Abstract
Background
Biennial screening is generally recommended for average-risk women aged 50–74 years, but tailored screening may provide greater benefits.
Objective
To estimate outcomes for varying screening intervals after age 50 based on breast density and risk.
Design
Collaborative simulation modeling using national incidence, breast density, and screening performance data.
Setting
U.S. population.
Patients
Women ages ≥50 with combinations of breast density and relative risk (RR: 1.0, 1.3, 2.0, 4.0).
Interventions
Annual, biennial, or triennial digital mammography screening from age 50 to 74 (versus no screening) and age 65 to 74 (versus biennial 50–64).
Measurements
Lifetime breast cancer deaths, life expectancy and quality-adjusted life years (QALYs), false-positives, benign biopsies, overdiagnoses, cost-effectiveness and ratio of false-positives to breast cancer deaths averted.
Results
Screening benefits and overdiagnosis increase with breast density and risk. False-positives and benign biopsies decrease with increasing risk. Among women with fatty or scattered fibroglandular breast density and RR=1.0–1.3, breast cancer deaths averted were similar for triennial versus biennial screening for both age groups (medians: age 50–74, 3.4–5.1 vs. 4.1–6.5; age 65–74, 1.5–2.1 vs. 1.8–2.6). Breast cancer deaths averted increased with annual versus biennial screening for ages 50–74 years with all levels of breast density and RR=4.0, and ages 65–74 years with heterogeneously or extremely dense breasts and RR=4.0, but harms were almost 2-fold higher. Triennial screening for average-risk and annual screening for highest-risk subgroups cost <$100,000 per QALY gained.
Limitations
Models did not consider ages <50, RR< 1, or other imaging modalities.
Conclusions
Average-risk/low-breast density women undergoing triennial screening and higher-risk/high-breast density women receiving annual screening will maintain a similar or better balance of benefits and harms compared to biennial screening of average-risk women.
Primary Funding Source
National Cancer Institute
Introduction
Despite on-going debate surrounding breast cancer screening for women in their 40s, there is a greater consensus about US guidelines for average-risk women 50 and older (1, 2), with groups now recommending biennial mammography from ages 50 or 55 to 74 years (3, 4). Biennial screening is supported by clinical trials (5, 6), observational studies (5, 7), and modeling results (8). Present recommendations also acknowledge that implementing screening in clinical practice should involve shared decision-making to consider preferences, risk-levels, and breast density (3, 4). However, there are limited data to guide clinicians and women in making personalized decisions about screening intervals based on such factors.
Observational data (7, 9) and modeling studies (10, 11) suggest that annual screening may be more effective than biennial screening for women at high risk due to dense breasts and other risk factors, and that triennial screening may retain most of the benefit of biennial screening but be less harmful and more cost-effective for women with low risk/low density. However, past empirical research on alternative screening intervals did not include mortality outcomes (12). Moreover, most prior modeling studies have relied on single models (10, 11), data on film-screen mammography and older treatment regimens (10, 11, 13), did not consider changes in breast density as women age (10), and/or did not consider triennial intervals (8).
To fill this gap, three well-established Cancer Intervention and Surveillance Modeling Network (CISNET) (14) models collaborated with the Breast Cancer Surveillance Consortium (BCSC), a longstanding network of six U.S. breast imaging registries with linkages to tumor and pathology registries (15), to evaluate varying screening intervals for digital mammography among subgroups of women based on age, risk, and breast density. Outcomes were projected for women who were either 50 (or 65) and deliberating whether to initiate (or continue) biennial screening until age 74 or, alternatively, to undergo annual or triennial screening. Study results are intended to inform discussions about implementing tailored breast cancer screening intervals to maximize the benefits while minimizing the harms of screening.
Methods
Overview of Breast Cancer Screening Strategies
The study included three microsimulation models—Model E (Erasmus Medical Center, Rotterdam, Netherlands), Model G-E (Georgetown University Medical Center, Washington, DC; Albert Einstein College of Medicine, Bronx, New York), and Model W (University of Wisconsin-Madison, Madison, Wisconsin; Harvard Medical School, Boston, Massachusetts)—and was either exempt from human subjects review or approved by review boards at each institution.
Models used a lifetime horizon to evaluate screening strategies for two populations—women aged 50 and starting screening for the first time, and women aged 65 who had undergone biennial screening from ages 50 to 64. We selected these ages because there is general consensus on screening in the 50s; and age 65 since increasing competing mortality and decreases in breast density might alter the balance of benefits and harms.
Strategies for each age group varied by screening interval (annual, biennial, and triennial) and were compared to no screening. These intervals were applied to population subgroups based on combinations of four breast density levels [Breast Imaging Reporting and Data System (BI-RADS) a=almost entirely fat, b=scattered fibroglandular density, c=heterogeneously dense, or d=extremely dense (16)] and four exemplar relative risk (RR) levels based on risk factors other than breast density. The risk levels represent common risk factors considered alone or in combination: 1.0 (“average”); 1.3 (e.g., postmenopausal obesity (17–27)); 2.0 (e.g., history of benign breast biopsy (25–28)); and 4.0 (history of lobular carcinoma in situ (25–29))(Appendix Table 1). Populations with risk suggestive of BRCA1/2 mutations were not included in these analyses.
Model Overview
The models shared common inputs but employed different structures and underlying assumptions (Appendix Table 2) (8, 14). Models started with estimates of age-specific breast cancer incidence (22) and stage- and estrogen receptor(ER)/human epidermal growth factor receptor 2(HER2)-specific survival trends (30) without screening or adjuvant treatment. Incidence in the absence of screening was calibrated from an age-period-cohort model that accounts for changes in underlying risk, e.g., secular patterns in postmenopausal hormone use (31). Tumors had a range of pre-clinical time periods during which they could be detected by screening (i.e., sojourn times). Data on screening and ER/HER2-specific adjuvant treatment were added to generate breast cancer-specific incidence and mortality (14). Models have been validated using the AGE trial (8). Screen detection of cancer during the preclinical detectable period could result in the identification and treatment of earlier stage or smaller tumors and lead to breast cancer mortality reduction (Appendix Figure 1). All models assumed that a portion of DCIS was non-progressive and not lethal; Model W also considered that some small invasive cancers would not progress.
Model Input Parameters
The models used a common set of age-specific variables for population demographics (32), breast cancer natural history and risk (30, 31, 33–36), digital mammography (37, 38), breast density, treatment (39–41), mortality (30), costs (42, 43), and quality of life (Table 1 and Appendix Table 2) (14, 44–46). Each model also included parameters to represent preclinical detectable times, lead-time, and age- and ER/HER2-specific stage distribution in screen- vs. non-screen-detected women on the basis of their specific model structure. These model-specific parameters were based on assumptions about combinations of values that reproduced US trends in incidence and breast cancer-specific mortality from 1975–2010 in the SEER Program (47). To isolate the efficacy of varying screening strategies, all models assumed 100% adherence to screening and receipt of the most effective treatment.
Table 1.
