Abstract
Purpose
To examine outcomes through 36 months in phakic eyes with newly diagnosed primary open-angle glaucoma (POAG) naïve to therapy randomized to treatment with two trabecular micro-bypass stents or topical prostaglandin.
Methods
Subjects with POAG naïve to therapy, with intraocular pressure (IOP) ≥21 and ≤40 mmHg, were randomized to implantation of two stents or travoprost. Additional medication was to be prescribed post-treatment for elevated IOP or glaucomatous optic nerve findings. Of 101 randomized subjects, 100 subjects were followed for 24 months and 73 subjects were followed for 36 months. Follow-up on all subjects is ongoing.
Results
In this randomized cohort of 101 POAG subjects, 54 subjects underwent 2-stent surgery and 47 received topical travoprost. Mean pre-treatment IOP was 25.5 ± 2.5 mmHg in stent-treated eyes and 25.1 ± 4.6 mmHg in medication-treated eyes. By 3 years, mean IOP was 14.6 mmHg in stent eyes (with medication added in 6 eyes) and 15.3 mmHg in travoprost eyes (with a second medication added in 11 eyes). In the subset of eyes that did not require additional medical therapy, mean IOP was 14.5 mmHg and 15.7 mmHg in the respective groups. Ninety-one percent of stent eyes had 3-year IOP ≤18 mmHg without additional therapy (62% ≤ 15 mmHg) and 79% of travoprost eyes had 3-year IOP ≤18 mmHg (21% ≤ 15 mmHg). Safety was favorable in both groups.
Conclusions
In this prospective, randomized comparison of subjects with newly diagnosed POAG naïve to therapy, substantial IOP reduction with a favorable low complication rate was shown through 3 years after either 2 trabecular stents implanted as the sole procedure or topical travoprost therapy. These data suggest 2-stent implantation may be a viable initial treatment option comparable to topical prostaglandin in newly diagnosed POAG patients.
Trial registration: ClinicalTrials.gov identifier, NCT01443988.
Funding
Glaukos Corporation, Laguna Hills, CA.
Electronic supplementary material
The online version of this article (doi:10.1007/s40123-016-0065-3) contains supplementary material, which is available to authorized users.
Keywords: Glaucoma, IOP, MIGS, POAG, Prostaglandin, Trabecular bypass
Introduction
Traditionally, treatment for newly diagnosed open-angle glaucoma (OAG) has started with topical ocular hypotensive medical therapy, followed by laser trabeculoplasty. These approaches have a lower risk profile than filtering or drainage device surgery. Further, these modalities preserve ocular tissues in case more invasive surgery is eventually required.
Despite these advantages, the efficacy of medical and laser therapy is limited. Various factors may hinder the proper use of topical medications, such as side effects (e.g., ocular hyperemia, iris hyperchromia, and periorbital atrophy), cost, intolerance to topical medications, and difficulty with drop instillation [1, 2]. In addition, patient adherence to medical therapy is frequently documented to be low [3–6]. In one study of 28,741 patients are naïve to glaucoma therapy, for example, 70% of patients discontinued their therapy after 1 year [3]. Such non-compliance limits the actual vs. expected effect of medical therapy and can increase the risk of disease progression, cost to patients, and cost to providers [7]. Laser procedures do not share the same challenges as topical medications, but their efficacy may be limited by short-term inflammation and long-term attrition [8].
Surgical methods addressing outflow via Schlemm’s canal have evolved over the last 15–20 years, from ab externo procedures such as trabeculectomy and viscocanalostomy to modern ab interno procedures such as trabecular micro-bypass stent implantation during micro-invasive glaucoma surgery (MIGS) [9]. In particular, the iStent (iStent® Trabecular Micro-Bypass, Glaukos Corporation, Laguna Hills, CA, USA) is a first-in-class treatment for mild-moderate OAG and is commercially available in the United States and 28 other countries [10]. Implantation of a single iStent in conjunction with cataract surgery has been shown to safely lower intraocular pressure (IOP) and medication usage through up to 5 years postoperatively in patients with glaucoma and cataract [11–17]. Implantation of multiple iStent devices during cataract surgery or as a standalone procedure also has shown effectiveness though up to 3 years postoperatively in patients with glaucoma not controlled on previous medication regimens [18–21]. MIGS with trabecular micro-bypass stents offers a highly favorable safety profile compared to more invasive traditional incisional glaucoma surgery or more recent suprachoroidal stent procedures [22–24].
