Abstract
There is a sufficient body of work documenting the distribution of 3-hydroxy oxylipins in microbes. However, there is limited information on the role of these compounds in microbial pathogenesis. When derived from mammalian cells, these compounds regulate patho-biological processes, thus an understanding of 3-hydroxy oxylipin function and metabolism could prove important in shedding light on how these compounds mediate cellular pathology and physiology. This could present 3-hydroxy oxylipin biosynthetic pathways as targets for drug development. In this minireview, we interrogate the relevant yeast and bacterial 3-hydroxy oxylipin literature in order to appreciate how these compounds may influence the inflammatory response leading to disease development.
Keywords: Bacteria, 3-Hydroxy oxylipins, Inflammation, Pathogenesis, Yeast
1. Biochemistry: definition, occurence and biosynthesis
The word “oxylipin” describes a group of secondary metabolites that originate from the oxidation or further conversion of polyunsaturated fatty acids [1]. These lipid-based molecules are pivotal signal molecules documented to act in a hormone-like manner where they mediate a number of complex biological processes across a number of life domains. In terrestrial higher plants, oxylipins play a role in host defence mechanisms against pathogens and pests [2]. In mammalian cells, these molecules regulate cellular homeostasis and immune responses [3–5]. In marine algae, it is hypothesised that they may be involved in defence mechanisms [6], while in bacteria and fungi they may regulate virulence, biofilm formation via quorum-sensing mechanism [7,8], and play a role in sexual and asexual development [9]. Oxylipins constitute among others the eicosanoids and hydroxy oxylipins [9]. Given the broad scope of oxylipins, their distribution and function, this minireview is dedicated to hydroxy oxylipins and in particular, 3-hydroxy oxylipins in fungi and bacteria. The reader is referred to excellent reviews paying special attention to other oxylipins and lipid mediators that regulate important biological processes in cellular physiology and pathology [1,2,9–13].
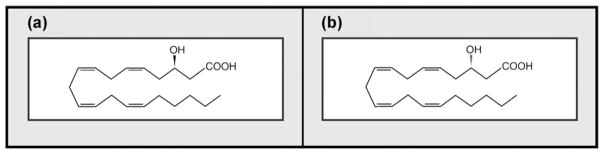
3-Hydroxy oxylipins (3-OH oxylipins) are fatty acid-based molecules characterised by a hydroxyl group on the beta-carbon atom, from the carboxylic group (Fig. 1). The carbon chain of 3-OH oxylipins may be branched and may vary considerably in length as well as in the degree of desaturation [9,14,15]. 3-Hydroxy oxylipins are also widely distributed in nature, occurring in mammals, bacteria and yeasts, including medically important pathogens [7,16–20]. In mammalian systems, production of 3-hydroxy oxylipins is mainly attributed to fatty acid oxidation disorders. Accumulation of these molecules in the blood is regarded as a major metabolic indicator of long chain hydroxyacyl coenzyme A dehydrogenase (LCHAD) deficiency in newborns and patients with liver failure [20].
Fig. 1.
The chemical structures of a typical 3-hydroxy oxylipin. (a) Depicts the R-enantiomer while; (b) depicts the S-enantiomer. Obtained with permission from Kock et al. [64].
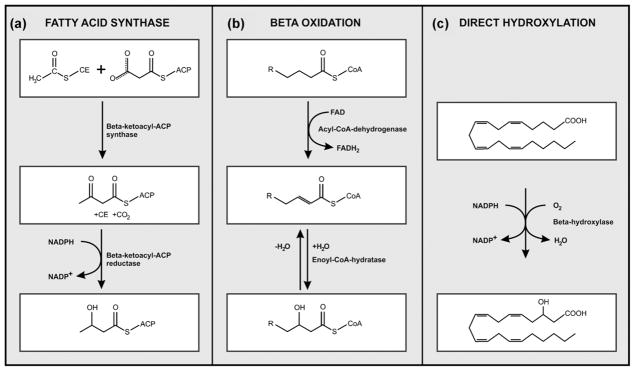
The biosynthetic pathways for 3-OH oxylipins vary and although some remain poorly described, three generally accepted enzymatic routes have been reported (Fig. 2):
Fig. 2.
