Abstract
Streptococcus pneumoniae is a leading cause of bacterial pneumonia and is the principal cause of morbidity and mortality worldwide. Previous studies suggested that excessive activation of neutrophils results in the release of neutrophil elastase, which contributes to lung injury in severe pneumonia. Although both pneumococcal virulence factors and neutrophil elastase contribute to the development and progression of pneumonia, there are no studies analysing relationships between these factors. Here, we showed that pneumolysin, a pneumococcal pore-forming toxin, induced cell lysis in primary isolated human neutrophils, leading to the release of neutrophil elastase. Pneumolysin exerted minimal cytotoxicity against alveolar epithelial cells and macrophages, whereas neutrophil elastase induced detachment of alveolar epithelial cells and impaired phagocytic activity in macrophages. Additionally, activation of neutrophil elastase did not exert bactericidal activity against S. pneumoniae in vitro. P2X7 receptor, which belongs to a family of purinergic receptors, was involved in pneumolysin-induced cell lysis. These findings suggested that infiltrated neutrophils are the primary target cells of pneumolysin, and that S. pneumoniae exploits neutrophil-elastase leakage to induce the disruption of pulmonary immune defences, thereby causing lung injury.
Streptococcus pneumoniae, which is also called pneumococcus, is a Gram-positive Diplococcus and a major human pathogen. This bacterium causes bacterial pneumonia, meningitis, otitis media, and septicaemia, and is the principal cause of morbidity and mortality in young children, elderly people, and patients with immunodeficiencies1. The primary treatment of pneumococcal diseases involves the use of effective antibiotics; however, with the widespread availability and overuse of antibiotics, multidrug-resistant strains of pneumococci have become increasingly prevalent2, with 30% of S. pneumoniae in pneumococcal diseases fully resistant to one or more antibiotics. Additionally, total medical costs associated with pneumococcal pneumonia are >$96 million per year in the United States3. Therefore, basic research is still necessary to provide additional therapeutic targets to prevent and treat pneumococcal pneumonia.
A variety of pneumococcal virulence factors, including pneumolysin (PLY) and the autolytic enzyme LytA, contribute to the development of pneumococcal diseases4. PLY is a potent intracellular toxin that causes inflammation and tissue damage and varies little with serotype. PLY toxicity is associated with its ability to induce ring-shaped pores in cholesterol-containing membranes that mediate cell death5, whereas LytA is responsible for the characteristic autolytic behaviour associated with pneumococcus. It was reported that LytA potentially contributes to pneumococcal pathogenesis by catalysing the release of intracellular toxins, such as PLY, and generating pro-inflammatory cell-wall fragments6,7.
The immune response to pneumococcal pneumonia is generally characterized as an intense inflammatory reaction induced mainly by cell-wall components of the bacterium8. Recognition of the bacterium by pattern-recognition receptors located on the cell surface of lung epithelium and alveolar macrophages activates the induction of pro-inflammatory cytokines and chemokines, leading to rapid and heavy infiltration of neutrophils into infected lungs9. Although neutrophils are one of the first lines of defence against microbial pathogens, it was recently reported that excessive neutrophil activation caused the release of elastase, which contributed to lung injury in pneumococcal pneumonia10,11.
Neutrophil elastase (NE) is a potent serine protease that acts as a powerful host-defence component and exerts antimicrobial activity against a wide variety of bacteria, including S. pneumoniae, which is phagocytosed by neutrophils12. However, NE degrades a variety of extracellular-matrix proteins13, as well as lung-surfactant proteins14, which act as important host-defence agents against lung infections. Therefore, excessive release of NE can damage surrounding tissues and contribute to lung dysfunction associated with pneumonia. Consequently, patients with severe pneumonia exhibit increased levels of NE in lung15 and plasma16, and in animal models, intratracheal inoculation of S. pneumoniae causes acute lung injury characterized by increased accumulation of neutrophils and elevation of NE activity in bronchoalveolar-lavage fluid17,18. However, the underlying mechanisms by which neutrophils release NE in response to S. pneumoniae infection remain elusive.
Although both pneumococcal virulence factors and NE contribute to the development and progression of pneumococcal pneumonia and lung injury, respectively, there are few studies analysing the relationship between these factors, which eventually damage lung cells. In this study, we hypothesized that pneumococcal PLY induces neutrophil cell death and subsequent release of NE in an autolysis-dependent manner. To test this hypothesis, we examined the cytotoxicity of pneumococcal-culture supernatant or PLY on primary isolated human neutrophils and investigated the sensitivity of alveolar epithelial cells and macrophages to PLY.
Results
S. pneumoniae induces cytotoxicity against human neutrophils in an autolysis-dependent manner
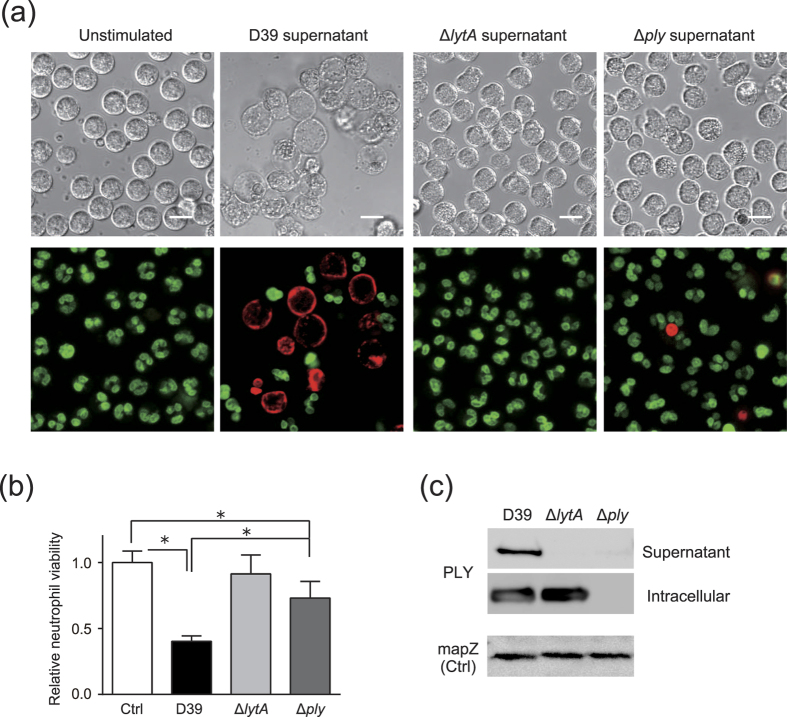
We first investigated whether pneumococcal-culture supernatant can induce cytotoxicity in human neutrophils. Figure 1a shows that treatment with culture supernatant from an S. pneumoniae wild-type strain D39 induced cell lysis against neutrophils, and cell-viability assays also indicated significant cytotoxicity induced by treatment with the culture supernatant (Fig. 1b). We next examined whether the culture supernatant from lytA-isogenic mutant (ΔlytA) and ply-isogenic mutant (Δply) strains induced cytotoxicity against neutrophils. Interestingly, treatment with ΔlytA-culture supernatant did not induce significant levels of cytotoxicity (Fig. 1a and b), and, although treatment with Δply-culture supernatant slightly decreased the number of viable neutrophils, the level of induced cytotoxicity was significantly lower than that induced by treatment with S. pneumoniae wild-type strain culture supernatant. These findings suggested that LytA or PLY expression induced neutrophil cell death. Western blot analysis demonstrated that PLY was detected in S. pneumoniae wild-type strain culture supernatant, whereas it was not detected in the supernatant of ΔlytA or Δply strains (Fig. 1c), suggesting that PLY release from S. pneumoniae is associated with its autolysis. When PLY level was analysed by densitometry, PLY protein level in the S. pneumoniae wild-type strain culture supernatant was 6.1 ± 0.4 μg/mL (Fig. S1).
Figure 1. Streptococcus pneumoniae culture supernatant induces cytotoxicity against human neutrophils in an autolysis-dependent manner.
