Abstract
The contribution of chorioamnionitis (CA) to mortality and morbidity in preterm infants is difficult to assess because observational studies frequently present significant differences in baseline characteristics of the infants exposed or non-exposed to CA. In an attempt to perform a thorough assessment of the possible association between CA and patent ductus arteriosus (PDA) in preterm infants, we conducted a meta-analysis in which adjusted odds ratios (ORs) were pooled and we analyzed the effects of potential confounders, such as gestational age (GA) or birth weight (BW). We identified 45 relevant studies (27186 patients, 7742 CA cases). Random effects meta-analysis of crude ORs showed a significant positive association between CA and PDA (OR 1.352, 95% CI 1.172 to 1.560). Adjusted ORs were reported in 11 studies (19577 infants). Meta-analysis of these studies showed a significant negative association between CA and PDA (OR 0.802, 95% CI 0.751 to 0.959). Meta-regression showed that the differences in GA or BW between the CA-exposed and non-exposed groups were significantly correlated with the effect size of the association between PDA and CA. In conclusion, our study confirms that confounders need to be taken into account when assessing the association between CA and clinical outcomes in preterm infants.
The term chorioamnionitis (CA) refers to an intrauterine status of infection/inflammation in tissues of either mixed fetal-maternal (choriodecidual space) or fetal origin (chorioamniotic membranes, amniotic fluid, umbilical cord)1,2,3,4. CA is considered to be one of the main causes of preterm labor and its incidence increases with decreasing gestational age (GA). Moreover, CA may induce a fetal inflammatory response which is thought to play an important role in short- and long-term morbidity after very preterm birth1,2,3,4,5,6,7,8,9,10,11,12,13. In recent years, numerous observational studies have been summarized in several meta-analyses attempting to clarify the association between CA and neonatal brain injury11, cerebral palsy6, bronchopulmonary dysplasia (BPD)7,10, necrotizing enterocolitis (NEC)12, and retinopathy of prematurity (ROP)13, among other adverse outcomes of prematurity. Nevertheless, since CA is a major risk factor for spontaneous preterm birth, the GA-independent contribution of CA to mortality and morbidity of preterm infants is very difficult to assess3.
Patent ductus arteriosus (PDA) is a common clinical problem among very preterm infants14,15. Very recently, Park et al. conducted a meta-analysis aiming to investigate the possible association between CA and PDA16. This meta-analysis was based on 23 studies (17.708 preterm infants, 4681 CA events) and showed a significant association between CA and PDA with an odds ratio (OR) of 1.43 and a 95% confidence interval (CI) of 1.19 to 1.7216. However, an important limitation of the study was that confounding factors, such as GA, were not taken into account. Noteworthy is that the three largest cohort studies reporting on the association between CA and PDA, showed a significant positive crude OR but a significant negative association when the OR was adjusted for confounding factors17,18,19. Unfortunately, this relevant finding was missed in the meta-analysis of Park et al.16.
According to McElrath et al., the pregnancy disorders that lead to very preterm delivery can be divided into two broad groups20. One group is characterized by the presence of signs of infection/inflammation, but absence of indicators of impaired placentation. This group is associated with preterm labor, premature rupture of membranes (PROM), placental abruption, and cervical insufficiency. The second group is characterized by the relative absence of inflammation, but presence of histologic features of dysfunctional placentation. This group is associated with preeclampsia and the entity identified as fetal indication/intrauterine growth restriction. Therefore, observational studies comparing the outcomes of infants with and without CA are, in fact, comparing the effects of placental infection/inflammation with vascular placental pathology10,21. This may result in significant differences between the CA and the “control” group in terms of, for example, GA, birth weight (BW), or use of antenatal corticosteroids10,20,21,22. These differences may exert an important influence in outcomes such as PDA.
In an attempt to perform a more thorough assessment of the possible association between CA and PDA in preterm infants, we conducted a systematic review and meta-analysis in which adjusted ORs, whenever available, were pooled. In addition, we analyzed the magnitude of the differences in potential confounders, such as GA or BW, between the infants of the CA and the control group. Finally, we performed a meta-regression in order to investigate the effect of confounders on the association between CA and PDA.
Results
Description of studies
We identified 1188 potentially relevant studies from which 45 (27186 patients, 7742 CA cases, 8033 PDA cases) met the inclusion criteria (Supplementary Figure 1). The main characteristics of the included studies are shown in Supplementary Table 1. While all studies provided data to measure the association between CA and PDA, none of the studies was primarily designed to assess this association. In 40 studies7,8,9,17,18,19,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56, the objective was to examine the outcomes, including PDA, of preterm infants with and without maternal CA. In 5 studies57,58,59,60,61, the objective was to examine the risk factors for PDA, including maternal CA. Ten studies17,19,23,24,25,57,58,59,60,61 dealt with clinical and 347,8,9,18,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55 dealt with histological CA. One study56 described intra-amniotic infection/inflammation. This study was pooled in the group of histological CA. The quality of each study according to the Newcastle-Ottawa Scale is summarized in the Supplementary Table 1. All studies included in the meta-analysis achieved at least six stars, indicating good quality.