To date, reports of trabecular micro-bypass stent implantation have focused on patients with mild to moderate OAG who have received prior medical or surgical treatment for their disease [11–22]. Due to its favorable safety and clinical effectiveness, however, it is possible that iStent implantation may be a suitable treatment option for patients with newly diagnosed glaucoma who are naïve to therapy and who require treatment long before they undergo cataract surgery.
To address the potential utility of iStent as initial therapy in these patients, we conducted a prospective, randomized study to evaluate the IOP-lowering effect and complication rates of implanting two iStents as a standalone procedure compared to primary medical therapy in patients recently diagnosed with OAG who had not undergone prior glaucoma treatment. An earlier report showed IOP control and favorable safety through 2 years [25]. The present report covers outcomes through 3 years.
Methods
This study was designed to enroll phakic subjects with newly diagnosed primary OAG (POAG), pseudoexfoliative glaucoma (PEX), or ocular hypertension that had not undergone prior treatment of any kind. Subjects were to present at the screening exam with IOP ≥21 and ≤40 mmHg, cup to disk (C:D) ratio ≤0.9, and normal angle anatomy. The study excluded subjects with uveitic, neovascular, or angle-closure glaucoma; glaucoma associated with vascular disorders; corneal pathology or prior corneal surgery; congenital or traumatic cataract or prior cataract surgery; retinal or optic nerve disorders; or any ocular disease or condition that, in the opinion of the investigator, would place the subject at significant risk, confound study results, or interfere with study participation. Subjects with fellow eyes in clinical trials and pregnant or nursing women also were excluded.
The study site was the S.V. Malayan Opthalmology Centre in Yerevan, Armenia. Ethics committee approval was secured for conduct of the study, and subjects signed informed consent documents. The study was conducted as per the principles governing clinical research as set in the Declaration of Helsinki 1964 (as revised in 2013) and applicable ISO/GCP guidelines. The ClinicalTrials.gov registration number for this study is NCT01443988 [26].
One-hundred qualified subjects were to be randomized in a 1:1 ratio under an open-label, unmasked strategy for implantation with either two iStent devices or topical travoprost (Travatan® 0.004%; Alcon®, Fort Worth, TX, USA). Stents were implanted by one staff surgeon and a team of visiting surgeons (Appendix 1). Following treatment, subjects were scheduled to return for evaluations at day 1, week 1, and at months 1, 3, 6, 12, 18, 24, 30, 36, 42, 48, 54 and 60. Pre-treatment and post-treatment examination at each scheduled visit included measurement of IOP via Goldmann applanation tonometry, best-corrected visual acuity (BCVA) via decimal chart, visual field via Humphrey 24-2 Swedish Interactive Thresholding Algorithm (SITA) standard perimetry, corneal thickness via pachymetry, slit-lamp evaluation, fundus examination and clinical assessment of nerve abnormalities, C:D ratio estimation, medication status, and assessment of complications and surgical interventions. In the event of elevated IOP (e.g., >21 mmHg) or optic nerve findings’ (e.g., worsened nerve appearance together with visual field progression) post-treatment, subjects were to be treated with additional therapy. This included initiating medical therapy in the stent group and administering additional medication(s) in the travoprost group.
The iStent device and implantation technique have been described in detail in previous work [12]. In brief, this single-piece, titanium, heparin-coated device has an L-shaped structure with a snorkel (inlet) on the short side which resides in the anterior chamber. The inlet opens to the half-pipe body which resides in Schlemm’s canal. The stent is 1.0 mm long and 0.33 mm in height. The inlet is 0.25 mm long with a bore diameter of 0.12 mm. The implant is provided to the surgeon pre-loaded in a disposable, single-use, stainless steel inserter that allows precise stent insertion ab internally into Schlemm’s canal. Once implanted, the stent is designed to create a bypass through the trabecular meshwork to Schlemm’s canal to improve aqueous outflow through the physiologic natural pathway with resultant decrease in IOP.