Biosynthetic pathways catalysing 3-hydroxy oxylipin production. (a) and (b) Depict enzymatic route similar to fatty acid synthase and beta oxidation, respectively while; (c) depicts direct hydroxylation of a fatty acid molecule.
fatty acid synthase (FAS) enzyme-system [21,22]. Here, the NADPH-dependent beta-ketoacyl-ACP reductase carries out the reduction of beta-ketoacyl-ACP to beta-hydroxyacyl-ACP,
an enzymatic pattern similar to mitochondrial beta oxidation, however, incomplete [23]. The oxygen of the hydroxyl group inserted in the fatty acid chain originates from water. In this case, the produced 3-D hydroxyacyl-CoA enantiomer, cannot be, or is poorly, metabolised further by 3-hydroxyacyl-CoA dehydrogenase [24], and consequently accumulates inside the mitochondria. This compound is then excreted as a 3-D hydroxy oxylipin [25],
direct hydroxylation of the fatty acid via a cytochrome P450 enzyme [26,27], with the oxygen molecule originating from the air.
2. Patho-biological functions of microbial 3-hydroxy oxylipins
Microbial cell walls perform two critical roles in immunity, namely to provide protection from the extracellular environment, and interaction with the environment [28]. 3-Hydroxy oxylipins have been reported to be closely associated with cell walls of pathogens [7,16,18,19]. In bacteria, they are attached or bound to cell wall components, whereas in yeasts, they are mainly in a free form - coating or deposited on cell wall surfaces. Literature suggests that during infection, microbial cell wall components mediate key processes that could modulate the immune response leading to development of disease [29–31]. This minireview pays special attention to the role of 3-OH oxylipins in modulating the inflammatory response. Inflammation, usually a result of cytokine production, is a complex biological response that attempts to clear and heal vascular tissue of infection or other forms of damage [32,33]. Disease outcome may determine a shift in the balance maintained by both pro-inflammatory and anti-inflammatory cytokines.
2.1. Bacterial 3-hydroxy oxylipins
3-Hydroxy oxylipins occur as unique structural components of the sepsis-causing endotoxin (lipopolysaccharide layer; LPS), which is characteristic of Gram-negative bacteria [34]. The 3-OH oxylipin-containing Lipid A fraction is documented to be responsible for the toxic and immuno-modulating properties of LPS [30,35,36]. Upon shedding, the endotoxin triggers an innate immune response characterised by cytokine production. Here, the endotoxin is first recognised by receptor protein i.e. cluster of differentiation (CD)-14 and in turn, presented to toll-like receptor (TLR)-4 on surfaces of innate cells resulting in intracellular signalling [37,38]. This leads to the production of pro-inflammatory cytokines, i.e. interleukin (IL-) 1 and tumour necrosis factor alpha (TNF-alpha), and activation of mononuclear cells. These cytokines can then induce synthesis of mediator molecules viz. cyclo-oxygenase 2, phospholipase A2 and nitric oxide (NO) synthase - which up regulate inflammation [32,39]. Subsequently, these cytokines together with mediator molecules, acting through specific G-protein-coupled receptors, promote inflammation, causing widespread endothelial injury and platelet activation [40,41], and at high endotoxin levels, septic shock can be induced [41].
Interestingly, 3-hydroxy oxylipins are used as biomarkers for estimating the amount of endotoxins and Gram-negative bacteria in atmospheric bioaerosols [42]. Inhalation of bioaerosols-containing 3-hydroxy oxylipins i.e. entotoxin, can also initiate infectious processes that elicit allergenic and immunological responses [43,44]. Peden et al. [45] reported that a nasal challenge with LPS causes an eosinophil influx in nasal airways of atopic subjects, suggesting exposure may increase allergen-induced bronchial inflammation in asthmatics [43].
3-OH oxylipins from Porphyromonas gingivalis constitute a major component of bioactive lipids reported to potentiate interleukin-1b-mediated secretory response in gingival fibroblasts. This organism is thought to be a major periodontal pathogen associated with inflammatory periodontal disease in adults [46].
3-Hydroxy oxylipins also occur as complex molecules such as mycolic acid, which are 3-OH oxylipins with long alpha alkyl branched chains [22]. Here too, 3-OH oxylipins are associated with pathogenicity of Mycobacterium tuberculosis, the causative agent of tuberculosis. These compounds confer the pathogen with the ability to grow within macrophages and to avoid detection [47]. When this bacterium is lysed, mycolic acid is released from the cell wall. Regarded as pathogen-associated molecular patterns (PAMP), the released mycolic acid may then invoke an immune response [48–51]. Most of the damage in the lungs during tuberculosis is thought to be due to the up regulated inflammatory response. Here, it is hypothesised that IL-1, TNF-alpha and NO may induce oxidative damage to mitochondria by inhibiting the electron transport chain [41]. This inhibitory action results in less cellular energy and dysoxia.