Analysis based on unadjusted data
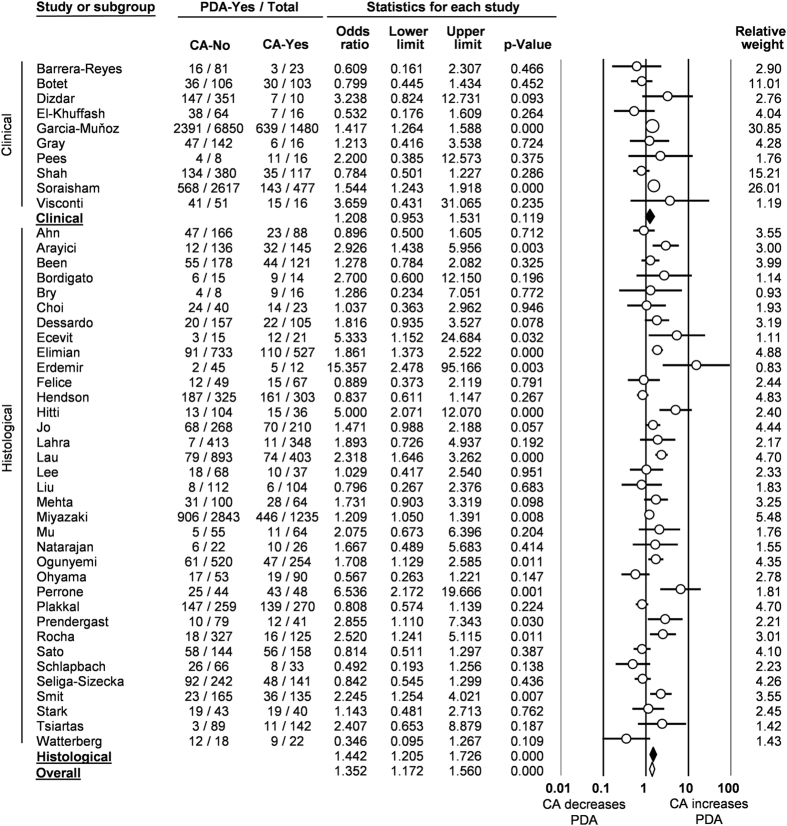
The pooled unadjusted OR from the 45 studies showed a significant positive association between CA exposure and PDA (OR 1.352, 95% CI 1.172 to 1.560; Heterogeneity: Q = 123,1, P < 0.001, I2 = 64.3) (Fig. 1). The association remained significant for histological CA (OR 1.442, 95% CI 1.205 to 1.726; Heterogeneity: Q = 105.0, P < 0.001, I2 = 67.6) but not for clinical CA (OR 1.208, 95% CI 0.953 to 1.531; Heterogeneity: Q = 17.6, P = 0.040, I2 = 48.9) (Fig. 1). Neither visual inspection of the funnel plot (Supplementary Figure 2) nor the regression test of Egger (P = 0.517) revealed evidence of publication bias.
Figure 1. Forest plot for association between chorioamnionitis (CA) and patent ductus arteriosus (PDA).
Unadjusted results.
In order to explore the possible differences in baseline characteristics between the groups of exposed and non-exposed to CA, we performed a number of additional meta-analyses. As summarized in Table 1, infants exposed to CA showed significantly lower GA (Supplementary Figure 3) and BW (Supplementary Figure 4), significantly higher rates of exposure to antenatal corticosteroids, significantly higher rates of premature rupture of membranes (PROM), significantly lower rates of cesarean delivery, and significantly lower rates of preeclampsia.
Table 1. Random effects meta-analyses of potential confounders.
| Meta-analysis | Chorioamnionitis | k | Effect size | 95% CI | Z | P | Heterogeneity |
||
|---|---|---|---|---|---|---|---|---|---|
| Q | P | I2 | |||||||
| Gestational age (weeks) | Clinical | 4 | MD -1.151 | −1.612 to −0.689 | −4.888 | <0.001 | 25.308 | <0.001 | 88.146 |
| Histological | 29 | MD -1.418 | −1.725 to −1.112 | −9.070 | <0.001 | 333.271 | <0.001 | 91.958 | |
| Any type | 33 | MD -1.336 | −1.592 to −1.081 | −10.260 | <0.001 | 369.827 | <0.001 | 91.347 | |
| Birth weight (g) | Clinical | 4 | MD -48 | −130 to 34 | −1.145 | 0.252 | 37.199 | <0.001 | 91.935 |
| Histological | 28 | MD -80 | −113 to −46 | −4.659 | <0.001 | 107.816 | <0.001 | 74.957 | |
| Any type | 32 | MD -75 | −106 to −44 | −4.745 | <0.001 | 147.878 | <0.001 | 79.037 | |
| Antenatal corticosteroids | Clinical | 3 | OR 1.498 | 1.024 to 2.191 | −1.504 | 0.133 | 11.743 | 0.003 | 82.969 |
| Histological | 26 | OR 1.234 | 1.049 to 1.451 | −4.549 | <0.001 | 60.735 | <0.001 | 58.837 | |
| Any type | 29 | OR 1.271 | 1.095 to 1.475 | 3.156 | 0.002 | 72.503 | <0.001 | 61.381 | |
| Cesarean section | Clinical | 3 | OR 0.434 | 0.389 to 0.485 | −14.725 | <0.001 | 1.695 | 0.428 | 0.000 |
| Histological | 17 | OR 0.373 | 0.297 to 0.469 | −8.483 | <0.001 | 83.466 | <0.001 | 80.830 | |
| Any type | 20 | OR 0.422 | 0.382 to 0.466 | −16.953 | <0.001 | 85.911 | <0.001 | 77.884 | |
| PROM | Any type | 16 | OR 2.884 | 2.085 to 3.989 | 6.401 | <0.001 | 107.419 | <0.001 | 86.036 |
| SGA | Any type | 11 | OR 0.341 | 0.211 to 0.549 | 4.423 | <0.001 | 58.549 | <0.001 | 82.920 |
| Preeclampsia | Any type | 6 | OR 0.143 | 0.084 to 0.243 | −7.182 | <0.001 | 13.948 | 0.016 | 64.153 |
K: number of studies; PDA: patent ductus arteriosus; MD: mean difference (chorioamnionitis-exposed minus unexposed); OR: odds ratio (OR > 1 means increased risk in infants exposed to chorioamnionitis);; PROM: premature rupture of membranes; SGA: small for gestational age.