Human neutrophils isolated from peripheral blood were exposed for 8 h to supernatant from S. pneumoniae wild-type strain D39, ΔlytA, or Δply cultures. (a) Representative differential interference contrast images and fluorescence images of cells stained by Hoechst 33342 (DNA; green) and ethidium homodimer III (necrotic cells; red) are shown. Scale bar: 10 μm. (b) Cell-viability assay was performed to evaluate supernatant cytotoxicity. Data represent the mean ± SD of quadruplicate experiments and were evaluated using one-way analysis of variance with Tukey’s multiple-comparisons test. *p < 0.05 between indicated groups. (c) Intracellular and extracellular (culture supernatant) expression of PLY protein in D39, ΔlytA, and Δply was determined by western blot analysis. mapZ, pneumococcal mid-cell-anchored protein Z; PLY, pneumolysin; SD, standard deviation.
Neutrophils are more susceptible to PLY-induced cell death than A549 and RAW264.7 cells
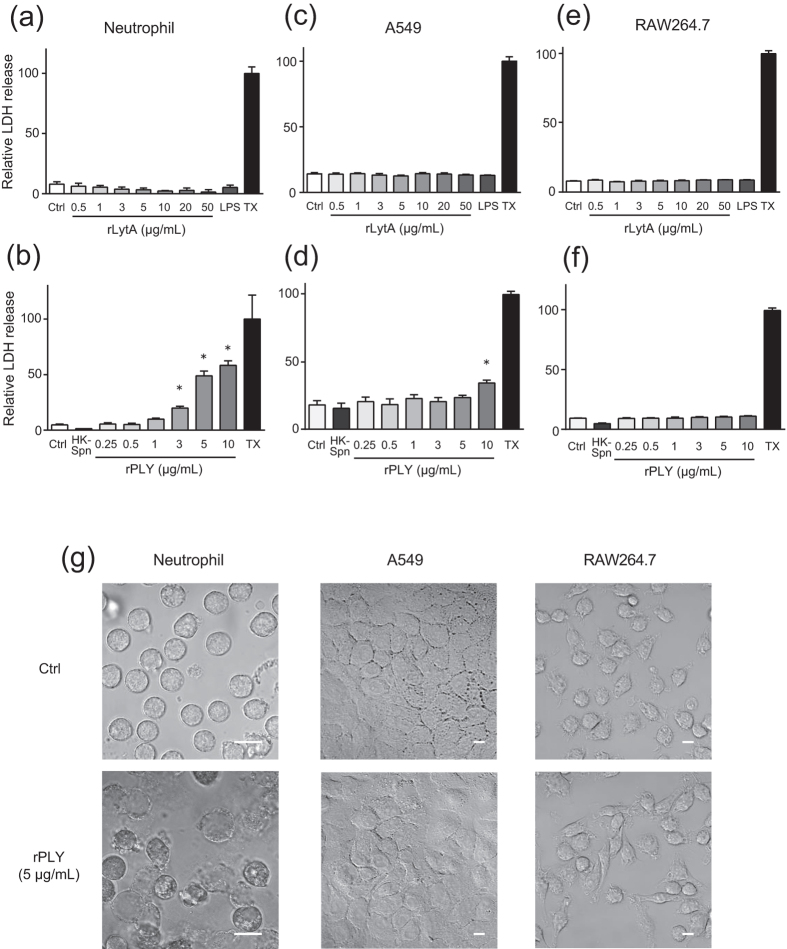
We next investigated whether LytA or PLY induces cytotoxicity against neutrophils. Administration of recombinant PLY (rPLY) caused lactate dehydrogenase (LDH) leakage from neutrophils in a dose-dependent manner, which was not observed following administration of recombinant LytA (rLytA) (Fig. 2a and b). We also examined the cytotoxicity of these proteins against A549 human lung epithelial cells and RAW264.7 mouse macrophages. As shown in Fig. 2c–f, rLytA did not exhibit cytotoxicity against these cells, and rPLY did so only at high concentrations (10 μg/mL), which induced low levels of LDH leakage from A549 cells. In addition, cell viability assay showed consistent results (Fig. S2). Microscopic analysis also revealed that rPLY administration induced cell lysis of neutrophils, while it did not induce morphological changes in A549 or RAW264.7 cells (Fig. 2g). These findings suggested that autolysis-induced release of PLY selectively eliminated neutrophils.
Figure 2. Neutrophils are susceptible to rPLY-induced cell death, whereas A549 and RAW264.7 cells are resistant.
(a,b) Human neutrophils, (c,d) A549 alveolar epithelial cells, or (e,f) RAW264.7 macrophages were exposed to various concentration of rLytA (0.5–50 μg/mL), LPS (100 ng/mL) (a,c,e), rPLY (0.25–10 μg/mL), or heat-killed Streptococcus pneumoniae D39 (HK-Spn) (b,d,f) for 6 h, followed by evaluation of cytotoxicity by LDH assay. Data represent the mean ± SD of quadruplicate experiments and were evaluated using one-way analysis of variance with Dunnett’s multiple-comparisons test. *Significantly different from the control group at p < 0.05. (g) Representative microscopic image of rPLY-treated (5 μg/mL) cells. Scale bar: 10 μm. LDH, lactate dehydrogenase; LPS, lipopolysaccharide; rPLY, recombinant pneumolysin; SD, standard deviation; TX, Triton X-100.
Neutrophils release NE in response to rPLY stimulation
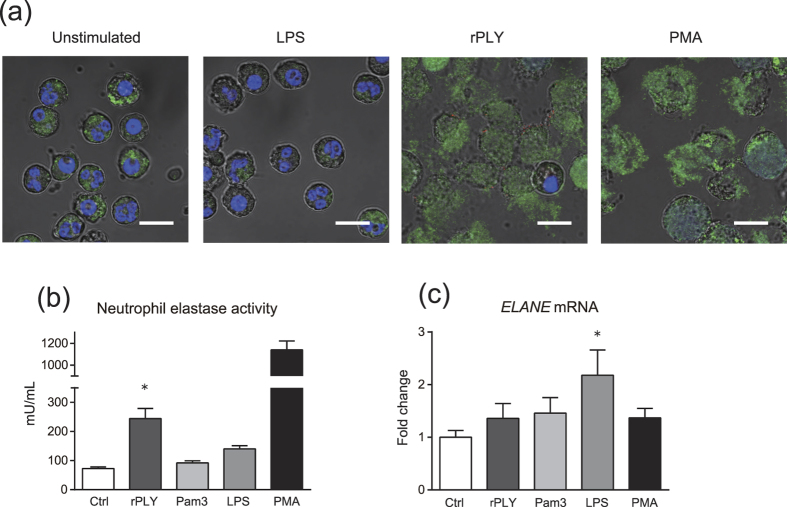
We hypothesized that rPLY-induced neutrophil cell death results in the release of NE and leads to lung injury. To test this hypothesis during acute inflammation in vivo, we increased neutrophil density in our in vitro assays to values typically accumulated in inflamed tissue (5 × 106 neutrophils/200 μL)19. Immunofluorescence confocal microscopy analyses showed that rPLY-treated neutrophils released NE (Fig. 3a). Consistently, rPLY stimulation significantly increased NE activity in neutrophil-culture supernatant, whereas toll-like receptor (TLR) agonists had little effect (Fig. 3b). However, rPLY treatment did not elicit upregulation in ELANE mRNA transcription, suggesting that rPLY-induced cell lysis resulted in NE release without promoting NE-gene transcription (Fig. 3c).
Figure 3. Neutrophils release NE in response to rPLY stimulation.
Human neutrophils (5 × 106 cells/200 μL) were exposed to rPLY (5 μg/mL), Pam3CSK4 (1 μg/mL), LPS (100 ng/mL), or PMA (40 nM) for 6 h. (a) Representative fluorescence-microscopy images of neutrophils stained for DNA (DAPI; blue), NE (green), and rPLY (red). Scale bar: 10 μm. (b) NE activity in the culture supernatant was evaluated using an elastase-activity assay kit. (c) Real-time PCR was performed to quantify NE (ELANE) mRNA in neutrophils exposed to these stimuli for 3 h. The relative quantity of ELANE mRNA was normalized to the relative quantity of GAPDH mRNA. (b,c) Data represent the mean ± SD of quadruplicate experiments and were evaluated using one-way analysis of variance with Dunnett’s multiple-comparisons test. *Significantly different from the control group at p < 0.05. LDH, lactate dehydrogenase; NE, neutrophil elastase; rPLY, recombinant pneumolysin; SD, standard deviation.