2.2. Yeast 3-hydroxy oxylipins
The lipopolysaccharide layer is not limited to bacteria. The presence of this cell wall component has been reported in a medically important higher basidiomycete, Antrodia camphorata [52]. Interestingly, this fungal LPS reverses immuno-regulating properties exerted by bacterial LPS.
Nigam and co-workers were the first to provide evidence concerning the biological function of 3-OH oxylipins in mammalian cells [53]. In their study, 3-OH oxylipins were observed to act as a strong chemotactic agent - the potency of which is comparable with those of leukotriene B4 or fMet-Leu-Phe. In addition, 3-OH oxylipins affected signal transduction processes in human neutrophils and tumour cells in multiple ways, possibly via a G-protein receptor.
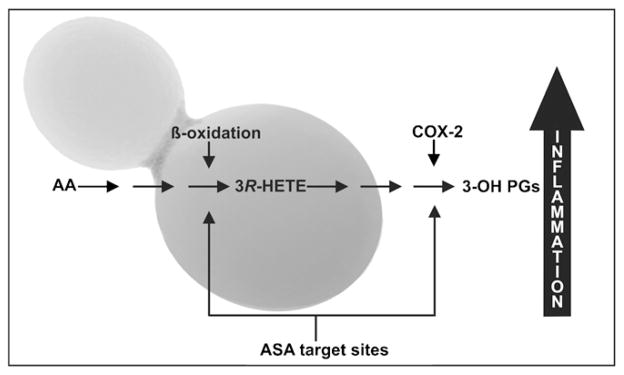
Fluorescence studies conducted using a specific immunological probe against 3-OH oxylipins, revealed these compounds to be deposited on hyphal cell surfaces of the pathogen, Candida albicans, the causative agent of candidiasis [16,54,55]. In 2005, Ciccoli and co-workers elucidated a novel acetylsalicylic acid (ASA; aspirin) sensitive patho-biological process in C. albicans [17]. They found that this yeast converts arachidonic acid, released from infected host cells, to a 3-OH oxylipin i.e. 3-hydroxy eicosatetraenoic acid (3-HETE) via incomplete mitochondrial beta-oxidation. This 3-OH oxylipin, which is stereo-chemically similar to arachidonic acid, then acts as substrate for the host cyclooxygenase-2 (COX-2), leading to the production of potent pro-inflammatory 3-OH prostaglandin E2 (3-OH-PG E2) (Fig. 3). This novel compound could signal the expression of IL-6 gene, via the EP 3 receptor (PGE2 receptor 3) and raise cAMP levels via the EP 4 receptor. These results led this group of researchers to conclude that, these compounds have strong biological activities similar to and in some cases even more potent than those of the normally produced mammalian eicosanoids. This organism can also employ its own endogenously produced PG E2 to mediate pathogenesis [12,56].
Fig. 3.
A diagram showing the formation of potent inflammatory 3-hydroxy prostaglandins in host cells from 3-HETE produced via incomplete beta-oxidation from host-released arachidonic acid (AA) by the yeast Candida albicans. ASA, acetyl-salicylic acid; COX-2, cyclooxygenase-2; 3(R)-HETE, 3(R) hydroxyeicosatetraenoic acid; 3-OH PGs, 3-hydroxy prostaglandins.
Obtained with permission from Kock et al. [64].
Recently, the Nigam group also showed that 3-OH oxylipins can effect quorum sensing in C. albicans [8], a function used by microorganisms to measure population density and to regulate pathogenicity [8,57]. This group demonstrated that this yeast utilises 3-OH oxylipins, i.e. 3-OH-14:2 produced from 18:2, as a signal for expression of genes responsible for accelerating cell morphogenesis at a certain population density.
Bio-prospecting studies into the presence of these compounds in other pathogenic yeasts led to the discovery of 3-OH oxylipins in capsules of Cryptococcus neoformans [18]. The cryptococcal capsule can inhibit phagocytosis and influence cytokine production, functions crucial for mounting an efficient immune response [58–60]. The study by Sebolai and co-workers [18,25] revealed a novel release mechanism of these compounds as “oily-droplets” into the surrounding environment. The release mechanism involved the participation of cell wall components namely, capsule and spiky capsular protuberances, as well mitochondria. This release mechanism was inhibited by ASA in a dose dependent manner. However, the function of these compounds upon release remains unknown. Could they also act as virulence factors alone or in association with the glucuronoxylomannans (GXM)? It has been established that GXM induces inflammation by activating TLRs [61,62].
