Abstract
Lipid signaling in pathogenic fungi has been studied to determine the role of these pathways in fungal biology and human infections. Owing to their unique nature, they may represent targets for future antifungal treatments. Farnesol signaling was characterized as a quorum-sensing molecule, with exposure inhibiting filamentation. Research has shown involvement in both the Ras1-adenylate cyclase and MAP kinase pathways. In species of Aspergillus, farnesol exposure induces apoptosis-like changes and alterations in ergosterol synthesis. Eicosanoid production has been characterized in several pathogenic fungi, utilizing host lipids in some cases. The role in virulence is not known yet, but it may involve modulation of host lipids. Sphingolipid signaling pathways seem to center around the production of diacylglycerol in the formation of inositol phosphorylceramide. Diacylglycerol activates both melanin production through laccase and transcription of antiphagocytic protein, both of which are involved in virulence.
Keywords: farnesol, prostaglandin, Cryptococcus, sphingolipid, Aspergillus, Candida
INTRODUCTION
Pathogenic fungi such as Candida, Aspergillus, and Cryptococcus continue to be a significant medical issue worldwide. The search for more effective and specific therapies for these infections continues to this day. Among the pathways and systems examined for potential new drug targets, lipid signaling has emerged as an attractive and interesting field. The lipid signaling pathways involving farnesol (FA), eicosanoids, and sphingolipids in these fungi have been the best characterized to date.
FARNESOL SIGNALING
One major player in lipid signaling pathways of pathogenic fungi is FA, a 15-carbon oxygenated lipid made up of isoprene moieties. FA has the distinction of being the first quorum-sensing molecule identified in eukaryotes. Hornby et al. (26) reported that a lipid-soluble molecule released into the media was responsible for inoculum size-dependent growth changes in Candida albicans, characterized by a reduction in yeast-to-mycelia transitions. Further evaluations of FA-related phenotypes revealed that both biofilm formation and hyphal development are suppressed in C. albicans. This phenomenon may not be limited to C. albicans, as there is evidence that FA reduces hyphal formation in C. dubliniensis (23) and alters gene expression and cell polarization in C. parapsilosis (55). FA secretion and biofilm formation have been documented for eight Candida species under a variety of conditions (66), although C. albicans seems to have the highest FA secretion.
FA-mediated hyphal blockage was evaluated under a variety of different conditions, including several defined media and serum-containing conditions (43). C. albicans grown in all defined media tested showed similar FA-related reduction in germ tube formation, with about 1 μM of FA needed to show a 50% decrease. FA susceptibility was greatly reduced in the presence of serum (albumin), implying that the dynamics of FA signaling in relation to pathophysiology may be more complex than in vitro studies suggest. Mosel et al. (43) also showed that FA had little effect on existing germ tubes, suggesting the main role of FA is prevention of formation initiation. In addition to the effects on hyphal formation, FA is partially protective against oxidative stress (67), but not in a manner that induces classical antioxidant genes such as catalase or superoxide dismutases.
A potential link between FA production and pathogenicity was alluded to by Navarathna et al. (45), who both increased FA production and enhanced virulence of C. albicans in mouse models of infection when the fungus was pretreated with low concentrations of azoles. Azoles are a class of antifungal drugs that inhibits the synthesis of ergosterol, a major component of fungal membranes. This azole-induced production of FA had been seen previously when it was treated with a variety of different azoles (27). Farnesyl pyrophosphate (FPP) is a common precursor to the synthesis of both FA and ergosterol, the major sterol component of fungal membranes. Because azole drugs disrupt ergosterol synthesis after the formation of FPP, treatment with azoles leads to an accumulation of FPP that then leads to an increased production of FA.
Since this observation, several aspects of virulence have been examined with respect to FA. Navarathna et al. (46) created a mutant strain of C. albicans with reduced endogenous FA production and showed that this strain had reduced virulence in mouse models compared with wild-type and reconstituted strains. Additionally, exogenous FA administration increased murine mortality (46) and altered host cytokine production (46, 47, 58) during infection. Shchepin et al. (58) looked at synthetic compounds comparable to FA in quorum-sensing attributes in relation to mouse models of C. albicans infection. They showed that although the synthetic compounds varied in their function as virulence factors, neither of the compounds tested protected mice from infection. This could imply a strict structural requirement of these molecules for function. Taken together, these data further support the link between FA and Candida pathogenesis and highlight the need for future investigations into the exploitation of this molecular process for therapies.
The various effects of FA on Candida are clear, but what signaling pathways underlie these phenotypes? The answer to this question may be the key to understanding the complex role of FA in the life cycle and pathogenesis of this fungus. Some clues to these processes come from microarray analyses. Cao et al. (6) looked at gene expression in C. albicans biofilms inhibited by FA exposure. They found that along with genes involved in processes such as drug resistance and heat shock, the expression of several genes associated with hyphal formation was altered when exposed to FA. Genes of interest in this group include TUP1 (dTMP uptake), PDE2 (phosphodiesterase), and CRK1 (Cdc2-related kinase). Tup1 is a transcriptional repressor that is upregulated in the presence of FA, whereas CRK1 and PDE2 are downregulated. TUP1 downregulation is associated with hyperfilamentation, while PDE2 and CRK1 have been hypothesized to positively effect filamentation. Taken together, FA affects the expression of these genes in a manner consistent with the effect of FA treatment on hyphal formation. Enjalbert et al. (14) studied effects of FA on gene expression profiles in strains of C. albicans that had resumed growth after stationary phase upon transfer to fresh media. In these cases, FA delayed this rebound of growth and changed the expression of genes that are normally affected (positively or negatively) during hyphal formation. Among the affected genes are cyclin genes HGC1, CLN3, and PCL2 and histone genes. For example, HGC1 is necessary for hyphal development (69) and the microarray data show that FA exposure downregulates HGC1. Another group examined the expression profile of C. albicans genes in the early yeast-to-hyphae transition period when grown in nonenriched media (8). They found that many genes affected by FA are also affected by high cell density, which would be expected from a quorum-sensing molecule. In general, they found that under these conditions expression profiles were similar to those seen in studies of gene expression C. albicans following phagocytosis (38).
