Abstract
Background and Aims:
Caudal epidural analgesia is the most commonly used method of post-operative analgesia in children undergoing subumbilical surgeries. Many additive drugs have been used to prolong the post-operative analgesia. The aim of this study was to compare the efficacy of tramadol or midazolam addition to caudal ropivacaine for post-operative analgesia in children undergoing subumbilical surgeries.
Methods:
In this prospective, randomised, double-blinded comparative study, sixty children of either gender, in the age group of 1–5 years and scheduled for elective subumbilical surgeries were randomly divided into three groups of twenty each. Children in Group R received an epidural injection of 1 mL/kg of 0.2% plain ropivacaine whereas children in Group RT received an epidural injection of 2 mg/kg of tramadol plus 1 mL/kg of 0.2% ropivacaine and Group RM received an epidural injection of 50 μg/kg midazolam plus 1 mL/kg of 0.2% ropivacaine. The primary outcome variable was the duration of time to rescue analgesia. The secondary outcome variables were motor block, sedation score and urinary retention. Statistical comparison among the three groups was performed using one-way ANOVA with post hoc analysis using Bonferroni. For qualitative variables, Chi-square test was used. Statistical significance was defined as P < 0.05.
Results:
The mean duration of time to rescue analgesia was significantly longer (P < 0.001) in Group RT (913 ± 315.5 min) and Group RM (769.2 ± 331.9 min) compared to Group R (437.75 ± 75.68 min). However, there was no significant difference in the duration of time to rescue analgesia between RT and RM groups. Motor block and sedation scores were comparable between groups.
Conclusions:
The addition of tramadol or midazolam to caudal epidural ropivacaine prolongs the duration of analgesia without causing significant side effects.
Keywords: Caudal epidural block, epidural midazolam, epidural ropivacaine, epidural tramadol, paediatric anaesthesia, post-operative analgesia
INTRODUCTION
Historically, children have been undertreated for pain and painful procedures. Although caudal epidural block was described by Campbell in 1933,[1] it has evolved to become the most popular regional anaesthetic technique for intra- and post-operative pain relief in children from the 1980s. Caudal block is probably the most easily learned and mastered technique of all regional anaesthetic procedures.[2,3] A major limitation of this technique is the relatively short duration of post-operative analgesia even with long-acting local anaesthetics. Catheter placement into the caudal space adds to the risk of infection and tends to prevent early mobilisation and hence is not very popular.[4]
Prolongation of analgesia with caudal technique has been achieved with addition of various adjuvants to local anaesthetic agents, opioids being the most widely used adjuvant medication. However, the restricted availability of opioids (as low as 0.4% for the needy in India),[5] due to strict regulations, and some of its unpleasant side effects compel the clinician to seek non-opioid drugs such as clonidine, s-ketamine, neostigmine, midazolam and dexmedetomidine as adjuvant for caudal epidural anaesthesia. Some of these newer agents such as dexmedetomidine have unwanted haemodynamic effects.
In this study, we have selected two easily available and inexpensive drugs as adjuvant with local anaesthetic ropivacaine that may have a better safety profile compared to bupivacaine. The aim of this study was to compare the efficacy of caudally administered tramadol and midazolam as adjuvant to ropivacaine, compared to ropivacaine alone for post-operative analgesia in paediatric patients undergoing subumbilical surgical procedures.
METHODS
After obtaining the institutional research and ethics committee approval, sixty American Society of Anesthesiologists Physical Status I children in the age group of 1–5 years of either gender scheduled for elective subumbilical surgeries were randomly divided into three groups. A random number generating software was used to allocate children into three groups, and blinding was assured by drug preparation by a consultant anaesthesiologist not involved in the further follow-up of the study. Patients were excluded if they had a history of allergy to local anaesthetic, bleeding disorder or any evidence of infection, either local or systemic, or there were any neurological deficit or spinal deformities were suspected.