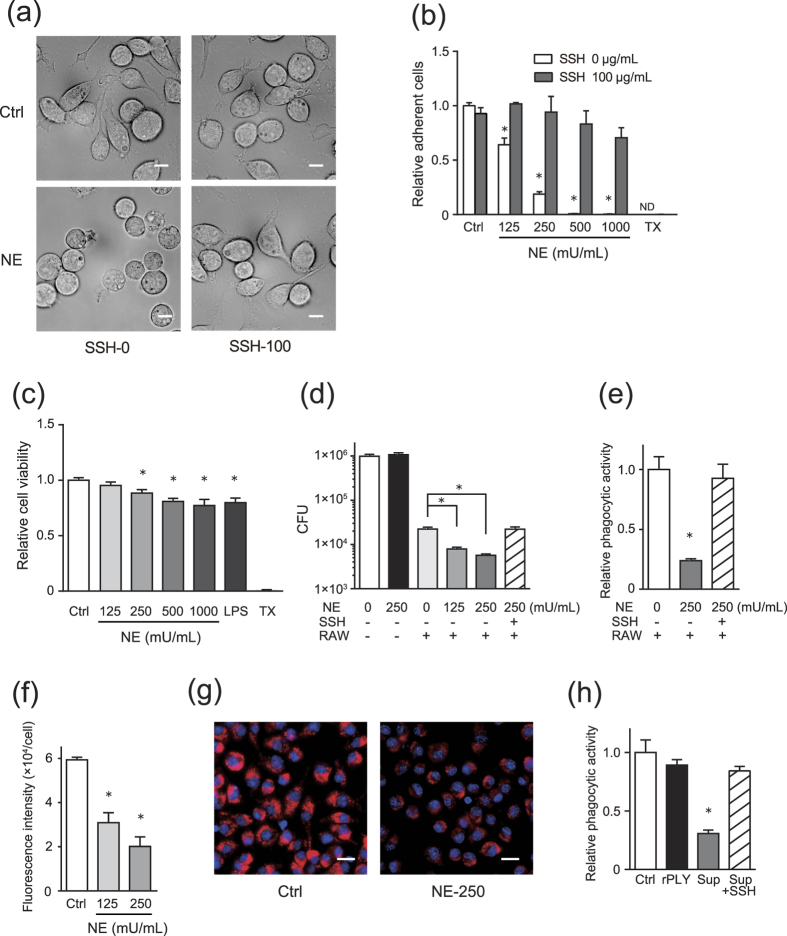
NE induces A549-cell detachment
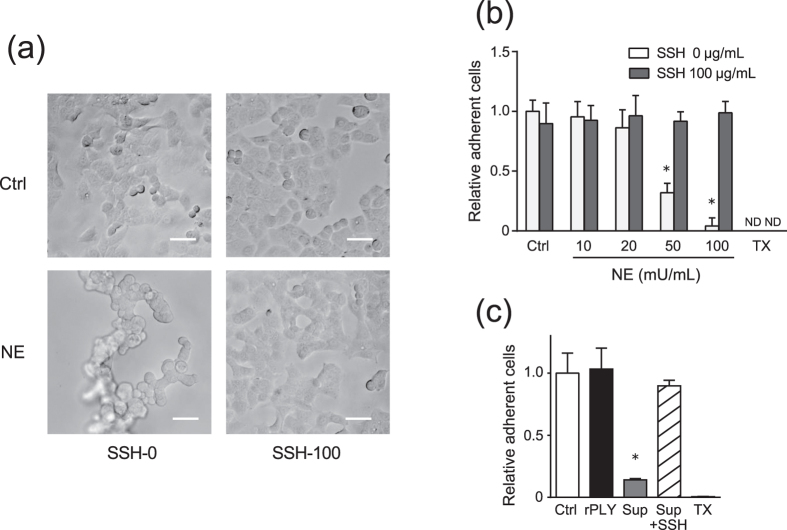
Our observations prompted us to further examine whether NE release induced cellular dysfunction in alveolar epithelial cells and macrophages, both of which play important roles in antimicrobial defence against pneumonia20,21. As shown in Fig. 4a and b, NE release induced A549-cell detachment within 6 h in a dose-dependent manner. Additionally, treatment with the culture supernatant from rPLY-treated neutrophils also induced A549-cell detachment, whereas rPLY treatment alone did not affect cell detachment (Fig. 4c). Furthermore, this effect was counteracted by administration of the NE inhibitor sivelestat (Fig. 4a–c).
Figure 4. NE induces A549-cell detachment.
A549 cells were cultured in serum-free DMEM and exposed to various concentrations (10–100 mU/mL) of NE in the presence or absence of the NE inhibitor sivelestat (100 μg/mL) for 6 h. (a) Morphologic changes in cells exposed to 100 mU/mL NE were observed by optical microscope. Scale bar: 100 μm. (b) MTT assay was performed to quantify A549-cell detachment. (c) A549 cells were exposed to supernatant (Sup) from rPLY-treated human neutrophils for 3 h, followed by MTT assay to quantify epithelial cell detachment. (b,c) Data represent the mean ± SD of quadruplicate experiments and were evaluated using one-way analysis of variance with Dunnett’s multiple-comparisons test. *Significantly different from the control group at p < 0.05. MTT, thiazolyl blue tetrazolium bromide; ND, not detected; NE, neutrophil elastase; rPLY, recombinant pneumolysin; SD, standard deviation; SSH-0, sivelestat sodium hydrate 0 μg/mL, SSH-100, sivelestat sodium hydrate 100 μg/mL.
Previous studies indicated that NE treatment elicited upregulation of interleukin (IL) 8 mRNA transcription in alveolar epithelial cells22,23; however, our results showed that IL-8-protein levels were not elevated in the supernatant of NE-treated A549 cells (Fig. S3). These results may be due to IL-8 degradation by NE under serum-free conditions (Fig. S4).
NE induces RAW264.7-cell detachment and impairs phagocytic activity
We next examined whether NE can induce macrophage detachment. Although NE treatment caused cell rounding and induced RAW264.7-cell detachment (Fig. 5a and b), 90% of the cells remained viable in Dulbecco’s modified Eagle’s medium (DMEM) containing 250 mU/mL NE (Fig. 5c). Therefore, we assessed the effect of NE on phagocytic activity in RAW264.7 cells. Interestingly, pre-treatment of the cells with NE for 6 h significantly decreased phagocytosed S. pneumoniae in RAW264.7 cells (Fig. 5d and e). We also measured phagocytic activity based on the uptake of indicators in NE-treated cells according to fluorescence intensity. The fluorescence intensity in NE-treated cells was significantly lower than that observed in control cells after a 30-min incubation with phagocytosis indicators (Fig. 5f and g). Additionally, administration of culture supernatant from rPLY-treated neutrophils also impaired phagocytic activity (Fig. 5h). Interestingly, this effect were counteracted by sivelestat treatment (Fig. 5a,b,d,e and h).
Figure 5. NE induces detachment of RAW264.7 cells and impairs phagocytic activity.
RAW264.7 cells were cultured in serum-free DMEM and exposed to various concentrations (125–1000 mU/mL) of NE in the presence or absence of the NE inhibitor sivelestat (100 μg/mL) for 6 h. (a) Morphologic changes in cells were observed by optical microscope. Scale bar: 10 μm. (b) MTT assay was performed to quantify macrophage detachment. (c) Cell-viability assays. (d,e) NE-treated RAW264.7 cells were infected with Streptococcus pneumoniae D39 (MOI = 10) for 1 h, and (d) viable counts of phagocytosed S. pneumoniae were determined by plating serially diluted macrophage lysates on blood-agar plates. (e) Relative phagocytic activity of NE-treated RAW264.7 cells compared to untreated cells against S. pneumoniae. (f,g) NE-treated RAW264.7 cells were incubated in DMEM containing pHrodo red Staphylococcus aureus bioparticles for 30 min, and (f) the fluorescence intensity per cell was calculated. (g) Representative fluorescence-microscopy images of cells and nuclei stained with the bioparticles (red) and DAPI (blue), respectively. (h) RAW264.7 cells were exposed to culture supernatant (Sup) from rPLY-treated neutrophils or rPLY (5 μg/mL) for 3 h, followed by infection with S. pneumoniae D39 (MOI = 10) for 1 h and measurement of relative phagocytic activity. (b–f,h) Data represent the mean ± SD of quadruplicate experiments and were evaluated using one-way analysis of variance with Dunnett’s multiple-comparisons test. *Significantly different from the control groups at p < 0.05. MTT, thiazolyl blue tetrazolium bromide; ND, not detected; NE, neutrophil elastase; rPLY, recombinant pneumolysin; SD, standard deviation; SSH-0, sivelestat sodium hydrate 0 μg/mL, SSH-100, sivelestat sodium hydrate 100 μg/mL.