Model Input Parameters
| Parameter | Description | Data Source |
|---|---|---|
| Population Demographics | ||
| Birth cohorts | 1970 birth cohort | (32) |
| Natural History of Breast Cancer | ||
| Incidence in the absence of screening | An age-period-cohort model is used as a starting point for calibration to observed SEER Program rates. | (31) |
|
|
||
| Stage distribution | Stage distribution among clinically-detected and digital screen-detected women by age group (<50, 50–64, ≥65 years), screening round (first, subsequent), and screening interval (annual, biennial, triennial). | BCSC data from 1994–2013 (digital from 2003–2013) |
|
|
||
| ER/HER2 joint distribution | Probability of ER/HER2 conditional on age and stage at diagnosis. | BCSC |
|
|
||
| Sojourn time | Sojourn time by joint ER/HER2 status and age. | (30) |
|
|
||
| Mean stage dwell time/tumor growth rates | Varies by models; can vary by age and/or ER/HER2 status. | (33–35) |
| Breast Cancer Screening | ||
| Mammography use | Assume all women are screened by digital mammography. | (37, 38) |
|
|
||
| Sensitivity/detection rates of digital screening | Sensitivity of initial and subsequent digital mammography by age group, screening interval (annual, biennial, triennial), and breast density. See Appendix Table 3. | BCSC |
|
|
||
| Specificity | False-positive mammograms are calculated as the difference between the overall number of positive mammograms in a screening scenario minus the number of positive mammograms among breast cancer cases. | BCSC |
|
|
||
| Prevalence of breast density | Prevalence of breast density (BI-RADS a, b, c, d) by age group. Density is assigned at age 40 years and can decrease by one level or remain the same at age 50 years and again at age 65 years. | BCSC |
|
|
||
| Risk levels for density | Risk of breast cancer based on BI-RADS relative to average density by age group. | BCSC |
|
|
||
| Risk levels for factors other than density | RR=1 is used at the referent for average population. RR=1.3, 2.0, and 4.0 are used as levels associated with common risk factors. | (36) |
| Breast Cancer Treatment | ||
| Treatment use | Assume receipt of and adherence to the most effective available treatment specific to age, stage and ER/HER2 status. | 1997–2010 (40, 41) |
|
|
||
| Treatment effects | Meta-analyses of clinical trial results. | (39) |
| Survival | ||
| Breast cancer survival | 26-year breast cancer survival before adjuvant treatment by joint ER/HER2 status, age group, and AJCC/SEER stage or tumor size | (30) |
|
|
||
| Non-breast cancer mortality | Age- and cohort-specific all-cause mortality rates by year. | Vanness D, Personal communication, 2015 |
| Costs | ||
| Screening mammogram | $138.28 | Medicare reimbursement |
|
|
||
| Work-up after false-positive mammogram | Imaging costs: $141.42 (all ages). Biopsy costs by age: $1,354.05 for ages 50–64; $1,361.39 for ages 65–74; and $1,442.19 for ages 75–100. Biopsies applied to 10.6% of women screened within each age group. | (42) |
|
|
||
| Work-up after true positive mammogram | By age: $2,154.58 for ages 50–64; $2,166.52 for ages 65–74; and $1,826.80 for ages 75–100. | (42) |
|
|
||
| Breast cancer treatment | By stage during initial treatment: $13,695.67 for DCIS and local stage; $25,893.77 for regional stage; and $39,990.86 for distant stage. During the last year of life among women with cancers that were not cured/progressed, depending on stage at diagnosis: $37,070.10 for DCIS and local stage; $43,878.64 for regional stage; and $61,544.91 for distant stage. | (43) |
| Utilities | ||
| Healthy women | Age-specific quality of life utilities among women without breast cancer. | (45) |
|
|
||
| Screening mammogram | 0.994 for 1 week | (44) |
|
|
||
| Diagnostics after positive mammogram | 0.895 for 5 weeks | (44) |
|
|
||
| Cancer treatment | By stage: 0.9 for 2 years for DCIS and local stage; 0.75 for 2 years for regional stage; and 0.6 until death for distant stage. | (46) |
Abbreviations: AJCC, American Joint Committee on Cancer; BCSC, Breast Cancer Surveillance Consortium; BI-RADS, Breast Imaging Reporting and Data System; DCIS, ductal carcinoma in situ; ER, estrogen receptor; HER2, human epidermal growth factor 2; RR, relative risk; SEER, Surveillance, Epidemiology, and End Results.
Note: Not all models use all parameters; some models use parameters as direct inputs and others use them as a target for calibration or other estimation (See Appendix Table 2).
The population included women born in 1970 and followed until death. This birth cohort was chosen since they experience modern conditions (e.g., digital mammography performance, treatment effectiveness, competing mortality, etc.) and for consistency with recent collaborative modeling reports (8). In each simulation, subgroups of women were followed from age 25 until death or age 100. Subgroups were defined based on combinations of four RR levels (1.0, 1.3, 2.0 and 4.0) and four breast density levels with the combination of risk from breast density and other factors treated multiplicatively. The risk level modified the underlying breast cancer incidence in the absence of screening. We assumed that risk-level was constant over age and did not affect other model parameters. Women were assigned to either the same breast density category or to the next lower category at ages 50 and 65 based on observed age-specific prevalence in the BCSC (27, 48). Density also affected mammography performance (Table 1 and Appendix Table 3).
Digital mammography sensitivity and specificity were based on age, initial or subsequent screen, screening interval, and breast density using BCSC data (Table 1 and Appendix Table 3). Models GE and W used these data for calibration, and Model E fit estimates from the BCSC and other sources (35). Specificity data were used to estimate false-positive mammogram rates. BCSC rates of biopsy recommendations were applied to these estimates to calculate the number of benign biopsies.
Treatment effectiveness was based on clinical trials and was modeled as a reduction in mortality risk (Model G-E) or increase in the proportion cured (Models E and W) as compared with age-, stage-, and ER/HER2-specific survival in the absence of therapy (39). Women died either of breast cancer or other causes.
Screening Outcomes
Primary outcomes were lifetime benefits and harms; secondary outcomes were use of services and costs. Benefits included breast cancer deaths averted and life-years (LY) and quality-adjusted life-years (QALY) gained. QALYs were based on utilities for the general US population estimated both with and without adjustments for undergoing a screening exam (−0.006 for 1 week per exam = −1 hour per exam) and having a positive screening result and undergoing diagnostic evaluation (−0.0105 for 5 weeks = −8.8 hours). Adjustments were also made for breast cancer treatment (Table 1).
Harms included false-positive mammograms, benign biopsies, and over-diagnosis. The rate of false-positive mammograms was the number of mammograms read as abnormal in women without cancer divided by the total number of screening mammograms. Benign biopsies were defined as a biopsy recommendation among women with false-positive screening results (49). Over-diagnosis was defined as screen-detected cancer that would not have been diagnosed in a woman’s lifetime in the absence of mammography (14, 50).
Costs were estimated based on the number of mammograms, evaluation of positive mammograms including additional imaging and/or biopsy among cancer cases and those with false-positives, and stage-specific cancer treatments based on Medicare reimbursement schedules and published studies, reported in 2014 US dollars (Table 1).
Analysis
For each age group modeled (≥50 and ≥65) there were 16 possible population subgroups based on combinations of risk and density. Benefits and harms for each strategy were compared to no screening for each 1,000 women screened. No screening was assumed to occur prior to age 50 in all analyses. Screening strategies for women aged 65–74 assumed women received biennial mammograms during ages 50–64. We report the median benefits and harms and the range across models as a measure of uncertainty. In secondary analyses, the ratio of false-positive mammograms to breast cancer deaths averted was calculated as one metric of the trade-offs of harms to benefits. Finally, we estimated the incremental costs per QALY for each strategy and population risk/density subgroup. For this estimate, the change in cost was divided by the change in benefit (e.g., QALYs) when each more costly screening strategy was compared with the strategy of next lowest cost within the subgroup. Costs and QALYs were discounted at 3% per year and QALYs included screening and work-up adjustments. Screening strategies were considered cost-effective with a common threshold of $100,000 per QALY gained (51).
Role of the Funding Source
The National Cancer Institute funded this research and had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Results
The results of all three models illustrate that across intervals and age groups, screening (vs. no screening; Appendix Table 4) had a greater absolute benefit in terms of breast cancer deaths averted, LYs, and QALYs among two groups of women: those with dense breasts and those at higher RR within each breast density group (Tables 2 and 3). Adjustments for screening harms did not affect the QALY ordering of screening strategies.
Table 2.
Lifetime benefits of screening annually, biennially or triennially per 1000 women screened by relative risk, breast density, and age group across 3 models.