Written informed parental consent was obtained with respect to type of anaesthesia, the study being conducted, mode of pain relief and nature of surgery. Adequate fasting – 6 h for solids, 4 h for breast milk and 2 h for clear fluids – was ensured. Premedication with syrup triclofos 75 mg/kg mixed with atropine 40 μg/kg was given orally 1 h before induction of anaesthesia. Baseline values for heart rate (HR), respiratory rate (RR), SpO2 and blood pressure (BP) were recorded once the child was brought to operation theatre. Intravenous access was secured with 22 gauge cannula after inhalational induction with 50% oxygen (O2) and 50% nitrous oxide (N2 O) with 8% sevoflurane. Anaesthesia was maintained with N2O and O2 in the ratio of 2:1 along with 0.5%–1% halothane by mask ventilation using Jackson Rees modification of Ayer's T-piece. Spontaneous ventilation was assisted whenever necessary. Intravenous Ringer's Lactate was used for deficit correction and maintenance.
Injection ketamine 1 mg/kg was given intravenously to all patients 1 min before caudal epidural needle placement. The child was then positioned in left lateral decubitus, and caudal epidural was placed under strict aseptic precautions using drugs prepared by primary consultant anaesthesiologist not involved in the further follow-up of the cases. Caudal epidural block was performed using a 23 gauge scalp vein set with drug volume of 1 mL/kg up to a maximum of 20 mL. Group R received 1 mL/kg of 0.2% epidural ropivacaine, Group RT received 1 mL/kg of 0.2% epidural ropivacaine with 2 mg/kg of tramadol and Group RM received 1 mL/kg of 0.2% epidural ropivacaine along with 50 μg/kg of midazolam. The tramadol and midazolam used as adjuvants were preservative-free preparations (Supridol and Mezolam from Neon Laboratories Ltd, Mumbai - 400 093, Maharashtra, India). All patients were monitored for HR, RR, BP and SpO2 and documented at 5 min interval. The surgical incision was done 10 min after administration of caudal epidural injection. An increase in HR or RR of 15% or more from baseline value was taken as inadequate analgesia and supplemented with 1–2 μg/kg fentanyl, and these children were excluded from the analysis. Anaesthesia was discontinued after skin closure and 100% O2 was given for 5 min.
The primary outcome variable was duration of time to rescue analgesia. Quality of pain relief was assessed by modified Children's Hospital of Eastern Ontario Pain Scale (mCHEOPS)[6] [Table 1].[6] The secondary outcome variables were sedation, motor block and urinary retention. Postoperatively, patients were monitored for HR, RR and SpO2 in the recovery room for 1 h. Respiratory depression was defined as RR <10/min or O2 saturation <94%.
Table 1.
Modified Children's Hospital of Eastern Ontario Pain Scale

In the post-operative ward, children were observed hourly up to 6 h and then 3 hourly for next 6 h and then at 24 h. Rescue analgesia was given when the mCHEOPS score was 6 or more and children were given oral paracetamol 15 mg/kg body weight. The time interval from the caudal block to the administration of rescue analgesic was taken as the duration of analgesia. A four-point sedation score was used to assess the level of sedation: 0 - awake, 1 - mild sedation, 2 - tending to sleep and 3 - deep sleep, unable to awaken. Time for spontaneous eye opening from the time of discontinuation of anaesthesia is taken as the duration of sedation. Motor block was assessed every 30 min for 3 h using modified Bromage scale as below: score 0 - no motor block, 1 - inability to raise extended legs, 2 - inability to flex knee and 3 - no movement possible. Time of spontaneous ambulation was also noted. The time of first spontaneous micturition was recorded. Side effects such as nausea, vomiting and pruritus were also noted.
In a study comparing bupivacaine and bupivacaine plus tramadol,[7] it was found that the difference in mean duration to rescue analgesic was 2.8 h between the two groups. Assuming similar difference at the minimum, the sample size calculated was 18 in each group. Statistical analysis was performed using the statistical package SPSS v19.0 (IBM India Pvt Ltd, Bangalore, India). Quantitative data were presented as mean and standard deviation and qualitative data as frequency. Statistical comparison among the three groups was carried out using one-way ANOVA with post hoc analysis using Bonferroni. For qualitative variables, Chi-square test was used. Significance was defined as P < 0.05.