Travoprost topical ophthalmic solution is a synthetic prostaglandin F analog. The drug works to increase uveoscleral outflow and provide subsequent IOP reduction. In this study, brand-name Travatan (Alcon, Fort Worth, TX, USA) was used. Patients were instructed to instill medication once daily in the evening at 8 pm.
We assessed mean IOP over time in all eyes regardless of additional medical therapy after the initial treatment, and mean IOP in eyes that had not received additional therapy after their initial stent or travoprost treatment. Additional efficacy analysis included the proportion of eyes that had postoperative IOP ≤18 and ≤15 mmHg without additional medical therapy. Analyses of safety consisted of assessment of adverse events and complications, visual field, C:D ratio, central corneal thickness, and BCVA through the 36-month post-treatment period.
The randomized cohort population consisted of subjects randomized to treatment with two stents or travoprost (n = 101). Analyses were performed on available eyes from the Randomized Cohort preoperatively, month 12, month 24 and month 36. Mean and standard deviation values were calculated for continuous variables.
Results
Subject Accountability, Demographics, and Pre-Study Parameters
A total of 101 subjects (101 POAG, 1 PEX) completed the screening examination and were randomized to treatment with either two stents or with travoprost. To eliminate different diagnoses as a potential confounder, the one subject with PEX was excluded from the current analysis. Of the 101 subjects with POAG, 100 subjects completed follow-up through month 24 and 73 subjects completed follow-up through month 36.
Demographics and pre-treatment parameters are shown in Table 1. The stent and travoprost groups were similar in age (64.5 ± 11.1 vs. 62.0 ± 11.3 years, respectively), screening IOP (25.5 ± 2.5 vs. 25.1 ± 4.6 mmHg), C:D ratio (0.7 ± 0.2 vs. 0.6 ± 0.1), visual field, central corneal thickness, and BCVA. There were fewer males in the 2-stent group (25/54 or 46%) than in the medication group (32/47 or 68%). All subjects were Caucasian.
Table 1.
Subject demographic and pre-study parameters, randomized cohort
| 2-Stent group (N = 54) | Travoprost group (N = 47) | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 64.5 (11.1) | 62.0 (11.3) |
| Gender | ||
| Male/female | 25/29 | 32/15 |
| Eye | ||
| OD/OS | 20/34 | 24/23 |
| C:D ratio | ||
| Mean (SD) | 0.7 (0.2) | 0.6 (0.1) |
| Corneal thickness (µm) | ||
| Mean (SD) | 552.6 (41.2) | 540.3 (59.2) |
| Preop medicated IOP (mmHg) | ||
| Mean (SD) | 25.5 (2.5) | 25.1 (4.6) |
| BCVA (snellen) | ||