| Density | RR | Breast cancer deaths averted vs. no screening, median (range across models) | Life years gained vs. no screening, median (range across models) * | ||||
|---|---|---|---|---|---|---|---|
| Triennial | Biennial | Annual | Triennial | Biennial | Annual | ||
| Ages 50–74† | |||||||
| Almost entirely fatty | 1 | 3.4 (1.8–3.6) | 4.1 (2.4–4.3) | 4.7 (3.2–5.6) | 50 (35–64) | 64 (47–73) | 84 (62–85) |
| 1.3 | 4.4 (2.4–4.6) | 5.3 (3.1–5.5) | 6.0 (4.1–7.1) | 64 (46–82) | 82 (60–94) | 108 (80–109) | |
| 2 | 6.4 (3.6–7.0) | 8.0 (4.8–8.0) | 9.1 (6.2–10.3) | 94 (69–124) | 120 (92–142) | 159 (122–163) | |
| 4 | 11.0 (7.2–13.1) | 13.8 (9.2–15.0) | 17.2 (12.0–17.7) | 164 (136–235) | 209 (177–269) | 277 (233–309) | |
| Scattered fibroglandular | 1 | 4.0 (2.9–5.9) | 5.2 (3.8–6.8) | 6.9 (5.1–7.9) | 59 (56–107) | 77 (74–123) | 106 (101–143) |
| 1.3 | 5.1 (3.7–7.5) | 6.5 (4.9–8.7) | 8.7 (6.6–10.1) | 75 (72–137) | 97 (95–158) | 134 (129–184) | |
| 2 | 7.2 (5.6–11.2) | 9.2 (7.4–12.9) | 12.3 (9.9–15.0) | 109 (107–204) | 144 (139–236) | 194 (191–275) | |
| 4 | 11.5 (10.8–20.2) | 14.7 (13.9–23.3) | 19.4 (18.4–27.0) | 207 (175–372) | 269 (227–430) | 360 (308–502) | |
| Hetero-geneously dense | 1 | 4.8 (3.3–8.4) | 6.3 (4.4–9.8) | 8.4 (6.1–11.7) | 72 (64–149) | 94 (86–175) | 130 (122–210) |
| 1.3 | 6.0 (4.2–10.7) | 7.7 (5.6–12.4) | 10.4 (7.8–14.8) | 90 (82–190) | 117 (110–223) | 161 (155–267) | |
| 2 | 8.3 (6.3–15.5) | 10.6 (8.3–18.1) | 14.3 (11.6–21.6) | 124 (122–278) | 162 (162–326) | 230 (224–392) | |
| 4 | 12.4 (11.4–26.5) | 15.8 (15.1–31.0) | 21.0 (20.8–37.1) | 221 (192–485) | 294 (248–568) | 411 (338–685) | |
| Extremely dense | 1 | 5.1 (3.1–9.9) | 6.5 (4.2–11.7) | 8.9 (6.0–14.4) | 75 (61–174) | 98 (82–206) | 138 (121–255) |
| 1.3 | 6.2 (4.0–12.5) | 8.0 (5.4–14.7) | 10.9 (7.7–18.1) | 93 (79–219) | 122 (106–261) | 170 (155–323) | |
| 2 | 8.4 (5.9–17.9) | 10.8 (7.9–21.1) | 14.7 (11.4–26.0) | 127 (115–317) | 166 (155–376) | 231 (226–468) | |
| 4 | 12.0 (10.4–29.3) | 15.4 (14.0–34.7) | 20.5 (20.2–42.9) | 204 (187–534) | 277 (242–634) | 402 (332–789) | |
| Ages 65–74‡ | |||||||
| Almost entirely fatty | 1 | 1.5 (0.8–1.6) | 1.8 (1.0–2.0) | 2.3 (1.4–2.4) | 16 (11–21) | 19 (15–26) | 26 (21–31) |
| 1.3 | 1.9 (1.0–2.0) | 2.3 (1.4–2.6) | 3.0 (1.9–3.1) | 20 (14–27) | 24 (19–34) | 34 (27–40) | |
| 2 | 2.7 (1.5–3.0) | 3.2 (2.1–3.9) | 4.3 (2.8–4.4) | 28 (20–40) | 33 (29–50) | 47 (41–59) | |
| 4 | 4.2 (2.6–5.4) | 5.1 (3.8–7.0) | 6.8 (5.0–8.0) | 44 (37–71) | 54 (52–92) | 73 (73–107) | |
| Scattered fibroglandular | 1 | 1.7 (1.1–2.3) | 2.1 (1.6–2.9) | 3.0 (2.2–3.4) | 18 (17–30) | 23 (22–39) | 33 (32–45) |
| 1.3 | 2.1 (1.5–2.9) | 2.6 (2.1–3.7) | 3.6 (2.9–4.3) | 22 (21–38) | 30 (27–49) | 42 (39–58) | |
| 2 | 2.9 (2.1–4.2) | 3.5 (3.0–5.4) | 4.9 (4.1–6.3) | 30 (29–55) | 43 (36–71) | 60 (53–84) | |
| 4 | 4.0 (3.6–7.2) | 5.3 (4.9–9.4) | 7.2 (6.8–10.9) | 50 (41–96) | 74 (50–124) | 102 (73–146) | |
| Hetero-geneously dense | 1 | 2.0 (1.2–3.6) | 2.5 (1.8–4.7) | 3.6 (2.5–5.7) | 21 (17–47) | 26 (25–62) | 38 (37–75) |
| 1.3 | 2.5 (1.5–4.5) | 3.0 (2.2–5.9) | 4.3 (3.2–7.1) | 26 (21–59) | 32 (31–77) | 47 (47–95) | |
| 2 | 3.2 (2.2–6.4) | 3.9 (3.2–8.4) | 5.5 (4.6–10.1) | 33 (31–84) | 46 (40–111) | 66 (60–135) | |
| 4 | 4.0 (3.6–10.1) | 5.4 (4.8–13.3) | 7.6 (6.7–16.1) | 50 (40–134) | 76 (49–176) | 109 (72–216) | |
| Extremely dense | 1 | 2.0 (1.1–4.3) | 2.5 (1.7–5.9) | 3.6 (2.4–7.3) | 21 (16–57) | 26 (24–77) | 39 (36–97) |
| 1.3 | 2.4 (1.4–5.4) | 3.0 (2.1–7.3) | 4.3 (3.1–9.1) | 25 (20–72) | 31 (30–96) | 46 (45–122) | |
| 2 | 3.0 (2.0–7.5) | 3.7 (3.0–10.1) | 5.3 (4.4–12.6) | 31 (29–99) | 43 (38–134) | 64 (57–170) | |
| 4 | 3.5 (3.3–11.2) | 4.9 (4.3–15.1) | 7.3 (6.0–18.9) | 46 (36–149) | 70 (43–202) | 105 (64–257) | |
Abbreviations: RR, relative risk.
Life years gained are undiscounted.
Screening is initiated at age 50.
Women who are currently 65 and have been screened biennially from 50–64.
Table 3.
Lifetime QALY benefits of screening annually, biennially or triennially per 1000 women screened by relative risk, breast density, and age group with and without screening and work-up adjustments.
| Density | RR | QALYs gained with screening and work-up adjustments vs. no screening, median (range across models) * | QALYs gained without screening and work-up adjustments vs. no screening, median (range across models)* | ||||
|---|---|---|---|---|---|---|---|
| Triennial | Biennial | Annual | Triennial | Biennial | Annual | ||
| Ages 50–74† | |||||||
| Almost entirely fatty | 1 | 32 (21–44) | 41 (29–49) | 51 (36–51) | 37 (26–50) | 48 (35–58) | 63 (47–66) |
| 1.3 | 43 (29–59) | 54 (39–66) | 69 (50–71) | 47 (34–65) | 61 (45–75) | 80 (61–86) | |
| 2 | 65 (46–93) | 82 (63–105) | 106 (81–116) | 70 (52–99) | 89 (69–114) | 118 (93–131) | |
| 4 | 118 (98–183) | 150 (128–209) | 194 (168–234) | 123 (103–190) | 157 (135–217) | 206 (180–249) | |
| Scattered fibroglandular | 1 | 36 (35–76) | 47 (47–86) | 60 (60–92) | 43 (43–85) | 57 (56–99) | 78 (78–115) |
| 1.3 | 48 (47–101) | 63 (62–114) | 82 (81–126) | 55 (55–110) | 73 (72–127) | 99 (98–148) | |
| 2 | 75 (71–155) | 100 (92–178) | 132 (123–200) | 83 (78–164) | 110 (102–190) | 149 (140–222) | |
| 4 | 153 (122–292) | 199 (158–336) | 264 (212–386) | 160 (129–301) | 209 (168–349) | 280 (228–407) | |
| Hetero-geneously dense | 1 | 44 (40–110) | 57 (55–126) | 75 (73–143) | 52 (49–120) | 69 (66–141) | 95 (94–169) |
| 1.3 | 57 (54–143) | 74 (74–165) | 100 (98–190) | 65 (63–153) | 85 (85–180) | 120 (119–216) | |
| 2 | 86 (83–215) | 114 (108–249) | 159 (145–292) | 94 (91–225) | 125 (119–263) | 178 (165–317) | |
| 4 | 164 (133–384) | 220 (173–448) | 305 (233–533) | 172 (141–394) | 230 (184–461) | 322 (251–556) | |
| Extremely dense | 1 | 47 (40–131) | 62 (54–154) | 84 (77–185) | 54 (47–140) | 71 (64–166) | 100 (94–206) |
