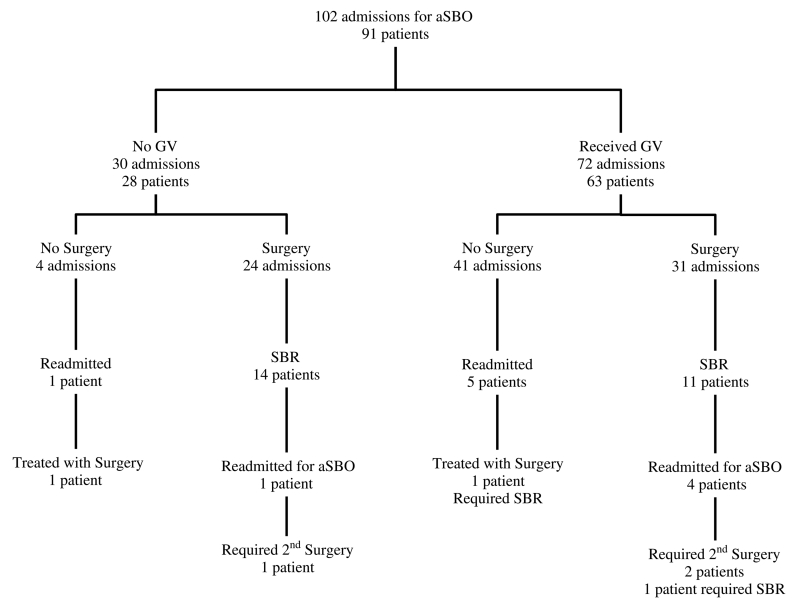
We observed a 99% rate of compliance to the protocol. Twenty-eight patients did not receive GV (Fig. 1). Four of these patients had resolution of obstruction following bowel rest, nasogastric decompression, and IV fluid hydration before the administration of GV. One of these four patients required readmission and adhesiolysis without bowel resection. The other 24 patients who did not receive GV underwent surgery, and 14 of these patients (58%) required small bowel resection (SBR). One of these patients had recurrence of aSBO within 1 month, was readmitted, and underwent adhesiolysis.
Figure 1.
Patient algorithm.
Of the 72 patient admissions directed into GV challenge group, 41 (57%) resulted in GV passage to the colon with resolution of obstructive symptoms without surgical intervention (Fig. 1). Five patients (7%) who were discharged after initially passing the GV challenge required readmission. Four of these patients were treated again with GV challenge with resolution of obstruction, and the remaining one patient underwent adhesiolysis and SBR. Thirty-one patient admissions (43%) resulted in failure to pass GV to the colon and were therefore considered to have failed the challenge. In each of the 31 cases of failure to pass the GV challenge, adhesiolysis was performed. Eleven (33%) of these operations included SBR. Four patients in this group were readmitted with recurrent aSBO, and two of these patients required a second surgery.
No statistically significant difference was identified for the age, sex, history of ventral hernia, history of small bowel obstruction, or body mass index for the GV group as compared with the no-GV group (Table 1). Patients who did not receive GV were more likely to have CT scan findings of bowel wall thickening (20% vs. 5.6%, p < 0.03). All other CT findings were not significantly different between the groups. Admission white blood cell, creatinine, lactate, base deficit, or bicarbonate levels were also similar between the groups. Patients who underwent SBR presented with higher heart rates (91 [IQR, 80–101] vs. 83.5 [IQR 70–28], p < 0.0450) and lower blood pressures (128.5 [IQR, 112–140] vs. 136.5 [IQR, 121–152], p < 0.0007) than those of patients who did not undergo SBR.
TABLE 1.