RESULTS
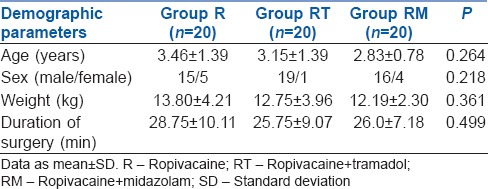
All the sixty children, enrolled in the three groups, were analysed. The groups were comparable with respect to patient age, sex, weight and duration of surgery [Table 2]. The baseline vital parameters were comparable between the groups. Intraoperatively, HR, RR, BP or SpO2 did not show any significant change in any group indicating haemodynamic stability.
Table 2.
Demographic data comparison between the three groups
Pain scores using mCHEOPS were comparable between the groups at all timeframes except at 9th h when there was a significant difference in the pain score. The mean value of mCHEOPS in Group R (3.79 ± 1.58) was higher than that in Group RT (2.45 ± 0.76) and the Group RM (2.65 ± 1.27) at 9th h (P = 0.010) [Figure 1]. The mean duration of time to rescue analgesia for Group R was 437.75 ± 75.68 min. For Group RT, it was 913.00 ± 315.50 min, and for Group RM, it was 769.25 ± 331.99 min. The differences in mean time for rescue analgesia were statistically significant between the Group R and the other two groups (P < 0.001) [Figure 2]. There was no significant difference in mean time for rescue analgesia between Group RT (913.00 ± 315.50 min) and Group RM (769.25 ± 331.99 min) (P = 0.361).
Figure 1.
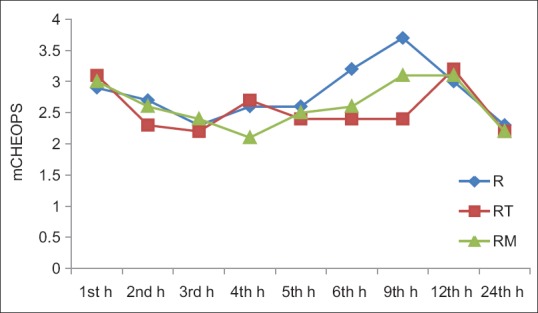
Comparison of pain scores at various time interval. There was significant difference in modified Children's Hospital of Eastern Ontario Pain Scale at 9th h (P = 0.010). R = Ropivacaine, RT = Ropivacaine + Tramadol, RM = Ropivacaine + Midazolam
Figure 2.
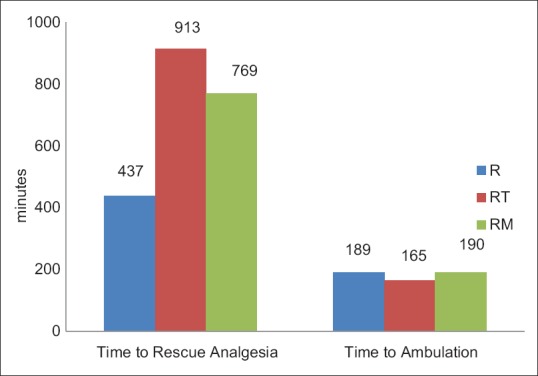
Comparison of Time to rescue analgesia and time to spontaneous ambulation. R = Ropivacaine, RT = Ropivacaine + Tramadol, RM = Ropivacaine + Midazolam
The mean duration of time to spontaneous ambulation for Group R was 189.25 ± 75.68 min, for Group RT 165.00 ± 67.98 min and for Group RM 190.75 ± 80.48 min (P value 0.479). The mean duration of time to spontaneous eye opening for Group R was 20.35 ± 8.89 min, for Group RT 17.00 ± 5.56 min and for Group RM 15.00 ± 5.88 min (P = 0.056). The mean duration to spontaneous micturition for Group R was 342.40 ± 62.70 min, for Group RT 331.25 ± 93.06 min and for Group RM 307.75 ± 65.08 min (P = 0.335) [Figure 3].