Although important to the molecular biology of signaling, gene expression alone cannot determine the specific roles of factors in the cellular processes underlying FA signaling. Further examination of some of the genes/proteins indicated in the microarray studies has yielded interesting results. Tup1 acts globally to repress hyphal formation (3). Tup1 interacts with other proteins such as Tcc1 (30) that function as corepressors after complexing with DNA binding proteins such as Nrg1 (5) and Rfg1 (29, 33), which are homologous to Saccharomyces cerevisiae proteins Nrg1p and Rox1p, respectively. When TUP1 is deleted, the strain shows constitutive filamentous growth with an inability to grow as yeast (3). Furthermore, activation of Tup1 and related factors results in a downregulation of genes that are involved in filament formation (4, 44). Nrg1 is a repressor of filament growth, and deletions of NRG1 have phenotypes similar to TUP1 deletions (5). nrg1−/− mutants show no virulence in mouse models of candidiasis. Another interesting aspect of the study examines hyphal genes that are affected by Nrg1, with Tup1-controlled genes ECE1 and HWP1 (4, 64) constitutively expressed in the nrg−/− mutant. RFG1 deletion showed a phenotype similar to the nrg−/−, and the double mutant showed colonies with a more pronounced wrinkled appearance than did the singular deletions. Examinations of the link between FA, Tup1, and these related DNA binding proteins have shown promising results. Kebaara et al. (31) recently established this link by examining the response of various knockout mutants to FA. The tup1−/− and nrg−/− mutants do not exhibit a morphological response to FA, whereas the rfg1−/− mutant does respond to FA. As seen before, treatment with FA increases TUP1 expression with a concomitant decrease in hyphae-specific genes such as HWP1 and RBT1. Interestingly, both tup1−/− and nrg1−/− mutants overproduced FA up to 19 times more than wild type did. The implications of this finding suggest a possible feedback mechanism between Tup1, Nrg1, and the production of FA.
Other pathways seem to be involved in FA-related changes in the morphology of C. albicans. Ras1, a GTPase, activates the enzyme adenylate cyclase (AC), which produces the common second messenger cyclic-AMP (cAMP). cAMP activates protein kinase A (PKA), which phosphorylates many downstream factors. One of these is a transcription factor called Efg1, which induces the morphological transition to hyphae (18, 35). Davis-Hanna et al. (12) established that this Ras1-AC-PKA-Efg1 pathway is inhibited byFA and rescued from that inhibition in an Efg1-dependent manner upon addition of a cAMP analog, dibutyryl-cAMP.
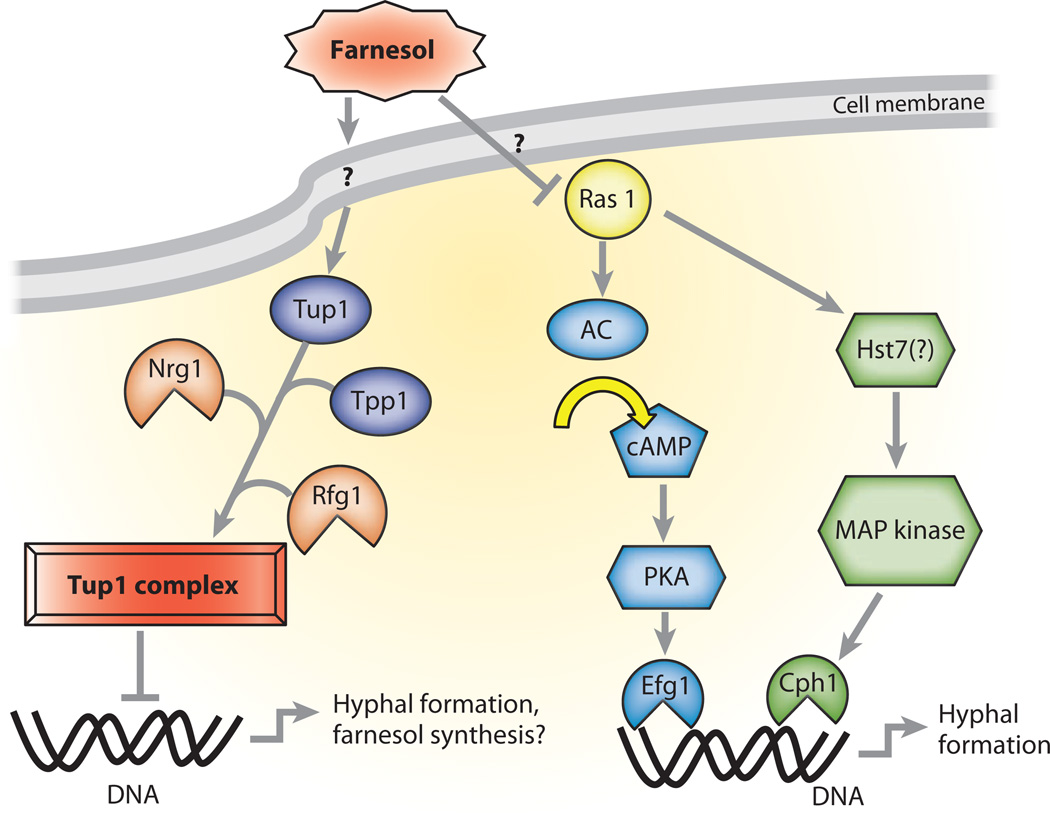
There is evidence that FA influences mitogen-activated protein (MAP) kinase cascades through reduced expression of genes such as HST7 (a MAP kinase kinase) and CPH1 (a transcription factor involved in hyphal formation) upon treatment, and the reduced cascade activity was measured indirectly by showing general amino acid permease 1 (GAP1) (56). Because Ras1 is a common factor of the MAP kinase pathway and the PKA-Efg1 pathway, it is possible that this is the molecule that FA acts upon. A summary of factors in farnesol signaling in C. albicans can be found in Figure 1.
Figure 1.
Known and hypothetical components of farnesol signaling in Candida albicans. Upon treatment with farnesol, Tup1 global transcriptional repressor associates with Tpp1, Nrg1, and Rfg1 to form a DNA binding complex. This complex inhibits gene expression of proteins involved in hyphal formation and may play a role in farnesol feedback pathways. Additionally, farnesol affects components of AC and MAP kinase signaling pathways. Farnesol may be through a common component, Ras, and inhibits these cascades that eventually lead to transcription of factors in hyphal formation. Abbreviations: AC, adenylate cyclase; cAMP, cyclic adenosine monophosphate; MAP, mitogen-activated protein; PKA, protein kinase A.