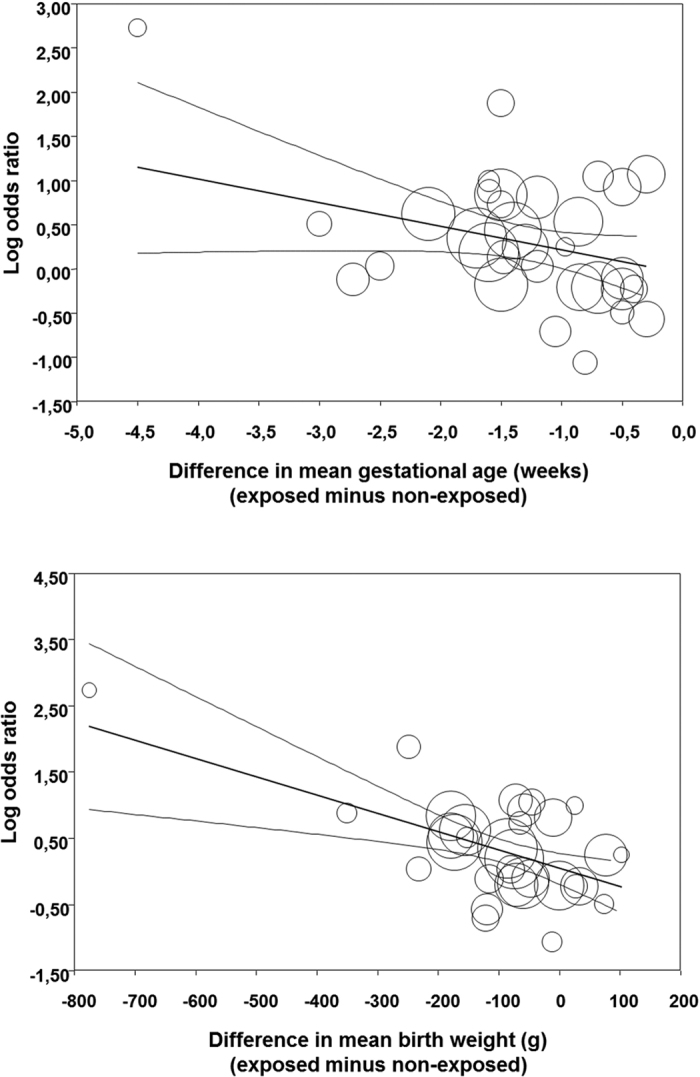
In order to analyze the possible influence of the above mentioned baseline characteristics on the unadjusted association between CA and PDA, we performed a meta-regression analysis. As depicted in Table 2 and Fig. 2, this analysis showed that the differences in GA or BW between the CA exposed and non-exposed groups were significantly correlated with the effect size of the association between PDA and CA. In contrast, meta-regression could not demonstrate a significant effect of the rate of use of antenatal corticosteroids, mode of delivery, rate of SGA, rate of PROM, or rate of preeclampsia on the effect size of the different studies (Table 2).
Table 2. Random effects meta-regression.
| Meta-regression | k | Coefficient | 95% CI | Z | P |
|---|---|---|---|---|---|
| Diff. mean gestational age (per week) | 32 | −0.266 | −0.501 to −0.032 | −2.22 | 0.026 |
| Diff. mean gestational age (significant yes/no) | 32 | 0.584 | 0.190 to 0.977 | 2.91 | 0.004 |
| Diff. mean birth weight (per 100 g) | 31 | −0.277 | −0.421 to −0.132 | −3.75 | 0.000 |
| Diff. mean birth weight (significant yes/no) | 31 | 0.123 | −0.208 to 0.455 | 0.73 | 0.466 |
| Chorioamnionitis type (clinical/histological) | 45 | 0.204 | −0.161 to 0.569 | 1.10 | 0.273 |
| Antenatal corticosteroids (log OR) | 28 | 0.143 | −0.194 to 0.480 | 0.83 | 0.406 |
| Cesarean section (log OR) | 21 | 0.083 | −0.176 to 0.341 | 0.63 | 0.530 |
| Early onset sepsis (log OR) | 16 | 0.022 | −0.124 to 0.168 | 0.29 | 0.770 |
| late onset sepsis(log OR) | 22 | 0.309 | −0.135 to 0.752 | 1.36 | 0.173 |
| Small for gestational age (log OR) | 11 | 0.188 | −0.266 to 0.643 | 0.81 | 0.416 |
| Premature rupture of membranes (log OR) | 16 | −0.264 | −0.579 to 0.051 | −1.65 | 0.099 |
K = number of studies.
Figure 2. Meta-regression plot of association between chorioamnionitis and PDA controlling for difference in gestational age and birth weight between exposed and non-exposed groups.
Finally, we performed an additional analysis aimed at evaluating the role of the presence of a fetal inflammatory response (i.e., funisitis) on the development of PDA. Eight studies8,9,26,27,33,35,40,41,46 reported on PDA in infants with histological CA with or without funisitis. The pooled OR for PDA of the group with CA and funisitis (1.613, 95% CI 0.935 to 2.786 P = 0.086) was not significantly different (meta-regression coefficient: 0.233, 95% CI -0.405 to 0.817, P = 0.473) from the pooled OR of the group with CA without funisitis (1.322, 95% CI 0.975 to 1.792, P = 0.073) (Supplementary Figure 5).
Analysis based on adjusted data
Adjusted ORs were reported in 8 studies17,18,19,31,37,40,44,56. Data on 3 additional studies8,24,27 were obtained from the authors. Therefore, a total of 11 studies (19577 infants) were included in these analysis that showed a significant negative association between CA and PDA (OR 0.802, 95% CI 0.751 to 0.959; Heterogeneity: Q = 35.0, P < 0.001, I2 = 71.4). This association remained significant for clinical (OR 0.849, 95% CI 0.703 to 0.916; Heterogeneity: Q = 0.7, P = 0.709, I2 = 0.0) but not for histological CA (OR 1.214, 95% CI 0.781 to 1.692; Heterogeneity: Q = 26.6, P < 0.001, I2 = 73.7) (Fig. 3, Table 3).
Figure 3. Forest plot for association between chorioamnionitis (CA) and patent ductus arteriosus (PDA).
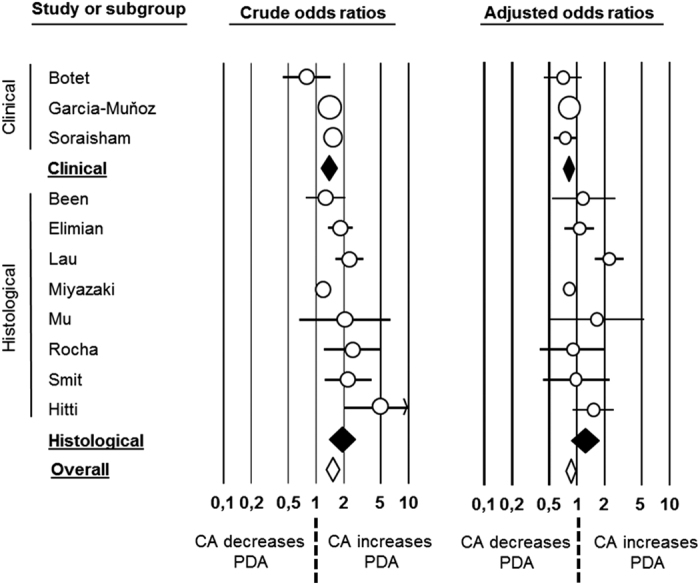
Unadjusted (left) and adjusted (right) results.
