Abstract
In mammals, hair follicles cover most of the body surface and exhibit precise and stereotyped orientations relative to the body axes. Follicle orientation is controlled by the planar cell polarity (PCP; or, more generally, tissue polarity) system, as determined by the follicle mis-orientation phenotypes observed in mice with PCP gene mutations. The present study uses conditional knockout alleles of the PCP genes Frizzled6 (Fz6), Vangl1, and Vangl2, together with a series of Cre drivers to interrogate the spatio-temporal domains of PCP gene action in the developing mouse epidermis required for follicle orientation. Fz6 is required starting between embryonic day (E)11.5 and E12.5. Eliminating Fz6 in either the anterior or the posterior halves of the embryo or in either the feet or the torso leads to follicle mis-orientation phenotypes that are limited to the territories associated with Fz6 loss, implying either that PCP signaling is required for communicating polarity information on a local but not a global scale, or that there are multiple independent sources of global polarity information. Eliminating Fz6 in most hair follicle cells or in the inter-follicular epidermis at E15.5 suggests that PCP signaling in developing follicles is not required to maintain their orientation. The asymmetric arrangement of Merkel cells around the base of each guard hair follicle dependents on Fz6 expression in the epidermis but not in differentiating Merkel cells. These experiments constrain current models of PCP signaling and the flow of polarity information in mammalian skin.
Keywords: Skin, Tissue polarity, Planar cell polarity, Mouse development, Merkel cell, Fz6, Vangl1, Vangl2
1. Introduction
In metazoan animals, the complex morphologies of cellular and multi-cellular structures appear to be genetically hard-wired as judged by their dramatic variation between species and their near constancy within species. One feature that characterizes many types of biological structures is polarity relative to local and/or global anatomic landmarks. A genetic system that controls polarity of this type has been extensively investigated in Drosophila, and is referred to as tissue polarity or planar cell polarity (PCP). PCP controls the chirality of ommatidia and the polarity of cuticular hairs and bristles, and it is mediated by a small number of genes that code for integral membrane proteins and membrane-associated cytoplasmic proteins (Adler, 2002; Jenny, 2010; Goodrich and Strutt, 2011). In epithelia, the asymmetric localization of PCP proteins marks each cell with an orientation vector within the plane of the epithelium.
In vertebrates, homologues have been identified for each of the Drosophila PCP genes. In mice, mutation of these genes demonstrates their central role in controlling polarity in a wide variety of contexts, including neural tube closure (Wang et al., 2006b; Torban et al., 2008; Curtin et al., 2003), axon guidance (Wang et al., 2002; Lyuksyutova et al., 2003; Tissir et al., 2005; Zhou et al., 2008; Hua et al., 2013, 2014b), and the orientations of motile cilia (Tissir et al., 2010; Vladar et al. 2012; Boutin et al., 2014; Ohata et al., 2014; Shi et al., 2014), inner ear sensory hair cells (Montcouquiol et al., 2003; Wang et al., 2005, 2006b; Jones et al., 2014), hair follicles (Guo et al., 2004; Wang et al., 2006a; Devenport and Fuchs, 2008; Ravni et al., 2009), and lingual papillae (Hua et al., 2014a).
Mammalian hair follicles form by invagination of surface epithelial cells into the dermis, and they generally exhibit an orientation that is oblique to the plane of the epithelium. Mutations in the PCP genes Fz6, Celsr1, and Vangl2 produce hair follicle orientation phenotypes. In Fz6−/− fetuses and neonates, the orientations of hair follicles in back skin appear to be nearly randomized, implying that PCP signaling is required for the initial orientations relative to the body axes (Wang et al., 2006a, 2010). As development proceeds, a Fz6-independent process causes follicles to progressively reorient within the dermis in a manner that minimizes angular differences between neighboring follicles. The result is a series of enlarging patterns, including whorls and cruciforms.
A set of central and unanswered questions in the PCP field relates to the source and timing of polarity information and the manner in which that information propagates across tissues and between distinct cellular structures. In the vertebrate epidermis, the identities of the anterior/posterior polarity signals on the trunk and the proximal/distal polarity signals on the limbs are currently unknown. With spatially and temporally defined manipulations of gene activity in mice it may be possible to constrain models concerning the nature of these signals and their relationship to PCP signaling. With this idea in mind, we have used anatomically localized and cell-type specific Cre-mediated recombination to create spatial and temporal patterns of Fz6 activity to determine when polarity information is acquired, and whether this information exhibits an obligatory flow from one part of the embryo to another, and whether Fz6 is required in developing follicles to maintain their orientation and in Merkel cells to produce an asymmetric cell cluster. The results imply that, in the mouse epidermis, Fz6-dependent polarity signaling begins at embryonic day (E)12 and that there are multiple sources of global polarity information.
2. Results
2.1. Timing of Fz6 expression for hair follicle orientation
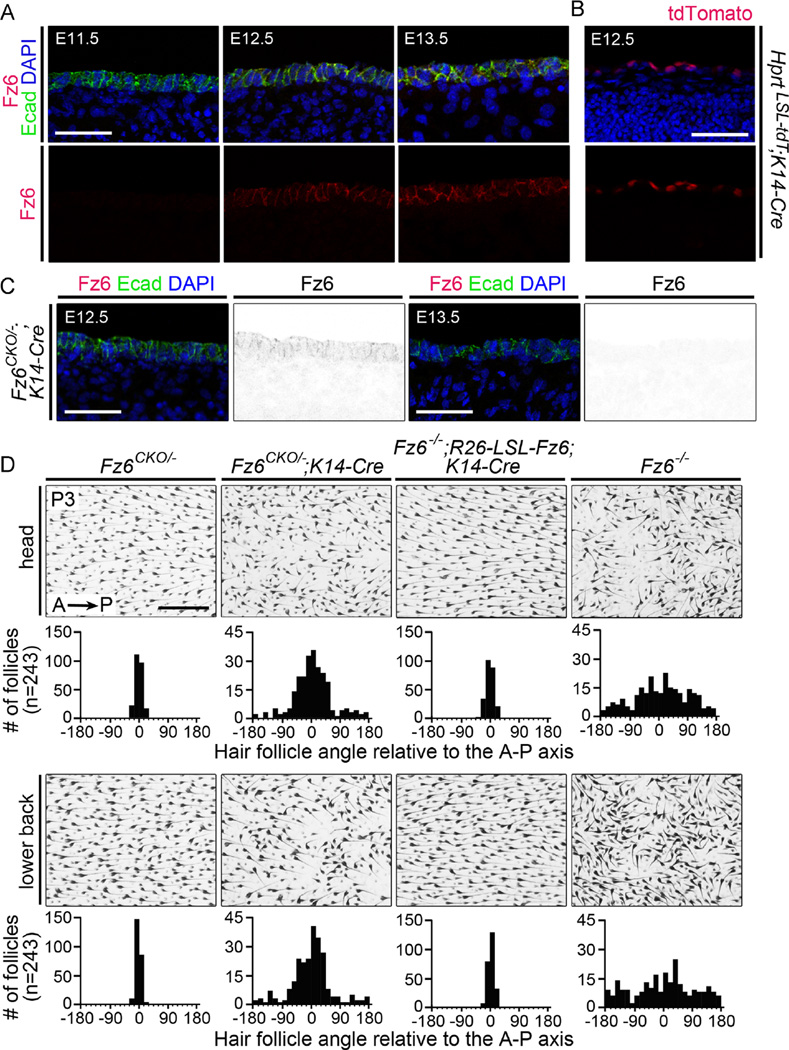
On the head and back of wild type (WT) mice, hair follicles point with high precision from anterior to posterior, and, as noted in the Introduction, this global polarity is largely absent in fetal and early postnatal Fz6−/− mice. In a first set of experiments, we investigated the critical time window for Fz6-dependent signaling, by eliminating or activating Fz6 expression with an epidermal-specific K14-Cre transgene (Dassule et al., 2000) that initiates Cre expression at nearly the same time as the endogenous Fz6 gene. In the developing epidermis on the back, Fz6 is initially detected at E12.5 and it continues to be expressed throughout fetal life (Fig. 1A and S1). One day later, at E13.5, placodes appear at the locations of future guard hair follicles (Ahn, 2015). Expression of the K14-Cre transgene begins between E11.5 and E12.5, as determined by crossing it to Hprt-LSL-tdT, a highly recombinogenic reporter that expresses a nuclear-localized 3HA-tagged tdTomato (Fig. 1B; LSL represents loxP-stop-loxP; Wu et al., 2014). [See Table 1 for a summary of the temporal patterns of Cre expression in the mouse lines used in this study.]
Fig. 1.