| 20/40 or better | 40 (74%) | 39 (83%) |
| 20/100 or better | 52 (96%) | 47 (100%) |
| 20/200 or better | 54 (100%) | 47 (100%) |
BCVA best-corrected visual acuity, C:D cup to disk, IOP intraocular pressure, OD/OS right eye/left eye, SD standard deviation
Efficacy
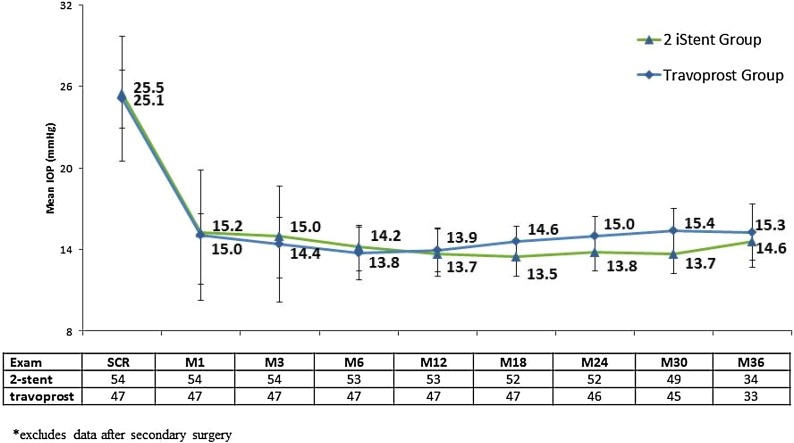
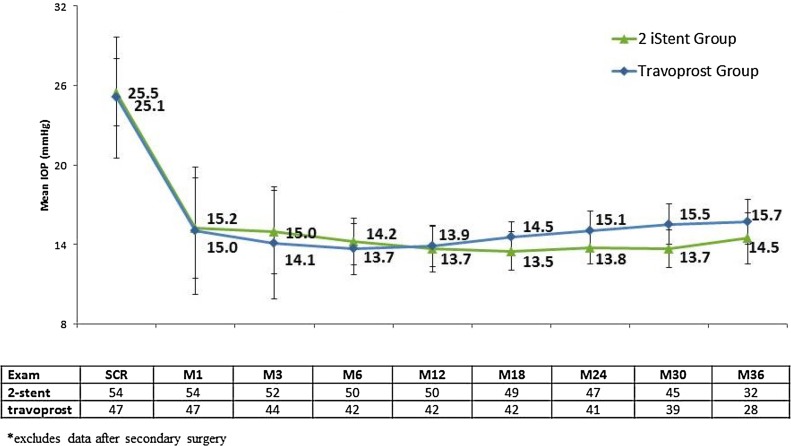
Subjects experienced notable reduction of IOP following implantation of two iStent trabecular micro-bypass devices or administration of topical travoprost (Fig. 1). For the stent group, mean IOP reduced from 25.5 mmHg preoperatively to 14.6 mmHg at 3 years. For the travoprost group, mean IOP reduced from 25.1 mmHg preoperatively to 15.3 mmHg at 3 years. By month 12, medication had been added to 8 subjects (3 in stent group, 5 in travoprost group) (Table 2). An additional 4 eyes (2 per group) had medication added at or before month 24. By the month 36 exam, an additional 5 eyes (1 in stent group and 4 in travoprost group) had medication added to their original treatment. In the subset of eyes that did not require additional medical therapy, mean IOP at 3 years was 14.5 mmHg in the stent group and 15.7 mmHg in the travoprost group (Fig. 2).
Fig. 1.
Mean IOP (mmHg) and number of subjects through 3 years, available eyes of randomized cohort*. IOP intraocular pressure
Table 2.
Subjects with post-treatment medical therapy, randomized cohort
| Subj # | Preop unmedicated IOP (mmHg) | Postop exam when med was addeda | IOP (mmHg) at exam when med was added | Medication added |
|---|---|---|---|---|
| 2-Stent group (N = 54) | ||||
| 37 | 30 | Month 1 | 30 | Brimonidine, timolol |
| 61 | 25 | Month 1 | 26 | Tafluprost |
| 72 | 26 | Month 3 | 23 | Travoprost, timolol |
| 7 | 24 | Month 18 | 16b | Tafluprost |
| 82 | 25 | Month 18 | 18b | Timolol |
| 34 | 28 | Month 36 | 19b | Timolol |
| Travoprost group (N = 47) | ||||
| 56 | 38 | Month 1 | 26 | Brinzolamide, timolol |
| 62 | 32 | Month 1 | 28 | Brinzolamide |
| 93 | 29 | Month 1 | 23 | Brinzolamide |
| 81 | 38 | Month 3 | 32 | Brinzolamide |
| 97 | 30 | Month 3 | 25 | Brinzolamide, timolol |
| 41 | 21 | Month 24 | 20b | Timolol |
| 76 | 26 | Month 24 | 18b | Timolol |
| 4 | 23 | Month 30 | 19b | Betoptic |
| 65 | 29 | Month 30 | 18b | Betoptic |
| 10 | 22 | Month 36 | 19b | Timolol |
| 14 | 22 | Month 36 | 19b | Timolol |
IOP intraocular pressure
aDoes not reflect all study visits following treatment. The complete schedule of postoperative visits for both groups was as follows: day 1, week 1, and at months 1, 3, 6, 12, 18, 24, 30, 36, 42, 48, 54 and 60
bMedication added due to optic nerve findings
Fig. 2.