NE did not exhibit bactericidal activity against S. pneumoniae
It has been reported that NE plays an important role in intracellular bacterial killing by neutrophils12. Here, we examined whether NE exerted bactericidal activity against S. pneumoniae and observed that NE did not decrease the number of viable S. pneumoniae in vitro (Fig. 6), suggesting that rPLY-induced NE release did not adversely affect S. pneumoniae viability.
Figure 6. NE does not exhibit bactericidal activity against Streptococcus pneumoniae in vitro.
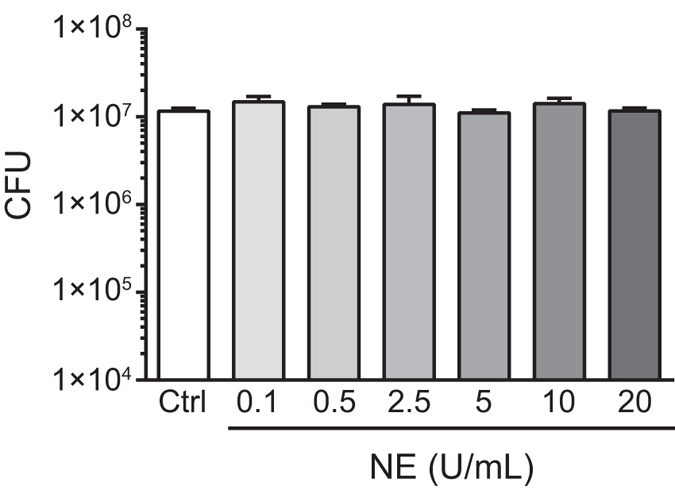
S. pneumoniae D39 was cultured in TS broth until reaching the exponential-growth phase, followed by exposure to various concentrations (0.1–20 U/mL) of NE for 6 h, dilution, and inoculation onto blood-agar plates. Plates were incubated under aerobic condition at 37 °C for 2 to 3 days. Data represent the mean ± SD of quadruplicate experiments. NE, neutrophil elastase; SD, standard deviation; TS, tryptic soy.
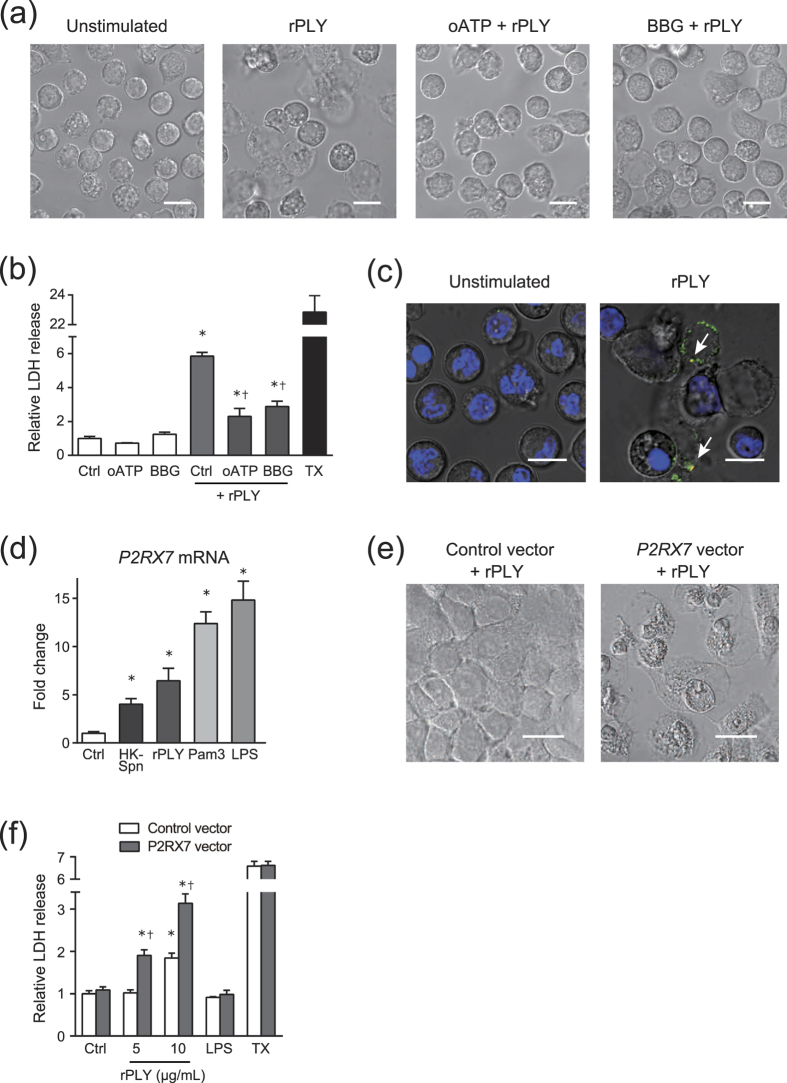
P2X7 receptor is a possible membrane target of PLY
Previous studies have demonstrated that pore-forming toxins, such as alpha toxin from Staphylococcus aureus and beta toxin from Clostridium perfringens, produce cytotoxicity by specific interactions with the P2X7 receptor24,25. Therefore, we investigated whether rPLY stimulation induced P2X7 receptor-mediated cytotoxicity. As shown in Fig. 7a, pre-treatment of neutrophils with selective P2X7 receptor antagonists (oxidized ATP and Brilliant Blue G) inhibited PLY-induced cell lysis, although slight morphological alterations were observed. Additionally, these antagonists partially but significantly inhibited rPLY-induced LDH leakage from neutrophils (Fig. 7b). To confirm that rPLY was bound to the P2X7 receptor, we performed co-localization studies in rPLY-treated neutrophils subjected to immunostaining for rPLY and the endogenous P2X7 receptor. Our results indicated that rPLY co-localized with the P2X7 receptor at the cell surface (Fig. 7c). Additionally, unstimulated neutrophils rarely expressed the P2X7 receptor, whereas the P2X7 receptor was highly expressed in PLY-treated dead neutrophils. We considered that the expression level of the P2X7 receptor provided a mechanism to explain the higher sensitivity of neutrophils to rPLY treatment than that observed in A549 cells. Indeed, P2RX7 mRNA was not detected in A549 cells (Fig. S5); however, P2RX7 mRNA was detected in neutrophils, with upregulated levels of P2RX7 transcription observed in the presence of heat-killed S. pneumoniae, TLR-agonist treatment, and rPLY stimulation (Fig. 7d). Figure S6 shows that rPLY stimulation did not significantly affect transcription of TNFα mRNA in neutrophils, suggesting that upregulation of P2RX7 mRNA transcription in rPLY-treated neutrophils was not due to endotoxin contamination or TLR-dependent signalling. Furthermore, we observed that overexpression of P2X7 receptor in A549 cells enhanced rPLY-induced cell lysis (Fig. 7e), and that rPLY stimulation significantly increased LDH release from overexpressed A549 cells (Fig. 7f). These findings suggest that rPLY treatment elicited upregulation of P2X7 receptor expression and subsequent interaction between rPLY and P2X7 receptor triggered the cytotoxicity.
Figure 7. rPLY stimulation upregulates P2X7 receptor expression and induces cytotoxicity.