Table 3. Crude and adjusted ORs and confounders.
| Study or subgroup | Crude OR (95% CI) | P | Adjusted OR (95% CI) | P | Confounders included in analysis | |
|---|---|---|---|---|---|---|
| Clinical | Botet | 0.799 (0.445–1.434) | 0.452 | 0.705 (0.443–1.122) | 0.140 | GA |
| Garcia-Muñoz | 1.417 (1.264–1.588) | 0.000 | 0.830 (0.710–0.970) | 0.019 | GA, BW | |
| Soraisham | 1.544 (1.243–1.918) | 0.000 | 0.750 (0.561–1.002) | 0.052 | GA, BW, delivery mode, ACS, maternal hypertension, 5 min Apgar | |
| Clinical | 1.383 (1.134–1.686) | 0.001 | 0.802 (0.703–0.915) | 0.001 | ||
| Histological | Been | 1.278 (0.784–2.082) | 0.325 | 1.172 (0.537–2.560) | 0.691 | GA, SGA, sex, multiple birth, delivery mode, preeclampsia, PROM, ACS |
| Elimian | 1.861(1.373–2.522) | 0.000 | 1.060(0.740–1.519) | 0.751 | GA, BW, BW percentile, 5 min Apgar | |
| Lau | 2.318 (1.646–3.262) | 0.000 | 2.218(1.552–3.170) | 0.000 | GA, BW, delivery mode, multiple births, ACS, maternal hypertension, SGA, 5 min Apgar <7, SNAP-II score, NTISS score | |
| Miyazaki | 1.209 (1.050–1.391) | 0.008 | 0.830(0.698–0.987) | 0.035 | GA, BW, SGA, sex, maternal age, parity, diabetes, preeclampsia, PROM, NRFS, ACS, delivery mode | |
| Mu | 2.075 (0.673–6.396) | 0.204 | 1.653 (0.510–5.358) | 0.402 | GA | |
| Rocha | 2.520(1.241–5.115) | 0.011 | 0.900 (0.400–2.025) | 0.799 | GA, BW | |
| Smit | 2.245 (1.254–4.021) | 0.007 | 0.979 (0.428–2.237) | 0.960 | GA, SGA, sex, multiple birth, delivery mode, preeclampsia, PROM, ACS | |
| Hitti | 5.000 (2.071–12.070) | 0.000 | 1.500 (0.900–2.500) | 0.120 | BW | |
| Histological | 1.925 (1.416–2.616) | 0.000 | 1.214(0.871–1.692) | 0.252 | ||
| Overall | 1.524 (1.29–1.80) | 0.000 | 0.849 (0.751–0.959) | 0.009 |
GA: Gestational age, BW: Birth weight, ACS: Antenatal corticosteroids, SGA: Small for GA, PROM: Premature rupture of membranes SNAP-II: Score for Neonatal Acute Physiology, PROM: Premature rupture of membranes, NTISS: Neonatal therapeutic intervention scoring system, NRFS: Non-reassuring fetal status.
To investigate the effect of adjustment, the crude data of the above mentioned 11 studies were pooled and compared with the pooled adjusted ORs from the same studies (Fig. 3 and Table 3). The meta-analysis of this subgroup of crude data showed a significant positive association between CA and PDA (OR 1.524, 95% CI 1.291 to 1.800). The association remained significant for clinical (OR 1.383, 95% CI 1.134 to 1.686) and histological CA (OR 1.925, 95% CI 1.416 to 2.616). Meta-regression showed that the pooled crude ORs were significantly different than the pooled adjusted ORs from the same studies (P < 0.000001, P = 0.043, and P < 0.0008 for clinical, histological and any type of CA respectively).
Discussion
There is a substantial body of evidence supporting that CA is a major risk factor for spontaneous preterm birth but the independent contribution of CA to prematurity-associated mortality and morbidity is much more difficult to assess3. The present study confirms that confounders need to be taken into account when assessing the association between CA and clinical outcomes in preterm infants. The meta-analysis of unadjusted data showed a significant positive association between CA and PDA, similar to the one reported in the study of Park et al.16. In contrast, the meta-analysis of adjusted data showed a significant negative association between CA and PDA. Moreover, our analyses provide data on the magnitude of the differences in GA, BW, rate of SGA, use of antenatal corticosteroids, and mode of delivery between infants exposed and non-exposed to CA. Meta-regression showed that differences in GA and BW between infants exposed and unexposed to CA may account for the higher risk of PDA observed when unadjusted data were pooled.
We used an extensive search strategy, which included not only studies describing PDA as outcome after exposure to CA, but also studies that assessed CA as potential risk factor for PDA. Through this search strategy, we identified 23 studies (9478 patients) which were not included in the study of Park et al.16. However, the key methodological limitation of the meta-analysis of Park et al. is the exclusive exploitation of unadjusted ORs16. Whereas descriptive analyses can still be done with such unadjusted data, meaningful statistical inference can be problematic62. When patient-level data are not available, confounding in meta-analysis can be reduced by using adjusted odds and/or hazard ratios from each source study62,63,64. By choosing to favor unadjusted ORs, Park et al. sacrificed patient-level data adjustment performed with a comprehensive set of predictors, leading to an overestimation in the strength of association between CA and PDA.
Meta-analysis of observational studies presents challenging methodological issues involving different study designs (i.e., cohort and case-control), variation in the quality of studies in terms of assessment of exposure and outcomes, missing data, control for confounding, or choice of controls in the case-control studies62,63,64. As underlined by Hartling et al. in their meta-analysis on the association between CA and BPD, studies reporting pooled data on outcomes of preterm infants exposed or unexposed to CA should take into account that the “control” group likely included infants with different baseline characteristics than the CA-exposed group10,21. As mentioned in the introduction, the pathophysiological processes that lead to very preterm delivery have been divided into two main categories20: intrauterine infection/inflammation and placental vascular dysfunction. In addition to distinct pathophysiological pathways, conditions of delivery are different between the two groups. In the vascular disease group, there is a higher incidence of caesarean section, growth restriction, and older GAs than in the infection/inflammation group20,22. Accordingly, our analyses showed that the infants exposed to CA were born significantly earlier (~1.3 weeks), were lighter (~75 g), presented growth restriction less frequently, had a higher rate of PROM, and had a lower rate of cesarean section. Meta-regression showed that the differences in GA and BW significantly influenced the association between CA and PDA. This is not surprising since both CA and PDA are inversely related to GA1,2,15,65,66 and highlights again the relevance of adequate correction, at least for this important confounder.