Figure 3.
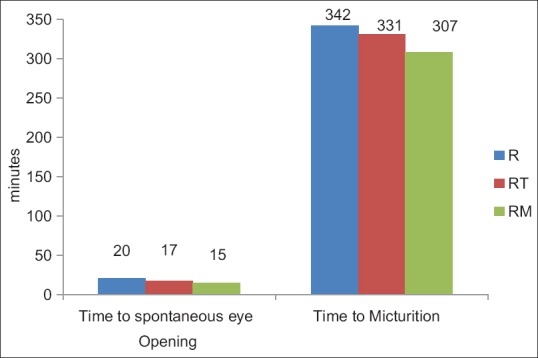
Comparison of time to spontaneous eye opening and time to micturition. R = Ropivacaine, RT = Ropivacaine + Tramadol, RM = Ropivacaine + Midazolam
No significant side effects were noted in any group. One child in Group R and one in Group RT had vomiting in the post-operative ward. No child had respiratory depression in the post-operative period.
DISCUSSION
Children are equally susceptible to pain as adults. Post-operative pain in children is mostly undertreated due to unique problems such as non-acceptance of injections, difficulty in correctly assessing pain and fear of side effects of opioids. With improvement in surgical technique and awareness of physiological processes, the number of surgical procedure and the need to treat paediatric pain is increasing day by day. Caudal epidural block is extensively used for post-operative analgesia in children undergoing lower abdominal, perineal and genitourinary surgeries.
The short duration of action (4–8 h)[8] of local anaesthetics alone in caudal block has forced the anaesthesiologists to search for additives to enhance the duration of analgesia in children. Continuous caudal analgesia with catheter technique has added risk of infection and delay in mobilisation. Although excellent analgesia with extradural opioids is well established, unpleasant side effects and restricted availability have been responsible for a decline in their popularity, especially in paediatrics. In a retrospective study, clinically significant respiratory depression was observed when 0.07 mg/kg morphine was given caudally.[9] As the time period of respiratory depression is unclear, all children after caudal opioids needed postoperative intensive care admission with 24 h SpO2 monitoring.[10] High incidence of other complications such as nausea-vomiting[11] and urinary retention[12] with extradural morphine was also reported.
In the present study, we have attempted to compare the two commonly used, easily available and relatively inexpensive agents, tramadol and midazolam, as adjuvant in caudal anaesthesia along with ropivacaine, an amino amide local anaesthetic agent. We have selected ropivacaine because of its safer neurological and cardiovascular toxicity profile.[13,14] Studies have established the safety of tramadol and midazolam as caudal adjuvant without significant adverse effects.[15,16,17,18] However, this is the first study to compare the two adjuvant drugs, midazolam and tramadol, with caudal ropivacaine on duration of analgesia. Most of the authors recommended a concentration of 0.2% ropivacaine for effective caudal epidural analgesia without unwanted motor block.[19,20] Studies comparing ropivacaine and bupivacaine show no significant difference in terms of duration of analgesia, and motor block was found to be less with ropivacaine.