3. Concluding remarks and perspectives
Over the years, microbial lipids have been shown to have bioactive functions mediating a number of cellular processes [10–13,63]. Most of our knowledge on 3-OH oxylipins stems from extensive studies conducted in non-pathogenic yeasts and studies focusing on bacterial endotoxins [9,35,64]. In yeast studies, the biological functions of these compounds were defined based on their role in facilitating cell aggregation, possibly for protection purposes [65], or for facilitating spore release from asci following sexual reproduction [9]. In addition, these molecules act as “toxins” secreted by lactic acid bacteria, where they are employed to appropriate environmental advantage against yeasts and molds in the bio-preserve of fermentation products [66]. As analysed in this minireview, we now can appreciate the role of 3-OH oxylipins, mainly associated with cell wall components or surfaces of medically important pathogens, as signal molecules, triggering inflammatory responses.
The role of mitochondria in cancer development and programmed-cell death is well established [67–70]. As reported in literature, microbial mitochondria “house” enzymatic pathways that catalyse the biosynthesis of patho-biologically active 3-OH oxylipins [17,21,23,24]. This exposes mitochondria as targets for controlling biosynthesis and effects of 3-OH oxylipins, hence further highlighting the critical role of this organelle in cellular pathogenesis. Since the study by Ciccoli et al. [17] demonstrated that during infection, mammalian cyclooxygenases can serve as additional enzymes catalysing synthesis of 3-OH oxylipins, further contributing to inflammation, it will be interesting to determine if infected host cell’s mitochondria could serve as another 3-OH oxylipin production site particularly, in persons without fatty acid oxidation disorders [20].
Other questions that need to be answered are, could the actions of aspirin, a known anti-mitochondrial and anti-fungal [25,71–76], now be extended to control bacterial infections caused by the highly aerobic M. tuberculosis? Can aspirin inhibit the production of mycolic acids based on the structural similarities between aspirin and acyl-portions of the FAS biosynthetic pathway? In answering these questions, consideration should be taken in order to realise efficacy against pathogens without adversely affecting human mitochondria.
In higher eukaryotic cell systems such as in humans, mitochondria are responsible for generation of cellular energy under strictly aerobic conditions [77,78], hence colonisation of lungs by highly aerobic pathogens such as M. tuberculosis and C. neoformans. During pulmonary cryptococcosis, cryptococcal phospholipase can degrade the phospholipid component of lung surfactants leading to increased inflammation via the production of eicosanoids [12]. And unlike in some lower eukaryotic cell systems, humans cannot switch to fermentation when oxygen is depleted thus mitochondrial damage can prove deadly.
Could the change in form and complexity of 3-OH oxylipins from bacteria (bound or attached to cell wall components) to yeasts (in a “free” form and deposited onto cell walls) be indicative of an evolutionary development? According to the endosymbiotic theory, it is proposed that mitochondria are descendents of ancient bacteria [79]. Therefore, is it possible that the present day mitochondria, ancestral descendant of ancient sepsis-causing bacteria through this theory, adapted and found a novel way to shed virulence factors i.e. 3-OH oxylipins, from a safe or protected environment within eukaryotic cells? Though the theory is controversial, there is molecular evidence including phylogeny studies, in support of the theory [80]. Therefore, it would be interesting to determine if genes encoding enzymes involved in the biosynthesis of mitochondrially-produced 3-OH oxylipins are related or even conserved in both yeasts and bacteria.
Acknowledgments
Our apologies to those authors whose work we may have overlooked in this minireview. The authors would also like to thank anonymous reviewers for their scholarly contributions. Our work on 3-OH oxylipins has been supported by the National Research Foundation of South Africa. Maurizio Del Poeta (MDP) is supported by National Institute of Health (NIH) awards AI056168, AI071142, AI078493, and AI087541. MDP is a Burroughs Welcome New Investigator in the Pathogenesis of Infectious Diseases.
Footnotes
Conflict of interest
There are no competing interests.