| 1.3 | 60 (54–169) | 79 (73–199) | 108 (104–240) | 67 (61–177) | 88 (82–211) | 124 (120–261) | |
| 2 | 85 (83–248) | 112 (112–293) | 161 (153–358) | 92 (90–257) | 121 (121–305) | 176 (169–379) | |
| 4 | 154 (129–425) | 210 (169–503) | 302 (231–622) | 161 (136–433) | 218 (178–514) | 317 (246–641) | |
| Ages 65–74‡ | |||||||
| Almost entirely fatty | 1 | 9 (6–15) | 11 (8–18) | 15 (11–20) | 11 (8–16) | 13 (10–21) | 19 (15–24) |
| 1.3 | 12 (8–19) | 15 (11–24) | 20 (16–27) | 14 (10–21) | 17 (14–27) | 24 (20–31) | |
| 2 | 18 (13–30) | 22 (19–38) | 29 (26–42) | 20 (15–31) | 24 (21–40) | 34 (30–47) | |
| 4 | 30 (25–55) | 37 (36–71) | 50 (49–81) | 32 (27–57) | 39 (38–73) | 54 (53–85) | |
| Scattered fibroglandular | 1 | 10 (10–21) | 13 (12–27) | 18 (17–29) | 13 (13–23) | 17 (16–30) | 24 (23–36) |
| 1.3 | 13 (13–28) | 18 (16–35) | 25 (22–39) | 16 (15–30) | 22 (19–39) | 31 (28–46) | |
| 2 | 19 (19–42) | 28 (23–53) | 38 (32–60) | 21 (21–44) | 31 (26–57) | 44 (38–67) | |
| 4 | 35 (28–75) | 52 (33–96) | 71 (47–111) | 37 (30–77) | 55 (37–99) | 77 (53–117) | |
| Hetero-geneously dense | 1 | 12 (10–35) | 15 (14–45) | 20 (20–52) | 15 (13–38) | 19 (18–49) | 27 (27–60) |
| 1.3 | 15 (13–45) | 20 (18–58) | 27 (26–68) | 18 (16–47) | 24 (22–62) | 35 (33–76) | |
| 2 | 21 (20–65) | 30 (25–85) | 43 (36–100) | 24 (23–67) | 34 (29–89) | 50 (43–108) | |
| 4 | 35 (27–105) | 54 (33–138) | 76 (46–167) | 38 (30–107) | 57 (36–142) | 82 (52–174) | |
| Extremely dense | 1 | 12 (10–44) | 15 (15–58) | 21 (21–72) | 15 (12–46) | 18 (18–62) | 27 (27–78) |
| 1.3 | 15 (13–55) | 20 (18–74) | 28 (27–91) | 18 (15–57) | 23 (21–77) | 34 (32–98) | |
| 2 | 20 (20–78) | 29 (24–104) | 43 (35–130) | 22 (22–80) | 32 (27–107) | 48 (41–136) | |
| 4 | 33 (24–118) | 51 (29–159) | 76 (43–201) | 35 (26–120) | 53 (32–162) | 80 (47–206) | |
Abbreviations: QALY, quality-adjusted life year; RR, relative risk.
QALYs gained are undiscounted.
Screening is initiated at age 50.
Women who are currently 65 and have been screened biennially from 50–64.
Women Starting Screening at Age 50
For all screening intervals, as risk and breast density increased, the benefits (breast cancer deaths averted, life expectancy and quality-adjusted life expectancy) of screening increased and the harms (false positives, benign biopsies and over-diagnosis) decreased with greater risk (Tables 2, 3 and 4).
Table 4.
Lifetime harms of screening annually, biennially or triennially per 1000 women screened by relative risk, breast density, and age group
| Density and RR | False-positives vs. no screening, median (range across models) | Benign biopsies vs. no screening, median (range across models) | Over-diagnosis vs. no screening, median (range across models)* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Triennial | Biennial | Annual | Triennial | Biennial | Annual | Triennial | Biennial | Annual | |
| Ages 50–74† | |||||||||
| Almost entirely fatty | |||||||||
| 1 | 489 (424–616) | 618 (613–858) | 1101 (1094–1548) | 79 (68–106) | 91 (91–136) | 127 (127–191) | 11 (9–17) | 12 (11–20) | 17 (12–24) |
| 1.3 | 484 (420–611) | 612 (606–851) | 1089 (1081–1536) | 78 (67–106) | 91 (90–135) | 126 (125–190) | 12 (11–21) | 15 (11–26) | 21 (12–31) |
| 2 | 471 (412–600) | 598 (590–836) | 1062 (1051–1507) | 76 (66–104) | 89 (88–133) | 123 (122–187) | 17 (11–31) | 22 (11–37) | 30 (12–44) |
| 4 | 438 (390–571) | 564 (547–794) | 996 (972–1429) | 71 (63–99) | 84 (81–126) | 116 (113–177) | 27 (11–53) | 35 (11–63) | 49 (12–75) |
| Scattered areas of fibroglandular density | |||||||||
| 1 | 781 (693–935) | 1009 (991–1326) | 1806 (1776–2440) | 126 (111–158) | 150 (147–206) | 209 (206–296) | 13 (11–22) | 17 (11–27) | 23 (12–35) |
| 1.3 | 767 (683–922) | 994 (972–1309) | 1776 (1740–2406) | 123 (110–156) | 148 (144–203) | 206 (202–292) | 16 (11–28) | 20 (11–34) | 29 (12–44) |
| 2 | 734 (662–894) | 963 (929–1267) | 1714 (1659–2329) | 118 (107–152) | 143 (138–197) | 199 (193–283) | 21 (10–39) | 28 (11–48) | 39 (12–62) |
| 4 | 649 (613–818) | 888 (818–1158) | 1568 (1452–2123) | 105 (99–140) | 132 (122–181) | 183 (169–259) | 31 (11–60) | 40 (12–74) | 56 (13–95) |
| Heterogeneously dense | |||||||||
| 1 | 917 (822–1064) | 1197 (1171–1524) | 2123 (2080–2829) | 163 (146–195) | 178 (174–235) | 266 (261–365) | 16 (10–20) | 20 (11–26) | 28 (12–38) |
| 1.3 | 894 (807–1043) | 1174 (1141–1493) | 2078 (2023–2771) | 159 (144–191) | 174 (169–230) | 261 (254–358) | 19 (10–25) | 24 (11–32) | 34 (12–46) |
| 2 | 842 (775–995) | 1125 (1073–1424) | 1984 (1896–2642) | 150 (138–183) | 167 (160–220) | 249 (238–342) | 25 (10–34) | 32 (11–44) | 45 (13–63) |
| 4 | 715 (703–875) | 1016 (906–1248) | 1778 (1585–2308) | 128 (126–162) | 152 (136–194) | 224 (200–301) | 32 (11–49) | 41 (12–63) | 57 (14–89) |
| Extremely dense | |||||||||
| 1 | 732 (652–849) | 939 (925–1200) | 1668 (1647–2225) | 130 (116–156) | 139 (137–185) | 209 (206–288) | 16 (10–17) | 21 (11–22) | 31 (12–32) |
| 1.3 | 712 (638–827) | 917 (898–1169) | 1626 (1597–2167) | 127 (113–152) | 136 (133–181) | 204 (200–281) | 19 (10–21) | 26 (11–27) | 37 (12–39) |
| 2 | 666 (608–780) | 872 (839–1102) | 1540 (1487–2039) | 119 (108–144) | 129 (125–171) | 193 (186–265) | 26 (10–26) | 34 (11–35) | 47 (13–53) |
| 4 | 555 (543–663) | 776 (697–933) | 1359 (1223–1719) | 99 (97–123) | 116 (104–146) | 171 (154–225) | 32 (10–37) | 41 (12–49) | 56 (15–74) |
| Ages 65–74‡ | |||||||||
| Almost entirely fatty | |||||||||
| 1 | 145 (137–169) | 209 (206–227) | 413 (395–459) | 22 (20–25) | 29 (29–32) | 45 (43–51) | 5 (4–8) | 6 (5–11) | 9 (5–13) |
| 1.3 | 142 (135–166) | 206 (202–224) | 405 (388–453) | 21 (20–25) | 29 (28–31) | 45 (43–50) | 7 (4–10) | 8 (5–14) | 11 (5–17) |
| 2 | 135 (130–160) | 198 (193–217) | 387 (373–438) | 20 (20–24) | 28 (27–30) | 43 (41–48) | 9 (4–15) | 11 (5–20) | 15 (6–25) |
| 4 | 119 (118–145) | 178 (169–197) | 340 (335–399) | 18 (18–22) | 25 (24–28) | 37 (37–44) | 14 (5–25) | 16 (6–34) | 22 (7–41) |