The critical time window for Fz6-dependent PCP signaling in the epidermis. (A) Immunostaining for Fz6 and E-cadherin shows that epithelial expression of Fz6 is first detected at E12.5 in WT back skin. (B) K14-Cre recombines the Hprt-LSL-tdT reporter in skin epithelial cells beginning at E12.5.(C) Immunostaining for Fz6 and E-cadherin in Fz6CKO/−;K14-Cre embryos shows that epithelial accumulation of Fz6 is detectable at low level at E12.5 and is undetectable at E13.5. Scale bar in A–C, 50 µm. (D) Flat mounts of head and lower back skin at P3 from Fz6CKO/− (phenotypically WT), Fz6CKO/−;K14-Cre, Fz6−/−;R26-LSL-Fz6;K14-Cre and Fz6−/− mice. Quantifications of follicle angles relative to the anterior-posterior (A–P) axis are shown for each genotype beneath a representative flat mount image (n = 3 mice per genotype; see Materials and Methods for further details). Zero degrees corresponds to the anterior-to-posterior vector; 180 and −180 degrees correspond to the posterior-to-anterior vector. Fz6CKO/−;K14-Cre mice show a partial hair follicle orientation phenotype, and Fz6−/−;R26-LSL-Fz6;K14-Cre mice show normal hair follicle orientations. Scale bar, 0.5 mm.
Table 1.
Location and timing of Cre expression determined with the Hprt-LSL-tdT reporter.
| Cre line | Location | Embryonic day |
|---|---|---|
| K14-Cre | epidermis on the back | E11.5 (−), E12.5 (+) |
| Cdx2-Cre | posterior, including the epidermis | E11.5 (+) |
| Emx1-Cre | limb bud ectoderm | E11.5 (+) |
| Shh-Cre | hair follicles | E14.5 (−), E15.5 (+) |
| Math1-Cre | Merkel cells | E14.5 (−), E15.5 (+) |
tdTomato fluorescence was assessed by visual inspection and scored as absent/undetectable (−) or present (+).
Using K14-Cre to inactivate a conditional Fz6 knockout allele (Fig. S2) in Fz6CKO/−;K14-Cre mice, we observed the transient appearance of Fz6 at E12.5, followed by its disappearance one day later (Fig. 1C). The phenotypic consequences of eliminating Fz6 from E13.5 onward is a follicle mis-orientation phenotype on the back and head at postnatal day (P)3 that is significantly milder than the Fz6−/− phenotype (quantified in Fig. 1D), suggesting that transient expression of Fz6 at E12.5 allowed the developing epidermis to partially acquire global polarity. We note that the possible perdurance of Fz6, at levels below the limit of detection, could extend the window of Fz6 action beyond E12.5. The same K14-Cre transgene was used to activate Fz6 expression from a Rosa(R)26-LSL-Fz6 knock-in allele on a Fz6−/− background. In earlier work, we observed that Cre-mediated recombination of the R26-LSL-Fz6 locus in the parental germline led to ubiquitous expression of Fz6 in the skin at approximately the same level as the endogenous Fz6 gene and to a full rescue the Fz6−/− phenotype (Hua et al., 2014a). Fig. 1D shows that Fz6−/−;R26-LSL-Fz6;K14-Cre mice have a WT hair orientation phenotype. These data imply that Fz6-mediated polarity signaling in the epidermis of the back and head begins between E11.5 and E12.5.
2.2. Effect of anterior or posterior expression of Fz6 on hair follicle orientation
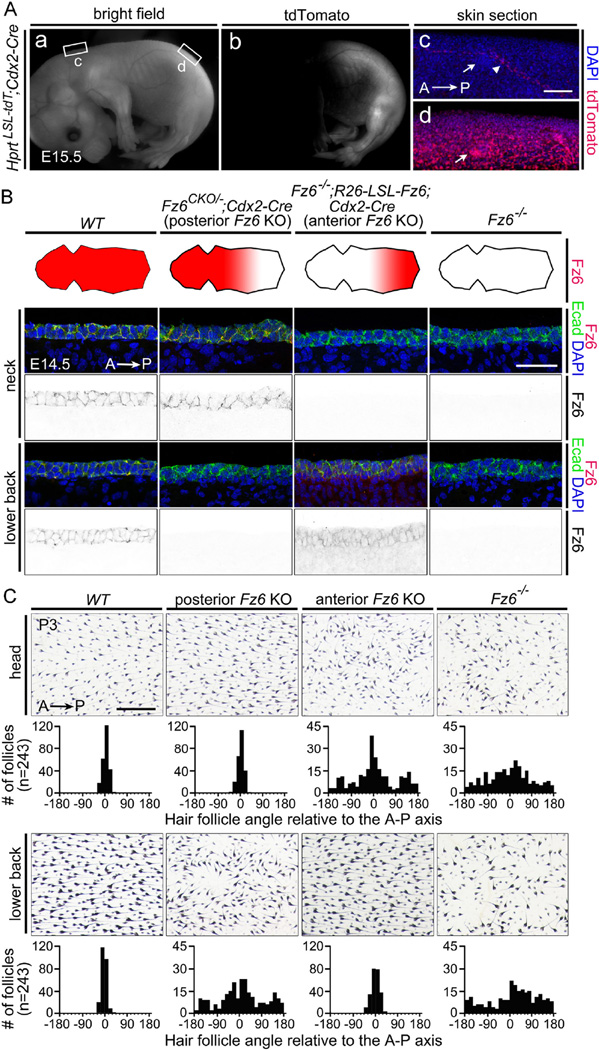
We next sought to test the hypothesis that global polarity information originates from a single source in either the anterior or the posterior of the embryo and propagates across the surface epithelium in a Fz6-dependent manner. The experimental strategy involved eliminating Fz6 exclusively in either the anterior or posterior halves of the embryo. For selective deletion of Fz6 in posterior territories we used a Caudal2 (Cdx2)-Cre transgene (Hinoi et al., 2007), which is expressed in all or nearly all embryonic tissues, including the ectoderm, posterior to the insertion of the umbilical vessels. Cdx2-Cre expression begins before E11.5, a time prior to the initiation of Fz6 expression (Fig. 2A and S3; Table 1). We will refer to Fz6CKO/−;Cdx2-Cre embryos as “posterior Fz6 KO”.
Fig. 2.
Selective deletion of Fz6 in the anterior or posterior of the embryo leads to regional loss of follicle orientation. (A) Cdx2-Cre recombines the Hprt-LSL-tdT reporter throughout the posterior embryo, with the boundary between expressing and non-expressing tissue approximately at the level of the umbilical vessels. Inset rectangles marked (c) and (d) are enlarged to the right. Arrows in panel (c) and (d) point to hair follicles; the arrowhead in (c) points to a blood vessels, the only structures that show Cre-mediated activation of the tdTomato reporter anterior to the mid-abdominal boundary. A, anterior; P, posterior. Scale bar, 100 µm.(B) Elimination of Fz6 in the anterior or posterior halves of the embryo. The four schematics show a flat-mounted dorsal mouse skin with the head to the left. The territory of Fz6 expression is indicated in red. Lower panels, immunostaining for Fz6 and E-cadherin confirms the territorial expression diagrammed above. Scale bar, 50 µm. (C) Follicle orientations in the head (upper panels) and lower back (lower panels) in P3 skin flat mounts from mice with the same four genotypes as shown in panel (B). See Methods section for details on quantification. Scale bar, 0.5 mm.
At present, there are no Cre lines that uniformly recombine anterior structures in a spatial pattern that is complementary to the pattern of Cdx2-Cre expression. Therefore, to eliminate Fz6 in the anterior of the embryo, we used an indirect strategy in which Cdx2-Cre -mediated recombination activated Fz6 expression from the R26-LSL-Fz6 locus in the posterior of Fz6−/− embryos. As this combination of ubiquitous loss of endogenous Fz6 and posterior-specific expression of ectopic Fz6 is equivalent to an anterior-specific elimination of Fz6, we will refer to it as “anterior Fz6 KO”.
In preliminary experiments, we compared the levels of Fz6 accumulation in cross-sections of skin at E14.5 in anterior and posterior locations (the neck and lower back, respectively) in WT, Fz6CKO/−;Cdx2-Cre (“posterior Fz6 KO”), Fz6−/−;R26-LSL-Fz6;Cdx2-Cre (“anterior Fz6 KO”), and Fz6−/− embryos (Fig. 2B). As expected, Fz6 is present in WT epidermis and absent in Fz6−/− epidermis. In the “posterior Fz6 KO” embryo, Fz6 is present in the anterior but not the posterior epidermis. In the “anterior Fz6 KO”, Fz6 is present in both the epidermis and dermis in the posterior of the embryo, consistent with expression of the R26 locus in most if not all cell types, but Fz6 is absent in the anterior of the embryo.
Hair follicle orientations were quantified in anterior (head) and posterior (lower back) regions in flat mounted skins from WT, “posterior Fz6 KO”, “anterior Fz6 KO”, and Fz6−/− mice at P3 (Fig. 2C). At this age, follicles in Fz6−/− mice exhibit a broad distribution of orientations with a subtle anterior-to-posterior bias. In contrast, WT mice show a narrow distribution centered on the anterior-to-posterior vector. “Posterior Fz6 KO” and “anterior Fz6 KO” mice show broad distributions of follicle orientations in the territories lacking Fz6 expression and normal anterior-to-posterior follicle orientations in those regions where Fz6 is expressed.