(a,b) Neutrophils were pre-incubated for 1 h with P2X7 receptor antagonist (500 μM oxidized ATP and 3 μM BBG), followed by exposure to rPLY (5 μg/mL) for 3 h and (a) observation of morphologic changes in cells by optical microscope (scale bar: 10 μm). (b) An LDH assay was performed to evaluate cytotoxicity. (c) Representative fluorescence-microscopy images of rPLY-treated (5 μg/mL) neutrophils stained for DNA (DAPI; blue), P2X7 receptor (green), and rPLY (red). Arrows indicate co-localization of P2X7 receptor and rPLY (scale bar: 10 μm). (d) Real-time PCR was performed to quantify P2RX7 mRNA in neutrophils stimulated with rPLY and TLR agonists for 3 h. The relative quantity of P2RX7 mRNA was normalized to the relative quantity of GAPDH mRNA. (e,f) A549 cells were transfected with the P2RX7 expression vector or empty vector, then stimulated with rPLY (5 μg/mL) for 6 h. (e) Morphologic changes in cells were observed by optical microscope (scale bar: 20 μm), and (f) an LDH assay was performed to evaluate cytotoxicity. Data represent the mean ± SD of quadruplicate experiments. (b) Data were evaluated using one-way analysis of variance with Tukey’s multiple-comparisons test. *Significantly different from the untreated-control group at p < 0.05. †Significantly different from the rPLY-treated control group at p < 0.05. (d) Data were evaluated using one-way analysis of variance with Dunnett’s multiple-comparisons test. *Significantly different from the control group at p < 0.05. (f) Data were evaluated using one-way analysis of variance with Tukey’s multiple-comparisons test. *Significantly different at p < 0.05 from the untreated control within the same vector-transfected group. †Significantly different at p < 0.05 from the control vector-transfected group using the same concentrations as those used in the rPLY-treated group. BBG, Brilliant Blue G; LDH, lactate dehydrogenase; NE, neutrophil elastase; rPLY, recombinant pneumolysin; SD, standard deviation; TX, Triton-X 100.
Discussion
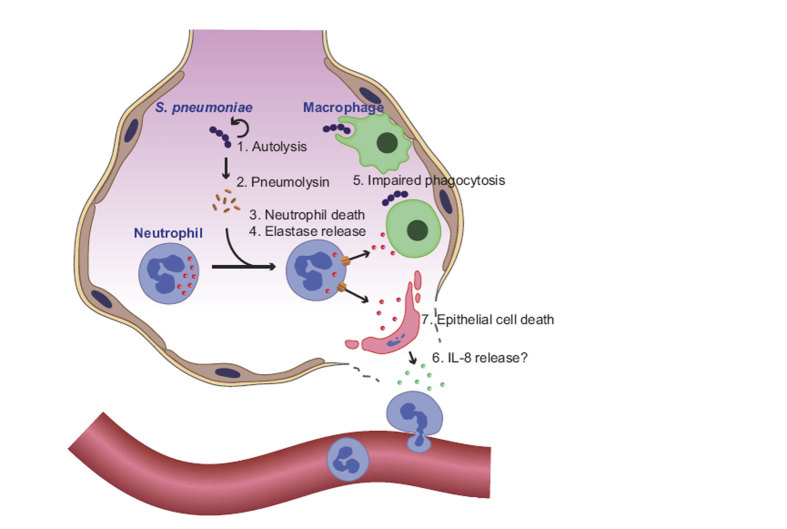
In this study, we showed that pneumococcal autolysis induces PLY release, which exerts cytotoxicity against infiltrating neutrophils through the P2X7 receptor. However, we also observed that PLY exhibited less cytotoxicity against alveolar epithelial cells and macrophages. Therefore, S. pneumoniae exploits NE leakage from degraded neutrophils to promote alveolar epithelial cell injury and impair the phagocytic function of macrophages (Fig. 8). Our findings demonstrated that pneumococcus may not simply attack lung tissue by its cytotoxic agent, but may also exploit host antimicrobial enzymes.
Figure 8. Summary of pneumococcus-induced lung injury.
Pneumococcal LytA causes (1) autolysis of the bacteria and (2) release of PLY, which promotes pore formation in the neutrophil membrane and (3) induction of cell death. (4) NE is released by dead neutrophils and (5) impairs phagocytic activity of macrophages. Additionally, (6) NE may induce IL-8 production from alveolar epithelial cells. Finally, (7) NE triggers cell death, but does not exert bactericidal activity against pneumococcus. NE, neutrophil elastase; PLY, pneumolysin.
LytA is an enzyme that degrades the peptidoglycan backbone of bacterial organisms26, leading to bacterial autolysis. This enzyme is located in the cell envelope and is involved in a variety of other physiological cell functions27. We observed that, although treatment with ΔlytA-culture supernatant exhibited significantly less cytotoxicity than that observed following treatment with wild-type strain culture supernatant, administration of LytA itself did not induce cytotoxicity, suggesting that LytA-induced autolysis indirectly causes the release of bacterial virulence factors, including PLY. However, treatment with Δply-culture supernatant slightly but significantly induced cytotoxicity. These findings suggested that other cytotoxic agents might also be released by pneumococcal autolysis. One possible candidate molecule for this cytotoxicity is α-enolase, which is a pneumococcal glycolytic enzyme and induces neutrophil death with NETs formation28.
Although several reports demonstrated PLY cytotoxicity against alveolar epithelial cells, monocytes, T cells, and neutrophils29,30,31,32, there are no studies comparing the sensitivity of neutrophils with that of alveolar epithelial cells to PLY stimulation. Here, we observed that human neutrophils were more sensitive to PLY-induced cell lysis than A549 cells, and that excessive doses of PLY induced only minimal cytotoxicity in A549 cells. However, RAW264.7 cells were resistant to PLY-induced cell lysis. Although RAW264.7 cell may not accurately reflect characteristics associated with human macrophages, human monocyte-derived macrophages isolated from whole blood are also resistant to PLY-induced cell lysis33, which was consistent with our experimental findings. While no studies have so far reported PLY-induced cytolytic activity on alveolar macrophages, our findings suggested that infiltrated neutrophils are the primary target cells of PLY.
A major reason for the differences in cellular sensitivity to PLY-induced cytolysis is different cholesterol and phospholipid compositions of plasma membrane between various cell types34,35. It has been reported that liposomes composed of sphingomyelin in combination with artificially high concentration of cholesterol efficiently bind pneumolysin and other pore-forming toxins36. However, liposomes composed of cholesterol and other phospholipid had less binding affinity. Another reason may involve expression level of the purinergic receptor P2X7 on the cell surface37. Previous studies reported variations in cell sensitivity against the C. perfringens-produced pore-forming beta toxin, which induced cytotoxicity dependent upon expression levels of the P2X7 receptor in human immune-cell lines25. Here, neutrophils did not constitutively express the P2X7 receptor, and for this reason, pre-treatment with selective P2X7 receptor antagonists did not completely abolish rPLY-induced cell lysis. However, PLY stimulation significantly upregulated P2X7 receptor expression and induced cell lysis. Rather than performing P2X7 receptor knockdown experiments in neutrophils, we overexpressed human P2X7 receptor in A549 cells due to the short lifespan of neutrophils. Consistent with our findings in neutrophils and those of a previous study25, we observed increased cell-lysis activity in PLY-treated A549 cells overexpressing the P2X7 receptor. The mechanisms by which the P2X7 receptor is involved in pore-forming toxin-induced cell lysis are unclear; however, one possible mechanism involves the release of ATP by cells through PLY-induced membrane pores38, followed by ATP binding to P2X7 receptors, the subsequent opening of additional P2X7-associated pores, and cell lysis37.
Recently, several studies showed that NE inhibition reduced lung injury in animal models17,18. Interestingly, these studies also demonstrated that treatment with NE inhibitors reduced the number of viable pneumococci in infected lungs, suggesting that extracellular NE induces disruption of pulmonary immune defences rather than exerting bactericidal activity. Here, we observed that administration of ≥50 mU/mL and ≥125 mU/mL NE induced alveolar epithelial cell detachment and impaired the phagocytic function of macrophages, respectively, but did not affect pneumococcal viability. Additionally, NE concentrations are significantly elevated in bronchoalveolar-lavage fluid during severe pneumonia (average: 400 mU/mL)15. Therefore, it is likely that NE induces these events in vivo. Although the molecular mechanisms associated with NE-induced cell detachment are complex and not completely known, NE is thought to be involved in the disruption of cell-matrix adhesion and cell-cell junctions39,40. Furthermore, mechanisms associated with NE-induced inhibition of phagocytic activity may be related to cell rounding and detachment, because pseudopodia extension in activated macrophages is essential for phagocytosis and cell adherence41,42. Phagocytosis can also be controlled by a number of factors, including pro-inflammatory cytokines43, extracellular-matrix proteins, such as fibronectin44, or cell-surface molecules45. NE-induced proteolytic digestion of inflammatory cytokines and fibronectin may also inhibit macrophage activation46,47,48. Each of these NE-induced deleterious effects was ameliorated by administration of an NE inhibitor; therefore, controlling NE activity constitutes an attractive therapeutic technique for preventing lung injury in pneumococcal pneumonia.