Patient Characteristics
| GV, n = 72 |
No GV, n = 30 |
||
|---|---|---|---|
| Mean (range) or n (%) |
Mean (range) or n (%) |
p (α = 0.05) | |
| Age | 60.2 (21–96) | 58.2 (22–91) | 0.62 |
| Male | 28 (38.9) | 11 (36.7) | 0.53 |
| History of ventral hernia | 15 (20.8) | 3 (10.0) | 0.15 |
| History of small bowel obstruction |
26 (36.1) | 13 (43.3) | 0.54 |
| Body mass index, kg/m2 | 28.4 (12.5–53.2) | 28.9 (16.3–68.0) | 0.88 |
| Heart rate | 89 (52–142) | 88 (57–140) | 0.80 |
| Systolic blood pressure | 133 (89–178) | 134 (90–193) | 0.86 |
| White blood cell count | 10.3 (0.8–27.0) | 10.3 (3.9–24.3) | |
| Serum creatinine | 1.3 (0.1–12.9) | 1.5 (0.5–11.5) | 0.69 |
| Lactic acid | 1.5 (0.4–5.5) | 1.4 (0.7–3.0) | 0.61 |
| Base deficit | 3.7 (−9–18) | 1.6 (−6–9) | 0.19 |
| Serum bicarbonate | 24.7 (12.1–38.3) | 24.6 (18.9–31.0) | 0.84 |
| CT, fecalization of small bowel |
10 (13.9) | 6 (20.0) | 0.40 |
| CT, free abdominal fluid | 9 (12.5) | 7 (23.3) | 0.18 |
| CT, bowel wall thickening | 4 (5.6) | 6 (20.0) | <0.03 |
| CT, closed-loop obstruction |
6 (8.3) | 5 (16.7) | 0.51 |
| CT, mesenteric swirl | 0 (0.0) | 1 (3.3) | 0.33 |
As demonstrated in Table 2, patients who received GV and did not require surgery had lower HLOS (3 days [IQR, 2–5] vs. 11 days [IQR, 9–16], p < 0.0001), faster passage of contrast into the colon (5 hours [IQR, 0.5–18] vs. 8 hours [IQR, 5.8–12], p < 0.0002), and lower mortality (0%, p < 0.0140). Patients undergoing SBR had an increased HLOS than patients who had surgery without SBR (9.3 days vs. 6.1 days, p < 0.0001). Patients in the GV group had significantly lower rates of operation (47% vs. 86%, p < 0.01) and SBR (31% vs. 56%, p < 0.04) as compared with the no-GV group.
TABLE 2.
Outcomes
| Outcomes |
GV + No Surgery (n = 41) Medians (IQR) or n (%) |
GV + Surgery (n = 31) | No GV + Surgery (n = 26) | No GV + No Surgery (n = 4) |
|---|---|---|---|---|
| HLOS | 3 (2–5) (*p < 0.0001) | 11 (9–16) | 9.5 (6.75–13.75) (**p = 0.9999) | 4.25 (3–5.25) (†p = 0.9999) |
| Time to colon, h | 5 (0.5–18) (*p < 0.0002) | 8 (5.8–12) | N/A | N/A |
| Time to surgery, d | N/A | 2(1–2) | 0.5(0–1.75) (**p = 0.9996) | N/A |
| Mortality | 0 (0%) (*p = 0.0140) | 1 (2.9%) (‡p = 0.9143) | 2 (5.8%) (**p = 0.1276) | 0 (0%) (°p = 0.0792) |
p annotates the comparison between the GV + No Surgery and GV + Surgery groups.
p annotates the comparison between the GV + Surgery group and the No GV + Surgery group.
p annotates the comparison between No GV + No Surgery group and GV + No Surgery group.
p annotates the comparison between the GV + Surgery group and all other groups.