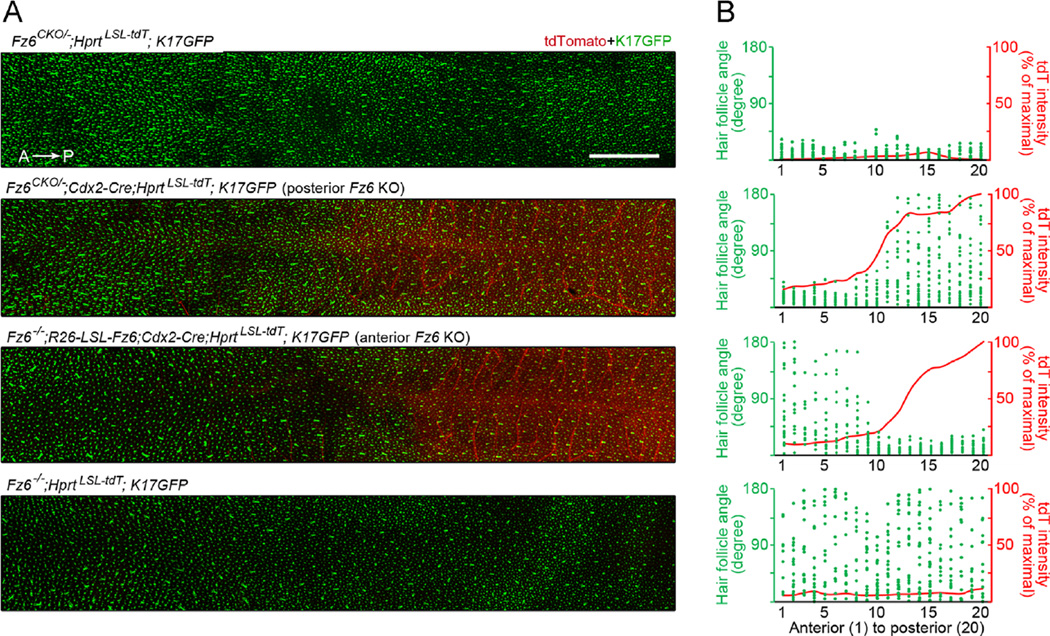
To visualize and quantify follicle orientations at the earliest stage of development (and prior to the accumulation of melanin within the follicle), back skins from the same four genotypes were examined at E17.5 in the presence of a Keratin(K)17-GFP transgene, which is expressed specifically in hair follicles (Bianchi et al., 2005). For this experiment, the Hprt-LSL-tdT reporter was also present to simultaneously demarcate the zone of Cdx2-Cre activity. At E17.5, the developing guard hair follicles are the only follicles that have elongated sufficiently to permit a determination of orientation. As seen in Fig. 3, the boundary between the anterior zone with undetectable Cre-mediated recombination and the posterior zone with high Cre-mediated recombination extends over several millimeters along the anterior-posterior axis. In the Fz6−/− back skin (bottom panel in Fig. 3), the distribution of follicle orientations shows the same subtle posterior bias as at P3, implying that loss of Fz6 does not lead to a complete randomization of the initial follicle orientations. In “posterior Fz6 KO” back skin, the territories of follicle mis-orientation and Cdx2-Cre are closely matched (second panel in Fig. 3). Interestingly, in the “anterior Fz6 KO” back skin (third panel in Fig. 3), follicles anterior to and within several millimeters of the zone of Cre-recombination are largely oriented in an anterior-to-posterior direction, despite the inference from the Hprt-LSL-tdT reporter that they are predominantly Fz6−/−. These data imply that polarity information can spread over a limited distance through Fz6−/− tissue or through tissue that contains a mixture of Fz6-expressing and Fz6-null epithelial cells.
Fig. 3.
Quantification at high spatial resolution of hair follicle orientations in back skin at E17.5 in response to anterior or posterior loss of Fz6. (A) Montage of confocal images of back skin flat mounts. Hair follicles are visualized with a K17-GFP reporter and the zone of Cdx2-Cre activity is visualized with a Hprt-LSL-tdT reporter. The Hprt-LSL-tdT reporter was shown previously to express most strongly in vascular endothelial cells (Wu et al., 2014), a pattern confirmed here. Scale bar, 2 mm. (B) The images in (A) were divided into 20 bins of equal width along the A–P axis, and within each bin the orientations of the most mature (guard) hair follicles were determined. The plots shows angles for each guard hair follicle (green dots) and the normalized tdT intensity (red line) within each bin.
The analysis of hair follicle orientations in the back skin of “anterior Fz6 KO” and “posterior Fz6 KO” fetuses and postnatal mice eliminates one model in which global polarity information originates from a unique source in either the anterior or the posterior poles of the embryo and then propagates across the surface epithelium in a Fz6-dependent manner. Such a model predicts that a zone of Fz6−/−tissue adjacent to the source of polarity information would block its propagation to more distal territories. However, these experiments do not eliminate models in which polarity information originates from both anterior and posterior poles or propagates across the embryo by a mechanism that is independent of Fz6.
2.3. Effect of limb-specific expression or knockout of Fz6 on hair follicle orientation
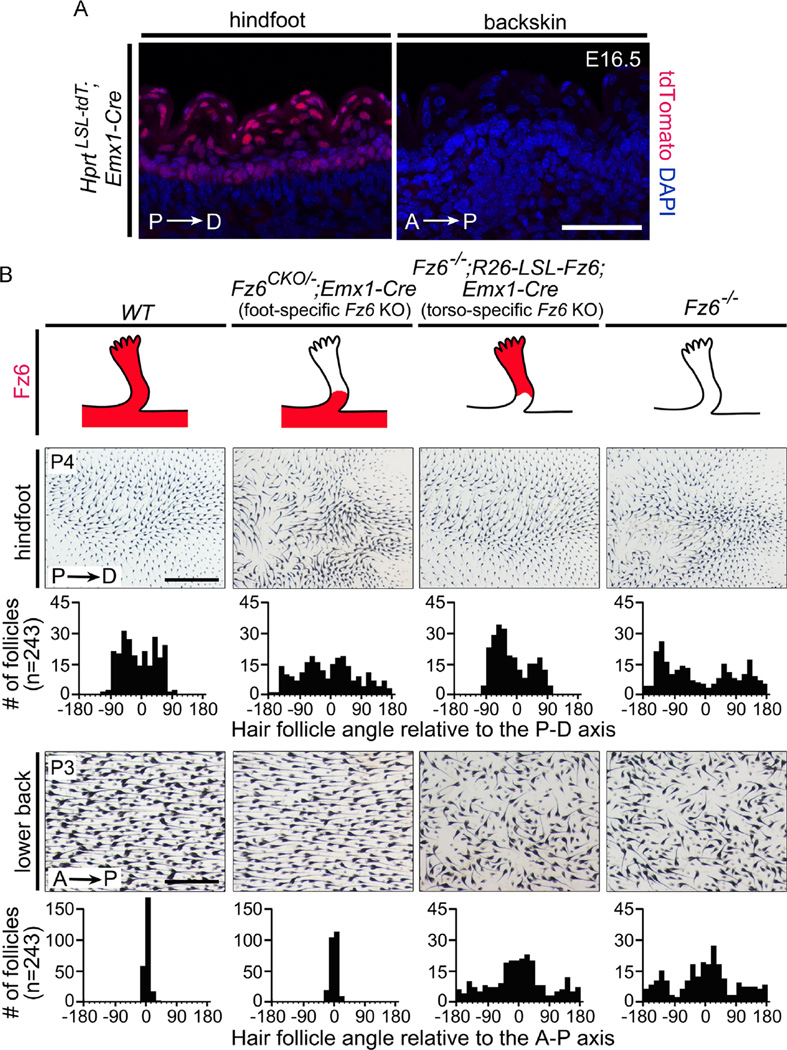
On the dorsal surface of the feet of WT mice, hair follicles at all stages of development point from the proximal limb distally toward the digits and the palmar surface, and no follicles exhibit a distal-to-proximal orientation. In contrast, in Fz6−/− mice, many early postnatal follicles on the dorsal surface of the feet exhibit a distal-to-proximal orientation, and during the first postnatal week these follicles reorganize to produce a single whorl on each hindfoot and a milder whorl-like pattern on each front foot (Guo et al., 2004; Wang et al., 2006a, 2010). To investigate the possible flow of polarity information from torso to limb, we employed an experimental strategy analogous to that described above for the anterior-posterior axis, but using instead an Emx1-Cre knock-in allele that is expressed specifically in the limb bud ectoderm prior to E11.5 (Fig. 4A and S3; Table 1; Gorski et al., 2002). To eliminate Fz6 in the distal limb ectoderm, we generated Fz6CKO/−;Emx1-Cre mice that we will refer to as “foot-specific Fz6 KO”. To eliminate Fz6 everywhere except in the distal limb ectoderm, we generated Fz6−/−;R26-LSL-Fz6;Emx1-Cre mice, which we will refer to as “torso-specific Fz6 KO”.