In conclusion, our in vitro study demonstrated that pneumococcal pore-forming toxin was a major mediator of neutrophil cell death and subsequent release of NE, which eventually disrupted pulmonary immune defences. NE is exploited by pneumococcus as an etiological agent during pneumococcal pneumonia. From a therapeutic viewpoint and in addition to antibiotic use, novel strategies for disease treatment might include blockage of PLY infiltration by engineered liposomes36, neutrophil inhibition by chemokine-related antibodies or antagonists49,50, or NE inhibition by specific inhibitors18. Our findings also suggested that evolutionary progression of pneumococcus has allowed the release of PLY by self-destruction to exploit host molecules for promoting pneumococcal infection.
Materials and Methods
Bacterial strains and reagents
S. pneumoniae D39 (NCTC 7466) was purchased from the National Collection of Type Cultures (Salisbury, UK). S. pneumoniae ΔlytA was kindly provided by Dr. Shigetada Kawabata (Osaka University, Osaka, Japan)46. S. pneumoniae was grown in tryptic soy (TS) broth (Becton Dickinson, Franklin Lakes, NJ, USA) or 5% sheep-blood plates, with 100 μg/mL spectinomycin (Sigma-Aldrich, St. Louis, MO, USA) added to the medium to allow for lytA-negative mutant-strain selection. rLytA protein was kindly provided by Dr. Yuuki Sakaue (Niigata University, Niigata, Japan). Thiazolyl blue tetrazolium bromide (MTT) was purchased from Sigma-Aldrich, oxidized ATP was purchased from Merck Millipore (Billerica, MA, USA), and Brilliant Blue G was purchased from Abcam (Cambridge, UK). Mid-cell-anchored-protein Z antibody was generated by Eurofins Genomics K.K. (Tokyo, Japan).
Construction of rPLY expression plasmid
The rPLY-expression plasmid was constructed using a pQE-30 vector (Qiagen, Hilden, Germany). The forward (5′- GGGGGATCCATGGCAAATAAAGCAGTAAA-3′) and reverse (5′- CCCCTCGAGCTAGTCATTTTCTACCTTATC-3′) primers were used to amplify the ply gene of S. pneumoniae D39 by polymerase chain reaction (PCR). The resulting PCR fragment was cloned into a pQE-30 vector, which was transformed into Escherichia coli strain M15 (Stratagene, San Diego, CA, USA) by electroporation using the Gene Pulser II Electroporation System (Bio-Rad Laboratories, Hercules, CA, USA). The M15 transformants were grown in Luria–Bertani broth (Nacalai Tesque, Kyoto, Japan) supplemented with 100 μg/mL ampicillin (Meiji Seika, Tokyo, Japan) to select for the pQE-30 vector and allowing its expression. The His-tagged rPLY protein was purified using a Ni-nitrilotriacetic acid column (Qiagen) under native conditions in accordance with the manufacturer’s instructions. The purified rPLY protein was dialyzed against phosphate buffered saline (PBS) and had a specific activity of 14,000 haemolytic units per mg of protein. A haemolytic unit is defined as the amount of rPLY contained in 1 ml of PBS which will cause 50% lysis of 1 ml of a 1% human erythrocyte suspension after 30 min at 37 °C. The amount of lipopolysaccharide (LPS) in 1 μg of purified rPLY protein was determined to be <2 pg according to a LPS-detection kit (GenScript, Piscataway Township, NJ, USA).
Integration mutagenesis using a targeted plasmid
The PCR product of the internal portion of the ply gene was amplified using 5′-GGGGGATCCACCAACGACAGTCGCCTCTAG-3′ and 5′-CCCTCTAGATTCCATGCTGTGAGCCGTTAT-3′ primers and ligated into the suicide vector pSF15151. The resulting plasmid was transformed into wild-type strain D39 by adding 100 ng of plasmid and 100 ng of competence-stimulating peptide to 1 mL of exponentially growing cells at an optical density at 600 nm (OD600) of 0.152. After incubation at 37 °C for 2 h, each culture was plated onto kanamycin (Meiji Seika)-containing blood-agar plates to select for the inactivated-mutant strain (Δply)53.
Preparation of bacterial supernatant
S. pneumoniae strains were grown statically in TS broth at 37 °C for 20 h under aerobic conditions. Overnight cultures were inoculated into fresh TS broth to allow continued bacterial growth until reaching the exponential-growth phase (OD600 = 0.2). Cells were collected by centrifugation at 3000 g for 10 min and suspended in RPMI1640 medium, followed by an additional 12-h incubation. Thereafter, bacterial supernatants were collected by additional centrifugation at 3000 g for 10 min and subsequent filtration (0.22-μm filter; Merck Millipore) prior to their being used for assays.
Human neutrophil isolation
Neutrophils were prepared as previously described54. Briefly, heparinized blood was obtained from healthy donors and mixed at a 1:1 ratio with PBS containing 3% dextran T500. After incubation at room temperature for 60 min, the supernatant was layered onto Ficoll-Paque (GE Healthcare, Little Chalfont, UK). After centrifugation at 450 g for 20 min, layers containing neutrophils were collected, and residual red-blood cells were lysed by hypotonic lysis. Viable cells were monitored using the Trypan-blue exclusion technique, and the cells were counted in a haemocytometer. The experimental protocol was approved by the Institutional Review Board of Niigata University, and the methods were carried out in accordance with the approved guidelines. Informed consent was obtained from all donors prior to their inclusion in this study (permit # 25-R9-07-13).
Cell lines
Human alveolar epithelial cell line A549 (ATCC CCL-185) and mouse macrophage cell line RAW264.7 (ATCC TIB-71) were obtained from RIKEN Cell Bank (Ibaraki, Japan) and DS Pharma Biomedical Co. Ltd. (Osaka, Japan), respectively. These cell lines were grown in DMEM (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% foetal bovine serum (Japan Bio Serum Co. Ltd., Hiroshima, Japan), 100 U/mL penicillin, and 100 μg/mL streptomycin (Wako Pure Chemical Industries) at 37 °C in 95% air and 5% CO2.
Cytotoxicity assay
Human neutrophils (2 × 105 in 200 μL) were seeded onto a 96-well plate (Becton Dickinson) and cultured in RPMI1640 medium containing 50% supernatant from S. pneumoniae D39, ΔlytA, or Δply cultures at 37 °C for 8 h. Cells were treated with Hoechst 33342 and ethidium homodimer III and visualised by a confocal laser-scanning microscope (Carl Zeiss, Jena, Germany). Also, Viable cells were determined using AlamarBlue cell-viability reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer instructions. Neutrophils, A549 cells (5 × 104 in 200 μL), or RAW264.7 cells (5 × 104 in 200 μL) were stimulated with various concentrations of rLytA (0.5–50 μg/mL), rPLY (0.25–10 μg/mL), LPS (100 ng/mL; Sigma-Aldrich), or heat-killed S. pneumoniae for 6 h. Additional cytotoxic analyses were performed using an LDH-measurement kit (Roche Diagnostics K.K., Tokyo, Japan).
Western blot analysis
The supernatant from S. pneumoniae strains were mixed with 2% SDS-sample buffer, heated at 99 °C for 3 min, separated by SDS-PAGE using 12.5% gels (Gellex International, Tokyo, Japan), and transferred to polyvinylidene difluoride membranes (Merck Millipore). The membranes were incubated with blocking reagent (Nacalai Tesque) to block nonspecific binding and probed with the anti-PLY antibody (Abcam) diluted in Tris-buffered saline containing 0.05% Tween 20 (TaKaRa, Shiga, Japan). The membrane was then incubated with a HRP-conjugated secondary antibody (Cell Signalling Technologies, Danvers, MA, USA) in Tris-buffered saline containing 0.05% tween 20. The membrane was treated with HRP substrates (GE Healthcare) and analysed by chemiluminescence detector (Fujifilm, Tokyo, Japan). As a loading control, whole-cell lysates of S. pneumoniae strains prepared through the process of bacterial-supernatant preparation were immunoblotted with an antibody specific for pneumococcal mid-cell-anchored protein Z, which is a key membrane protein involved in bacterial-cell division and morphogenesis55.