Previous meta-analyses on the relationship between CA and BPD10, cerebral palsy6, or ROP13 showed that the positive association observed with unadjusted data was significantly reduced, or became non-significant, when adjusted data were pooled. The differences that we observed herein are even more marked since the significant positive association between CA and PDA became a significant negative association when only adjusted data were taken into consideration. This suggests that CA may even exert a protective effect on the occurrence of PDA. A possible protective effect of CA in outcomes such as IRDS, or BPD has been reported in several individual studies and it has been suggested that CA exposure may protect the infants by promoting lung maturation and reducing the need for surfactant and mechanical ventilation7,18,21,22,41,67,68. On the other hand, the possible beneficial effect of CA might be erased by the frequent occurrence of postnatal pro-inflammatory events and complications such as sepsis21,67,68. Nonetheless, the relationship between PDA and respiratory condition in preterm infants is complex and bidirectional. In many instances, the presence of a large ductal shunt is suspected only on the basis of respiratory findings, such as increasing requirements for supplemental oxygen, or inability to reduce mechanical ventilator support15. Conversely, stimuli that alter pulmonary precapillary tone, such as surfactant administration or mechanical ventilation can alter the left-to-right PDA shunt15. Therefore, the possible effect of CA on PDA development might be mediated through the effects of CA on the clinical respiratory condition of the infants.
That the fetal inflammatory response induced by CA might specifically influence the closure of the ductus arteriosus (DA) is a biologically plausible hypothesis. In fact, neonatal sepsis is recognized as an important risk factor for developing a hemodynamically significant PDA. As reviewed by Vucovich et al.66, the possible mechanisms linking neonatal inflammation/infection and PDA include (i) hypoxia-induced DA relaxation due to respiratory insufficiency secondary to the inflammatory process; (ii) ductal relaxation mediated by components of bacteria, cytokines, or endogenous vasoactive mediators, such as prostaglandins, NO or CO; (iii) increased fluid administration in order to treat the increasing third space volume that often accompanies the inflammatory response; and (iv) administration of drugs such as aminoglycosides that are known relaxants of the DA66,69. Nevertheless, it should be considered that not all intraamniotic infections will lead to an inflammatory process extending to the fetal component4. Funisitis is considered the histologic counterpart of the fetal inflammatory response syndrome4. The present analysis showed that the presence of funisitis combined with CA did not significantly change the odds of having PDA, when compared with CA in the absence of funisitis. This is an argument against the fetal inflammatory response as etiopathogenic factor for PDA.
Maternal administration of corticosteroids in case of anticipated preterm delivery reduces neonatal mortality and morbidity and has become standard of care in current obstetric practice70,71. However, several concerns exist, or have existed, regarding the administration of antenatal steroids in cases of suspected intrauterine infection. Given their immunosuppressive effects, corticosteroids could theoretically activate or worsen infections and, therefore, some guidelines delineate CA as a contraindication for antenatal steroids70,71. Surprisingly, our meta-analysis shows that the rate of use of antenatal corticosteroids is higher in preterm infants exposed to CA when compared with the non-exposed infants (Table 2). Nevertheless, the higher rate of exposure to corticosteroids was only significant for the group of histological CA. Thus, it can be assumed that when the decision of starting corticosteroids was taken, clinicians did not suspect the presence of CA, at least in a number of patients. Of note is that two meta-analyses showed that administration of antenatal corticosteroids in patients with histological CA was linked to a significant reduction in PDA as well as in in mortality, RDS, and IVH8,72. In the present study, meta-regression could not demonstrate a significant influence of the rate of use of antenatal corticosteroids on the association between CA and PDA. However, the higher use of antenatal steroids in CA-exposed infants should be taken into account in future meta-analyses investigating the relationship between CA and neonatal outcomes.
Limitations of the literature and of our systematic review and meta-analysis deserve comment. First, the published literature showed great heterogeneity in definition of exposure, outcome, and in assessment of confounders. Second, we found no studies having the evaluation of the association between CA and PDA as main objective. Third, adjusted data were available only from 11 of the 45 studies included in the meta-analysis However, it should be noted that these 11 studies accounted for 72% of the infants and they were the studies with the highest quality. Nevertheless, we had to rely on the adjusted analyses as presented in the published reports and the variables which they included, which were not consistent across studies. On the other hand, the main strength of the present study is the use of rigorous methods including extensive and comprehensive search; duplicate screening, inclusion, and data extraction to reduce bias; and meta-regression to control for potential confounders.
In conclusion, the current meta-analysis demonstrates that the previously reported increased risk of PDA among preterm infants exposed to CA16, depends more on CA as etiological factor for preterm birth than on the possible effects of infection/inflammation on DA pathobiology. Our present results underscore the need for including all potential confounding factors in future observational studies on the outcomes of CA and performing analyses that adjust for these confounders and the possible interactions among them.
Methods
The study was conducted according to the MOOSE guidelines for systematic review and meta-analysis of observational studies73. A protocol was developed prospectively that detailed the specific objectives, criteria for study selection, the approach to assessing study quality, clinical outcomes, and statistical methodology.
Sources and search strategy
A comprehensive literature search was undertaken using the PubMed/MEDLINE and EMBASE databases from their inception to December 1, 2015. The search terms involved various combinations of the following keywords: “chorioamnionitis”, “intrauterine infection” “intrauterine inflammation” “prenatal infection” “prenatal inflammation”, “antenatal infection” “antenatal inflammation” “ductus arteriosus” “patent ductus arteriosus”, “risk factors”, “outcome”, “cohort”, and “case-control”. No language limit was applied. We performed additional searches by screening reference lists from articles of interest as well as citations to articles of interest, using the ISI Web of Knowledge and Google Scholar. We also contacted topic specialists to identify additional potentially relevant studies.
Study selection
Studies were included if they had a CA and a comparison group, examined preterm or low BW infants, and reported primary data that could be used to measure the association between exposure to CA and the presence of a PDA. To identify relevant studies, two reviewers (EB, EV) independently screened the results of the searches and applied inclusion criteria using a structured form. Discrepancies were resolved through discussion or in consultation with a third reviewer (PD).