Fig. 4.
Expression or elimination of Fz6 in the epidermis of the feet leads to regional retention or loss, respectively, of hair follicle orientation. (A) Emx1-Cre recombines the Hprt-LSL-tdT reporter in the epidermis of the hindfoot but not the back. P, proximal; D, distal. Scale bar, 50 µm. (B) Upper panels: genotypes that express or eliminate Fz6 in the epidermis of the feet. The four schematics show a hindfoot with the territory of Fz6 expression indicated in red. Lower panels: follicle orientations on the hindfeet at P4 and the back at P3 in skin flat mounts. See Methods section for details on quantification. Scale bars, 1 mm (feet) and 0.5 mm (back).
Hair follicle orientations were quantified in flat mounts of foot skin at P4 and back skin at P3 from WT, Fz6CKO/−;Emx1-Cre (“foot-specific Fz6 KO”), FZ6−/−;R26-LSL-Fz6;Emx1-Cre (“torso-specific Fz6 KO”), and Fz6−/− mice (Fig. 4B). At P4, WT foot skins show a distribution of orientations from −90 to +90 degrees, reflecting the range of follicle orientations that point from the dorsal surface of the foot toward the palmar surface, especially around the periphery of the foot. In contrast, P4 Fz6−/− foot skins have many follicles with distal-to-proximal orientations (90–180 degrees and from −180 to −90 degrees). In “torso-specific Fz6 KO” mice at P3, as well as at later ages (data not shown), follicle orientations on the feet are indistinguishable from WT. Similarly, in “foot-specific Fz6 KO” mice at P3, as well as at later stages (data not shown), follicle orientations on the feet are indistinguishable from the orientations observed on Fz6−/− feet. These data imply that epidermal polarity on the feet is dependent on the local activity of Fz6 and is independent of Fz6 activity in the torso.
2.4. Effects of hair follicle-specific versus epidermis-specific Fz6 expression and knockout
If polarity information for determining hair follicle orientation is propagated across the epidermis by PCP signaling, then disrupting PCP signaling specifically in the inter-follicular epidermis should block the flow of information and cause follicle mis-orientation. Additionally, depending on the manner in which follicles receive and process polarity information, disruption of PCP signaling specifically within follicles might also lead to a follicle mis-orientation phenotype. These predictions are compatible with the timing of Fz6 expression that we observe in the epidermis: as noted above in the context of Fig. 1, Fz6 expression begins at E11.5-E12.5, and by E13.5, when guard hair placodes first appear, it is expressed uniformly in the epidermis on the back.
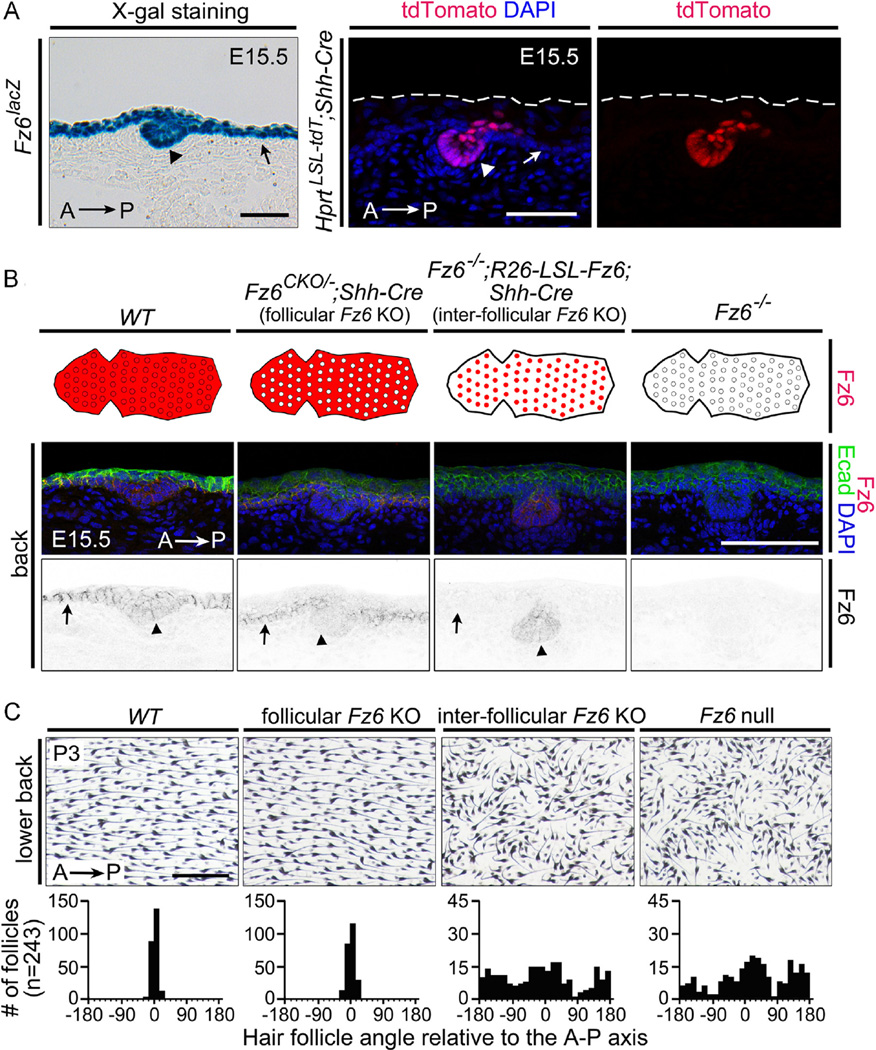
At present, there is no mouse line that expresses Cre exclusively in all inter-follicular epidermal cells or exclusively in all hair follicle epithelial cells. However, Shh is expressed in a subset of epithelial cells within hair follicles as early as E14.5 (Bitgood and McMahon, 1995), and Shh-Cre (a Cre knock-in at the Shh locus; Harfe et al., 2004) recombines the Hprt-LSL-tdT reporter in > 50% of developing guard hair follicle epithelial cells by E15.5 (Fig. 5A, right panels). Shh-Cre-induced expression of the tdTomato reporter was not observed in hair follicles prior to E15.5 or in the inter-follicular epidermis at any time (Table 1). By E15.5, Fz6 expression is readily detected in both developing guard hair follicles and inter-follicular epidermis, and developing guard hair follicles are already correctly oriented along the anterior-posterior axis (Fig. 5A, left).
Fig. 5.
Dependence of hair follicle orientation on Fz6 expression in the inter-follicular epidermis. (A) Left panel: expression of Fz6 in both hair follicle cells and inter-follicular epidermis assessed by X-gal staining of a cross-section from an E15.5 Fz6lacZ embryo. Right panels: Shh-Cre recombines the Hprt-LSL-tdT reporter in hair follicle epithelial cells but not in the surface epidermis. The skin surface is marked by dashed lines. Scale bars, 50 µm. (B) Exclusive expression or knockout of Fz6 in hair follicle epithelial cells by Shh-Cre. The schematics show a whole-mount view of back skin with hair follicles (circles) and the surrounding surface epidermis. Anterior is to the left, and the territory of Fz6 expression is indicated in red. Lower panels: Immunostaining for Fz6 and E-cadherin confirms the territorial expression patterns diagrammed above. Arrows, epidermis; arrowheads, hair follicles. Scale bar, 100 µm. (C) Flat mount images of back skin at P3 from mice with the same four genotypes as shown in panel (B). Beneath each image is the quantification of follicle orientations relative to the anterior-posterior (A–P) axis. Scale bar, 0.5 mm.
To investigate the role of Fz6 in maintaining follicle orientation after E15.5, we designed an experimental strategy analogous to that described above for the anterior-posterior axis and the feet. To eliminate Fz6 in a subset of follicle epithelial cells starting at E15.5, we generated Fz6CKO/−;Shh-Cre mice, which we will refer to as “follicular Fz6 KO”, and to eliminate Fz6 in the inter-follicular epithelium at all times, we generated Fz6−/−;R26-LSL-Fz6;Shh-Cre mice, which we will refer to as “inter-follicular Fz6 KO”. In preliminary experiments, we compared the levels of Fz6 accumulation in cross-sections of E15.5 back skin in WT, Fz6CKO/−;Shh-Cre (“follicular Fz6 KO”), Fz6−/−;R26-LSL-Fz6;Shh-Cre (“inter-follicular Fz6 KO”), and Fz6−/− embryos (Fig. 5B). As expected, Fz6 is observed throughout the epidermis and in developing guard hair follicles in WT embryos, and it is absent in Fz6−/− embryos. In the “follicular Fz6 KO”, Fz6 is detected at higher levels in the inter-follicular epidermis than in follicles. In the “inter-follicular Fz6 KO”, Fz6 is detected only in hair follicles.