Another kinase gene involved in FA response and quorum sensing in C. albicans is CHK1, which encodes a histidine kinase. The deletion of CHK1 led to a strain (chk1−/−) that was unresponsive to FA treatment even at higher doses, whereas deletion of other histidine kinases such as Sln1p and Nik1p produced strains that responded to FA normally (34). This is possibly connected to previous findings in which CHK1 interacts with MAP kinase, one of its downstream regulators, or another yet unidentified mediator. Chk1 does not appear to be involved in quorum-related antifungal resistance (52).
The focus of FA signaling thus far has been on C. albicans, but other pathogenic fungi have shown responses to FA as well. Recently, biofilm formation in Pneumocystis carinii has been described and is inhibited by exposure to FA in this pathogenic fungus as well (11). Of note, some Aspergillus species have shown various effects upon FA treatment. FA does not affect the hyphal morphology of A. nidulans, but it does inhibit conidiation of A. niger (37). Along with this conidiation inhibition, FA treatment decreases the intracellular levels of cAMP, which is produced by AC. Treatment with inhibitors of AC induces a phenotype similar to that induced by FA, which suggests that cAMP may be involved in the mechanism of reduced conidiation. A. nidulans does show an interesting phenotype upon FA treatment, which is consistent with morphological changes associated with apoptosis (57). This phenotype includes DNA fragmentation and nuclear condensation. Recent studies have further examined this phenotype and found a decrease in mRNA transcripts for proteins involved in transcription, translation, ergosterol biosynthesis, and ribosomal biogenesis along with an increase in transcripts for mitochondrial proteins. These studies found that treatment with FA induces autophagy and mitochondrial fragmentation. Ergosterol biosynthesis in A. nidulans seems to be affected, as evidenced by a punctate distribution of filipin staining (fluorescent compound that binds ergosterol) as opposed to the uniform distribution of untreated fungi. Because the studies of ergosterol polarization have involved sphingolipid-rich lipid rafts in C. albicans (41), it would be worthwhile to further explore the links between FA exposure and characteristics of lipid raft domains. Of interest to signaling studies is the observation that the protein kinase C (PKC)-deficient strain of A. nidulans (calC2) showed resistance to FA treatment. Some studies have shown links between PKC and autophagy (51), though not in Aspergillus. Could these FA-related observations be explained by a common cause involving PKC?
One notable aspect of the research described above relating to Aspergillus species and FA is that Aspergillus species do not seem to produce detectable amounts of FA. This could mean that the phenotypes described are laboratory artifacts and that these phenomena do not occur in nature. More likely is the idea that FA, in addition to quorum sensing, is produced by Candida to compete with other organisms such as Aspergillus. This idea adds a different dimension to the FA studies, implying a complex system of regulating not only the growth of Candida itself but also its competition with other fungi and microbes for resources. The FA-mediated interaction between Candida and Aspergillus is not the only documented case of different microorganisms interacting through FA. Pseudomonas aeruginosa is a gram-negative bacteria that often coexists with Candida species in clinical situations such as cystic fibrosis. FA inhibits swarming motility in P. aeruginosa (42) and reduces the production of pyocyanin, a known virulence factor (10).
In summary, many signaling genes and factors influenced by FA lead to the morphological changes observed. Although it can seem daunting, important common factors that appear in these studies suggest an overall theme of signaling and phenotype. The Ras1-cAMP connection appears to be common not only in Candida, but also in several pathways implicated in the FA response, including Aspergillus. In the past few years, significant advancements have furthered our understanding of this signaling molecule. Future research will revolve around the mechanism by which FA activates these pathways, alternative pathways involved in these morphological changes, the role of FA in complex microbial interactions, and whether FA can be exploited therapeutically.
EICOSANOID SIGNALING
Although a major player in fungal lipid signaling, FA is not the only lipid molecule that affects signaling pathways in fungi. Eicosanoids are one such family of lipids that plays roles in a number of pathogenic fungi. Eicosanoids are 20-carbon-long oxygenated lipids derived from fatty acids such as arachidonic acid. Included in the family of eicosanoids are groups of lipids, such as prostaglandins and leukotrienes, that have various effects related to immune function and inflammation in humans.
Noverr et al. (49) first reported the occurrence of prostaglandins and leukotrienes in culture supernatants of Cryptococcus neoformans and C. albicans. Synthesis of the prostaglandins appeared to be from both de novo pathways and salvage of precursors (such as arachidonic acid) from the media. However, Wright et al. (68) confirmed that there is no arachidonic acid produced endogenously by Cryptococcus. C. albicans takes up arachidonic acid (13).Other pathogenic fungi synthesize these eicosanoids. Noverr et al. (50) showed that several different strains and species of pathogenic fungi produce both leukotrienes and prostaglandins in culture. This production was dramatically increased when arachidonic acid was added to culture media. Future studies confirmed that the prostaglandins purified from C. neoformans (15) and C. albicans (17) were of the prostaglandin E2 (PGE2) subtype. Once purified, these prostaglandins exhibited effects on mammalian cells (including cytokine modulation) similar to effects exhibited by mammalian PGE2. Inhibitors of cyclooxygenase (COX), a class of enzymes that synthesize prostaglandins from arachidonic acid, have varied effects on these fungi. Paracoccidioides brasiliensis shows reduced production of PGE2 when treated with COX inhibitors (2). In C. albicans, synthesis of PGE2 is stopped by COX and lipoxygenase inhibitors that are nonspecific with respect to the isoenzyme targeted (17). However, compounds that specifically inhibit the COX2 isoenzyme do no affect PGE2 production. Alem et al. (1) found a difference between PGE2 production by suspended Candida and Candida associated with a biofilm, with the latter producing more. Both of these conditions showed a block in PGE2 synthesis upon treatment with nonspecific COX inhibitors. Because C. albicans has no homolog to COX enzymes, alternative synthesis strategies were examined. Two such enzymes, the fatty acid desaturase Ole2 and the copper oxidase Fet3, were implicated when deletion of these enzymes produced strains with greatly reduced PGE2 synthesis. Given that deletion of either enzyme did not completely abolish PGE2 production, it seems that other synthesis pathways may exist. Taken together, the synthesis of PGE2 in C. albicans might occur through an enzyme with some general structural similarity but little homology to the known COX enzymes. As for the effect of COX inhibitors on C. neoformans, the answer is not as clear. Early reports (49) showed that COX inhibitor treatment decreased the amount of PGE2 in cultures; however, this seemed to result from a decrease in the number of viable cells.