The analgesic effect of extradurally administered midazolam is through γ-amino butyric acid (GABA)/benzodiazepine system of spinal cord, and previous studies have recommended an optimal dose of 50 μg/kg for extradural administration.[10] Tramadol, a weak opioid, exerts its analgesic effect through μ receptors and also inhibits serotonin uptake and is devoid of any respiratory depressant effect.[21] The dose of tramadol used in caudal epidural block in various studies was 1–2 mg/kg.[15,17,18,22,23]
In our study, the mean duration of analgesia was longer with addition of tramadol (Group RT 913.00 ± 315.50 min) and midazolam (Group RM 769.25 ± 331.99 min) compared to ropivacaine alone (Group R 437.75 ± 75.68 min). A study on fifty children undergoing herniotomy showed that addition of midazolam to caudal bupivacaine 0.25% significantly prolonged the duration of analgesia without any side effect compared to bupivacaine alone.[24] When used as a sole agent, midazolam in caudal block was not associated with any respiratory depression or prolonged sedation. A randomised controlled study comparing the effects of addition of midazolam, neostigmine or ketamine to caudal bupivacaine in eighty children undergoing inguinal herniotomy showed a significant prolongation of post-operative analgesia with addition of midazolam (376 ± 24 min), ketamine (336 ± 16 min) or neostigmine (442 ± 31 min) to caudal bupivacaine.[25]
We have observed a significant prolongation of rescue analgesia with addition of tramadol to caudal ropivacaine (913.00 ± 315.50 min) compared to ropivacaine alone (437.75 ± 75.68 min). This was comparable with previously reported studies.[16,17] Another study confirmed the safety of caudal tramadol for post-operative analgesia. The unpleasant side effects of tramadol, nausea and vomiting were not significantly observed in our study similar to previous reports.[17]
In our study, we have established the efficacy of tramadol and midazolam as effective adjuvant with ropivacaine for prolonging the duration of post-operative analgesia. There was no incidence of respiratory depression or sedation, and the motor block was also minimal. Only two patients had vomiting in the post-operative period. There was no incidence of pruritus or bladder retention in any group.
In this study, injection ketamine was used in all patients before caudal anaesthesia keeping in mind the analgesic effect of ketamine. As it was used in all the three groups and the duration of action is about 30 min, it was considered not to compound the post-operative analgesia duration.
CONCLUSION
Addition of tramadol or midazolam to caudal epidural block with ropivacaine showed significant prolongation of post-operative analgesia compared to ropivacaine alone. The mean duration of analgesia in tramadol group was more than the midazolam group though this difference was statistically not significant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Campbell MF. Caudal anesthesia in children. Am J Urol. 1933;30:245–9. [Google Scholar]
- 2.Dalens B, Hasnaoui A. Caudal anesthesia in pediatric surgery: Success rate and adverse effects in 750 consecutive patients. Anesth Analg. 1989;68:83–9. [PubMed] [Google Scholar]
- 3.Rowney DA, Doyle E. Epidural and subarachnoid blockade in children. Anaesthesia. 1998;53:980–1001. doi: 10.1046/j.1365-2044.1998.00527.x. [DOI] [PubMed] [Google Scholar]
- 4.Turan A, Memis D, Basaran UN, Karamanlioglu B, Süt N. Caudal ropivacaine and neostigmine in pediatric surgery. Anesthesiology. 2003;98:719–22. doi: 10.1097/00000542-200303000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Rajagopal MR, Joranson DE. India: Opioid availability. An update. J Pain Symptom Manage. 2007;33:615–22. doi: 10.1016/j.jpainsymman.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Gaitini LA, Somri M, Vaida SJ, Yanovski B, Mogilner G, Sabo E, et al. Does the addition of fentanyl to bupivacaine in caudal epidural block have an effect on the plasma level of catecholamines in children? Anesth Analg. 2000;90:1029–33. doi: 10.1097/00000539-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Doda M, Mukherjee S. Postoperative analgesia in children- comparative study between caudal bupivacaine and bupivacaine plus tramadol. Indian J Anaesth. 2009;53:463–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Thomas AR. Pain management in paediatric patients. Br J Anaesth. 1990;64:85–104. doi: 10.1093/bja/64.1.85. [DOI] [PubMed] [Google Scholar]