| Scattered areas of fibroglandular density | |||||||||
| 1 | 230 (225–278) | 343 (333–375) | 667 (648–757) | 34 (34–42) | 48 (47–52) | 73 (71–83) | 7 (4–10) | 8 (5–15) | 12 (5–20) |
| 1.3 | 223 (220–271) | 335 (322–366) | 645 (632–741) | 33 (33–41) | 47 (45–51) | 71 (69–81) | 8 (4–13) | 10 (5–19) | 14 (6–24) |
| 2 | 209 (206–257) | 317 (298–348) | 597 (597–704) | 31 (31–39) | 44 (42–49) | 66 (66–77) | 11 (5–18) | 13 (6–26) | 18 (6–34) |
| 4 | 180 (166–225) | 276 (239–299) | 520 (480–607) | 27 (25–34) | 39 (33–42) | 57 (53–67) | 14 (5–27) | 17 (7–38) | 23 (8–50) |
| Heterogeneously dense | |||||||||
| 1 | 273 (260–329) | 407 (397–432) | 794 (760–875) | 46 (44–56) | 57 (56–61) | 95 (91–105) | 8 (4–10) | 10 (5–14) | 14 (6–20) |
| 1.3 | 262 (250–319) | 394 (381–417) | 762 (735–845) | 45 (43–54) | 55 (53–58) | 91 (88–101) | 10 (5–12) | 12 (6–17) | 17 (7–25) |
| 2 | 238 (230–298) | 367 (346–384) | 693 (684–779) | 41 (39–51) | 51 (48–54) | 83 (82–93) | 12 (5–16) | 15 (7–23) | 21 (8–33) |
| 4 | 182 (181–254) | 302 (264–311) | 580 (528–617) | 31 (31–43) | 42 (37–44) | 70 (63–74) | 13 (6–22) | 16 (8–32) | 22 (10–46) |
| Extremely dense | |||||||||
| 1 | 202 (187–239) | 295 (291–312) | 583 (553–631) | 34 (32–41) | 41 (41–44) | 70 (66–76) | 7 (4–9) | 10 (6–11) | 15 (7–17) |
| 1.3 | 193 (179–231) | 284 (279–298) | 559 (532–604) | 33 (30–39) | 40 (39–42) | 67 (64–72) | 9 (5–10) | 12 (6–13) | 18 (7–20) |
| 2 | 175 (161–214) | 263 (253–268) | 507 (491–544) | 30 (27–36) | 37 (35–37) | 61 (59–65) | 12 (5–12) | 15 (7–17) | 21 (9–27) |
| 4 | 133 (118–180) | 197 (191–221) | 404 (383–412) | 23 (20–31) | 28 (27–31) | 49 (46–49) | 12 (6–16) | 14 (9–24) | 20 (11–38) |
Abbreviations: RR, relative risk.
Over-diagnosed cases are defined as cases that would not have been clinically detected in the absence of screening. The value includes DCIS and invasive over-diagnosis. Over-diagnosis is calculated by comparing cases detected in the screening scenario to those detected in the unscreened scenario.
Per 1000 women compared to no screening at any age.
Per 1000 women compared to biennial mammograms 50–64 with no subsequent screening.
Among women at average risk (RR= 1.0–1.3) and fatty (BI-RADS=a) or scattered fibroglandular (BI-RADS=b) density, screening biennially from 50–74 compared to no screening averted a median of 4.1–6.5 breast cancer deaths in 1000 women screened, respectively (Table 2). Screening outcomes were similar for triennial screening of average-risk and low-breast density groups compared to no screening; the median breast cancer deaths averted were 3.4–5.1 for every 1000 women screened. Screening triennially compared to biennially for the average-risk and low-density groups resulted in a median of 21–23% fewer false-positive mammograms, 13–17% fewer benign biopsies, and 8–20% fewer over-diagnosed cases, respectively (Table 4). If RR increased to 2 among those with fatty or scattered fibroglandular density breasts, then triennial screening resulted in a median of 1.6 or 2 fewer breast cancer deaths averted per 1000 screened, respectively, compared to biennial screening. Thus, 1000 women would undergo 9 rounds of screening to avert 6.4–7.2 deaths from breast cancer with 471–734 false-positives and 76–118 biopsies for triennial screening versus 13 rounds of screening to avert 8.0–9.2 deaths from breast cancer with 598–963 false-positives and 89–143 biopsies.
The benefits of more frequent screening increased as density increased and as RR increased to 2 or more. For instance, biennial screening from ages 50–74 years among subgroups with a RR=2 and heterogeneously dense breasts (BI-RADS=c) resulted in a median of 10.6 breast cancer deaths averted and 1125 false-positive mammograms per 1000 women screened compared to no screening. If this group of women had been screened annually rather than biennially, a median of 3.7 more deaths could have been averted but with almost 2-fold more false-positive mammograms (1984 vs. 1125 per 1000 screened). Breast cancer deaths averted per 1000 screens were highest with annual screening for women ages 50–74 years with all levels of breast density and RR of 4.0 ranging from 17.2 breast averted for women with fatty breasts to 20.5 for women with extremely dense breasts (BI-RADS=d).
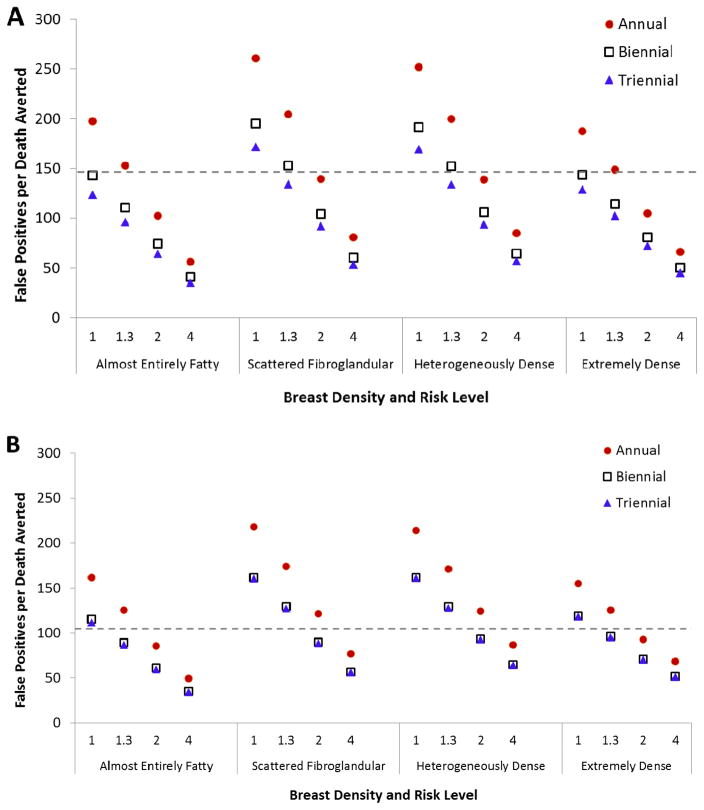
The ratio of harms to benefits for subgroups with different levels of risk and density screened during ages 50–74 is graphically illustrated for an exemplar model on Figure 1 (panel A). Compared to the ratios projected for biennial screening of average-risk women from ages 50–74 years irrespective of breast density, annual screening has a comparable or better ratio when RR is ≥2 across all density groups. Triennial screening has comparable or better ratios of harms to benefits than biennial screening for average risk women irrespective of breast density for nearly all of the risk-density subgroups because false-positive mammograms are reduced with triennial screening and the magnitude of breast cancer deaths averted is similar or slightly lower than with biennial screening.
Figure 1.
False-positives mammograms per breast cancer death averted for women (A) aged 50–74 and (B) aged 65–74 according to screening frequency and risk level (relative risk group, breast density) using an exemplar model (Model E). Values for all screening frequencies compared to the scenario with no mammography screening. Values for ages 65–74 assume all women received biennial screening during ages 50–64. Dashed lines show this value for women with average density and average risk receiving biennial screening (147.7 for ages 50–74 and 105.8 for ages 65–74). Having fewer false-positives per death averted than this level, i.e., a value below the dashed line, would be more favorable.