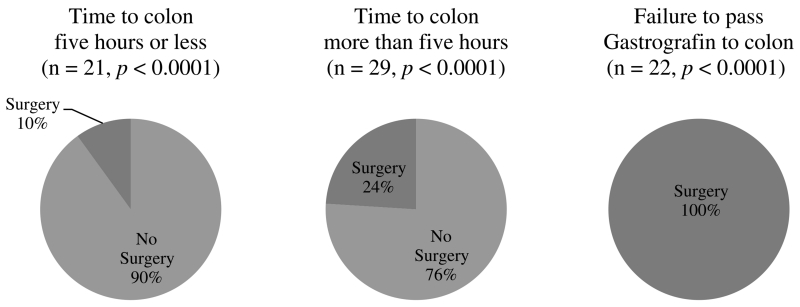
The relationship between GV progression to the colon and the need for surgical intervention was significant (Fig. 2). The presence of GV in the colon less than 5 hours following administration resulted in a 90% rate of resolution of obstruction (n = 19 of 21, p < 0.0001). Seven of the patients who had progression of GV to the colon after 5 hours (24%) failed clinically with feeding intolerance, fever, leukocytosis, and/or physical signs of peritonitis and therefore required surgical intervention (n = 7 of 29, p < 0.0001).
Figure 2.
Association between GV progression and operative intervention.
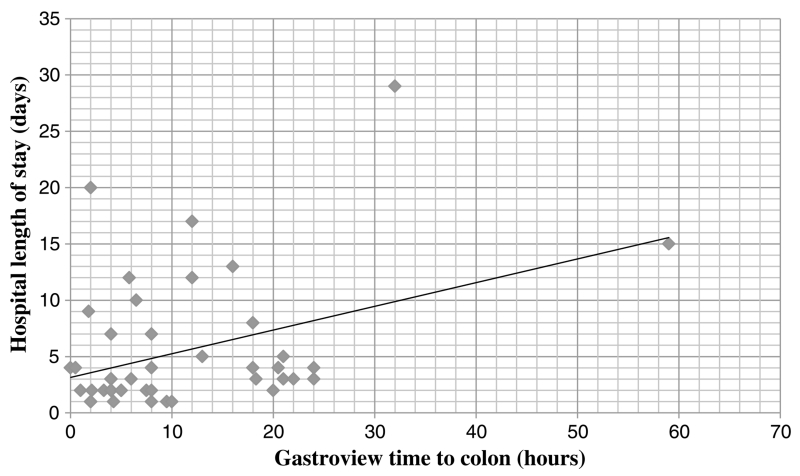
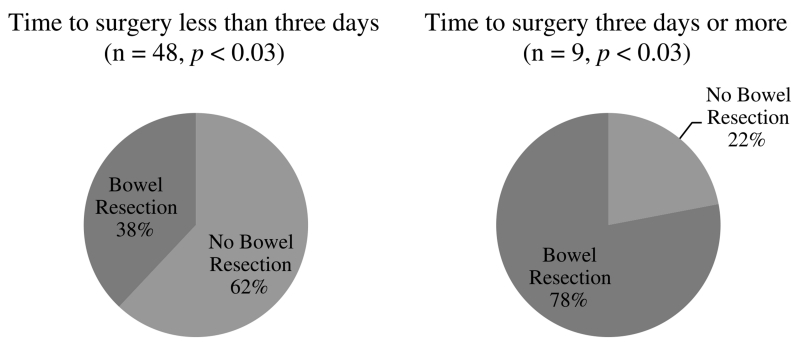
There was a direct relationship between the duration of time before passing GV to the colon and overall HLOS (r2 = 0.459) (Fig. 3). Seven patients underwent an operation 3 days or more after admission (Fig. 4). These patients had a statistically significant increase in bowel resection rate (78%) compared with patients undergoing surgery within 3 days of admission (38%) (p < 0.03). One of these patients was allowed to progress without an operation because of operative risks associated with ventilator dependence and sepsis secondary to pneumonia, and one patient initially failed to pass GV from the stomach to the duodenum, and EGD identified a gastric ulcer. This patient passed GV to the colon after this procedure was performed.
Figure 3.
The duration of time before passing GV to the colon versus overall HLOS.
Figure 4.
Time to surgery versus bowel resection rates.