References
- 1.Brodhun F, Feussner I. Oxylipins in fungi. FEBS J. 2011;278:1047–63. doi: 10.1111/j.1742-4658.2011.08027.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsitsigiannis DI, Keller NP. Oxylipins as developmental and host-fungal communication signals. Trends Microbiol. 2007;15:109–18. doi: 10.1016/j.tim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–75. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 4.Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–72. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 5.Curtis-Prior P. The eicosanoids. West Sussex: John Wiley and Sons Ltd; 2004. [Google Scholar]
- 6.Bouarab K, Adas F, Gaquerel E, Kloareg B, Salaün J-P, Potin P. The innate immunity of a marine red algae involves oxylipins from both the eicosanoids and octadecanoid pathways. Plant Physiol. 2004;135:1838–48. doi: 10.1104/pp.103.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–40. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nigam S, Ciccoli R, Ivanov I, Sczepanski M, Deva R. On mechanism of quorum sensing in Candida albicans by 3(R)-hydroxy-tetradecaenoic acid. Curr Microbiol. 2010;62:55–63. doi: 10.1007/s00284-010-9666-6. [DOI] [PubMed] [Google Scholar]
- 9.Kock JLF, Strauss CJ, Pohl CH, Nigam S. The distribution of 3-hydroxy oxylipins in fungi. Prostaglandins Other Lipid Mediat. 2003;71:85–96. doi: 10.1016/s1098-8823(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 10.Shea JM, Del Poeta M. Lipid signaling in pathogenic fungi. Curr Opin Microbiol. 2006;9:352–8. doi: 10.1016/j.mib.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Singh A, Del Poeta M. Lipid signaling in pathogenic fungi. Cell Microbiol. 2011;13:177–85. doi: 10.1111/j.1462-5822.2010.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noverr MC, Erb-Downward JR, Huffnagle GB. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin Microbiol Rev. 2003;16:517–33. doi: 10.1128/CMR.16.3.517-533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erb-Downward JR, Huffnagle GB. Role of oxylipins and other lipid mediators in fungal pathogenesis. Future Microbiol. 2006;192:219–27. doi: 10.2217/17460913.1.2.219. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt RK, Falck JR, Nigam S. Enantiospecific total synthesis of a novel arachidonic acid metabolite 3-hydroxyeicosatetraenoic acid. Tetrahedron Lett. 1998;39:249–52. [Google Scholar]
- 15.Groza NV, Ivanov IV, Romanov SG, Myagkova GI, Nigam S. A novel synthesis of 3(R)-HETE, 3(R)-HTDE and enzymatic synthesis of 3(R), 15(S)-DiHETE. Tetrahedron. 2002;58:9859–63. [Google Scholar]
- 16.Deva R, Ciccoli R, Schewe T, Kock JLF, Nigam S. Arachidonic acid stimulates cell growth and forms a novel oxygenated metabolite in Candida albicans. Biochim Biophys Acta. 2000;1486:299–311. doi: 10.1016/s1388-1981(00)00073-1. [DOI] [PubMed] [Google Scholar]
- 17.Ciccoli R, Sahi S, Singh S, et al. Oxygenation by cyclooxygenase-2 (COX-2) of 3-hydroxyeicosatetraenoic acid (3-HETE), a fungal mimetic of arachidonic acid, produces a cascade of novel bioactive 3-hydroxy-eicosanoids. Biochem J. 2005;390:737–47. doi: 10.1042/BJ20041995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebolai OM, Pohl CH, Botes PJ, et al. 3-Hydroxy fatty acids found in capsules of Cryptococcus neoformans. Can J Microbiol. 2007;53:809–12. doi: 10.1139/W07-045. [DOI] [PubMed] [Google Scholar]
- 19.Marrakchi H, Bardou F, Lancèlle M-A, Daffè M. A comprehensive overview of mycolic acid structure and biosynthesis. In: Daffè M, Reyrat J-M, editors. The mycobacterial cell envelope. Washington: ASM Press; 2008. pp. 41–62. [Google Scholar]
- 20.Jones PM, Bennett MJ. Clinical applications of 3-hydroxy fatty acids analysis by gas chromatography–mass spectrometry. Biochim Biophys Acta. doi: 10.1016/j.bbalip.2011.06.026. in press. [DOI] [PubMed] [Google Scholar]
- 21.Hiltunen JK, Okubo F, Kursu KJ, Autio KJ, Kastaniotis AJ. Mitochondrial fatty acid synthesis and maintenance of respiratory competent mitochondria in yeast. Biochem Soc Trans. 2005;33:1162–5. doi: 10.1042/BST20051162. [DOI] [PubMed] [Google Scholar]