More recent studies (15) have shown that COX inhibitor treatment does not affect cryptococcal production of PGE2. Like C. albicans, C. neoformans has no homolog to known COX enzymes. These two observations suggest that an entirely different mechanism of PGE2 synthesis occurs in these fungi. Erb-Downward et al. (16) followed up on these ideas and found that several polyphenolic chemicals that typically inhibit lipoxygenase activity inhibit production of PGE2. Laccase, the enzyme in Cryptococcus responsible for melanin synthesis, binds phenolic substances and therefore was examined for a role in eicosanoids production. Deletion or inhibition of laccase resulted in a lack of PGE2 production, but laccase alone was unable to synthesize PGE2 from either arachidonic acid or prostaglandin H2. Interestingly, laccase converted prostaglandin G2 to PGE2. The multicopper oxidase Fet3 is homologous to laccase (17). These findings indicate that we are just beginning to understand prostaglandin production in Cryptococcus.
Little is known about the direct effect of PGE2 on these fungi, though Levitin et al. (36) reported several alterations in the transcription profile of C. albicans upon exogenous exposure. Another class of lipids derived from omega-3-polyunsaturated fatty acids like arachidonic acid is the resolvins. In human cells, these compounds are anti-inflammatory and affect migration of neutrophils in later stages of inflammation. Recently, Haas-Stapleton et al. (22) described the synthesis of fungal resolvins in C. albicans. The synthesis of these resolvins was inhibited by lipoxygenase inhibitors, and production of these lipids by C. albicans affected neutrophil chemotaxis, phagocytosis, and intracellular killing at different concentrations. This finding is more evidence for the role of eicosanoid-derived lipids in fungal modulation of the host immune system.
Phospholipases may also play a role in the eicosanoid signaling story. Phospholipases cleave fatty acid moieties from larger lipid molecules. Freeing arachidonic acid and other eicosanoids precursors from these molecules is the first step toward prostaglandin synthesis. Cryptococcus phospholipase (PLB1) is cell-wall-bound secreted, and deletion mutants deficient in this gene show cell wall defects (60) and reduced virulence in murine models of cryptococcosis (9). Noverr et al. (48) showed that this strain was deficient in production of certain prostaglandins and leukotrienes when given phospholipid precursors but not when given arachidonic acid directly. Owing to their observations that alveolar macrophages showed growth inhibition of the plb1 strain upon phagocytosis, they hypothesized that Plb1 is required for growth inside macrophages. Wright et al. (68) found that the prostaglandins synthesized during the macrophage-Cryptococcus interaction were derived from host (macrophage) arachidonic acid. Treatments that reduced production of phospholipases in Cryptococcus such as tipranavir have shown therapeutic effects on models of cryptococcosis (7). In summary, phospholipases may play an important role in prostaglandin metabolism in pathogenic fungi and should be further evaluated for their role in virulence and host-fungi interactions.
As mentioned, Candida and Cryptococcus are not the only pathogenic fungi to produce eicosanoids. Genes identified in Aspergillus nidulans and A. fumigatus that are similar to COX genes also encode fatty acid oxygenases (63). The strains produced when these genes (ppoA, ppoB, and ppoC) were deleted showed deficits in PGE2 production. Further, a triple deletion mutant lacking all three of the PPO genes was resistant to oxidative stress and hypervirulent in a murine model of pulmonary aspergillosis. The authors hypothesize that the host immune system is further activated by the fungal prostaglandins in wild-type Aspergillus and that the triple deletion mutant is hypervirulent due to a lack of this activation.
Eicosanoid production and signaling in pathogenic fungi has made interesting advances in recent years. Studies presented here have shown that these oxylipins are produced in many fungi that are pathogenic to humans, and that their production may be involved in the pathogenesis of these fungal infections. Future studies focused on signaling mechanisms in these fungi upon autocrine/paracrine exposure to self-produced eicosanoids would be interesting, especially studies examining more complex effects of these prostaglandins on other commensal microbes. Another aspect of the research that focuses on fungi-host interactions is the idea that the fungi are hijacking host lipids for their own processes. (This phenomenon is not unheard of; see for example research on Chlamydia trachomatis in Reference 65). The exact role that these prostaglandins play in pathogen virulence and host response would be crucial for the development of therapies that target these pathways.
SPHINGOLIPID SIGNALING
A class of lipids gaining new prominence in both mammalian and fungal research is the sphingolipid family. In mammals, these lipids, which contain a sphingoid backbone often bound to an acyl chain by an N-amide linkage, have shown numerous effects in processes such as apoptosis, stress responses, and cell proliferation. In recent years, the role of sphingolipids in pathogenic fungi, in terms of signaling, growth, and virulence, has become a rapidly growing field. Because many of these enzymes and products are structurally distinct from their mammalian counterparts, they have the potential for therapeutic targeting with minimal effects on the host. Advanced mathematical models have been created and experimentally tested using the sphingolipid biosynthetic pathway of C. neoformans and its relation to pathogenesis (19).
One such enzyme that is unique to the fungal sphingolipid synthesis pathway is inositol phosphorylceramide (IPC) synthase. IPC synthase is responsible for the removal of an inositol-phosphate group from phosphatidylinositol and the transfer of that group to the terminal hydroxy group of phytoceramide. This reaction forms IPC and diacylglycerol (DAG) as a byproduct. Examinations of this enzyme in virulence of C. neoformans have shown that IPC synthase downregulation causes defects in melanin production (a known virulence factor in C. neoformans) and reduced growth inside alveolar macrophages. Both of these phenotypes may be responsible for the reduced virulence of strains with impaired IPC synthase (39, 40). The enzyme involved in the reverse process, inositol phosphosphingolipidphospholipase C (Isc1), is also involved in virulence. Isc1 removes the phosphorylinositol moiety from IPC, forming phytoceramide. When this enzyme is deleted, the resulting strain (Δisc1) is hypercapsulated and hypovirulent in immunocompromised mouse models of cryptococcosis and seems to be an obligate extracellular pathogen (59). The role of Isc1 likely revolves around macrophage interactions, as macrophage depletion results in the dissemination of the Δisc1 strain to the central nervous system.