Patients who were readmitted with recurrent aSBO had been discharged after an initial average HLOS of 4.3 days. The average time to readmission was 47.8 days. Forty-five percent of these patients required adhesiolysis, and 60% of these operations included bowel resection. There were three mortalities in our series. One death was caused by non-ST elevation myocardial infarction in a patient who underwent surgery without bowel resection after presenting with peritonitis. The second death occurred following withdrawal of care for a patient with postoperative dependence on mechanical ventilation via tracheostomy in the context of multiple myeloma and preoperative life expectancy of less than 1 year. This patient underwent surgery with resection of bowel involved in a closed-loop obstruction on the day of admission. A third death occurred in a patient who initially passed GV into the colon and then developed recurrent symptoms of obstruction and underwent surgery with bowel resection on hospital Day 4. This patient also had a preoperative life expectancy of less than 1 year because of metastatic bladder cancer and developed multiple organ system failure, and the family elected to withdraw care. None of the deaths were attributable to the operative procedure itself. There were no aspirations or other adverse events related to the administration of GV.
DISCUSSION
Clinical outcomes for patients presenting with aSBO are largely dependent on early operative intervention for patients who fail to resolve with medical management.3–5 Quickly and accurately identifying this group of patients is a difficult task. Signs and symptoms of compromised perfusion of the small bowel including continuous abdominal pain, fever, leukocytosis, tachycardia, signs of peritoneal irritation, hyperamylasemia, and metabolic acidosis are not reliable for diagnosing intestinal ischemia or complete bowel obstruction.10 Our goal was to initiate a standardized protocol designed for early differentiation between partial and complete bowel obstruction. Our data suggest that GV may be safely administered to patients presenting with aSBO and facilitates early identification of patients with a high likelihood of failing medical management.
Of all hemodynamic, laboratory, and imaging metrics measured, the only factor that was associated with the need for urgent operation was bowel wall thickening on CT scan. CT scan findings of closed-loop obstruction and mesenteric swirl were infrequently identified and inconsistently reported. Our protocol was successful in facilitating operative intervention within 72 hours of admission, consistent with contemporary recommendations for early surgical management.5–7 The difference in time to operation from 2.0 days for patients receiving GV to 0.5 days for patients not receiving GV is likely caused by the fact that all patients with peritonitis on examination or CT scan findings consistent with bowel compromise underwent an operation immediately following volume resuscitation and did not receive GV.
Administration of GV provided substantial prognostic information. Only 1 of 10 patients passing GV to the colon within 5 hours of administration required surgical management during that admission. In comparison, 24% of patients passing GV to the colon after a period of 5 hours required an operation. The decision to operate on patients who had passed GV to the colon was based on worsening abdominal pain, recurrent symptoms with feeding challenge, or fever and leukocytosis, as established in published literature.5 The duration of time before passing GV to the colon was directly proportional to overall HLOS, as would be expected for the natural history of this disease process. In our experience, patients underwent urgent operation when clinical or radiographic signs of peritonitis or bowel ischemia were evident and were not typically delayed by a lack of attending availability, operating room availability, or time of the day. Previously described risks associated with water-soluble contrast administration include aspiration and anaphylactoid reactions, which are likely caused by flavoring agents and preservatives in the contrast solution.18–20 However, there were no aspiration or anaphylactoid events in our series. Upon review of the patients who were readmitted with recurrent aSBO, it does not seem that these patients had been discharged prematurely as the average time to readmission was more than 1 month.
Previous studies have had similar results. A systematic review of 14 prospective trials found that the presence of contrast in the colon predicted resolution of the obstruction with 96% sensitivity, 98% specificity, 99% positive predictive value, and 90% negative predictive value. These values were unaffected by the duration of time before the appearance of contrast in the colon, and the authors concluded that there is no diagnostic advantage in waiting longer than 8 hours for contrast to reach the colon.21 Nonoperative management has been endorsed for patients with no persistent vomiting, peritonitis, or CT scan findings of free fluid, mesenteric edema, devascularized bowel, or lack of feces sign with the caveat that nonoperative management should be discontinued if the patient becomes febrile with leukocytosis greater than 15,000/μL.5
A review of our data compared with previous trials demonstrates higher operative rates, bowel resection rates, and length of stay compared with other institutions.22–28 One possible explanation is that we excluded only one patient admission of 103 admissions in 2013 and therefore included patients with bowel obstruction in conjunction with other acute medical and surgical conditions, which were often excluded from previous studies. The rationale for this approach was to improve the generalizability of our findings. We also observed that a substantial proportion of our patient population had ventral hernias and that most of these hernias were involved in the obstructive process in conjunction with adhesive disease. Data regarding these phenomena are not consistently available in the studies used for comparison.