Women at Age 65
The different screening intervals during ages 65–74 had similar patterns of benefits and harms across subgroups as observed for screening during 50–74 but with lower absolute magnitudes (Tables 2–4; Figure 1, panel B). If women changed from biennial to triennial screening at age 65, there were fewer than a median of 1 less death averted per 1000 women screened for all RR and density subgroups, except the RR of 4 and heterogeneously or extremely dense subgroups where there was a median of 1.4 fewer breast cancer deaths averted (Table 2). For example, continuing biennial screening among women with average-risk (RR= 1.0–1.3) and fatty breasts or scattered fibroglandular density breast density averted a median of 1.8–2.6 deaths, respectively for every 1000 women screened (Table 2) whereas switching to triennial screening averted a median of 1.5–2.1 deaths. Switching from biennial to annual screening increased the median breast cancer deaths averted to 2 or more for women with heterogeneously or extremely dense breasts and RR of 4.
As was the case for screening from 50–74, the ratio of harms (measured as false-positives) to benefits (breast cancer deaths averted) for annual screening during 65–74 was similar to—or better (lower) than—screening average risk populations biennially if RR=2 or greater in all density subgroups; exceptions were rare (Figure 1, panel B). Triennial screening also had a lower or more favorable ratio than biennial screening, because false-positive mammograms are reduced and the magnitude of breast cancer deaths averted is similar or slightly lower; continuing biennial screening has a similar balance as triennial for most subgroups as seen for average-risk groups, irrespective of breast density.
Cost-Effectiveness
Using a common threshold of $100,000 per QALY, triennial strategies were the only cost-effective strategies for subgroups with both average-risk and low-density (fatty breasts or scattered fibroglandular density) at both ages (Table 5). Biennial strategies were cost-effective for most density subgroups and average/intermediate risk (RR=1.3, 2.0). Annual strategies were only consistently cost-effective across models for subgroups with RR=4 and any density, or RR≥2 and heterogeneously or extremely dense breasts.
Table 5.
Incremental costs per quality-adjusted life year gained* by breast density, risk level, screening interval, and age for 3 models.
| Density | RR | Screening Frequency | Age 50–74 | Age 65–74 | ||||
|---|---|---|---|---|---|---|---|---|
| Model E | Model W | Model GE | Model E | Model W | Model GE | |||
| Fatty | 1.0 | Triennial | 68,777 | 117,753 | 43,098 | 100,058 | 131,294 | 27,639 |
| Biennial | 122,007 | 123,132 | 232,710 | 109,587 | 212,665 | 104,235 | ||
| Annual | 389,195 | 586,116 | Dom | 435,881 | 516,979 | >1,000,000 | ||
|
|
||||||||
| 1.3 | Triennial | 50,231 | 83,220 | 27,022 | 70,716 | 92,938 | 15,785 | |
| Biennial | 83,577 | 86,426 | 133,826 | 75,433 | 135,221 | 67,152 | ||
| Annual | 231,495 | 309,654 | >1,000,000 | 258,193 | 286,643 | 799,501 | ||
|
|
||||||||
| 2.0 | Triennial | 30,910 | W Dom | 10,364 | 42,229 | 58,276 | 3,004 | |
| Biennial | 50,526 | 50,084† | 65,297 | 46,300 | 70,911 | 32,912 | ||
| Annual | 122,540 | 148,375 | 392,745 | 141,183 | 146,961 | 263,493 | ||
|
|
||||||||
| 4.0 | Triennial | 14,969 | 22,663 | † | 19,130 | W Dom | † | |
| Biennial | 22,802 | 23,295 | 19,932 | 21,242 | 30,054 | 5,331 | ||
| Annual | 54,906 | 56,451 | 95,362 | 69,089 | 62,251 | 76,840 | ||
|
| ||||||||
| Scattered | 1.0 | Triennial | 69,714 | 72,156 | 18,509 | W Dom | 55,051 | 14,112 |
| Biennial | 111,605 | 75,673 | 104,454 | 101,612 | Dom | 61,723 | ||
| Annual | 317,991 | 288,199 | Dom | 382,578 | 612,349 | >1,000,000 | ||
|
|
||||||||
| 1.3 | Triennial | 50,010 | 51,493 | 9,683 | W Dom | 60,785 | 5,449 | |
| Biennial | 75,416 | 53,967 | 63,057 | 72,488 | 73,479 | 39,636 | ||
| Annual | 186,322 | 171,038 | 488,376 | 224,322 | 201,088 | 450,818 | ||
|
|
||||||||
| 2.0 | Triennial | 31,053 | 29,757 | 641 | W Dom | 38,299 | † | |
| Biennial | 43,721 | 31,198 | 28,182 | 42,160 | 42,347 | 15,956 | ||
| Annual | 96,584 | 85,607 | 144,723 | 120,188 | 104,553 | 159,293 | ||
|
|
||||||||
| 4.0 | Triennial | 15,414 | 12,179 | † | W Dom | 17,507 | Dom | |
| Biennial | 19,733 | 13,116 | 5,116 | 20,076 | 17,977 | ‡ | ||
| Annual | 44,019 | 32,452 | 39,105 | 61,818 | 39,362 | 42,660 | ||
|
| ||||||||
| Het. dense | 1.0 | Triennial | 57,924 | W Dom | 8,016 | W Dom | 75,197 | 611 |
| Biennial | 85,241 | 60,333† | 50,421 | 85,145 | 96,863 | 23,104 | ||
| Annual | 222,789 | 185,805 | 268,798 | 279,586 | 290,534 | 179,689 | ||
|
|
||||||||
| 1.3 | Triennial | 42,324 | 41,815 | 2,179 | 60,235 | 54,355 | † | |
| Biennial | 61,309 | 42,551 | 31,442 | 61,760 | 60,225 | 11,809 | ||
| Annual | 137,983 | 116,700 | 134,915 | 169,196 | 174,243 | 97,850 | ||
|
|
||||||||
| 2.0 | Triennial | 26,726 | 23,375 | † | W Dom | 31,637 | † | |
| Biennial | 35,235 | 26,574 | 12,543 | 36,446 | 36,762 | 478 | ||
| Annual | 75,747 | 57,557 | 56,331 | 99,035 | 85,503 | 44,784 | ||
|
|
||||||||
| 4.0 | Triennial | 13,432 | 8,534 | Dom | W Dom | 13,298 | Dom | |
| Biennial | 16,745 | 9,256 | ‡ | 18,673 | 13,814 | ‡ | ||
| Annual | 36,845 | 22,339 | 14,716 | 57,264 | 32,355 | 8,752 | ||
|
| ||||||||
| Dense | 1.0 | Triennial | 50,563 | 52,953 | 3,017 | W Dom | 63,918 | † |
| Biennial | 68,216 | 55,420 | 27,942 | 75,917 | 77,061 | 8,555 | ||
| Annual | 148,014 | 129,536 | 89,425 | 187,329 | 203,860 | 60,177 | ||
|
|
||||||||
| 1.3 | Triennial | 37,937 | 36,486 | † | W Dom | 45,929 | † | |
| Biennial | 49,172 | 40,051 | 16,293 | 55,033 | 52,754 | 2,547 | ||
| Annual | 101,399 | 87,230 | 56,264 | 130,774 | 130,339 | 36,740 | ||
|
|
||||||||
| 2.0 | Triennial | 24,715 | 20,626 | † | W Dom | 26,367 | Dom | |
| Biennial | 30,291 | 23,683 | 4,631 | 35,097 | 32,766 | ‡ | ||
| Annual | 60,577 | 47,687 | 25,753 | 82,794 | 71,187 | 15,070 | ||
|
|
||||||||
| 4.0 | Triennial | 13,169 | 7,130 | Dom | W Dom | 10,180 | Dom | |
| Biennial | 14,856 | 7,823 | ‡ | 19,207 | 11,669 | Dom | ||
| Annual | 31,433 | 18,224 | 4,407 | 52,645 | 26,834 | § | ||
Note: Incremental ratios bold if values are <$100,000, a common threshold for least costly and most effective strategies (dominant). Unless otherwise indicated, triennial strategies are compared to no screening. Breast density categories shown as: fatty, almost entirely fat; scattered, scattered fibroglandular density; het. dense, heterogeneously dense; and dense, extremely dense.
Abbreviations: RR, relative risk; Dom, more expensive and less effective (strongly dominated); W Dom, more expensive and more effective but less efficient (weakly dominated).
Costs and quality-adjusted life years discounted at 3% per year. Quality-adjusted life years include disutility from participation in screening mammography.
Strategy with no screening is strongly dominated. Triennial is the least costly strategy for comparison.
Strategy with biennial screening is the least costly.