The production of DAG by IPC synthase is a major component of this enzyme’s role in virulence. DAG seems to regulate cryptococcal virulence factors in two ways. First, DAG is a component in the melanin synthesis pathway. DAG activates PKC in mammalian cells through binding to the C1 domain of PKC (28). Heung et al. (25) found that this activation occurs in C. neoformans as well, with DAG produced from IPC synthase reactions binding to the C1 domain of the cryptococcal PKC (Pkc1) (24) and causing an increase in kinase activity. Deletion of the specific C1 domain stops the DAG-dependent activation of Pkc1. This disruption of Pkc1 activity causes alterations in the cell wall, preventing the enzyme laccase (melanin-producing enzyme) from properly associating. It was shown here that this is the mechanism by which IPC synthase regulates melanin synthesis. Aside from the effects on laccase, the involvement of Pkc1 in cell wall integrity is another aspect of this pathway. Cell wall integrity is crucial for fungal growth and virulence, often becoming the target of antifungal therapies. Gerik et al. (21) deleted 10 genes in the Pkc1 signaling pathway and found that phosphatase Ppg1 and kinases Bck1 and Mkk2 are all required for proper cell wall integrity. Along with these defects were predictable impairments in melanin production and capsule synthesis, as well as protection from oxidative and nitrosative stresses (20).
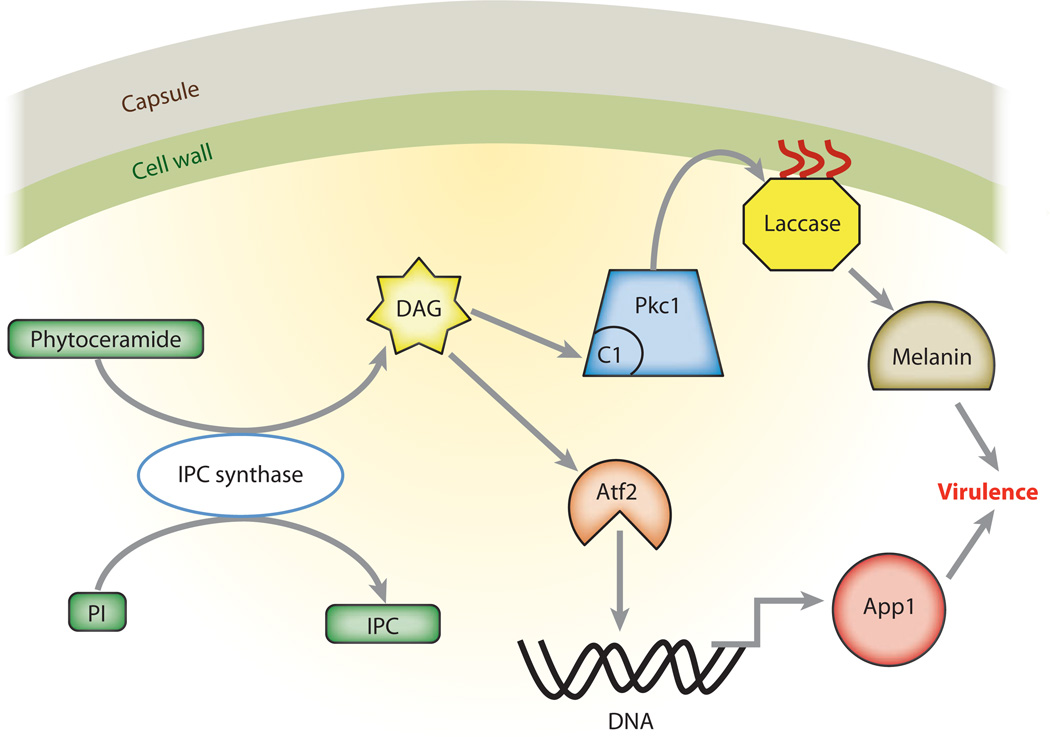
The second way that DAG production by IPC synthase regulates virulence factors is through the production of antiphagocytic protein (App1). App1 is not only involved in protection from phagocytosis, but also binds complement receptors CR2 and CR3 (61). The importance of App1 in virulence is clear from the phenotype of the deletion strain Δapp1, which is hypervirulent in complement-deficient mice but hypovirulent in mice with T-cell deficiencies (39). IPC synthase was indirectly involved in the transcription of APP1. DAG produced by IPC synthase activates the activating transcription factor 2 (Atf2), which binds to an ATF consensus sequence in the promoter region of APP1 (62). Upon binding, Atf2 upregulates the expression of App1, confirming another pathway by which DAG from IPC synthase regulates virulence of C. neoformans. Figure 2 summarizes the IPC regulation of virulence factors in C. neoformans.
Figure 2.
Sphingolipid signaling in Cryptococcus neoformans. IPC synthase in C. neoformans produces DAG as a byproduct of IPC synthesis. DAG binds to the C1 domain of protein kinase A, which is important for cell wall integrity. This integrity is crucial for localization of laccase, the enzyme responsible for melanin synthesis. In addition, DAG also activates the transcription factor Atf2, which leads to transcription of App1. Both App1 and melanin are virulence factors in C. neoformans. Abbreviations: IPC, inositol phosphorylceramide; PI, phosphatidylinositol; App1, antiphagocytic protein 1; Pkc1, protein kinase C1 (with C1 domain); DAG, diacylglycerol.
The future of sphingolipid pathway involvement in fungal disease is promising. Fluctuations in the pathway can be predicted with sophisticated mathematical models (19) and applied to pathogenesis. The IPC synthase-related pathways dominate the field of lipid signaling in pathogenic fungi to date, but other components of the fungal sphingolipid pathways are related to virulence. The deletion of the gene that encodes glucosylceramide synthase (GCS1) in C. neoformans yields a strain (Δgcs1) that is avirulent in inhalational mouse models of infection (54). This strain disseminates in a manner similar to Δisc1 when macrophages are depleted (32). Though the mechanism of this attenuated virulence is under examination (53), it is possible that lipid signaling plays a role in this phenotype as well. In C. albicans, enzymes known as Δ8-desaturases are responsible for the unique introduction of a double bond at the eighth carbon of the backbone of the fungal ceramide species. The deletion of this desaturase enzyme resulted in decreased hyphal growth and morphological alterations. Many enzymes in the sphingolipid pathway are still being characterized and deleted and may provide many more links between these lipids and virulence.