Our HLOS may be affected by the fact that open surgery is the standard approach to operative management of aSBO at our institution as opposed to laparoscopy. A laparoscopic approach to adhesiolysis may be appropriate for patients with mild abdominal distension, proximal obstruction, partial obstruction, and obstructions that seem to be caused by a single adhesive band.29 Potential advantages of a laparoscopic approach include an earlier return of bowel function as well as a decrease in wound complications, postoperative pain, length of stay, time to return to full activity, and postoperative adhesion formation.30,31 A National Inpatient Sample of 6,165 patients concluded that there was a significant decrease in postoperative complications, HLOS, and overall costs for cases of aSBO managed with laparoscopic adhesiolysis.32 In this study, 11.4% of patients underwent laparoscopic lysis of adhesions, with a 17.2% rate of conversion to open surgery.32 Although prospective randomized control trials are needed to assess the role of laparoscopic management of adhesive bowel obstruction, it seems that this approach is safe and effective for a select patient population.
Application of a standardized protocol for the management of aSBO at our institution facilitated early recognition of complete obstruction. Administration of GV had both diagnostic and therapeutic value and did not increase HLOS, morbidity, or mortality. Consistent with established recommendations,21,29–32 we plan to modify this protocol to encourage earlier intervention and laparoscopic adhesiolysis when appropriate. Patients who fail to pass GV to the colon after 8 hours will be examined and reassessed by the attending surgeon and/or chief resident. These modifications could create an effective platform for a prospective trial to establish the efficacy of this protocol as compared with traditional management.
Footnotes
AUTHORSHIP
T.L. contributed to the data collection, statistical analysis, data interpretation, manuscript writing, and manuscript revision. F.M. contributed to the study design, data interpretation, manuscript revision, and final approval. E.V. contributed to the statistical analysis. T.B. contributed to the data collection. S.B., C.C., L.L., W.R., D.M., L.A, and A.M. contributed to the study design, manuscript revision, and final approval. J.J. contributed to the study design, data collection, statistical analysis, data interpretation, manuscript writing, manuscript revision, and final approval.
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Ten Broek R, Issa Y, van Santbrink E, Bouvy N, Kruitwagen R, Jeekel J, Bakkum E, Rovers M, van Goor H. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. Br Med J. 2013;347:f5588. doi: 10.1136/bmj.f5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ray N, Denton W, Thamer M, Henderson S, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:1–9. doi: 10.1016/s1072-7515(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 3.Schrufnagel D, Rajaee S, Millham F. How many sunsets? Timing of surgery in adhesive small bowel obstruction: a study of the Nationwide Inpatient Sample. J Acute Care Surg. 2013;74(1):181–187. doi: 10.1097/TA.0b013e31827891a1. [DOI] [PubMed] [Google Scholar]