Data extraction
Two investigators (EB, PD) independently extracted data from relevant studies using a predetermined data extraction form and another two investigators (EV-M, EV) checked data extraction for accuracy and completeness. Discrepancies were resolved by consulting the primary report. Data extracted from each study included citation information, language of publication, country where research was conducted, objectives, study design, definitions of CA and PDA, inclusion/exclusion criteria, patient characteristics, and results (including raw numbers and adjusted analyses on CA and PDA where available).
Quality assessment
Methodological quality was assessed using the Newcastle-Ottawa Scale for cohort or case-control studies74. This scale uses a star rating system (range: 0–9 stars) scoring three aspects of the study: selection (0–4), comparability (0–2) and exposure/outcome (0–3). Two reviewers (PD and EV) independently assessed the methodological quality of each study. Discrepancies were resolved through discussion.
Statistical Analysis
Studies were combined and analyzed using comprehensive meta-analysis V 3.0 software (Biostat Inc., Englewood, NJ, USA). For dichotomous outcomes, the OR with 95% CI was calculated from the data provided in the studies. ORs adjusted for potential confounders were extracted from the studies reporting these data. For continuous outcomes, the mean difference (MD) with 95% CI was calculated. When studies reported continuous variables as median and range or interquartile range, we estimated the mean and standard deviation using the method of Wan et al.75. Due to anticipated heterogeneity, summary statistics were calculated with a random-effects model. This model takes into account variability between studies as well as within studies. Subgroup analyses were conducted according to the mixed-effects model76. In this model a random-effects model is used to combine studies within each subgroup and a fixed-effect model is used to combine subgroups and yield the overall effect. The study-to-study variance (tau-squared) is not assumed to be the same for all subgroups. This value is computed within subgroups and not pooled across subgroups. Statistical heterogeneity was assessed by Cochran’s Q statistic and by the I2 statistic, which is derived from Q and describes the proportion of total variation that is due to heterogeneity beyond chance77. We used the Egger’s regression test and funnel plots to assess publication bias. To explore differences between studies that might be expected to influence the effect size, we performed univariate random-effects meta-regression (method of moments)78. The potential sources of variability defined a priori were: CA type (clinical or histological), differences in GA and BW between the infants with and without CA, use of antenatal corticosteroids, mode of delivery, rate of SGA, rate of PROM, and rate of preeclampsia. A probability value of less than 0.05 (0.10 for heterogeneity) was considered statistically significant.
Additional Information
How to cite this article: Behbodi, E. et al. Chorioamnionitis appears not to be a Risk Factor for Patent Ductus Arteriosus in Preterm Infants: A Systematic Review and Meta-Analysis. Sci. Rep. 6, 37967; doi: 10.1038/srep37967 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank J. Been, J. Figueras, and F. Botet for kindly providing additional data and clarifications on their studies.
Footnotes
Author Contributions E.B. contributed to the literature search, the data extraction, analysis and interpretation of data, and the initial draft of the manuscript. E.V.-M. contributed to the data extraction, analysis and interpretation of data, draft of the manuscript, administrative, and technical support. P.D. contributed to the design, the oversight of data extraction, analysis and interpretation of data, the initial draft and manuscript revisions, and study supervision. E.V. conceived the idea for the study, designed the study methodology, contributed to the literature search, the oversight of data extraction, analysis and interpretation of data, the initial draft and manuscript revisions, and study supervision. E.V. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
References
- Tita A. T. & Andrews W. W. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol 37, 339–354 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugni L. et al. Chorioamnionitis and neonatal outcome in preterm infants: a clinical overview. J Matern Fetal Neonatal Med, 29, 1525–1529 (2015). [DOI] [PubMed] [Google Scholar]
- Thomas W. & Speer C. P. Chorioamnionitis: important risk factor or innocent bystander for neonatal outcome? Neonatology 99, 177–187 (2011). [DOI] [PubMed] [Google Scholar]
- Revello R., Alcaide M. J., Dudzik D., Abehsera D. & Bartha J. L. Differential amniotic fluid cytokine profile in women with chorioamnionitis with and without funisitis. J Matern Fetal Neonatal Med, 29, 2161–2165 (2015). [DOI] [PubMed] [Google Scholar]
- Gantert M. et al. Chorioamnionitis: a multiorgan disease of the fetus? J Perinatol 30 Suppl, S21–30 (2010). [DOI] [PubMed] [Google Scholar]
- Wu Y. W. & Colford J. M. Jr. Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA 284, 1417–1424 (2000). [DOI] [PubMed] [Google Scholar]
- Watterberg K. L., Demers L. M., Scott S. M. & Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 97, 210–215 (1996). [PubMed] [Google Scholar]
- Been J. V. et al. Histologic chorioamnionitis, fetal involvement, and antenatal steroids: effects on neonatal outcome in preterm infants. Am J Obstet Gynecol 201, 587 e581-588 (2009). [DOI] [PubMed] [Google Scholar]
- Liu Z., Tang Z., Li J. & Yang Y. Effects of placental inflammation on neonatal outcome in preterm infants. Pediatr Neonatol 55, 35–40 (2014). [DOI] [PubMed] [Google Scholar]
- Hartling L., Liang Y. & Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 97, F8–F17 (2012). [DOI] [PubMed] [Google Scholar]
- De Felice C. et al. Early neonatal brain injury in histologic chorioamnionitis. J Pediatr 138, 101–104 (2001). [DOI] [PubMed] [Google Scholar]
- Been J. V., Lievense S., Zimmermann L. J., Kramer B. W. & Wolfs T. G. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J Pediatr 162, 236–242 e232 (2013). [DOI] [PubMed] [Google Scholar]