Hair follicle orientations were quantified in flat mounted skins from P3 mice of the same four genotypes. “Inter-follicular Fz6 KO” mice exhibit a follicle mis-orientation phenotype equal in severity to that of Fz6−/− mice, whereas, “follicular Fz6 KO” mice exhibit a distribution of follicle orientations similar to that of WT controls. These data are consistent with the observations of Devenport and Fuchs (2008) with WT:Vangl2Lp/Lp chimeras showing that when a WT follicle is flanked by Vangl2Lp/Lp epidermis, the follicle exhibits the vertical Vangl2Lp/Lp phenotype. In interpreting this experiment, we note that Shh-Cre does not act until E14.5-E15.5, 1–2 days after guard hair placodes appear, at which time developing guard hair follicles are already oriented along the anterior-to-posterior axis (Fig. 5A and B). Thus, this experiment is relevant to the maintenance but not to the initiation of follicle orientation. With the additional caveat that Shh-Cre does not eliminate Fz6 expression in all follicle cells in the “follicle Fz6 KO” or activate Fz6 expression in all follicle cells in the “inter-follicular Fz6 KO”, the data suggest that the maintenance of the anterior-to-posterior orientation during follicle elongation does not require Fz6 expression in more than a minority of follicle cells after E15.5. The data are also consistent with a model in which PCP signaling prior to E14.5-E15.5 sets the orientation of follicle growth.
2.5. Effects of Merkel cell-specific Fz6 expression and knockout
Hair follicles are associated with a variety of non-follicle structures, each of which normally exhibits a polarity that matches the polarity of its associated follicle. These structures include sebaceous glands, arrector pili muscles, sensory nerve endings, and Merkel cell clusters (Ross and Pawlina, 2011). In Fz6−/− mice, sebaceous glands, arrector pili muscles, and sensory nerve endings reorient to track the aberrant orientation of their associated follicles (Chang and Nathans, 2013). In contrast, Merkel cell clusters lose their polarity in the absence of Fz6. The ~30 Merkel cells at the base of each guard hair follicle are arranged in a semi-circle with an anterior opening in WT skin, but they form a closed circle in Fz6−/− skin (Chang and Nathans, 2013). We note that Merkel cells are epidermal derivatives, and therefore it is not surprising that they are directly influenced by PCP signals.
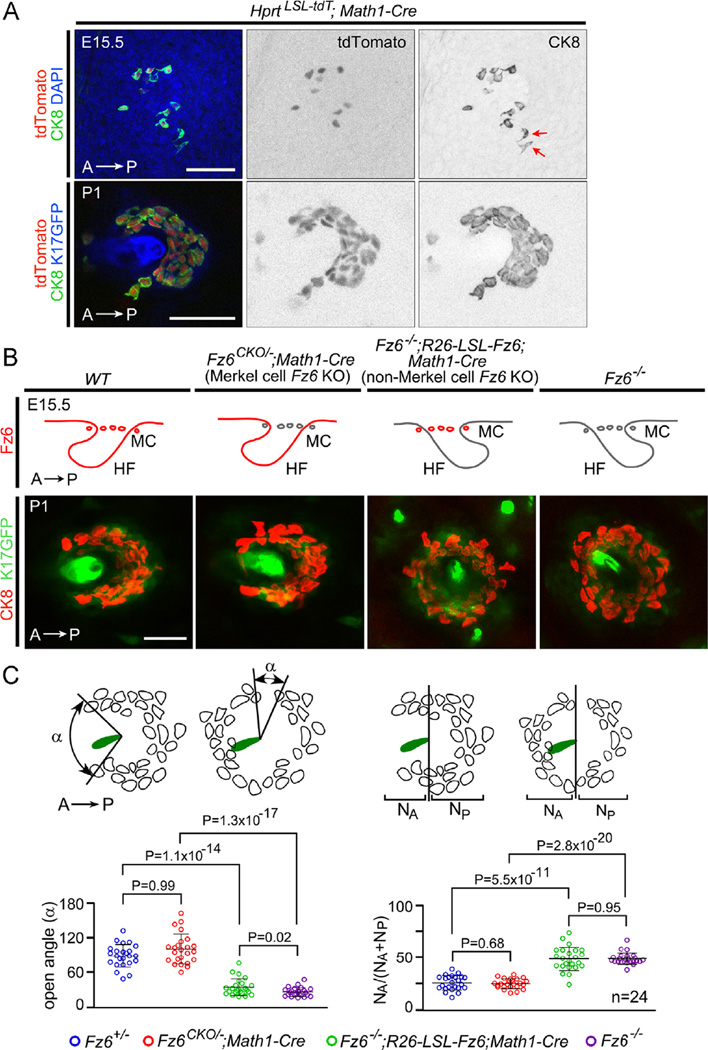
To investigate the flow of polarity information to Merkel cells, we utilized a Math1(Atoh1)-Cre knock-in line that expresses Cre in Merkel cells starting at E15.5 when Merkel cells begin to differentiate (Fig. 6A; Yang et al., 2010). Using the Math1-Cre line, we designed an experimental strategy analogous to that described above for hair follicles and inter-follicular epidermis (Fig. 6B). To eliminate Fz6 in Merkel cells, we generated Fz6CKO/−;Math1-Cre mice, which we will refer to as “Merkel cell Fz6 KO”, and to eliminate Fz6 in all epidermal derivatives other than Merkel cells, we generated Fz6−/−;R26-LSL-Fz6;Math1-Cre mice, which we will refer to as “non-Merkel cell Fz6 KO”.
Fig. 6.
Dependence of Merkel cell cluster polarity on Fz6 expression in the surrounding epidermis. (A) Math1-Cre recombines the Hprt-LSL-tdT reporter selectively in Merkel cells. In Hprt-LSL-tdJ;Math1-Cre fetuses at E15.5, Merkel cells begin to differentiate [determined by immunostaining for cytokeratin 8 (CK8)] and to express Math1-Cre as determined by tdTomato accumulation The presence of two CK8+ tdTomato- Merkel cells (red arrows) may reflect the time required for reporter accumulation after Cre-mediated recombination In Hprt-LSL-tdT;Math1-Cre at P1, tdTomato is expressed by all Merkel cells (marked by CK8), which are arranged in a semicircle around a central guard hair follicle (marked by K17-GFP expression). Scale bar, 50 um (B) Top, genotypes for exclusive expression or knock out of Fz6 in Merkel cells. The four sets of schematics show a transverse section of an E15.5 hair follicle (HF) with its associated Merkel cells (MC). Hair follicles are shown either correctly oriented (i.e. with an anterior to posterior polarity as they approach the skin surface; first and second panels) or inappropriately oriented (third and fourth panels). Anterior is to the left, and the territory of Fz6 expression is indicated in red. Bottom, examples of flat mount images of a P1 hair follicle (marked by K17-GFP expression) with its associated Merkel cells (marked by CK8). Scale bar, 50 µm. (C) Diagrams showing the measurement of the open angles of Merkel cell clusters and the distributions of Merkel cells around guard follicles in flat mount skin at P1. Left, for each Merkel cell cluster, the opening angle was measured by connecting the two most distantly separated Merkel cells to the center of the hair shaft within the same Z-plane. Right, number of Merkel cells anterior (NA) or posterior (NP) to a line bisecting the follicle at right angles to the A–P axis. Scatterplots show results for individual Merkel cell clusters together with mean +/− SD. P-values, student t-test.
In flat mounts of skin at P1, a time when Merkel cell clusters are fully developed, the arrangement of Merkel cells in “Merkel cell Fz6 KO” mice was indistinguishable from WT mice, and the arrangement of Merkel cells in “non-Merkel cell Fz6 KO” mice was indistinguishable from Fz6−/− mice (Fig. 6C). The data were quantified by measuring the size of the largest angular opening in each Merkel cell cluster and the fraction of Merkel cells that reside anterior to the center of each cluster (Fig. 6C; Chang and Nathans, 2013). In interpreting these experiments we note the possibility that the WT polarity of Merkel cell clusters in “Merkel cell Fz6 KO” mice might reflect the perdurance of Fz6 proteins that had accumulated prior to Math1-Cre-mediated Fz6 deletion between E14.5-E15.5. While keeping this caveat in mind, this experiment suggests is that the anterior-facing semi-circular arrangement of Merkel cells does not require Fz6 expression in Merkel cells during the time when the semi-circle is being formed.
2.6. Roles of Vangl1 and Vangl2 in hair follicle orientation
To test the generality of the spatial and temporal effects of Fz6 deletion, we asked whether deleting Vangl1 and Vangl2 - the only two mammalian orthologues of the Drosophila core PCP gene Vang - using K14-Cre or Cdx2-Cre would produce the same phenotypes as observed with deletion of Fz6. In previous work, we and others observed a loss of follicle polarity in fetuses homozygous for the semi-dominant Vangl2 Looptail (Lp) allele (Devenport and Fuchs, 2008; Wang et al., 2010). As Vangl2Lp/Lp mice die at birth, this follicle phenotype could only be examined during prenatal life. Curiously, prenatal Vangl2Lp/Lp follicles are oriented perpendicular to the plane of the epithelium. This phenotype is distinct from the Fz6−/− phenotype in which follicles are mis-oriented but retain their oblique angle to the epithelial plane.