- 22.Takayama K, Wang C, Besra G. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin Microbiol Rev. 2005;18:81–91. doi: 10.1128/CMR.18.1.81-101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venter P, Kock JLF, Kumar S, et al. Production of 3R-hydroxy-polyenoic fatty acids by the yeast Dipodascopsis uninucleata UOFS-Y128. Lipids. 1997;32:1277–83. doi: 10.1007/s11745-006-0164-3. [DOI] [PubMed] [Google Scholar]
- 24.Finnerty WR. Microbial lipid metabolism. In: Ratledge C, Wilkinson SG, editors. Microbial lipids. Vol. 2. London: Academic Press; 1989. pp. 525–58. [Google Scholar]
- 25.Sebolai OM, Pohl CH, Botes PJ, van Wyk PWJ, Kock JLF. The influence of acetyl-salicylic acid on oxylipin migration in Cryptococcus neoformans var. neoformans UOFS Y-1378. Can J Microbiol. 2008;54:91–6. doi: 10.1139/w07-114. [DOI] [PubMed] [Google Scholar]
- 26.Harayama S, Kok M, Neidle EL. Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol. 1992;46:565–71. doi: 10.1146/annurev.mi.46.100192.003025. [DOI] [PubMed] [Google Scholar]
- 27.van Dyk MS, Kock JLF, Botha A. Hydroxy long-chain fatty acids in fungi. World J Microbiol. 1994;10:495–504. doi: 10.1007/BF00367653. [DOI] [PubMed] [Google Scholar]
- 28.Pommerville JC. Cell structure and function in the bacteria and archaea. In: Pommerville JC, editor. Alcamo’s fundamentals of microbiology: body systems edition. Sudbury: Jones and Bartlett Publishers; 2010. pp. 98–128. [Google Scholar]
- 29.Barry CE, III, Lee RE, Mdluli K, et al. Mycolic acids: structure, biosynthesis, and physiological functions. Prog Lipid Res. 1998;37:143–79. doi: 10.1016/s0163-7827(98)00008-3. [DOI] [PubMed] [Google Scholar]
- 30.Seydel U, Oikawa M, Fukase K, Kusumoto S, Brandenburg K. Intrinsic conformation of lipid A is responsible for agonistics and antagonistic activity. Eur J Biochem. 2000;276:3032–9. doi: 10.1046/j.1432-1033.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 31.Bose I, Reese AJ, Ory JJ, Janbon G, Doering TL. A yeast under cover: the capsule of Cryptococcus neoformans. Eukaryot Cell. 2003;2:655–63. doi: 10.1128/EC.2.4.655-663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinarello CA. Pro-inflammatory cytokines. Chest. 2000;118:503–8. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 33.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation inflammation and well-being. Neuroimmunomodulation. 2005;12:255–69. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 34.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–72. [PubMed] [Google Scholar]
- 35.Rietschel ET, Kirikae T, Schade FU, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–25. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 36.Silipo A, de Castro C, Lanzetta R, Parrilli M, Molinaro A. Lipopolysaccharides. In: König H, Claus H, Varma A, editors. Prokaryotic cell wall compounds: structure and biochemistry. Berlin: Springer-Verlag; 2010. pp. 133–53. [Google Scholar]
- 37.Kitchens RL. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chem Immunol. 2000;74:61–82. doi: 10.1159/000058750. [DOI] [PubMed] [Google Scholar]
- 38.Triantafilou M, Triantafilou K. The dynamics of LPS recognition: complex orchestration of multiple receptors. J Endotoxin Res. 2005;11:5–11. doi: 10.1179/096805105225006641. [DOI] [PubMed] [Google Scholar]
- 39.Hopkins SJ. The pathophysiological role of cytokines. Legal Med. 2003;5:S45–57. doi: 10.1016/s1344-6223(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 40.Pober JS. Endothelial activation: intracellular signaling pathways. Arthritis Res. 2002;4:S109–16. doi: 10.1186/ar576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Annane D, Bellissant E, Cavaillon J. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 42.Lee AKY, Chan CK, Fang M, Lau APS. The 3-hydroxy fatty acids as biomarkers for quantification and characterization of endotoxins and Gram-negative bacteria in atmospheric aerosols in Hong Kong. Atmos Environ. 2004:6307–17. [Google Scholar]