- Mitra S., Aune D., Speer C. P. & Saugstad O. D. Chorioamnionitis as a risk factor for retinopathy of prematurity: a systematic review and meta-analysis. Neonatology 105, 189–199 (2014). [DOI] [PubMed] [Google Scholar]
- Simon S. R. et al. Platelet Counts and Patent Ductus Arteriosus in Preterm Infants: A Systematic Review and Meta-Analysis. Neonatology 108, 143–151 (2015). [DOI] [PubMed] [Google Scholar]
- Clyman R. I. in The role of patent ductus arteriosus and its treatments in the development of bronchopulmonary dysplasia. Semin Perinatol 37, 102–107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. W., Choi Y. S., Kim K. S. & Kim S. N. Chorioamnionitis and Patent Ductus Arteriosus: A Systematic Review and Meta-Analysis. PLoS One 10, e0138114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Munoz Rodrigo F., Galan Henriquez G., Figueras Aloy J. & Garcia-Alix Perez A. Outcomes of very-low-birth-weight infants exposed to maternal clinical chorioamnionitis: a multicentre study. Neonatology 106, 229–234 (2014). [DOI] [PubMed] [Google Scholar]
- Miyazaki K. et al. Impact of chorioamnionitis on short- and long-term outcomes in very low birth weight preterm infants: the Neonatal Research Network Japan. J Matern Fetal Neonatal Med 29, 331–337 (2016). [DOI] [PubMed] [Google Scholar]
- Soraisham A. S. et al. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol 200, 372 e371-376 (2009). [DOI] [PubMed] [Google Scholar]
- Egger M., Schneider M. & Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ 316, 140–144 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. et al. Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issues. Cancer Causes Control 17, 759–770 (2006). [DOI] [PubMed] [Google Scholar]
- Biondi-Zoccai G., Agostoni P., Abbate A., D’Ascenzo F. & Modena M. G. Potential pitfalls of meta-analyses of observational studies in cardiovascular research. J Am Coll Cardiol 59, 292–293 (2012). [DOI] [PubMed] [Google Scholar]
- McElrath T. F. et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol 168, 980–989 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaze-Masmonteil T. That chorioamnionitis is a risk factor for bronchopulmonary dysplasia–the case against. Paediatr Respir Rev 15, 53–55 (2014). [DOI] [PubMed] [Google Scholar]
- Durrmeyer X. et al. Perinatal risk factors for bronchopulmonary dysplasia in extremely low gestational age infants: a pregnancy disorder–based approach. J Pediatr 160, 578–583. e572 (2012). [DOI] [PubMed] [Google Scholar]
- Barrera-Reyes R. H., Ruiz-Macias H. & Segura-Cervantes E. [Neurodevelopment at one year of age in preterm newborns with history of maternal chorioamnionitis]. Ginecol Obstet Mex 79, 31–37 (2011). [PubMed] [Google Scholar]
- Botet F., Figueras J., Carbonell-Estrany X. & Narbona E. The impact of clinical maternal chorioamnionitis on neurological and psychological sequelae in very-low-birth weight infants: a case-control study. J Perinat Med 39, 203–208 (2011). [DOI] [PubMed] [Google Scholar]
- Pees C., Walch E., Obladen M. & Koehne P. Echocardiography predicts closure of patent ductus arteriosus in response to ibuprofen in infants less than 28 week gestational age. Early Hum Dev 86, 503–508 (2010). [DOI] [PubMed] [Google Scholar]
- Gray P. H. et al. Survival and neonatal and neurodevelopmental outcome of 24-29 week gestation infants according to primary cause of preterm delivery. Aust N Z J Obstet Gynaecol 37, 161–168 (1997). [DOI] [PubMed] [Google Scholar]
- Tsiartas P. et al. The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 26, 1332–1336 (2013). [DOI] [PubMed] [Google Scholar]
- Smit A. L. et al. Automated auditory brainstem response in preterm newborns with histological chorioamnionitis. J Matern Fetal Neonatal Med 15, 1864–9 (2015). [DOI] [PubMed] [Google Scholar]
- Seliga-Siwecka J. P. & Kornacka M. K. Neonatal outcome of preterm infants born to mothers with abnormal genital tract colonisation and chorioamnionitis: a cohort study. Early Hum Dev 89, 271–275 (2013). [DOI] [PubMed] [Google Scholar]
- Schlapbach L. J. et al. Impact of chorioamnionitis and preeclampsia on neurodevelopmental outcome in preterm infants below 32 weeks gestational age. Acta Paediatr 99, 1504–1509 (2010). [DOI] [PubMed] [Google Scholar]
- Sato M. et al. Severity of chorioamnionitis and neonatal outcome. J Obstet Gynaecol Res 37, 1313–1319 (2011). [DOI] [PubMed] [Google Scholar]
- Rocha G., Proenca E., Quintas C., Rodrigues T. & Guimaraes H. Chorioamnionitis and brain damage in the preterm newborn. J Matern Fetal Neonatal Med 20, 745–749 (2007). [DOI] [PubMed] [Google Scholar]
- Prendergast M. et al. Chorioamnionitis, lung function and bronchopulmonary dysplasia in prematurely born infants. Arch Dis Child Fetal Neonatal Ed 96, F270–274 (2011). [DOI] [PubMed] [Google Scholar]
- Plakkal N., Soraisham A. S., Trevenen C., Freiheit E. A. & Sauve R. Histological chorioamnionitis and bronchopulmonary dysplasia: a retrospective cohort study. J Perinatol 33, 441–445 (2013). [DOI] [PubMed] [Google Scholar]
- Perrone S. et al. Perinatal outcome and placental histological characteristics: a single-center study. J Matern Fetal Neonatal Med 25 Suppl 1, 110–113, 10.3109/14767058.2012.664344 (2012). [DOI] [PubMed] [Google Scholar]
- Ohyama M. et al. Re-evaluation of chorioamnionitis and funisitis with a special reference to subacute chorioamnionitis. Hum Pathol 33, 183–190 (2002). [DOI] [PubMed] [Google Scholar]
- Ogunyemi D., Murillo M., Jackson U., Hunter N. & Alperson B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Matern Fetal Neonatal Med 13, 102–109 (2003). [DOI] [PubMed] [Google Scholar]
- Mu S. C. et al. Impact on neonatal outcome and anthropometric growth in very low birth weight infants with histological chorioamnionitis. J Formos Med Assoc 107, 304–310 (2008). [DOI] [PubMed] [Google Scholar]
- Mehta R., Nanjundaswamy S., Shen-Schwarz S. & Petrova A. Neonatal morbidity and placental pathology. Indian J Pediatr 73, 25–28 (2006). [DOI] [PubMed] [Google Scholar]
- Lee S. Y. et al. Chorioamnionitis with or without funisitis increases the risk of hypotension in very low birthweight infants on the first postnatal day but not later. Arch Dis Child Fetal Neonatal Ed 91, F346–348 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J. et al. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am J Obstet Gynecol 193, 708–713 (2005). [DOI] [PubMed] [Google Scholar]