Strategy with annual screening is the least costly
DISCUSSION
This collaborative modeling study demonstrates that risk and density level can be useful for guiding tailored screening recommendations. For average risk women in the low density subgroups, which comprise a large proportion of the population, triennial screening provides a reasonable balance of benefits and harms and is cost-effective. Annual screening has a favorable balance for subgroups of women at age 50 with risk levels two to four times the average and heterogeneously or extremely dense breasts, and also would be considered cost-effective for these subgroups. Benefits of screening women with heterogeneously dense breasts (at any interval) were greater than screening women with extremely dense breasts within each risk level, reflecting increased risk but fewer missed cancers compared with screening women with extremely dense breasts. The same patterns are seen for women at age 65, such that subgroups with average-risk and low density can consider triennial screening, while the small number of women that remain at higher risk might benefit from annual screening. Notably, biennial screening maintains an acceptable balance of screening outcomes and is also cost-effective for women of RR=1.3 or 2 as long as they are not in the highest density groups. Finally, screening benefits and harms exist on a continuum across age, risk, and density with the optimal screening interval depending on patient values and preferences for benefits and harms.
Current US screening guidelines focus on the average-risk population and generally recommend biennial screening for women in their 50s or older (3, 4). These new modeling results support this recommendation for women in the population who do not have either higher than average risk and high density or average/low risk and density. Annual screening has been suggested for high-risk women (4). The current results provide further guidance on the specific combinations of RRs and breast density after age 50 that identify the subgroups where annual screening should be considered; these subgroups are estimated to constitute <1% of the population at both age 50 and 65 (Personal communication, BCSC, 2016).
While triennial screening is routinely employed in several countries (52, 53), this interval has not been considered in the US. Our modeling suggests that triennial screening has a comparable balance of benefits and harms as biennial screening in some groups. Decisions about using triennial vs. biennial screening for average risk women in the low density groups results in fewer false-positives, biopsies, and over-diagnosis with minimal impact on breast cancer deaths averted. Others have noted that triennial screening can be cost-effective for women aged 60–79 years with fatty or scattered fibroglandular density at average risk or RR≤2 (10, 11). Subgroups with low density (fatty and scattered fibroglandular densities) and RR=1.0–1.3 are 12% of 50-year old women and 20% of 65-year-old women (Personal communication, BCSC, 2016).
Breast cancer screening guidelines include an upper limit based on age or life expectancy (3, 4, 54). While we did not evaluate comorbidity, our study results suggest that screening intervals for older women should consider their competing causes of mortality, breast cancer risk and changes in density associated with aging.
The ability to tailor screening based on density may become increasingly feasible with the trend towards mandated standard reporting of breast density to women after a mammogram. Since our results demonstrate that the RR of breast cancer in combination with breast density has a strong influence on the net benefit of mammography at all screening intervals, evaluation of different risk assessment tools will be important in this context.
While the models provide new data and have consistent conclusions, several caveats should be considered. First, while the three models used common inputs, they varied in how these data were implemented based on model structure. These variations led to differences in the absolute values for outcome metrics. For instance, based on assumptions about temporal trends in underlying incidence, models with the lowest projected incidence estimate fewer breast cancer deaths averted than the models with higher incidence. This analysis includes three of six CISNET breast models and is an extension of work conducted by all six groups (8). Second, since the analytic goal was to determine screening efficacy, the models assumed 100% adherence to screening and use of the most effective modern treatments. Actual benefits will fall short of those projected under these assumptions. Next, we did not explicitly consider lower-than-average risks (i.e., RR<1). It will be important to extend our analyses to lower risk groups since most U.S. women have RR<1 across all density subgroups (70% of women at age 50 and 66% at age 65; Personal communication, BCSC, 2016). By extension our current findings suggest that triennial screening would be a reasonable option for lower-than-average risk subgroups among women with fatty or scattered fibroglandular density. We also did not model the impact of screening from ages 40–49, other combinations of ages and intervals, or BRCA1 /2 carriers. It is unclear whether the lack of strategies incorporating screening women in their forties would impact the balance of benefits and harms against longer (or shorter) screening intervals after age 50. While two age groups and change in density between age groups was considered, these results do not provide guidance for women who experience a change in risk over time; modeling change in risk with aging is an important area for future research. In addition, we used RR rather than absolute risk level since our simulation models were better suited for this approach. Absolute risk calculators are commonly available (27, 55–57) and the suitability of these calculators to assign risk to personalize screening intervals should continue to be evaluated. Finally, we did not evaluate alternative or supplemental imaging.
Overall, this comparative modeling study illustrates consistent patterns in benefits and harms that could be useful for guiding shared decision-making and tailoring screening intervals. The results demonstrate that for all screening intervals, benefits and harms change with risk and breast density and the threshold to decide on screening interval will depend on individual women’s preferences (1). Assessing breast density and breast cancer risk can identify subgroups of average-risk/low-density women who can consider triennial screening and higher-risk/high-density women who may benefit from annual screening.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Rocky Feuer, Sandra Lee, Hui Huang, Donald Berry, Kathleen Cronin, Eveline A. Heijnsdijk, Allison Kurian, and Donald Weaver for their advice regarding this project.
This work was supported by the National Institutes of Health under National Cancer Institute Grants P01 CA154292, P30 CA014520, and U01 CA152958. Data collection for model inputs from the Breast Cancer Surveillance Consortium (BCSC) was supported by the National Cancer Institute Grant P01 CA154292, contract HSN261201100031C and Grant U54 CA163303. The collection of BCSC cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the US. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html.
Footnotes
Reproducible Research Statement: Study protocol: Not available. Statistical code: Detailed information about the models is available online at http://cisnet.cancer.gov/breast/profiles.html and in reference (14). Data set: Input and output data from the models are available at reference (14) and by contacting Dr. Trentham-Dietz at trentham@wisc.edu.
This work was done by three independent modeling teams from Erasmus Medical Center (PI: de Koning); Georgetown University Medical Center, Lombardi Comprehensive Cancer Center and A. Einstein College of Medicine (PIs: Mandelblatt and Schechter); and University of Wisconsin-Madison and Harvard Medical School-Harvard Pilgrim Health Care (PIs: Trentham-Dietz, Alagoz and Stout).