Mean IOP (mmHG) and number of subjects through 3 years in Eyes without additional medical therapy, available eyes of randomized cohort*. IOP intraocular pressure
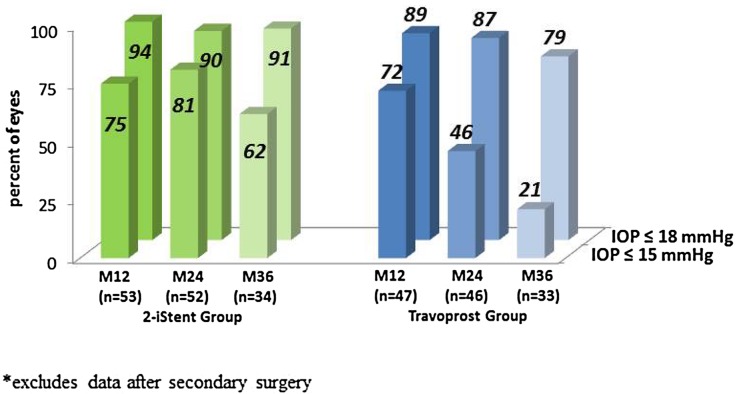
Proportional analyses showed that 94%, 90%, and 91% of stent-treated eyes had IOP ≤18 mmHg without the need for postoperative medication at 1, 2 and 3 years, respectively, while 89%, 87% and 79% of eyes in the travoprost group achieved IOP ≤18 mmHg at these timepoints without requiring additional medical therapy. The majority (62%) of stent eyes maintained IOP ≤15 mmHg through 3 years, while a decreasing percentage (21%) of travoprost eyes maintained IOP ≤15 mmHg over the 3-year follow-up period (Fig. 3).
Fig. 3.
Proportional analysis of post-treatment IOP, percent ≤15, ≤18 mmHg without additional therapy, available eyes of randomized cohort*. IOP intraocular pressure
Safety
Safety was favorable in both groups. Two complications were reported during stent insertion in the surgery group, both of which were attributed to subject movement during surgery: one of these subjects had hyphema which resolved by day 1 and one subject had a small iridodialysis which resulted in no postoperative ocular sequelae. No other operative complications were reported.
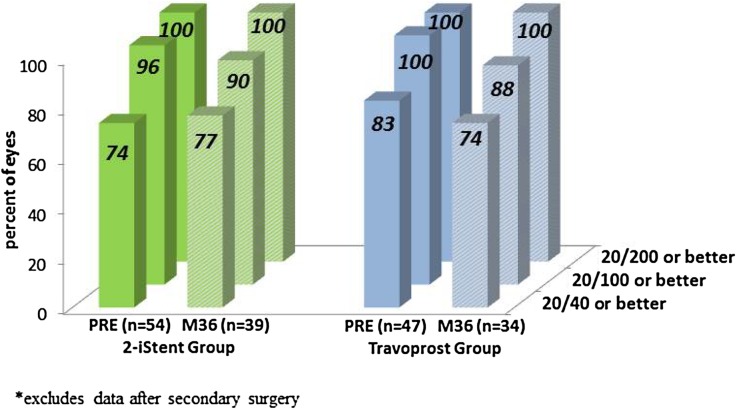
In general, BCVA was stable over time for both groups (Fig. 4). Progression of cataract over the 3-year follow-up period was reported in 11 eyes (20%) in the stent group and 8 eyes (17%) in the travoprost group. Of these, cataract surgery was performed in 6 eyes (5 in stent group and 1 in travoprost group), with last reported BCVA of 20/40 or better in these operated eyes. In the remaining non-operated subjects, three-year BCVA was 20/40 or better in 6 eyes (2 in stent group and 4 in med group), 20/100 in 1 eye (stent group), and 20/200 in 6 eyes (3 per group). No other post-treatment adverse events were reported in either group.
Fig. 4.
Proportional analysis of BCVA through 3 years, available eyes of randomized cohort*. BCVA best-corrected visual acuity, PRE preoperative
As shown in Table 3, C:D ratio, visual field, and central corneal thickness were stable through 36 months in both groups compared to pre-treatment values.