- 4.Keenan J, Turley R, McCoy C, Migaly J, Shapiro M, Scarborough J. Trials of nonoperative management exceeding 3 days are associated with increased morbidity in patients undergoing surgery for uncomplicated adhesive small bowel obstruction. J Trauma Acute Care Surg. 2014;76(6):1367–1372. doi: 10.1097/TA.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 5.Di Saverio S, Coccolini F, Galati M, Smerieri N, Biffl L, Ansaloni L, Tugnoli G, Velmahos G, Sartelli M, Catena F, et al. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2013 update of the evidence-based guidelines from the world society of emergency surgery ASBO working group. World J Emerg Surg. 201310;8(1):42. doi: 10.1186/1749-7922-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielinski M, Brannon M. Current management of small bowel obstruction. Adv Surg. 2011;45:1–29. doi: 10.1016/j.yasu.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Goussous N, Eiken P, Bannon M, Zielinski M. Enhancement of a small bowel obstruction model using the Gastrografin challenge test. J Gastrointest Surg. 2013;17:110–117. doi: 10.1007/s11605-012-2011-6. [DOI] [PubMed] [Google Scholar]
- 8.Zielinski M, Eiken P, Heller S, Lohse C, Huebner M, Sarr M, Bannon M. Prospective observational validation of a multivariate small bowel obstruction model to predict the need for operative intervention. J Am Coll Surg. 2011;212:1068–1076. doi: 10.1016/j.jamcollsurg.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Zielinski M, Eiken P, Brannon M, Heller S, Lohse C, Huebner M, Sarr M. Small bowel obstruction-who needs an operation? A multivariate production model. World J Surg. 2010;34:910–919. doi: 10.1007/s00268-010-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarr M, Bulkley G, Zuidema G. Preoperative recognition of intestinal strangulation. Am J Surg. 1983;145:176–182. doi: 10.1016/0002-9610(83)90186-1. [DOI] [PubMed] [Google Scholar]
- 11.Daneshmand S, Hedley C, Stain S. The utility and reliability of computed tomography scan in the diagnosis of small bowel obstruction. Am Surg. 1999;65:922–926. [PubMed] [Google Scholar]
- 12.Makita O, Ikushima I, Matsumoto N, Arikawa K, Yamashita Y, Takahashi M. CT differentiation between necrotic and nonnecrotic small bowel in closed loop and strangulating obstruction. Abdom Imaging. 1999;24:120–124. doi: 10.1007/s002619900458. [DOI] [PubMed] [Google Scholar]
- 13.Obuz F, Terzi C, Sokmen S, Yilmaz E, Yildiz D, Fuzun M. The efficacy of helical CT in the diagnosis of small bowel obstruction. Eur J Radiol. 2003;48:299–304. doi: 10.1016/s0720-048x(02)00382-0. [DOI] [PubMed] [Google Scholar]
- 14.Zalcman M, Sy M, Donckier V, Closset J, Gansbeke D. Helical CT signs in the diagnosis of intestinal ischemia in small-bowel obstruction. AJR Am J Roentgenol. 2000;175:1601–1607. doi: 10.2214/ajr.175.6.1751601. [DOI] [PubMed] [Google Scholar]
- 15.Assalia A, Schein M, Kopelman D, Hirshberg A, Hashmonai M. Therapeutic effect of oral Gastrografin in adhesive, partial small-bowel obstruction: a prospective randomized trial. Surgery. 1994 Apr;115(4):433–437. [PubMed] [Google Scholar]
- 16.Choi H, Chu K, Law W. Therapeutic value of Gastrografin in adhesive small bowel obstruction after unsuccessful conservative treatment: a prospective randomized trial. Ann Surg. 2002;236(1):1–6. doi: 10.1097/00000658-200207000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S. Small intestine transit time in the normal small bowel study. AJR Am J Roentgenol. 1968;104(3):522–524. doi: 10.2214/ajr.104.3.522. [DOI] [PubMed] [Google Scholar]