NE activity assay
Human neutrophils (5 × 106 cells in 200 μL) were exposed to rPLY (5 μg/mL), Pam3CSK4 (1 μg/mL), LPS (100 ng/mL), or PMA (40 nM) for 8 h. NE activity in culture supernatant was evaluated using an elastase-activity assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to manufacturer instructions.
Quantitative real-time PCR
Gene expression in neutrophils or A549 cells was quantified using quantitative real-time PCR. Briefly, RNA was extracted from cell lysates using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA) and quantified by spectrometry at 260 and 280 nm. The RNA was reverse transcribed using SuperScript VILO Master Mix (Thermo Fisher Scientific), and quantitative real-time PCR with cDNA was performed with the StepOnePlus real-time PCR system (Thermo Fisher Scientific) according to manufacturer protocol. TaqMan probes, sense primers, and antisense primers for expression of a housekeeping gene (GAPDH), NE (ELANE), or P2X7 receptor (P2RX7) were purchased from Thermo Fisher Scientific.
Immunofluorescence analysis
rPLY-treated neutrophils were fixed and permeabilized using a cell fixation and permeabilization kit (Thermo Fisher Scientific) according to manufacturer instructions, followed by incubation of the cells in a blocking solution (Thermo Fisher Scientific) for 30 min. For NE detection, samples were stained with rabbit anti-NE antibody (Abcam) in the blocking solution. After overnight incubation at 4 °C, the secondary AlexaFluor 488-conjugated goat anti-rabbit IgG antibody (Thermo Fisher Scientific) in blocking buffer was added, followed by a 2-h incubation in the dark. For detection of the P2X7 receptor and PLY, samples were stained with goat anti-P2X7 receptor (Abcam) and mouse anti-PLY antibodies (Abcam). After overnight incubation at 4 °C, the secondary AlexaFluor 488-conjugated bovine anti-goat IgG antibody (Jackson ImmunoResearch, West Grove, PA, USA) was added, followed by incubation for 2 h. Samples were then washed with PBS and incubated with AlexaFluor 594-conjugated goat anti-mouse IgG antibody (Thermo Fisher Scientific) and observed with a confocal laser-scanning microscope (Carl Zeiss).
Cell detachment assay
A549 and RAW264.7 cells (5 × 104 cells in 200 μL) were cultured in serum-free DMEM containing various concentrations of NE (10–100 mU/mL and 125–1000 mU/mL, respectively; Innovative Research, Novi, MI, USA) or supernatant from rPLY-treated neutrophils in the presence or absence of the NE inhibitor sivelestat sodium hydrate (sivelestat, ONO-5046, N-[2-[4-(2,2-Dimethylpropionyloxy)phenylsulfonylamino]benzoyl] aminoacetic acid; ONO Pharmaceutical Co., Osaka, Japan) at 37 °C for 6 h. The cells were washed with DMEM to remove detached cells. Thereafter, MTT assays were performed to detect non-detached cells as previously described56.
Phagocytic activity assay
S. pneumoniae D39 was cultured until reaching the exponential growth phase (OD600 = 0.2). RAW264.7 cells were cultured in serum-free DMEM and exposed to various concentration of NE (125–1000 mU/mL) or supernatant from rPLY-treated neutrophils in the presence or absence of the NE inhibitor sivelestat at 37 °C for 4 h. RAW264.7 cells were infected with S. pneumoniae (MOI = 10) for 1 h at 37 °C, followed by washing to remove extracellular non-adherent bacteria and a 1-h treatment with antibiotics (gentamicin [200 μg/mL] and penicillin [20 U/mL]) to eliminate residual or extracellular adherent bacteria. The macrophages were subsequently washed and lysed in sterile distilled water. Viable counts of phagocytized S. pneumoniae were determined by plating serially diluted macrophage lysates onto blood-agar plates subjecting them to aerobic culture.
For a quantitation of phagocytic activity, pHrodo red S. aureus bioparticles conjugate (Thermo Fisher Scientific), an indicator of phagocytic activity which increases in fluorescence as the pH of its surroundings, in Hank’s balanced-salt solution (Thermo Fisher Scientific) containing 20 mM HEPES (Nacalai Tesque) was added to each well containing NE-treated RAW264.7 cells, and the plate was incubated at 37 °C for 30 min. The cells stained with the indicator were counted by fluorescence microscopy (Keyence, Osaka, Japan), and fluorescence intensity per cell was calculated.
Gene overexpression
A549 cells were transfected with the P2RX7-expression vector (Origene, Rockville, MD, USA) or empty vector using Lipofectamine 3000 reagent (Thermo Fisher Scientific) according to manufacturer protocol. Overexpression of P2RX7 at the mRNA level was confirmed by quantitative PCR.
Statistical analysis
Data were analysed statistically by one-way analysis of variance with Tukey’s or Dunnett’s multiple-comparisons test using Graph Pad Prism Software version 6.05 (GraphPad Software, Inc., La Jolla, CA, USA).
Additional Information
How to cite this article: Domon, H. et al. Streptococcus pneumoniae disrupts pulmonary immune defence via elastase release following pneumolysin-dependent neutrophil lysis. Sci. Rep. 6, 38013; doi: 10.1038/srep38013 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We acknowledge Dr. Shigetada Kawabata and Dr. Masaya Yamaguchi (Osaka University) for kindly providing the S. pneumoniae ΔlytA strain. We also thank Dr. Yuuki Sakaue (Niigata University) for kindly providing rLytA. This work was supported by JSPS KAKENHI grant numbers 26861570, 26293390, 26305034, and 16K11439.
Footnotes
Author Contributions H.D. and Y.T. designed the study. H.D., T.M., K.N. and W.T. performed all of the experiments. H.D., M.O. and K.N. analyzed the data. H.D., M.O. and Y.T. wrote the paper. All authors discussed the results and approved of the manuscript.
References
- Bogaert D., De Groot R. & Hermans P. W. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4, 144–154 (2004). [DOI] [PubMed] [Google Scholar]
- Appelbaum P. C. Resistance among Streptococcus pneumoniae: Implications for drug selection. Clin. Infect. Dis. 34, 1613–1620 (2002). [DOI] [PubMed] [Google Scholar]
- The White house, National strategy for combating antibiotic-resistant bacteria Available at: https://www.whitehouse.gov/sites/default/files/docs/carb_national_strategy.pdf (September 2014) (2014).