Table 3.
C:D ratio, visual field, and central corneal thickness through 3 years, available eyes of randomized cohort
| Screening | M12 | M24 | M36 | |
|---|---|---|---|---|
| 2-Stent group | ||||
| N | 54 | 53 | 53 | 39 |
| C:D ratio | ||||
| Mean (SD) | 0.7 (0.2) | 0.7 (0.1) | 0.7 (0.1) | 0.7 (0.1) |
| VF: mean deviation (dB) | ||||
| Mean (SD) | −7.5 (8.8) | −7.7 (8.9) | −6.0 (9.7) | −6.8 (7.4) |
| VF: pattern standard deviation (dB) | ||||
| Mean (SD) | 4.6 (3.3) | 4.4 (3.1) | 4.7 (3.2) | 4.3 (3.1) |
| Corneal thickness (µm) | ||||
| Mean (SD) | 552.6 (41.2) | 547.1 (41.6) | 549.0 (43.9) | 555.1 (44.5) |
| Travoprost group | ||||
| N | 47 | 47 | 47 | 34 |
| C:D ratio | ||||
| Mean (SD) | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) |
| VF: mean deviation (dB) | ||||
| Mean (SD) | −5.8 (7.7) | −6.3 (7.6) | −5.5 (7.7) | −6.2 (6.0) |
| VF: pattern standard deviation (dB) | ||||
| Mean (SD) | 3.5 (2.6) | 3.5 (2.6) | 3.4 (2.4) | 3.4 (2.4) |
| Corneal thickness (µm) | ||||
| Mean (SD) | 540.3 (59.2) | 544.6 (59.3) | 545.2 (59.2) | 545.3 (61.8) |
C:D cup to disk, SD standard deviation, VF visual field
Discussion
The goal of this prospective, randomized study was to compare the IOP reduction and safety profile of two ab interno trabecular micro-bypass stents (iStent) vs. topical prostaglandin medication in eyes with newly diagnosed POAG. Both therapies demonstrated substantial IOP reduction through 3 years post-treatment. Without starting additional medications, IOP ≤15 mmHg was maintained in the majority of stent eyes through 3 years of follow-up, but in only a minority of medication eyes. In addition, a lower percentage of stent eyes (11.1%; 6/54) compared to travoprost eyes (23.4%; 11/47) required additional medication by 3 years. Both groups had similarly favorable safety profiles, including stable BCVA, C:D ratio, visual fields, and central corneal thickness. These data support consideration of multiple iStent implantations as a first-line treatment option comparable to topical prostaglandin for newly diagnosed POAG patients who face decisions about the management of their chronic disease.
Prior studies of trabecular micro-bypass stent implantation have focused on patients with mild to moderate OAG who have received previous medical or surgical treatment [11–18]. There is a relative paucity of glaucoma studies in newly diagnosed glaucoma patients who are naïve to treatment. Thus, the present study showing clinical outcomes of 2-iStent implantation in treatment-naïve eyes fills a key gap in the literature. These findings may be increasingly relevant as more surgeons are considering iStent implantation as initial treatment for their newly diagnosed glaucoma patients.
iStent implantation as initial therapy may offer several benefits over topical medications, whose utility may be limited by issues such as side effects and patient compliance [1–7, 27–30]. Furthermore, the cost of both brand-name and generic medications places a considerable financial burden on the newly diagnosed glaucoma patient [31], thus making a longstanding surgical solution even more appealing. This may be particularly important in glaucoma patients, the majority of whom have at least one additional chronic condition requiring medication [32].
In this study, the IOP decrease in the travoprost group was greater than the 25–35% IOP reductions reported in prior work [33–35]. This may be due to medication-naïve eyes, high compliance due to study participation, and/or regression to the mean. The greater IOP reduction also may be attributed to the pre-treatment IOP level (i.e., greater IOP reduction with higher preoperative IOP), a phenomenon which has been reported previously in both treated and treatment-naïve glaucoma patients [35, 36].