- 18.Skucas J. Anaphylactoid reactions with gastrointestinal contrast media. AJR Am J Roentgenol. 1997;168:962–964. doi: 10.2214/ajr.168.4.9124150. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Chang K, Lee P, Wang S, Chen K, Lin F. Oral Urografin in postoperative small bowel obstruction. World J Surg. 1999;23:1051–1054. doi: 10.1007/s002689900622. [DOI] [PubMed] [Google Scholar]
- 20.Trulzsch D, Penmetsa A, Karim A, Evans D. Gastrografin-induced aspiration pneumonia: a lethal complication of computed tomography. South Med J. 1992;85:1255–1256. doi: 10.1097/00007611-199212000-00025. [DOI] [PubMed] [Google Scholar]
- 21.Branco B, Barmparas G, Schnüriger B, Inaba K, Chan LS, Demetriades D. Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstruction. Br J Surg. 2010;97(4):470–478. doi: 10.1002/bjs.7019. [DOI] [PubMed] [Google Scholar]
- 22.Haule C, Ongom P, Kimuli T. Efficacy of Gastrografin® compared with standard conservative treatment in management of adhesive small bowel obstruction at Mulago National Referral Hospital. J Clin Trials. 2013;3(4) doi: 10.4172/2167-0870.1000144. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahmani N, Mohammadpour R, Khoshnood P, Ahmadi A, Assadpour S. Prospective evaluation of oral Gastrografin® in the management of postoperative adhesive small bowel obstruction. Indian J Surg. 2013;75(3):195–199. doi: 10.1007/s12262-012-0479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biondo S, Parés D, Mora L, Martí Ragué J, Kreisler E, Jaurrieta E. Randomized clinical study of Gastrografin administration in patients with adhesive small bowel obstruction. Br J Surg. 2003;90(5):542–546. doi: 10.1002/bjs.4150. [DOI] [PubMed] [Google Scholar]
- 25.Farid M, Fikry A, Nakeeb A, Fouda E, Elmetwally T, Yousef M, Omar W. Clinical Impacts of Oral Gastrografin Follow-Through in Adhesive Small Bowel Obstruction (SBO) J Surg Res. 2010:170–176. doi: 10.1016/j.jss.2009.03.092. [DOI] [PubMed] [Google Scholar]
- 26.Di Saverio S, Catena F, Ansaloni L, Gavioli M, Valentino M, Pinna A. Water-soluble contrast medium (Gastrografin) value in adhesive small intestine obstruction (ASIO): a prospective, randomized, controlled, clinical trial. World J Surg. 2008;32(10):2293–2304. doi: 10.1007/s00268-008-9694-6. [DOI] [PubMed] [Google Scholar]
- 27.Burge J, Abbas S, Roadley G, Donald J, Connolly A, Bissett I, Hill A. Randomized controlled trial of Gastrografin in adhesive small bowel obstruction. ANZ J Surg. 2005;75(8):672–674. doi: 10.1111/j.1445-2197.2005.03491.x. [DOI] [PubMed] [Google Scholar]
- 28.Assalia A, Kopelman D, Bahous H, Klein Y, Hashmonai M. Gastrografin for mechanical partial, small bowel obstruction due to adhesions. Harefuah. 1997;132(9):629–633. [PubMed] [Google Scholar]
- 29.Diaz J, Bokhari F, Mowery N, Acosta J, Block E, Bromberg W, Collier B, Cullinane D, Dwyer K, Griffen M, et al. Guidelines for management of small bowel obstruction. J Trauma. 2008;64:1651–1664. doi: 10.1097/TA.0b013e31816f709e. [DOI] [PubMed] [Google Scholar]
- 30.Nagle A, Ujiki M, Denham W, Murayama K. Laparoscopic adhesiolysis for small bowel obstruction. Am J Surg. 2004;187(4):464–470. doi: 10.1016/j.amjsurg.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 31.Szomstein S, Lo Menzo E, Simpfendorfer C, Zundel N, Rosenthal R. Laparoscopic lysis of adhesions. World J Surg. 2006;30:535–540. doi: 10.1007/s00268-005-7778-0. [DOI] [PubMed] [Google Scholar]
- 32.Macini G, Petroski G, Lin W, Sporn E, Miedema B, Thaler K. Nationwide impact of laparoscopic lysis of adhesions in the management of intestinal obstruction in the US. J Am Coll Surg. 2008;207(4):520–526. doi: 10.1016/j.jamcollsurg.2008.04.026. [DOI] [PubMed] [Google Scholar]