CONCLUSIONS
Lipid signaling in pathogenic fungi is a constantly growing field. New factors and fungal species are being studied constantly and the understanding of existing pathways is always improving. Aside from giving new insights into the biology of these important human pathogens, these pathways may represent a new class of antifungal treatments, if properly targeted. The discoveries presented here will likely be the foundation of many new advances in the years to come.
SUMMARY POINTS.
FA, a fungal quorum-sensing molecule, inhibits hyphal formation. FA exposure appears to alter gene expression in Candida and involve Ras, MAP kinase, and histidine kinase signaling pathways.
In Aspergillus, FA treatment induces apoptosis and alters production of membrane lipids such as ergosterol.
Eicosanoid production occurs in many pathogenic fungi. The prostaglandins are produced using host lipids and may be synthesized to manipulate the host immune system.
Sphingolipid signaling affects transcription of antiphagocytic proteins and the production of melanin in Cryptococcus through the production of DAG by IPC synthase.
Acknowledgments
This work was supported in part by the Burroughs Wellcome Fund; by grants AI56168, AI71142, and AI78493 (to M.D.P.) from the National Institute of Health; by RR17677 Project 2 (to M.D.P.) from the Centers of Biomedical Research Excellence Program of the National Center for Research Resources; by NIH grant C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. R.R. is supported in part by a Medical Scientist Training Grant from the National Institutes of Health (GM08716) and by the Graduate Assistance in Areas of National Need (GAANN) training grant in Lipidology and New Technologies from the U.S. Department of Education. Dr. Maurizio Del Poeta is a Burroughs Wellcome New Investigator in Pathogenesis of Infectious Diseases.
Glossary
- Farnesol (FA)
isoprenoid lipid involved in quorum sensing in Candida and apoptosis in Aspergillus
- Eicosanoids
a class of lipids derived from fatty acids like arachidonic acid that are involved in inflammation and immunomodulation in humans
- Sphingolipids
a class of lipids including species with N-linked acylation of a sphingosine backbone and several variations of head groups
- Hyphae
long branching filamentous cells in fungi and the major type of vegetative growth
- AC
adenylate cyclase
- MAP
mitogen-activated protein
- PGE2
prostaglandin E2
- COX
cyclooxygenase
- IPC
inositol phosphorylceramide
- DAG
diacylglycerol
- Pkc1
protein kinase C1
- APP1
antiphagocytic protein 1
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Ryan Rhome, Email: rhome@musc.edu.
Maurizio Del Poeta, Email: delpoeta@musc.edu.
LITERATURE CITED
- 1.Alem MA, Douglas LJ. Prostaglandin production during growth of Candida albicans biofilms. J. Med. Microbiol. 2005;54:1–5. doi: 10.1099/jmm.0.46172-0. [DOI] [PubMed] [Google Scholar]
- 2.Bordon AP, Dias-Melicio LA, Acorci MJ, Biondo GA, Fecchio D, et al. Prostaglandin E(2) production by high and low virulent strains of Paracoccidioides brasiliensis. Mycopathologia. 2007;163:129–135. doi: 10.1007/s11046-007-0098-1. [DOI] [PubMed] [Google Scholar]
- 3.Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 4.Braun BR, Johnson AD. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics. 2000;155:57–67. doi: 10.1093/genetics/155.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun BR, Kadosh D, Johnson AD. NRG1, a repressor of filamentous growth in C. albicans, is down-regulated during filament induction. EMBO J. 2001;20:4753–4761. doi: 10.1093/emboj/20.17.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao YY, Cao YB, Xu Z, Ying K, Li Y, et al. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob. Agents Chemother. 2005;49:584–589. doi: 10.1128/AAC.49.2.584-589.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cenci E, Francisci D, Belfiori B, Pierucci S, Baldelli F, et al. Tipranavir exhibits different effects on opportunistic pathogenic fungi. J. Infect. 2008;56:58–64. doi: 10.1016/j.jinf.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Cho T, Aoyama T, Toyoda M, Nakayama H, Chibana H, Kaminishi H. Transcriptional changes in Candida albicans genes by both farnesol and high cell density at an early stage of morphogenesis in N-acetyl-D-glucosamine medium. Nippon Ishinkin Gakkai. Zasshi. 2007;48:159–167. doi: 10.3314/jjmm.48.159. [DOI] [PubMed] [Google Scholar]
- 9.Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, et al. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 2001;39:166–175. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- 10.Cugini C, Calfee MW, Farrow JM, 3rd, Morales DK, Pesci EC, Hogan DA. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol. Microbiol. 2007;65:896–906. doi: 10.1111/j.1365-2958.2007.05840.x. [DOI] [PubMed] [Google Scholar]
- 11.Cushion MT, Collins MS, Linke MJ. Biofilm formation by Pneumocystis. Eukaryot. Cell. 2008;8:197–206. doi: 10.1128/EC.00202-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signaling pathway and the regulation of morphogenesis. Mol. Microbiol. 2008;67:47–62. doi: 10.1111/j.1365-2958.2007.06013.x. Showed farnesol role in Ras pathway.