There are several limitations to this study. Given the surgical vs. medical therapy study design, neither subjects nor clinicians were masked to treatment. Diurnal measurements of IOP were not performed. A pre-treatment grading of the crystalline lens was not used, and guidelines for when to perform cataract surgery were not standardized. Future work could incorporate such measures to address these study limitations, and also could encompass postoperative follow-up past 3 years. In addition, future analyses may examine the long-term cost effectiveness of iStent implantation vs. topical medication administration.
Conclusions
In summary, data from this prospective, randomized, controlled study provide a direct comparison of two trabecular micro-bypass stents vs. prostaglandin medical therapy in newly diagnosed POAG. In both groups, patients showed substantial IOP reduction and favorable safety through 3 years. These findings support the viability of multiple iStent implantations as an initial treatment option comparable to topical prostaglandin in newly diagnosed POAG.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The sponsor, Glaukos Corporation, Laguna Hills, CA, provided study devices, sponsorship for performing this study, publication charges, data collection, data management, data analysis, and editorial assistance in the preparation of this manuscript. Editorial assistance in the preparation of this manuscript was provided by Dana Hornbeak and Jane Ellen Giamporcaro of Glaukos Corporation. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
S D. Vold, L. Tetz, G. Auffarth, I. Masood, L. Au, I. I. K. Ahmed, and H. Saheb received non-financial support from Glaukos for their work as investigators in the study. S. D. Vold, L. Voskanyan, and I. I. K. Ahmed received financial support from Glaukos for their work as investigators in this study. I. I. K. Ahmed received non-study financial support from Glaukos.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of applicable ISO/GCP guidelines on human experimentation and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/8D76F060234F3623.
An erratum to this article is available at http://dx.doi.org/10.1007/s40123-016-0068-0.
References
- 1.Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009;116(11 Suppl):S30–S36. doi: 10.1016/j.ophtha.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122:1308–1316. doi: 10.1016/j.ophtha.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reardon G, Schwartz GF, Mozaffari E. Patient persistency with topical ocular hypotensive therapy in a managed care population. Am J Ophthalmol. 2004;137:3–12. doi: 10.1016/j.ajo.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Nordstrom BL, Friedman DS, Mozaffari E, et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140:598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS) Invest Ophthalmol Vis Sci. 2007;48:5052–5057. doi: 10.1167/iovs.07-0290. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GF, Reardon G, Mozaffari E. Persistency with latanoprost or timolol in primary open angle glaucoma suspects. Am J Ophthalmol. 2004;137:S13–S16. doi: 10.1016/j.ajo.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Mansberger SL. Are you compliant with addressing glaucoma adherence? Am J Ophthalmol. 2010;149(1):1. doi: 10.1016/j.ajo.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagar M, Ogunyomade A, O’Brart DP, et al. A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2005;89:1413–1417. doi: 10.1136/bjo.2004.052795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104. doi: 10.1097/ICU.0b013e32834ff1e7. [DOI] [PubMed] [Google Scholar]
- 10.Regulatory Affairs Division, Glaukos Corporation, Laguna Hills, CA, USA. Data on file.