- 43.Sohy C, Pons F, Casset A, et al. Low-dose endotoxin in allergic asthmatics: effect on bronchial and inflammatory responses to cat allergen. Clin Exp Allergy. 2006;36:795–802. doi: 10.1111/j.1365-2222.2006.02500.x. [DOI] [PubMed] [Google Scholar]
- 44.Poole JA, Dooley GP, Saito R, et al. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A. 2010;73:684–90. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peden DB, Tucker K, Murphy P, et al. Eosinophil influx to the nasal airway after local, low-level LPS challenge in humans. J Allergy Clin Immunol. 1999;104:388–94. doi: 10.1016/s0091-6749(99)70383-0. [DOI] [PubMed] [Google Scholar]
- 46.Mun J-Y, Onorato A, Nichols FC, et al. Structural confirmation of the dihydrosphinganine and fatty acid constituents of the dental pathogen Porphyromonas gingivalis. Org Biomol Chem. 2007;5:3826–33. doi: 10.1039/b712707c. [DOI] [PubMed] [Google Scholar]
- 47.Korf J, Stoltz A, Verschoor J, de Baetselier P, Grooten J. The Mycobacterium tuberculosis cell wall component mycolic acid elicits pathogen-associated host innate immune responses. Eur J Immunol. 2005;35:890–900. doi: 10.1002/eji.200425332. [DOI] [PubMed] [Google Scholar]
- 48.Fenton MJ. Macrophages and tuberculosis. Curr Opin Hematol. 1998;5:72–8. doi: 10.1097/00062752-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–7. [PubMed] [Google Scholar]
- 50.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–30. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 51.Modlin RL, Hans D, Brightbill HD, Godowski PJ. The toll of innate immunity on microbial pathogens. N Engl J Med. 1999;340:1834–5. doi: 10.1056/NEJM199906103402312. [DOI] [PubMed] [Google Scholar]
- 52.Cheng J-JY, Yang C-J, Cheng C-H, Wang YT, Huang NK, Lu M-K. Characterization and functional study of Antrodia camphorata lipopolysaccharide. J Agric Food Chem. 2005;53:469–547. doi: 10.1021/jf049281a. [DOI] [PubMed] [Google Scholar]
- 53.Nigam S, Schewe T, Kock JLF. 3(R)-Hydroxy oxylipins - a novel family of oxygenated polyenoic fatty acids of fungal origin. Adv Exp Med Biol. 1999;469:663–8. doi: 10.1007/978-1-4615-4793-8_95. [DOI] [PubMed] [Google Scholar]
- 54.Deva R, Ciccoli R, Kock JLF, Nigam S. Involvement of aspirin-sensitive oxylipins in vulvovaginal candidiasis. FEMS Microbiol Lett. 2001;198:37–43. doi: 10.1111/j.1574-6968.2001.tb10616.x. [DOI] [PubMed] [Google Scholar]
- 55.Deva R, Shankaranarayanan P, Ciccoli R, Nigam S. Candida albicans induces selectively transcriptional activation of cyclooxygenase-2 in HeLa cells: pivotal roles of Toll-like receptors, p38 mitogen-activated protein kinase, and NF-kB. J Immunol. 2003;171:3047–55. doi: 10.4049/jimmunol.171.6.3047. [DOI] [PubMed] [Google Scholar]
- 56.Erb-Downward JR, Noverr MC. Characterization of prostaglandin E2 production by Candida albicans. Infect Immun. 2007;75:3498–505. doi: 10.1128/IAI.00232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karatuna O, Yagci A. Analysis of quorum sensing-dependent virulence factor production and its relation with antimicrobial susceptibility in Pseudomonas aeruginosa respiratory isolates. Eur Soc Clin Microbiol Infect Dis. 2010;16:1770–5. doi: 10.1111/j.1469-0691.2010.03177.x. [DOI] [PubMed] [Google Scholar]
- 58.McClelland EE, Casadevall A, Eisenman CH, Kavanagh K, editors. New insights in medical mycology. New York: Springer; 2007. Pathogenesis of Cryptococcus neoformans; pp. 131–57. [Google Scholar]
- 59.Monari C, Bistoni F, Casadevall A, et al. Glucuronoxylomannan, a microbial compound, regulates expression of costimulatory molecules and production of cytokines in macrophages. J Infect Dis. 2005;191:127–37. doi: 10.1086/426511. [DOI] [PubMed] [Google Scholar]
- 60.Monari C, Bistoni F, Vecchiarelli A. Glucuronoxylomannan exhibits potent immunosuppressive properties. FEMS Yeast Res. 2006;6:537–42. doi: 10.1111/j.1567-1364.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- 61.Shoham S, Huang C, Chen JM, Golenbock DT, Levitz SM. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. J Immunol. 2001;166:4620–6. doi: 10.4049/jimmunol.166.7.4620. [DOI] [PubMed] [Google Scholar]