- 13.Deva R, Ciccoli R, Schewe T, Kock JL, Nigam S. Arachidonic acid stimulates cell growth and forms a novel oxygenated metabolite in Candida albicans. Biochim. Biophys. Acta. 2000;1486:299–311. doi: 10.1016/s1388-1981(00)00073-1. [DOI] [PubMed] [Google Scholar]
- 14.Enjalbert B, Whiteway M. Release from quorum-sensing molecules triggers hyphal formation during Candida albicans resumption of growth. Eukaryot. Cell. 2005;4:1203–1210. doi: 10.1128/EC.4.7.1203-1210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erb-Downward JR, Huffnagle GB. Cryptococcus neoformans produces authentic prostaglandin E2 without a cyclooxygenase. Eukaryot. Cell. 2007;6:346–350. doi: 10.1128/EC.00336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erb-Downward JR, Noggle RM, Williamson PR, Huffnagle GB. The role of laccase in prostaglandin production by Cryptococcus neoformans. Mol. Microbiol. 2008;68:1428–1437. doi: 10.1111/j.1365-2958.2008.06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erb-Downward JR, Noverr MC. Characterization of prostaglandin E2 production by Candida albicans. Infect. Immun. 2007;75:3498–3505. doi: 10.1128/IAI.00232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Q, Summers E, Guo B, Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 1999;181:6339–6346. doi: 10.1128/jb.181.20.6339-6346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia J, Shea J, Alvarez-Vasquez F, Qureshi A, Luberto C, et al. Mathematical modeling of pathogenicity of Cryptococcus neoformans. Mol. Syst. Biol. 2008;4:183. doi: 10.1038/msb.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerik KJ, Bhimireddy SR, Ryerse JS, Specht CA, Lodge JK. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot. Cell. 2008;7:1685–1698. doi: 10.1128/EC.00146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerik KJ, Donlin MJ, Soto CE, Banks AM, Banks IR, et al. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol. Microbiol. 2005;58:393–408. doi: 10.1111/j.1365-2958.2005.04843.x. Identifies PKC as crucial for cell wall integrity.
- 22.Haas-Stapleton EJ, Lu Y, Hong S, Arita M, Favoreto S, et al. Candida albicans modulates host defense by biosynthesizing the proresolving mediator resolvin E1. PLoS ONE. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriques M, Martins M, Azeredo J, Oliveira R. Effect of farnesol on Candida dubliniensis morphogenesis. Lett. Appl. Microbiol. 2007;44:199–205. doi: 10.1111/j.1472-765X.2006.02044.x. [DOI] [PubMed] [Google Scholar]
- 24. Heung LJ, Kaiser AE, Luberto C, Del Poeta M. The role and mechanism of diacylglycerol-protein kinase C1 signaling in melanogenesis by Cryptococcus neoformans. J. Biol. Chem. 2005;280:28547–28555. doi: 10.1074/jbc.M503404200. Describes the mechanism of IPC-melanin-virulence connection through PKC and laccase.
- 25.Heung LJ, Luberto C, Plowden A, Hannun YA, Del Poeta M. The sphingolipid pathway regulates Pkc1 through the formation of diacylglycerol in Cryptococcus neoformans. J. Biol. Chem. 2004;279:21144–21153. doi: 10.1074/jbc.M312995200. [DOI] [PubMed] [Google Scholar]
- 26. Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001;67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. First identifies farnesol as a quorum sensing molecule.
- 27.Hornby JM, Nickerson KW. Enhanced production of farnesol by Candida albicans treated with four azoles. Antimicrob. Agents Chemother. 2004;48:2305–2307. doi: 10.1128/AAC.48.6.2305-2307.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurley JH, Newton AC, Parker PJ, Blumberg PM, Nishizuka Y. Taxonomy and function of C1 protein kinase C homology domains. Protein Sci. 1997;6:477–480. doi: 10.1002/pro.5560060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadosh D, Johnson AD. Rfg1, a protein related to the Saccharomyces cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in Candida albicans. Mol. Cell Biol. 2001;21:2496–2505. doi: 10.1128/MCB.21.7.2496-2505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneko A, Umeyama T, Utena-Abe Y, Yamagoe S, Niimi M, Uehara Y. Tcc1p, a novel protein containing the tetratricopeptide repeat motif, interacts with Tup1p to regulate morphological transition and virulence in Candida albicans. Eukaryot. Cell. 2006;5:1894–1905. doi: 10.1128/EC.00151-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kebaara BW, Langford ML, Navarathna DH, Dumitru R, Nickerson KW, Atkin AL. Candida albicans Tup1 is involved in farnesol-mediated inhibition of filamentous-growth induction. Eukaryot. Cell. 2008;7:980–987. doi: 10.1128/EC.00357-07. Proved the mechanistic connection with farnesol and Tup1.
- 32.Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect. Immun. 2007;75:4792–4798. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalaf RA, Zitomer RS. The DNA binding protein Rfg1 is a repressor of filamentation in Candida albicans. Genetics. 2001;157:1503–1512. doi: 10.1093/genetics/157.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruppa M, Krom BP, Chauhan N, Bambach AV, Cihlar RL, Calderone RA. The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot. Cell. 2004;3:1062–1065. doi: 10.1128/EC.3.4.1062-1065.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leberer E, Harcus D, Dignard D, Johnson L, Ushinsky S, et al. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signaling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 2001;42:673–687. doi: 10.1046/j.1365-2958.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- 36.Levitin A, Whiteway M. The effect of prostaglandin E2 on transcriptional responses of Candida albicans. Microbiol. Res. 2007;162:201–210. doi: 10.1016/j.micres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Lorek J, Poggeler S, Weide MR, Breves R, Bockmuhl DP. Influence of farnesol on the morphogenesis of Aspergillus niger. J. Basic Microbiol. 2008;48:99–103. doi: 10.1002/jobm.200700292. [DOI] [PubMed] [Google Scholar]
- 38.Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell. 2004;3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luberto C, Martinez-Marino B, Taraskiewicz D, Bolanos B, Chitano P, et al. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J. Clin. Investig. 2003;112:1080–1094. doi: 10.1172/JCI18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luberto C, Toffaletti DL, Wills EA, Tucker SC, Casadevall A, et al. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev. 2001;15:201–212. doi: 10.1101/gad.856001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin SW, Konopka JB. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot. Cell. 2004;3:675–684. doi: 10.1128/EC.3.3.675-684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAlester G, O’Gara F, Morrissey JP. Signal-mediated interactions between Pseudomonas aeruginosa and Candida albicans. J. Med. Microbiol. 2008;57:563–569. doi: 10.1099/jmm.0.47705-0. [DOI] [PubMed] [Google Scholar]
- 43.Mosel DD, Dumitru R, Hornby JM, Atkin AL, Nickerson KW. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl. Environ. Microbiol. 2005;71:4938–4940. doi: 10.1128/AEM.71.8.4938-4940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murad AM, Leng P, Straffon M, Wishart J, Macaskill S, et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2001;20:4742–4752. doi: 10.1093/emboj/20.17.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navarathna DH, Hornby JM, Hoerrmann N, Parkhurst AM, Duhamel GE, Nickerson KW. Enhanced pathogenicity of Candida albicans pretreated with subinhibitory concentrations of fluconazole in a mouse model of disseminated candidiasis. J. Antimicrob. Chemother. 2005;56:1156–1159. doi: 10.1093/jac/dki383. [DOI] [PubMed] [Google Scholar]
- 46.Navarathna DH, Hornby JM, Krishnan N, Parkhurst A, Duhamel GE, Nickerson KW. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. Infect. Immun. 2007;75:1609–1618. doi: 10.1128/IAI.01182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navarathna DH, Nickerson KW, Duhamel GE, Jerrels TR, Petro TM. Exogenous farnesol interferes with the normal progression of cytokine expression during candidiasis in a mouse model. Infect. Immun. 2007;75:4006–4011. doi: 10.1128/IAI.00397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noverr MC, Cox GM, Perfect JR, Huffnagle GB. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 2003;71:1538–1547. doi: 10.1128/IAI.71.3.1538-1547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 2001;69:2957–2963. doi: 10.1128/IAI.69.5.2957-2963.2001. First reports prostaglandin synthesis in Cryptococcus and Candida.