Vangl1 and/or Vangl2 recessive loss-of-function mutations have been studied previously in the early embryo in the context of left-right asymmetry, in neural tube closure, and in inner ear development (Torban et al., 2008; Song et al., 2010; Copley et al., 2013; Pryor et al., 2014), but they have not been studied in the skin. To eliminate Vangl1 and/or Vangl2 in various allelic combinations in the epidermis we constructed a Vangl1CKO allele (Fig. S4) and combined it with a Vangl2CKO allele (Yin et al., 2012) and the K14-Cre transgene to generate mice in which different numbers of Vangl1 and Vangl2 alleles were deleted.
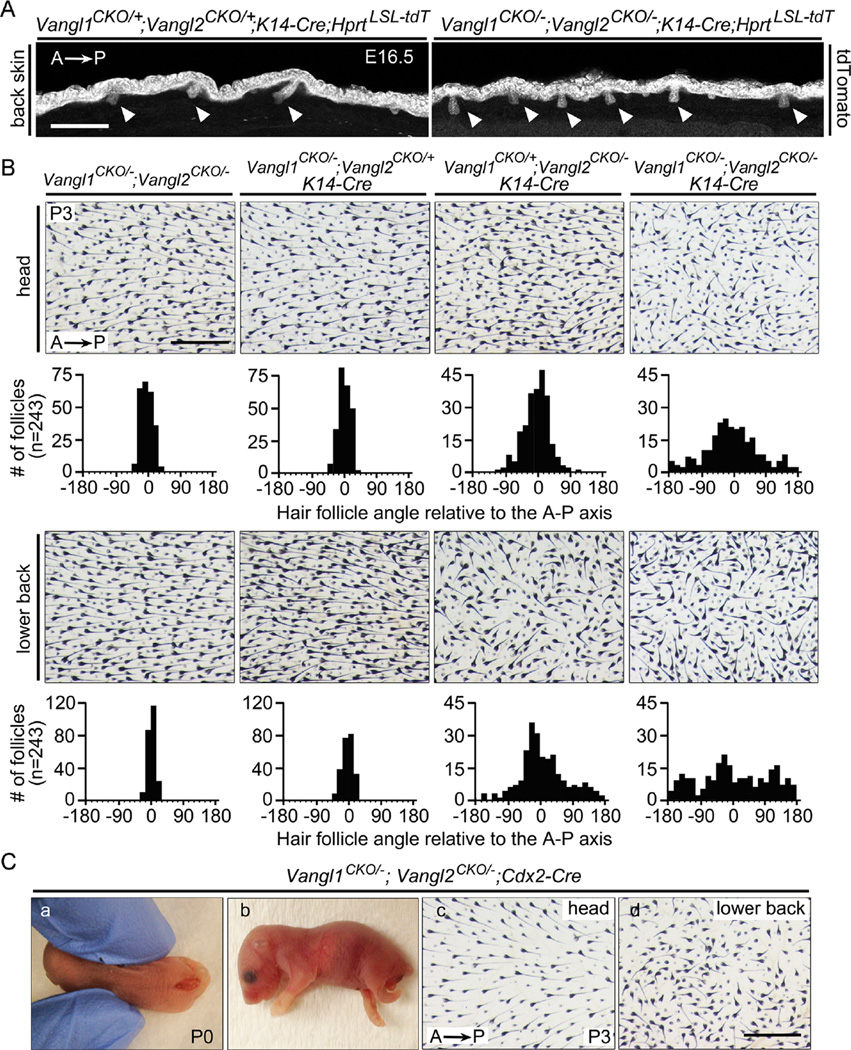
Eliminating one allele each of Vangl1 and Vangl2 in the epidermis at E12.5 with K14-Cre (Vangl1CKO/+;Vangl2CKO/+;K14-Cre) or ubiquitously (Vangl1CKO/−;Vangl2CKO/−, without Cre) had no effect on follicle orientations. However, eliminating all four Vangl alleles in the epidermis (Vangl1CKO/−;Vangl2CKO/−;K14-Cre) led to vertically oriented hair follicles in prenatal skin at E16.5 that closely resembled the phenotype observed with Vangl2Lp/Lp (Fig. 7A). By P3, follicles in Vang/1CKO/−;Vang/2CKO/−;K14-Cre mice had acquired an oblique angle relative to the epithelial surface and exhibited largely randomized orientations on the back and partially randomized orientations on the head (Fig. 7B). A comparison of follicle angles at P3 between Fz6CKO/−;K14-Cre mice (Fig. 1D, second from left) and Vangl1CKO/−;Vangl2CKO/−;K14-Cre mice (Fig. 7B, far right) revealed a more severe phenotype in Vang/1CKO/−;Vangl2CKO/−;K14-Cre mice. Together with the distinctive phenotype of vertically oriented follicles, these observations suggest that a more extensive loss of PCP signaling is produced by the combined loss of Vangl1 and Vangl2 than by loss of Fz6.
Fig. 7.
Relative importance of Vangl1 vs. Vangl2 in hair follicle orientation. (A) Surface epithelial cells and developing hair follicles (arrowheads) in back skin visualized with the Hprt-LSL-tdT reporter at E16.5. In the absence of epithelial Vangl1 and Vangl2, developing follicles are oriented perpendicular to the plane of the epidermis. Scale bar, 200 µm. (B) Hair follicle orientation in head and back skin at P3 following epidermis-specific loss of two, three, or four alleles of Vangl1 and Vangl2, beginning at E11.5-E12.5. Scale bar, 0.5 mm. (C) Phenotypes caused by posterior deletion of Vangl1 and Vangl2. Panels (a) and (b) show neural tube closure and hindlimb defects at P0 observed in ~95% of Vangl1CKO/−;Vangl2CKO/−;Cdx2-Cre. Panels (c) and (d) show a skin flat mount from a rare Vangl1CKO/−;Vangl2CKO/−;Cdx2-Cre mouse at P3 with a closed neural tube. Hair follicle orientations are normal on the head and aberrant on the lower back. Scale bar, 0.5 mm.
At P3, Vangl1CKO/−;Vangl2CKO/− mice (loss of one allele each of Vangl and Vangl2) and Vangl1CKO/−;Vangl2CKOI+;K14-Cre mice (loss of two alleles of Vang1 and one allele Vangl2) exhibit head and back follicle orientations that closely resemble those of the WT control, whereas Vangl1CKO/+;Vangl2CKO/−;K14-Cre mice (loss of one alleles of Vangl and two alleles of Vangl2) exhibit a phenotype intermediate between Vangl1CKO/−;Vangl2CKO/−;K14-Cre mice (loss of all four alleles) and WT mice (Fig. 7B). From these data we conclude that Vangl2 plays a larger role than Vangl1 in follicle orientation.
When Vangl1 and Vangl2 were deleted in the posterior of the body with Cdx2-Cre (Vangl1CKO/−,Vangl2CKO/−,Cdx2-Cre), > 95% of mice died shortly after birth, with most showing a caudal neural tube defect, tail truncation, and retarded growth of the hind limbs (Fig. 7C and S5). The single Vangl1CKO/−;Vangl2CKO/−;Cdx2-Cre mouse that did not have an open neural tube was analyzed at P3 and exhibited hair follicle orientations that were largely randomized on the lower back but not on the head (Fig. 7C, right panels), consistent with the pattern observed in Fz6CKO/−;Cdx2-Cre mice. Additionally, Vangl1CKO/−;Vangl2CKO/−;K14-Cre mice show the same circular arrangement of Merkel cells as observed in Fz6−/− mice, Vangl1CKO/−;Vangl2CKO/−;Shh-Cre show normal hair follicle orientations as observed in Fz6CKO/−;Shh-Cre mice, and Vangl1CKO/−;Vangl2CKO/−;Math1-Cre mice show a normal arrangement of Merkel cells as observed in Fz6CKO/−;Math1-Cre mice (data not shown). These observations support the view that Fz6 and Vangl1/Vangl2 work together to mediate PCP signaling in the epithelium.
3. Discussion
The body of work presented here represents the first systematic study of the spatiotemporal characteristics of mammalian PCP signaling in epithelial development. Utilizing a series of Cre driver lines with well-defined spatiotemporal patterns of expression, we have conditionally deleted Fz6 or Vangl1 and Vangl2 in different anatomic patterns and at different times in the mouse epidermis and in various epidermal derivatives. To compare the polarity phenotypes associated with Fz6 loss in anatomically reciprocal pairs of territories or cell types, we devised a strategy in which the phenotype produced by Fz6 deletion using a particular Cre driver is compared to the phenotype produced by ectopic Fz6 activation using the same Cre driver in the absence of endogenous Fz6. As described below, the results of these experiments constrain potential models of PCP signaling in the mammalian epidermis.