References
- 1.Kerlikowske K. Progress Toward Consensus on Breast Cancer Screening Guidelines and Reducing Screening Harms. JAMA Intern Med. 2015;175(12):1970–1. doi: 10.1001/jamainternmed.2015.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siu AL, Bibbins-Domingo K, Grossman DC, LeFevre ML Force USPST. Convergence and Divergence Around Breast Cancer Screening. Ann Intern Med. 2016;164(4):301–2. doi: 10.7326/M15-3065. [DOI] [PubMed] [Google Scholar]
- 3.Siu AL Force USPST. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279–96. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA. 2015;314(15):1599–614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers ER, Moorman P, Gierisch JM, Havrilesky LJ, Grimm LJ, Ghate S, et al. Benefits and Harms of Breast Cancer Screening: A Systematic Review. JAMA. 2015;314(15):1615–34. doi: 10.1001/jama.2015.13183. [DOI] [PubMed] [Google Scholar]
- 6.Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of Breast Cancer Screening: Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):256–67. doi: 10.7326/M15-0970. [DOI] [PubMed] [Google Scholar]
- 7.Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. Breast-cancer screening--viewpoint of the IARC Working Group. N Engl J Med. 2015;372(24):2353–8. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 8.Mandelblatt JS, Stout NK, Schechter CB, van den Broek JJ, Miglioretti DL, Krapcho M, et al. Collaborative Modeling of the Benefits and Harms Associated With Different U.S. Breast Cancer Screening Strategies. Ann Intern Med. 2016;164(4):215–25. doi: 10.7326/M15-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerlikowske K, Zhu W, Hubbard RA, Geller B, Dittus K, Braithwaite D, et al. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–16. doi: 10.1001/jamainternmed.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilaprinyo E, Forne C, Carles M, Sala M, Pla R, Castells X, et al. Cost-effectiveness and harm-benefit analyses of risk-based screening strategies for breast cancer. PloS one. 2014;9(2):e86858. doi: 10.1371/journal.pone.0086858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schousboe JT, Kerlikowske K, Loh A, Cummings SR. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Annals of Internal Medicine. 2011;155(1):10–20. doi: 10.7326/0003-4819-155-1-201107050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerlikowske K, Zhu W, Tosteson AN, Sprague BL, Tice JA, Lehman CD, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162(10):673–81. doi: 10.7326/M14-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Ravesteyn NT, Miglioretti DL, Stout NK, Lee SJ, Schechter CB, Buist DS, et al. Tipping the Balance of Benefits and Harms to Favor Screening Mammography Starting at Age 40 Years: A Comparative Modeling Study of Risk. Annals of Internal Medicine. 2012;156(9):609–17. doi: 10.1059/0003-4819-156-9-201205010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandelblatt J, Cronin KA, De Koning H, Miglioretti DL, Schechter C, Stout N, et al. Collaborative Modeling of U.S. Breast Cancer Screening Strategies. Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services; Rockville, MD: Dec, 2015. [Accessed May 2016]. AHRQ Publication No. 14-05201-EF-4. Available from: http://www.uspreventiveservicestaskforce.org/Home/GetFile/1/16255/collabmodelingbc/pdf. [Google Scholar]
- 15.Breast Cancer Surveillance Consortium. Rockville, MD: National Cancer Institute; 2015. Jul 06, [Internet] [cited 2016 May 17]. Available from: http://breastscreening.cancer.gov. [Google Scholar]
- 16.American College of Radiology. The American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) 4. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 17.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36(1):114–36. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–51. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Jr, et al. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87(11):1234–45. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol Consumption and Ethyl Carbamate. Lyon, France: World Health Organization; 2010. [PMC free article] [PubMed] [Google Scholar]
- 21.Chlebowski RT, Anderson GL, Aragaki AK, Prentice R. Breast Cancer and Menopausal Hormone Therapy by Race/Ethnicity and Body Mass Index. J Natl Cancer Inst. 2016;108(2) doi: 10.1093/jnci/djv327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–92. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results. From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 24.Trentham-Dietz A, Sprague BL, Hampton JM, Miglioretti DL, Nelson HD, Titus LJ, et al. Modification of breast cancer risk according to age and menopausal status: a combined analysis of five population-based case-control studies. Breast Cancer Res Treat. 2014;145(1):165–75. doi: 10.1007/s10549-014-2905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358(9291):1389–99. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 26.Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer. 1997;71(5):800–9. doi: 10.1002/(sici)1097-0215(19970529)71:5<800::aid-ijc18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast Density and Benign Breast Disease: Risk Assessment to Identify Women at High Risk of Breast Cancer. J Clin Oncol. 2015;33(28):3137–43. doi: 10.1200/JCO.2015.60.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyrstad SW, Yan Y, Fowler AM, Colditz GA. Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. Breast Cancer Res Treat. 2015;149(3):569–75. doi: 10.1007/s10549-014-3254-6. [DOI] [PubMed] [Google Scholar]
- 29.Mavaddat N, Pharoah PD, Michailidou K, Tyrer J, Brook MN, Bolla MK, et al. Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst. 2015;107(5) doi: 10.1093/jnci/djv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014;106(11) doi: 10.1093/jnci/dju289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gangnon RE, Sprague BL, Stout NK, Alagoz O, Weedon-Fekjaer H, Holford TR, et al. The contribution of mammography screening to breast cancer incidence trends in the United States: an updated age-period-cohort model. Cancer Epidemiol Biomarkers Prev. 2015;24(6):905–12. doi: 10.1158/1055-9965.EPI-14-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter SB, Gartner SS, Haines MR, Olmstead AL, Sutch R, Wright G. Historical Statistics of the United States, Volume One: Population. New York: Cambridge University Press; 2006. [Google Scholar]
- 33.Fryback DG, Stout NK, Rosenberg MA, Trentham-Dietz A, Kuruchittham V, Remington PL. The Wisconsin Breast Cancer Epidemiology Simulation Model. J Natl Cancer Inst Monogr. 2006;(36):37–47. doi: 10.1093/jncimonographs/lgj007. [DOI] [PubMed] [Google Scholar]
- 34.Mandelblatt J, Schechter CB, Lawrence W, Yi B, Cullen J. The SPECTRUM population model of the impact of screening and treatment on U.S. breast cancer trends from 1975 to 2000: principles and practice of the model methods. J Natl Cancer Inst Monogr. 2006;(36):47–55. doi: 10.1093/jncimonographs/lgj008. [DOI] [PubMed] [Google Scholar]
- 35.Tan SY, van Oortmarssen GJ, de Koning HJ, Boer R, Habbema JD. The MISCAN-Fadia continuous tumor growth model for breast cancer. J Natl Cancer Inst Monogr. 2006;(36):56–65. doi: 10.1093/jncimonographs/lgj009. [DOI] [PubMed] [Google Scholar]
- 36.Nelson HD, Zakher B, Cantor A, Fu R, Griffin J, O’Meara ES, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Ann Intern Med. 2012;156(9):635–48. doi: 10.1059/0003-4819-156-9-201205010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cronin KA, Mariotto AB, Clarke LD, Feuer EJ. Additional common inputs for analyzing impact of adjuvant therapy and mammography on U.S. mortality. J Natl Cancer Inst Monogr. 2006;(36):26–9. doi: 10.1093/jncimonographs/lgj005. [DOI] [PubMed] [Google Scholar]
- 38.Cronin KA, Yu B, Krapcho M, Miglioretti DL, Fay MP, Izmirlian G, et al. Modeling the dissemination of mammography in the United States. Cancer Causes Control. 2005;16(6):701–12. doi: 10.1007/s10552-005-0693-8. [DOI] [PubMed] [Google Scholar]
- 39.Early Breast Cancer Trialists’ Collaborative Group. Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–44. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mariotto AB, Feuer EJ, Harlan LC, Abrams J. Dissemination of adjuvant multiagent chemotherapy and tamoxifen for breast cancer in the United States using estrogen receptor information: 1975–1999. J Natl Cancer Inst Monogr. 2006;(36):7–15. doi: 10.1093/jncimonographs/lgj003. [DOI] [PubMed] [Google Scholar]
- 41.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. 2015. [Google Scholar]
- 42.Stout NK, Lee SJ, Schechter CB, Kerlikowske K, Alagoz O, Berry D, et al. Benefits, harms, and costs for breast cancer screening after US implementation of digital mammography. J Natl Cancer Inst. 2014;106(6):dju092. doi: 10.1093/jnci/dju092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 44.de Haes JC, de Koning HJ, van Oortmarssen GJ, van Agt HM, de Bruyn AE, van Der Maas PJ. The impact of a breast cancer screening programme on quality-adjusted life-years. Int J Cancer. 1991;49(4):538–44. doi: 10.1002/ijc.2910490411. [DOI] [PubMed] [Google Scholar]
- 45.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26(4):391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 46.Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98(11):774–82. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- 47.Surveillance Epidemiology and End Results (SEER) Program. (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2014 Sub (1973–2012) <Katrina/Rita Population Adjustment> - Linked to County Attributes - Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.
- 48.Sprague BL, Gangnon RE, Burt V, Trentham-Dietz A, Hampton JM, Wellman RD, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10) doi: 10.1093/jnci/dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg RD, Yankaskas BC, Abraham LA, Sickles EA, Lehman CD, Geller BM, et al. Performance benchmarks for screening mammography. Radiology. 2006;241(1):55–66. doi: 10.1148/radiol.2411051504. [DOI] [PubMed] [Google Scholar]
- 50.van Ravesteyn NT, Stout NK, Schechter CB, Heijnsdijk EA, Alagoz O, Trentham-Dietz A, et al. Benefits and harms of mammography screening after age 74 years: model estimates of overdiagnosis. J Natl Cancer Inst. 2015;107(7) doi: 10.1093/jnci/djv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 52.Giordano L, von Karsa L, Tomatis M, Majek O, de Wolf C, Lancucki L, et al. Mammographic screening programmes in Europe: organization, coverage and participation. J Med Screen. 2012;19(Suppl 1):72–82. doi: 10.1258/jms.2012.012085. [DOI] [PubMed] [Google Scholar]
- 53.Von Karsa L, Antilla A, Ronco G, Ponti A, Malila N, Arbyn M, et al. Cancer Screening in the European Union: Report on the implementation of the Council Recommendation on cancer screening. First Report. European Communities. 2008 [Google Scholar]
- 54.Lansdorp-Vogelaar I, Gulati R, Mariotto AB, Schechter CB, de Carvalho TM, Knudsen AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161(2):104–12. doi: 10.7326/M13-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 56.Rosner B, Colditz GA. Nurses’ health study: log-incidence mathematical model of breast cancer incidence. J Natl Cancer Inst. 1996;88(6):359–64. doi: 10.1093/jnci/88.6.359. [DOI] [PubMed] [Google Scholar]
- 57.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–30. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.