- 50.Noverr MC, Toews GB, Huffnagle GB. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect. Immun. 2002;70:400–402. doi: 10.1128/IAI.70.1.400-402.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozpolat B, Akar U, Mehta K, Lopez-Berestein G. PKC delta and tissue transglutaminase are novel inhibitors of autophagy in pancreatic cancer cells. Autophagy. 2007;3:480–483. doi: 10.4161/auto.4349. [DOI] [PubMed] [Google Scholar]
- 52.Perumal P, Mekala S, Chaffin WL. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob. Agents Chemother. 2007;51:2454–2463. doi: 10.1128/AAC.01237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhome R, McQuiston T, Kechichian T, Bielawska A, Hennig M, et al. Biosynthesis and immunogenicity of glucosylceramide in Cryptococcus neoformans and other human pathogens. Eukaryot. Cell. 2007;6:1715–1726. doi: 10.1128/EC.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rittershaus PC, Kechichian TB, Allegood JC, Merrill AH, Jr, Hennig M, et al. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Investig. 2006;116:1651–1659. doi: 10.1172/JCI27890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossignol T, Logue ME, Reynolds K, Grenon M, Lowndes NF, Butler G. Transcriptional response of Candida parapsilosis following exposure to farnesol. Antimicrob. Agents Chemother. 2007;51:2304–2312. doi: 10.1128/AAC.01438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato T, Watanabe T, Mikami T, Matsumoto T. Farnesol, a morphogenetic autoregulatory substance in the dimorphic fungus Candida albicans, inhibits hyphae growth through suppression of a mitogen-activated protein kinase cascade. Biol. Pharm. Bull. 2004;27:751–752. doi: 10.1248/bpb.27.751. [DOI] [PubMed] [Google Scholar]
- 57. Semighini CP, Hornby JM, Dumitru R, Nickerson KW, Harris SD. Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol. Microbiol. 2006;59:753–764. doi: 10.1111/j.1365-2958.2005.04976.x. Showed farnesol induces apoptosis in Aspergillus.
- 58.Shchepin R, Navarathna DH, Dumitru R, Lippold S, Nickerson KW, Dussault PH. Influence of heterocyclic and oxime-containing farnesol analogs on quorum sensing and pathogenicity in Candida albicans. Bioorg. Med. Chem. 2008;16:1842–1848. doi: 10.1016/j.bmc.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Shea JM, Kechichian TB, Luberto C, Del Poeta M. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect. Immun. 2006;74:5977–5988. doi: 10.1128/IAI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siafakas AR, Sorrell TC, Wright LC, Wilson C, Larsen M, et al. Cell wall-linked cryptococcal phospholipase B1 is a source of secreted enzyme and a determinant of cell wall integrity. J. Biol. Chem. 2007;282:37508–37514. doi: 10.1074/jbc.M707913200. [DOI] [PubMed] [Google Scholar]
- 61.Stano P, Williams V, Villani M, Cymbalyuk ES, Qureshi A, et al. App1: an antiphagocytic protein that binds to complement receptors 3 and 2. J. Immunol. 2009;182:84–91. doi: 10.4049/jimmunol.182.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tommasino N, Villani M, Qureshi A, Henry J, Luberto C, Del Poeta M. Atf2 transcription factor binds to the APP1 promoter in Cryptococcus neoformans: stimulatory effect of diacylglycerol. Eukaryot. Cell. 2008;7:294–301. doi: 10.1128/EC.00315-07. Showed link between DAG and App1 expression through Atf2.
- 63.Tsitsigiannis DI, Bok JW, Andes D, Nielsen KF, Frisvad JC, Keller NP. Aspergillus cyclooxygenase-like enzymes are associated with prostaglandin production and virulence. Infect. Immun. 2005;73:4548–4559. doi: 10.1128/IAI.73.8.4548-4559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuchimori N, Sharkey LL, Fonzi WA, French SW, Edwards JE, Jr, Filler SG. Reduced virulence of HWP1-deficient mutants of Candida albicans and their interactions with host cells. Infect. Immun. 2000;68:1997–2002. doi: 10.1128/iai.68.4.1997-2002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Ooij C, Kalman L, van Ijzendoorn Nishijima M, Hanada K, et al. Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis. Cell Microbiol. 2000;2:627–637. doi: 10.1046/j.1462-5822.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- 66.Weber K, Sohr R, Schulz B, Fleischhacker M, Ruhnke M. Secretion of E,E-farnesol and biofilm formation in eight different Candida species. Antimicrob. Agents Chemother. 2008;52:1859–1861. doi: 10.1128/AAC.01646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westwater C, Balish E, Schofield DA. Candida albicans-conditioned medium protects yeast cells from oxidative stress: a possible link between quorum sensing and oxidative stress resistance. Eukaryot. Cell. 2005;4:1654–1661. doi: 10.1128/EC.4.10.1654-1661.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright LC, Santangelo RM, Ganendren R, Payne J, Djordjevic JT, Sorrell TC. Cryptococcal lipid metabolism: Phospholipase B1 is implicated in transcellular metabolism of macrophage-derived lipids. Eukaryot. Cell. 2007;6:37–47. doi: 10.1128/EC.00262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng X, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]