- Lahra M. M., Beeby P. J. & Jeffery H. E. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics 123, 1314–1319 (2009). [DOI] [PubMed] [Google Scholar]
- Hendson L. et al. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J Pediatr 158, 397–402 (2011). [DOI] [PubMed] [Google Scholar]
- Erdemir G. et al. Histological chorioamnionitis: effects on premature delivery and neonatal prognosis. Pediatr Neonatol 54, 267–274 (2013). [DOI] [PubMed] [Google Scholar]
- Elimian A. et al. Histologic chorioamnionitis, antenatal steroids, and perinatal outcomes. Obstet Gynecol 96, 333–336 (2000). [DOI] [PubMed] [Google Scholar]
- Ecevit A. et al. Association of respiratory distress syndrome and perinatal hypoxia with histologic chorioamnionitis in preterm infants. Turk J Pediatr 56, 56–61 (2014). [PubMed] [Google Scholar]
- Dessardo N. S. et al. Chronic lung disease of prematurity and early childhood wheezing: is foetal inflammatory response syndrome to blame? Early Hum Dev 90, 493–499 (2014). [DOI] [PubMed] [Google Scholar]
- Choi C. W. et al. Decreased expression of transforming growth factor-beta1 in bronchoalveolar lavage cells of preterm infants with maternal chorioamnionitis. J Korean Med Sci 23, 609–615 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bry K. J., Jacobsson B., Nilsson S. & Bry K. Gastric fluid cytokines are associated with chorioamnionitis and white blood cell counts in preterm infants. Acta Paediatr 104, 575–580 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordigato M. et al. Asymmetric dimethylarginine in ELBW newborns exposed to chorioamnionitis. Early Hum Dev 87, 143–145 (2011). [DOI] [PubMed] [Google Scholar]
- Arayici S. et al. The effect of histological chorioamnionitis on the short-term outcome of preterm infants ≤ 32 weeks: a single-center study. J Matern Fetal Neonatal Med 27, 1129–1133 (2014). [DOI] [PubMed] [Google Scholar]
- Ahn H. M., Park E. A., Cho S. J., Kim Y. J. & Park H. S. The association of histological chorioamnionitis and antenatal steroids on neonatal outcome in preterm infants born at less than thirty-four weeks’ gestation. Neonatology 102, 259–264 (2012). [DOI] [PubMed] [Google Scholar]
- De Felice C. et al. Histologic chorioamnionitis and severity of illness in very low birth weight newborns. Pediatr Crit Care Med 6, 298–302 (2005). [DOI] [PubMed] [Google Scholar]
- Jo H. S. et al. The effect of histologic chorioamnionitis on the development of respiratory distress syndrome and chronic lung disease in preterm infants. Kor J Pediatr 47, 150–156 (2004). [Google Scholar]
- Natarajan G., Glibetic M., Thomas R. L. & Aranda J. V. Chorioamnionitis and ontogeny of circulating prostaglandin and thromboxane in preterm infants. Am J Perinatol 25, 491–497 (2008). [DOI] [PubMed] [Google Scholar]
- Stark M. J., Hodyl N. A., Belegar V. K. & Andersen C. C. Intrauterine inflammation, cerebral oxygen consumption and susceptibility to early brain injury in very preterm newborns. Arch Dis Child Fetal Neonatal Ed 101, F137–142 (2016). [DOI] [PubMed] [Google Scholar]
- Hitti J. et al. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks’ gestation or less. Obstet Gynecol 98, 1080–1088 (2001). [DOI] [PubMed] [Google Scholar]
- Dizdar E. et al. Low platelet count is associated with ductus arteriosus patency in preterm newborns. Early Hum Dev 88, 813–816, doi: 10.1016/j.earlhumdev.2012.05.007 (2012). [DOI] [PubMed] [Google Scholar]
- El-Khuffash A. F. & Molloy E. J. Influence of a patent ductus arteriosus on cardiac troponin T levels in preterm infants. J Pediatr 153, 350–353, doi: 10.1016/j.jpeds.2008.04.014 (2008). [DOI] [PubMed] [Google Scholar]
- Shah N. A. et al. Relationship between circulating platelet counts and ductus arteriosus patency after indomethacin treatment. J Pediatr 158, 919–923 e911-912 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti L. F. et al. Clinical and echocardiographic characteristics associated with the evolution of the ductus arteriosus in the neonate with birth weight lower than 1,500 g. Einstein (Sao Paulo) 11, 317–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N. et al. Histological severity of fetal inflammation is useful in predicting neonatal outcome. Placenta 36, 1490–1493 (2015). [DOI] [PubMed] [Google Scholar]
- Vucovich M. M. et al. Aminoglycoside-mediated relaxation of the ductus arteriosus in sepsis-associated PDA. Am J Physiol Heart Circ Physiol 307, H732–H740 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe A. H. Effects of chorioamnionitis on the fetal lung. Clin Perinatol 39, 441–457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. & Speer C. P. Chorioamnionitis: important risk factor or innocent bystander for neonatal outcome? Neonatology 99, 177–187 (2010). [DOI] [PubMed] [Google Scholar]
- Reese J., Veldman A., Shah L., Vucovich M. & Cotton R. B. Inadvertent relaxation of the ductus arteriosus by pharmacologic agents that are commonly used in the neonatal period. Semin Perinatol 34, 222–230 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle X., Di Renzo G. C., Stark A., Fanaroff A. & Carbonell-Estrany X. Guideline for the use of antenatal corticosteroids for fetal maturation. J Perinat Med 36, 191–196 (2008). [DOI] [PubMed] [Google Scholar]
- Gilstrap L. C. et al. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA 273, 413–418 (1995). [DOI] [PubMed] [Google Scholar]
- Amiya R. M. et al. Antenatal Corticosteroids for Reducing Adverse Maternal and Child Outcomes in Special Populations of Women at Risk of Imminent Preterm Birth: A Systematic Review and Meta-Analysis. PloS one 11, e0147604 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Jama 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Wells G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Date of access: 25/03/2016) (2012).
- Wan X., Wang W., Liu J. & Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14, 135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M., Hedges L. V., Higgins J. & Rothstein H. R. Subgroup analyses in Introduction to Meta-analysis, 149–186 (Wiley, 2009). [Google Scholar]
- Borenstein M., Hedges L., Higgins J. & Rothstein H. Identifying and quantifying heterogeneity in Introduction to Meta-Analysis, 107–125 (Wiley, 2009). [Google Scholar]
- Borenstein M., Hedges L. V., Higgins J. & Rothstein H. R. Meta‐Regression in Introduction to meta-analysis, 187–203 (Wiley, 2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.