- 62.Fonseca FL, Nohara LL, Radames JB, et al. Immunomodulatory effects of serotype B glucuronoxylomannan from Cryptococcus gattii correlate with polysaccharide diameter. Infect Immun. 2010;78:3861–70. doi: 10.1128/IAI.00111-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69:2957–63. doi: 10.1128/IAI.69.5.2957-2963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kock JLF, Sebolai OM, Pohl CH, van Wyk PWJ, Lodolo EJ. Oxylipin studies expose aspirin as antifungal. FEMS Yeast Res. 2007;7:1207–17. doi: 10.1111/j.1567-1364.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 65.Sebolai O, Kock JLF, Pohl CH, et al. Bioprospecting for novel hydroxyoxylipins in fungi: presence of 3-hydroxy palmitic acid in Saccharomycopsis malanga. Antonie Van Leeuwenhoek. 2001;80:311–5. doi: 10.1023/a:1013089817318. [DOI] [PubMed] [Google Scholar]
- 66.Sjogren J, Magnusson J, Broberg A, Schnurer J, Kenne L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl Environ Microbiol. 2003;69:7554–7. doi: 10.1128/AEM.69.12.7554-7557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crow MT, Mani K, Nam Y-J, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–70. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 68.Owens KM, Modica-Napolitano JS, Singh KK. Mitochondria and cancer. In: Singh KK, Castello LC, editors. Mitochondria and cancer. New York: Springer; 2009. pp. 1–22. [Google Scholar]
- 69.Pastorino JG, Hoek JB. Integration of energy metabolism and control of apoptosis. In: Singh KK, Castello LC, editors. Mitochondria and cancer. New York: Springer; 2009. pp. 103–30. [Google Scholar]
- 70.Ralph SJ, Neuzil J. Mitochondria as targets for cancer therapy. In: Singh KK, Castello LC, editors. Mitochondria and cancer. New York: Springer; 2009. pp. 211–50. [Google Scholar]
- 71.Somasundaram S, Rafi S, Hayllar J, et al. Mitochondrial damage: a possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut. 1997;41:344–53. doi: 10.1136/gut.41.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glasgow JFT, Middleton B, Moore R, Gray A, Hill J. The mechanism of inhibition of β-oxidation by aspirin metabolites in skin fibroblasts from Reye’s syndrome patients and controls. Biochim Biophys Acta. 1999;1454:115–25. doi: 10.1016/s0925-4439(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 73.Norman C, Howell KA, Millar H, Whelan JM, Day DA. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol. 2004;134:492–501. doi: 10.1104/pp.103.031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leeuw NJ, Swart CW, Ncango DM, et al. Acetylsalicylic acid as antifungal in Eremothecium and other yeasts. Antonie Van Leeuwenhoek. 2007;91:393–5. doi: 10.1007/s10482-006-9124-4. [DOI] [PubMed] [Google Scholar]
- 75.Sebolai OM, Pohl CH, Botes PJ, et al. Distribution of 3-hydroxy oxylipins and acetylsalicylic acid sensitivity in Cryptococcus species. Can J Microbiol. 2008;54:111–8. doi: 10.1139/w07-116. [DOI] [PubMed] [Google Scholar]
- 76.Kock JLF, Swart CW, Ncango DM, et al. Development of a yeast bio-assay to screen anti-mitochondrial drugs. Curr Drug Discov Technol. 2009;6:186–91. doi: 10.2174/157016309789054960. [DOI] [PubMed] [Google Scholar]
- 77.Corcoran CA, Huang Y, Sheikh MS. Energy generating pathways and the tumor suppressor p53. In: Singh KK, Castello LC, editors. Mitochondria and cancer. New York: Springer; 2009. pp. 131–50. [Google Scholar]
- 78.Scatena R, Bittoni P, Giardina B. Mitochondrial respiration and differentiation. In: Singh KK, Castello LC, editors. Mitochondria and cancer. New York: Springer; 2009. pp. 93–102. [Google Scholar]
- 79.Gray MW, Burger G, Lang BF. The origin and early evolution of mitochondria. Genome Biol. 2001;2:10181–5. doi: 10.1186/gb-2001-2-6-reviews1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersson GE, Karlberg O, Canback B, Kurland CG. On the origin of mitochondria: a genomics perspective. Philos Trans R Soc Lond B. 2003;358:165–79. doi: 10.1098/rstb.2002.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]