- 11.Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma. J Cataract Refract Surg. 2010;36:407–412. doi: 10.1016/j.jcrs.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Samuelson TW, Katz LJ, Wells JM, Duh Y-J, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–467. doi: 10.1016/j.ophtha.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38:1339–1345. doi: 10.1016/j.jcrs.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Arriola-Villalobos P, Martinez-de-la-Casa J, Diaz-Valle D, et al. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012;96:645–649. doi: 10.1136/bjophthalmol-2011-300218. [DOI] [PubMed] [Google Scholar]
- 15.Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma ocular hypertension: long-term results. J Cataract Refract Surg. 2015;41:2664–2671. doi: 10.1016/j.jcrs.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Fea AM, Consolandi G, Zola M, et al. Micro-bypass implantation for primary open-angle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmol. 2015. Article ID 795357. 10.1155/2015/795357. Accessed July 12, 2016. [DOI] [PMC free article] [PubMed]
- 17.Spiegel D, Wetzel W, Neuhann T, et al. Coexistent primary open-angle glaucoma and cataract: interim analysis of a trabecular micro-bypass stent and concurrent cataract surgery. Eur J Ophthalmol. 2009;19:393–399. doi: 10.1177/112067210901900311. [DOI] [PubMed] [Google Scholar]
- 18.Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed II. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911–1917. doi: 10.1016/j.jcrs.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed II, Katz LJ, Chang DF, et al. Prospective evaluation of microinvasive glaucoma surgery with trabecular microbypass stents and prostaglandin in open-angle glaucoma. J Cataract Refract Surg. 2014;40:1295–1300. doi: 10.1016/j.jcrs.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Donnenfeld ED, Solomon KD, Voskanyan L, et al. A prospective 3-year follow-up trial of implantation of two trabecular microbypass stents in open-angle glaucoma. Clin Ophthalmol. 2015;9:2057–2065. doi: 10.2147/OPTH.S91732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz LJ, Erb C, Carceller Guillamet AC, et al. Prospective, randomized study of one, two, or three trabecular bypass stents in open-angle glaucoma subjects on topical hypotensive medication. Clin Ophthalmol. 2015;9:2313–2320. doi: 10.2147/OPTH.S96695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rulli E, Biagioli E, Riva I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures. A systematic review and meta-analysis. JAMA Ophthalmol. 2013;131:1573–1582. doi: 10.1001/jamaophthalmol.2013.5059. [DOI] [PubMed] [Google Scholar]
- 23.Höh H, Grisanti S, Grisanti S, Rau M, Ianchulev S. Two-year clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary micro-stent. Klin Monbl Augenheilkd. 2014;231(4):377–381. doi: 10.1055/s-0034-1368214. [DOI] [PubMed] [Google Scholar]
- 24.Sarkisian S. Combined cataract surgery and supraciliary microstent implantation for open-angle glaucoma: multicenter 3-year results. Presented at 2016 American Society of Cataract and Refractive Surgeons, May, 2016, New Orleans, LA, USA.
- 25.Vold S. Prospective, randomized evaluation of microinvasive glaucoma surgery (MIGS) with two trabecular microbypass stents vs. prostaglandin in open-angle or pseudoexfoliative glaucoma or ocular hypertension naïve to therapy. Poster presented at 2015 Annual Meeting of the American Glaucoma Society, February 26—March 1, 2015, Coronado, CA.
- 26.http://www.clintrials.gov. Accessed July 20, 2016.
- 27.Terminology and guidelines for glaucoma. ISBN 978-88-98320-05-09. Copyright 2014 European Glaucoma Society. http://www.eugs.org. Accessed July 22, 2016.
- 28.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically. The Travatan dosing aid study. Ophthalmology. 2009;116:191–199. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Robin AL, Novack GD, Covert DW, et al. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144:533–540. doi: 10.1016/j.ajo.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009;116:S30–S36. doi: 10.1016/j.ophtha.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Red Book ™. Ann Arbor: Truven Health Analytics; 2013. http://micromedex.com/products/product-suites/clinical-knowledge/redbook. Accessed July 6, 2016.
- 32.Salim S, Shields MB. Glaucoma and systemic diseases. Surv Ophthalmol. 2010;55:64–77. doi: 10.1016/j.survophthal.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Peace J, Ahlberg P, Wagner M, et al. Equivalent IOP lowering found when travoprost is preserved with polyquaternium or benzalkonium chloride. Am J Ophthalmol. 2015;160:266–274. doi: 10.1016/j.ajo.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 34.Parrish RK, Palmberg P, Sheu W-P, XLT Study Group A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135:688–703. doi: 10.1016/S0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 35.Lanzl I, Hamacher T, Rosbach K, et al. Preservative-free tafluprost in the treatment of naive patients with glaucoma and ocular hypertension. Clin Ophthalmol. 2013;7:901–910. doi: 10.2147/OPTH.S41640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown RH, Gibson Z, Zhong L, Lynch MG. Intraocular pressure reduction after cataract surgery with implantation of a trabecular microbypass device. J Cataract Refract Surg. 2015;41(6):1318–1319. doi: 10.1016/j.jcrs.2015.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.