3.1. Timing of Fz6 expression and action
In the absence of Fz6, the initial follicle polarities on the head are largely randomized and those on the back are almost completely randomized. One model consistent with this phenotype posits that Fz6 acts as part of a system to propagate polarity information across the surface epithelium from source(s) at the extreme anterior and/or posterior of the embryo. Alternatively, one can envision models in which Fz6 only communicates polarity information locally, in which case other polarity systems would need to be invoked to set-up polarity on the scale of the entire embryo. In Drosophila, experiments with marked clones clearly demonstrate a role for PCP signaling in the local spread of polarity information, but they are also consistent with a role for PCP genes in setting up polarity on a larger scale (Adler, 2002; Strutt and Strutt, 2005; Goodrich and Strutt, 2011; Struhl et al., 2012).
In mice, the earliest developing hair follicles do not begin to appear until E13.5, when the embryo is ~12 mm in length (Kaufman, 1992). It would be remarkable if global polarity information could propagate across an embryo of this size. Instead, it seems more reasonable to suppose that anterior–posterior polarity information is generated when the embryo is far smaller and that this information is preserved and made available to the epidermis at later times. Following this line of reasoning, the experiments described here to determine the time window during which Fz6 acts can potentially constrain models of local vs. global roles for Fz6.
It has long been known that Fz6 is expressed in the epidermis and in hair follicles during late fetal and adult life (Guo et al., 2004). Here we show that Fz6 is not detectable in the epidermis by immunostaining prior to E12.5. We note, however, that physical analyses of gene expression - by immunostaining, in situ hybridization, detection of knock-in reporters, etc. - cannot be considered definitive methods for assessing the time and place of gene action because extremely low levels of gene expression can potentially provide biological function. Indeed, earlier experiments with Fz3−/−;Fz6−/− embryos demonstrated a redundant role for Fz3 and Fz6 in neural tube closure, even though Fz6 gene expression was undetectable, as determined by X-gal staining with a lacZ reporter knock-in, at the time of neural tube closure (~E8.5; Wang et al., 2006b).
Using K14-Cre, we have studied embryos with timed deletion or activation of Fz6. These experiments show that the critical time window for Fz6 function in hair follicle orientation begins at E11.5-E12.5 and continues at least through E13.5. Epidermal deletion of Vangl1 and Vangl2 at E11.5-E12.5 leads to a similar hair orientation phenotype. These results imply that Fz6-dependent PCP signaling begins early in epidermal development when the embryo is ~ 6 mm in length, 1–2 days prior to the appearance of hair follicle placodes. Compared to the most fundamental embryologic events (e.g. gastrulation), E11.5-E12.5 is relatively late, and the data therefore seem most compatible with a model in which Fz6 acts within the epidermis to locally interpret pre-existing polarity information.
3.2. Anatomic domains of Fz6 action: local influence within a global pattern
Direct tests of a role for Fz6 in propagating polarity information from a discrete source are described here in the contexts of anterior-posterior polarity on the head and torso and proximal-distal polarity in the limbs. In both contexts, early acting (i.e., before E11.5) and anatomically-localized Cre drivers allowed Fz6 gene activity to be selectively eliminated in complementary regions of the epithelium: anterior vs. posterior halves of the embryo, and limb vs. torso. The resulting phenotypes show that (1) hair follicles require local expression of Fz6 to attain their correct initial orientations, and (2) they are unaffected by the absence of Fz6 in adjacent territories. Moreover, the intermediate PCP phenotype produced by transient expression of Fz6 prior to K74-Cre-mediated inactivation in Fz6CKO/−;K14-Cre embryos showed no anterior-posterior variation in severity, as might have been predicted if Fz6 effects the propagation of polarity information from one end of the embryo to the other over the course of hours to days. Taken together, the data are most compatible with a model in which Fz6 - and, by inference, PCP signaling generally - acts to locally communicate, stabilize, and/or transduce polarity information, but it does not constitute the sole means for transmitting that information over long distances.
We note that the preceding conclusions refer to the initial orientations of hair follicles. Later, during the first 8–10 days of postnatal life, a Fz6-independent system of communication generates and refines patterns of follicle orientation that eventually encompass many thousands of follicles (Wang et al., 2006a, 2010). This later refinement process can be accurately modeled as a local interaction between follicles that favors a convergence of orientations among neighbors (Wang et al., 2006a). Although its molecular and cellular mechanisms are unknown, this refinement process exemplifies one way in which polarity information evolves and propagates across a vector field. A conceptually analogous system underlies the cooperative alignment of magnetic dipole moments among individual atoms in ferromagnetic materials (Feynman et al., 1963).
3.3. Acquisition of polarity information by Merkel cells and hair follicles
A major distinction between PCP in the mammalian epidermis and the Drosophila wing is that the former involves the polarized architectures of multicellular structures (hair follicles and Merkel cell clusters) whereas the latter only affects the orientations of subcellular structures (the actin-rich bundles that form the core of the wing hairs produced by each epithelial cell; Adler, 2002; Goodrich and Strutt, 2011). How individual cells within a multicellular structure acquire and differentially interpret polarity information to generate a coordinated spatial response is, at present, unknown.
The experiments described here with follicle-specific expression or deletion of Fz6 and Merkel cell-specific expression or deletion of Fz6 are consistent with a model in which polarity information from the surrounding epidermis influences the orientations of multi-cellular epithelium-derived structures. In the future it would be interesting to examine the way in which cell movements, cell proliferation and/or cell death shape architectural asymmetry in hair follicles and Merkel cell clusters. With the development of two-photon microscopic techniques for chronic imaging of identified cells in mammalian hair follicles (Mesa et al., 2015), it might be possible to investigate this question by monitoring epithelial cell behaviors longitudinally in ex vivo preparations of mouse embryos that are either WT or mutant for various PCP genes.
4. Materials and methods
4.1. Mouse lines
The Fz6CKO and Vangl1CKO alleles were generated by homologous recombination in mouse embryonic stem (ES) cells using standard techniques. In brief, targeting constructs (Fig. S2 and S4) were electroporated into R1 mouse ES cells; colonies were grown in medium containing G418 and ganclovir, and were screened by Southern blot hybridization; positive clones with a normal karyotype were injected into C57BL/6 blastocysts to generate chimeric founders; and germline transmission was confirmed by Southern blot hybridization and PCR.
The following mouse alleles were also used: Fz6− (Guo et al., 2004), R26-LSL-FZ6 (Hua et al., 2014a,b), Hprt-LSL-tdT (Wu et al., 2014), K17-GFP (Bianchi et al., 2005), Sox2-Cre (Hayashi et al., 2002), K14-Cre (Dassule et al., 2000; JAX 004782), Cdx2-Cre (Hinoi et al., 2007; JAX 009350), Emx1-Cre (Gorski et al., 2002; JAX 005628), Shh-Cre (Harfe et al., 2004; JAX 005622), Math1(Atoh1)-Cre (Yang et al., 2010; a kind gift of Dr. Lin Gan, University of Rochester), Vangl2CKO (Copley et al., 2013), and Vangl2− (Smallwood, Williams, and Nathans, unpublished). Mice were handled and housed according to the approved Institutional Animal Care and Use Committee (IACUC) protocol M013M469 of the Johns Hopkins Medical Institutions.
4.2. Immunostaining
For immunostaining of sagittal sections, embryos were embedded in OCT, fresh frozen, cryosectioned (14 µm) and fixed for 10 min in 4% formaldehyde in PBS. Sections were washed with PBST (0.1% Triton in PBS) for 10 min and blocked for 1 h with 5% normal donkey or goat serum in PBST. Primary antibodies were incubated for 2 h at room temperature or overnight at 4 degree. The following primary antibodies were used: goat anti-Fz6 (AF1526; R and D Systems; 1:400), rat anti-E-cadherin (ab11512-100; Abcam; 1:400) and rat anti-Cytokeratin8 (TROMA-I-c; Developmental Studies Hybridoma Bank; 1:500). Secondary antibodies in PBST were incubated for one hour at RT. Secondary antibodies were Alexa Fluor 488-, 594-, or 647-conjugated donkey anti-goat, donkey anti-rat, or goat anti-rat IgG antibodies (Invitrogen; Grand Island, NY). Finally, sections were washed three times in PBST and mounted on slides with Fluoromount-G (Southern Biotech; Birmingham, AL).
For visualizing tdTomato's native fluorescent signal, embryos were fixed for 30 min with 4% formaldehyde in PBS and treated with progressively increasing sucrose concentrations (10%, 20%, 30% in PBS) before cryosectioning. Immunostained samples were imaged using a Zeiss LSM700 confocal microscope with Zen software.