- Morales M., Martín-Galiano A. J., Domenech M. & García E. Insights into the evolutionary relationships of LytA autolysin and Ply pneumolysin-like genes in Streptococcus pneumoniae and related streptococci. Genome Biol. Evol. 7, 2747–2761 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley S. J., Orlova E. V., Gilbert R. J., Andrew P. W. & Saibil H. R. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell 121, 247–256 (2005). [DOI] [PubMed] [Google Scholar]
- Berry A. M., Lock R. A., Hansman D. & Paton J. C. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect. Immun. 57, 2324–2330 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran P., Hollingshead S. K., Paton J. C. & Briles D. E. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183, 3108–3116 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbo E. & Garau J. Of mice and men: innate immunity in pneumococcal pneumonia. Int. J. Antimicrob. Agents. 35, 107–113 (2010). [DOI] [PubMed] [Google Scholar]
- Kadioglu A. & Andrew P. W. The innate immune response to pneumococcal lung infection: the untold story. Trends. Immunol. 25, 143–149 (2004). [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N. Engl. J. Med. 320, 365–376 (1989). [DOI] [PubMed] [Google Scholar]
- Fox E. D., Heffernan D. S., Cioffi W. G. & Reichner J. S. Neutrophils from critically ill septic patients mediate profound loss of endothelial barrier integrity. Crit. Care 17, R226 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standish A. J. & Weiser J. N. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J. Immunol. 183, 2602–2609 (2009). [DOI] [PubMed] [Google Scholar]
- Belaaouaj A. et al. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 4, 615–618 (1998). [DOI] [PubMed] [Google Scholar]
- Hirche T. O. et al. Neutrophil serine proteinases inactivate surfactant protein D by cleaving within a conserved subregion of the carbohydrate recognition domain. J. Biol. Chem. 279, 27688–27698 (2004). [DOI] [PubMed] [Google Scholar]
- Wilkinson T. S. et al. Ventilator-associated pneumonia is characterized by excessive release of neutrophil proteases in the lung. Chest 142, 1425–1432 (2012). [DOI] [PubMed] [Google Scholar]
- Tagami T. et al. Plasma neutrophil elastase correlates with pulmonary vascular permeability: a prospective observational study in patients with pneumonia. Respirology 16, 953–958 (2011). [DOI] [PubMed] [Google Scholar]
- Hagio T. et al. Inhibition of neutrophil elastase reduces lung injury and bacterial count in hamsters. Pulm. Pharmacol. Ther. 21, 884–891 (2008). [DOI] [PubMed] [Google Scholar]
- Yanagihara K. et al. Effects of specific neutrophil elastase inhibitor, sivelestat sodium hydrate, in murine model of severe pneumococcal pneumonia. Exp. Lung Res. 33, 71–80 (2007). [DOI] [PubMed] [Google Scholar]
- Schauer C. et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 20, 511–517 (2014). [DOI] [PubMed] [Google Scholar]
- Cleaver J. O. et al. Lung epithelial cells are essential effectors of inducible resistance to pneumonia. Mucosal Immunol. 7, 78–88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Majlessi L., Deriaud E., Leclerc C. & Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 31, 761–771 (2009). [DOI] [PubMed] [Google Scholar]
- Chen H. C. et al. Neutrophil elastase induces IL-8 synthesis by lung epithelial cells via the mitogen-activated protein kinase pathway. J. Biomed. Sci. 11, 49–58 (2004). [DOI] [PubMed] [Google Scholar]
- Kuwahara I. et al. Neutrophil elastase induces IL-8 gene transcription and protein release through p38/NF-κB activation via EGFR transactivation in a lung epithelial cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L407–416 (2006). [DOI] [PubMed] [Google Scholar]
- Skals M., Leipziger J. & Praetorius H. A. Haemolysis induced by α-toxin from Staphylococcus aureus requires P2X receptor activation. Pflügers Arch. 462, 669–679 (2011). [DOI] [PubMed] [Google Scholar]
- Nagahama M. et al. Role of P2X7 receptor in Clostridium perfringens beta-toxin-mediated cellular injury. Biochim. Biophys. Acta. 1850, 2159–2167 (2015). [DOI] [PubMed] [Google Scholar]
- Jedrzejas M. J. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65, 187–207 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann. N. Y. Acad. Sci. 235, 439–447 (1974). [DOI] [PubMed] [Google Scholar]
- Mori Y. et al. α-Enolase of Streptococcus pneumoniae induces formation of neutrophil extracellular traps. J. Biol. Chem. 287, 10472–10481 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins J. B. et al. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect. Immun. 61, 1352–1358 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst R. A. et al. Sensitivities of human monocytes and epithelial cells to pneumolysin are different. Infect. Immun. 70, 1017–1022 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. et al. Genetic conjugation of components in two pneumococcal fusion protein vaccines enhances paediatric mucosal immune responses. Vaccine 33, 1711–1718 (2015). [DOI] [PubMed] [Google Scholar]
- Cockeran R. et al. Proinflammatory interactions of pneumolysin with human neutrophils. J. Infect. Dis. 183, 604–611 (2001). [DOI] [PubMed] [Google Scholar]
- Bewley M. A. et al. Pneumolysin activates macrophage lysosomal membrane permeabilization and executes apoptosis by distinct mechanisms without membrane pore formation. mBio 5, e01710–01714 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried E. L. Lipids of human leukocytes: relation to celltype. J. Lipid Res. 8, 321–327 (1967). [PubMed] [Google Scholar]
- Zachowski A. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem. J. 294, 1–14 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B. D. et al. Engineered liposomes sequester bacterial exotoxins and protect from severe invasive infections in mice. Nat. Biotechnol. 33, 81–88, (2015). [DOI] [PubMed] [Google Scholar]
- Yang D., He Y., Muñoz-Planillo R., Liu Q. & Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43, 923–932 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegen T. et al. The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release. J. Immunol. 187, 5440–5451 (2011). [DOI] [PubMed] [Google Scholar]
- Ayars G. H., Altman L. C., Rosen H. & Doyle T. The injurious effect of neutrophils on pneumocytes in vitro. Am. Rev. Respir. Dis. 129, 964–973 (1984). [DOI] [PubMed] [Google Scholar]
- Liu C. Y. et al. Apoptotic neutrophils undergoing secondary necrosis induce human lung epithelial cell detachment. J. Biomed. Sci. 10, 746–756 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun C. D., Han M. K., Kim U. H. & Chung H. T. Nitric oxide induces ADP-ribosylation of actin in murine macrophages: association with the inhibition of pseudopodia formation, phagocytic activity, and adherence on a laminin substratum. Cell. Immunol. 174, 25–34 (1996). [DOI] [PubMed] [Google Scholar]
- Swanson J. A. et al. A contractile activity that closes phagosomes in macrophages. J. Cell Sci. 112 (Pt 3), 307–316 (1999). [DOI] [PubMed] [Google Scholar]
- Ren Y. & Savill J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J. Immunol. 154, 2366–2374 (1995). [PubMed] [Google Scholar]
- McCutcheon J. C. et al. Regulation of macrophage phagocytosis of apoptotic neutrophils by adhesion to fibronectin. J. Leukoc. Biol. 64, 600–607 (1998). [DOI] [PubMed] [Google Scholar]
- Hart S. P., Dougherty G. J., Haslett C. & Dransfield I. CD44 regulates phagocytosis of apoptotic neutrophil granulocytes, but not apoptotic lymphocytes, by human macrophages. J. Immunol. 159, 919–925 (1997). [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Morgenthaler J. J., Chevallier I. & Schnebli H. P. Fibronectin-cleaving activity in bronchial secretions of patients with cystic fibrosis. J. Infect. Dis. 158, 89–100 (1988). [DOI] [PubMed] [Google Scholar]
- Kirkham P. A., Spooner G., Rahman I. & Rossi A. G. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem. Biophys. Res. Commun. 318, 32–37 (2004). [DOI] [PubMed] [Google Scholar]
- Gresnigt M. S. et al. Neutrophil-mediated inhibition of proinflammatory cytokine responses. J. Immunol. 189, 4806–4815 (2012). [DOI] [PubMed] [Google Scholar]
- Mukaida N., Matsumoto T., Yokoi K., Harada A. & Matsushima K. Inhibition of neutrophil-mediated acute inflammation injury by an antibody against interleukin-8 (IL-8). Inflamm. Res. 47 Suppl 3, S151–157 (1998). [DOI] [PubMed] [Google Scholar]
- Tazzyman S. et al. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int. J. Cancer. 129 (2011). [DOI] [PubMed] [Google Scholar]
- Tao L., LeBlanc D. J. & Ferretti J. J. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene 120, 105–110 (1992). [DOI] [PubMed] [Google Scholar]
- Håvarstein L. S., Coomaraswamy G. & Morrison D. A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 92, 11140–11144 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Terao Y., Mori Y., Hamada S. & Kawabata S. PfbA, a novel plasmin- and fibronectin-binding protein of Streptococcus pneumoniae, contributes to fibronectin-dependent adhesion and antiphagocytosis. J. Biol. Chem. 283, 36272–36279 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M. et al. Streptococcus pneumoniae invades erythrocytes and utilizes them to evade human innate immunity. PloS one 8, e77282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleurie A. et al. MapZ marks the division sites and positions FtsZ rings in Streptococcus pneumoniae. Nature 516, 259–262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa M. et al. Streptococcus pyogenes CAMP factor attenuates phagocytic activity of RAW 264.7 cells. Microbes. Infect. 18, 118–127 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.