4.3. Skin whole mounts
The procedures for preparation and processing of skin whole mounts for Merkel cell immunostaining and for imaging of hair follicles based on melanin content are described in Chang and Nathans (2013) and Chang et al. (2014). P3 whole mount skins processed for melanin were imaged using a Zeiss Stemi V11 microscope with a color Axiocam CCD in combination with Openlab software. Follicle orientations relative to the anterior-posterior (A–P) axis were quantified for three mice per genotype, except for the Vangl1CKO/−;Vangl2CKO/−;Cdx2-Cre genotype shown in Fig. 7C, for which only a single postnatal mouse survived. For each skin image, orientations relative to the A–P axis were determined for a set of 81 follicles closest to the grid points on a 9 × 9 grid encompassing 3.2 × 2.5 mm2.
4.4. Skeleton staining and X-gal staining
The procedures for X-gal staining and whole-mount alcian blue and alizarin red staining of cartilage and bone from embryos are described by Nagy et al. (2003).
Supplementary Material
Acknowledgments
Supported by the Howard Hughes Medical Institute. The authors thank Dr. Amir Rattner for helpful comments on the manuscript.
Footnotes
Author contributions
HC designed, conducted, and analyzed all of the experiments; JN designed and analyzed the experiments; HC and JN wrote the paper; JW constructed the Vangl1CKO targeting plasmid; PMS constructed the Fz6CKO targeting plasmid, and performed the ES cell targeting and initial characterization of the knockout lines.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.Org/10.1016/j.ydbio.2015.10.027.
References
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev. Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Ahn Y. Signaling in tooth, hair, and mammary placodes. Curr. Top. Dev. Biol. 2015;111:421–459. doi: 10.1016/bs.ctdb.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Bianchi N, Depianto D, McGowan K, Gu C, Coulombe P. Exploiting the keratin 17 gene promoter to visualize live cells in epithelial appendages of mice. Mol. Cell. Biol. 2005;25:7249–7259. doi: 10.1128/MCB.25.16.7249-7259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Boutin C, Labedan P, Dimidschstein J, Richard E, Cremer H, Andre P, Yang Y, Montcouquiol M, Goffinet AM, Tissir E. A dual role for planar cell polarity genes in ciliated cells. Proc. Natl. Acad. Sci. USA. 2014;111:E3129–E3138. doi: 10.1073/pnas.1404988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Nathans J. Responses of hair follicle-associated structures to loss of planar cell polarity signaling. Proc. Natl. Acad. Sci. USA. 2013;110:E908–E917. doi: 10.1073/pnas.1301430110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Wang Y, Wu H, Nathans J. Whole mount imaging of mouse skin and its application to the analysis of hair follicle patterning and sensory axon morphology. J. Vis. Exp. 2014:e51749. doi: 10.3791/51749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley CO, Duncan JS, Liu C, Cheng H, Deans MR. Postnatal refinement of auditory hair cell planar polarity deficits occurs in the absence of Vangl2. J. Neurosci. 2013;33:14001–14016. doi: 10.1523/JNEUROSCI.1307-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat. Cell. Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feynman RP, Leighton RB, Sands ML. The Feynman Lectures on Physics. Vol. 2. Reading, MA: Addison Wesley; 1963. Magnetic Materials. [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;128:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proc. Natl. Acad. Sci. S A. 2004;101:9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr. Patterns. 2002;2:93–97. doi: 10.1016/s0925-4773(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Hinoi T, Akyol A, Theisen BK, Ferguson DO, Greenson JK, Williams BO, Cho KR, Fearon ER. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 2007;67:9721–9730. doi: 10.1158/0008-5472.CAN-07-2735. [DOI] [PubMed] [Google Scholar]
- Hua ZL, Chang H, Wang Y, Smallwood PM, Nathans J. Partial inter-changeability of Fz3 and Fz6 in tissue polarity signaling for epithelial orientation and axon growth and guidance. Development. 2014a;141:3944–3954. doi: 10.1242/dev.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua ZL, Smallwood PM, Nathans J. Frizzled3 controls axonal development in distinct populations of cranial and spinal motor neurons. Elife. 2013;2:e01482. doi: 10.7554/eLife.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua ZL, Jeon S, Caterina MJ, Nathans J. Frizzled3 is required for the development of multiple axon tracts in the mouse central nervous system. Proc. Natl. Acad. Sci. USA. 2014b;111:E3005–E3014. doi: 10.1073/pnas.1406399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A. Planar cell polarity signaling in the Drosophila eye. Curr. Top. Dev. Biol. 2010;93:189–227. doi: 10.1016/B978-0-12-385044-7.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Cyan D, Kim SM, Li S, Ren D, Knapp L, Sprinzak D, Avraham KB, Matsuzaki F, Chi F, Chen P. Ankrd6 is a mammalian functional homolog of Drosophila planar cell polarity gene diego and regulates coordinated cellular orientation in the mouse inner ear. Dev. Biol. 2014;395:62–72. doi: 10.1016/j.ydbio.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. San Diego: Academic Press; 1992. [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Mesa KR, Rompolas P, Zito G, Myung P, Sun TY, Brown S, Gonzalez DG, Blagoev KB, Haberman AM, Greco V. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature. 2015;522:94–97. doi: 10.1038/nature14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- Ohata S, Nakatani J, Herranz-Perez V, Cheng J, Belinson H, Inubushi T, Snider WD, Garcia-Verdugo JM, Wynshaw-Boris A, Alvarez-Buylla A. Loss of Dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron. 2014;83:558–571. doi: 10.1016/j.neuron.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor SE, Massa V, Savery D, Andre P, Yang Y, Greene ND, Copp AJ. Vangl-dependent planar cell polarity signalling is not required for neural crest migration in mammals. Development. 2014;141:3153–3158. doi: 10.1242/dev.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravni A, Cm Y, Goffinet AM, Tissir F. Planar cell polarity cadherin Celsr1 regulates skin patterning in the mouse. J. Invest. Dermatol. 2009;129:2507–2509. doi: 10.1038/jid.2009.84. [DOI] [PubMed] [Google Scholar]
- Ross MN, Pawlina W. Histology: a Text and Atlas. Philadelphia: Lippincott, Williams, and Wilkins; 2011. Integumentary System; pp. 488–524. [Google Scholar]
- Shi D, Komatsu K, Hirao M, Toyooka Y, Koyama H, Tissir F, Goffinet AM, Uemura T, Fujimori T. Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development. 2014;141:4558–4568. doi: 10.1242/dev.115659. [DOI] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Casal J, Lawrence PA. Dissecting the molecular bridges that mediate the function of Frizzled in planar cell polarity. Development. 2012;139:3665–3674. doi: 10.1242/dev.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. Bioessays. 2005;27:1218–1227. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- Tissir F, Bar I, Jossin Y, De Backer O, Goffinet AM. Protocadherin Celsr3 is crucial in axonal tract development. Nat. Neurosci. 2005;8:451–457. doi: 10.1038/nn1428. [DOI] [PubMed] [Google Scholar]
- Tissir F, Cm Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, Poumay Y, Uemura T, Goffinet AM. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat. Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- Torban E, Patenaude AM, Lederc S, Rakowiecki S, Gauthier S, Andelfinger G, Epstein DJ, Gros P. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc. Natl. Acad. Sci. USA. 2008;105:3449–3454. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. Microtubules enable the planar cell polarity of airway cilia. Curr. Biol. 2012;22:2203–2212. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Ojan D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Badea T, Nathans J. Order from disorder: self-organization in mammalian hair patterning. Proc. Natl. Acad. Sci. USA. 2006a;103:19800–19805. doi: 10.1073/pnas.0609712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chang H, Nathans J. When whorls collide: the development of hair patterns in frizzled 6 mutant mice. Development. 2010;137:4091–4099. doi: 10.1242/dev.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 2006b;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Thekdi N, Smallwood PM, Macke JP, Nathans J. Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J. Neurosci. 2002;22:8563–8573. doi: 10.1523/JNEUROSCI.22-19-08563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Luo J, Yu H, Rattner A, Mo A, Wang Y, Smallwood PM, Erlanger B, Wheelan SJ, Nathans J. Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron. 2014;81:103–119. doi: 10.1016/j.neuron.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xie X, Deng M, Chen X, Can L. Generation and characterization of Atohl-Cre knock-in mouse line. Genesis. 2010;48:407–413. doi: 10.1002/dvg.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Copley CO, Goodrich LV, Deans MR. Comparison of phenotypes between different vangl2 mutants demonstrates dominant effects of the Looptail mutation during hair cell development. PLOS One. 2012;7:e31988. doi: 10.1371/journal.pone.0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Bar I, Achouri Y, Campbell K, De Backer O, Hebert JM, Jones K, Kessaris N, de Rouvroit CL, O’Leary D, Richardson WD, Goffinet AM, Tissir F. Early forebrain wiring: genetic dissection using conditional Celsr3 mutant mice. Science. 2008;320:946–949. doi: 10.1126/science.1155244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.