- 9.Valley RD, Bailey AG. Caudal morphine for postoperative analgesia in infants and children: A report of 138 cases. Anesth Analg. 1991;72:120–4. doi: 10.1213/00000539-199101000-00022. [DOI] [PubMed] [Google Scholar]
- 10.de Beer DA, Thomas ML. Caudal additives in children - Solutions or problems? Br J Anaesth. 2003;90:487–98. doi: 10.1093/bja/aeg064. [DOI] [PubMed] [Google Scholar]
- 11.Wolf AR, Hughes D, Wade A, Mather SJ, Prys-Roberts C. Postoperative analgesia after paediatric orchidopexy: Evaluation of a bupivacaine-morphine mixture. Br J Anaesth. 1990;64:430–5. doi: 10.1093/bja/64.4.430. [DOI] [PubMed] [Google Scholar]
- 12.Krane EJ. Delayed respiratory depression in a child after caudal epidural morphine. Anesth Analg. 1988;67:79–82. [PubMed] [Google Scholar]
- 13.Ray M, Mondal SK, Biswas A. Caudal analgesia in paediatric patients: Comparison between bupivacaine and ropivacaine. Indian J Anaesth. 2003;47:275–8. [Google Scholar]
- 14.Ivani G, De Negri P, Lonnqvist PA, L’Erario M, Mossetti V, Difilippo A, et al. Caudal anesthesia for minor pediatric surgery: A prospective randomized comparison of ropivacaine 0.2% vs. levobupivacaine 02% Paediatr Anaesth. 2005;15:491–4. doi: 10.1111/j.1460-9592.2004.01536.x. [DOI] [PubMed] [Google Scholar]
- 15.Ozcengiz D, Gunduz M, Ozbek H, Isik G. Comparison of caudal morphine and tramadol for postoperative pain control in children undergoing inguinal herniorrhaphy. Paediatr Anaesth. 2001;11:459–64. doi: 10.1046/j.1460-9592.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- 16.Prakash S, Tyagi R, Gogia AR, Singh R, Prakash S. Efficacy of three doses of tramadol with bupivacaine for caudal analgesia in paediatric inguinal herniotomy. Br J Anaesth. 2006;97:385–8. doi: 10.1093/bja/ael155. [DOI] [PubMed] [Google Scholar]
- 17.Senel AC, Akyol A, Dohman D, Solak M. Caudal bupivacaine-tramadol combination for postoperative analgesia in pediatric herniorrhaphy. Acta Anaesthesiol Scand. 2001;45:786–9. doi: 10.1034/j.1399-6576.2001.045006786.x. [DOI] [PubMed] [Google Scholar]
- 18.Batra YK, Prasad MK, Arya VK, Chari P, Yaddanapudi LN. Comparison of caudal tramadol vs bupivacaine for post-operative analgesia in children undergoing hypospadias surgery. Int J Clin Pharmacol Ther. 1999;37:238–42. [PubMed] [Google Scholar]
- 19.Deng XM, Xiao WJ, Tang GZ, Luo MP, Xu KL. The minimum local anesthetic concentration of ropivacaine for caudal analgesia in children. Anesth Analg. 2002;94:1465–8. doi: 10.1097/00000539-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Locatelli B, Ingelmo P, Sonzogni V, Zanella A, Gatti V, Spotti A, et al. Randomized, double-blind, phase III, controlled trial comparing levobupivacaine 0.25%, ropivacaine 0.25% and bupivacaine 0.25% by the caudal route in children. Br J Anaesth. 2005;94:366–71. doi: 10.1093/bja/aei059. [DOI] [PubMed] [Google Scholar]
- 21.Vickers MD, O’Flaherty D, Szekely SM, Read M, Yoshizumi J. Tramadol: Pain relief by an opioid without depression of respiration. Anaesthesia. 1992;47:291–6. doi: 10.1111/j.1365-2044.1992.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 22.Murthy BV, Pandya KS, Booker PD, Murray A, Lintz W, Terlinden R. Pharmacokinetics of tramadol in children after i.v. or caudal epidural administration. Br J Anaesth. 2000;84:346–9. doi: 10.1093/oxfordjournals.bja.a013437. [DOI] [PubMed] [Google Scholar]
- 23.Prosser DP, Davis A, Booker PD, Murray A. Caudal tramadol for postoperative analgesia in pediatric hypospadias surgery. Br J Anaesth. 1997;79:293–6. doi: 10.1093/bja/79.3.293. [DOI] [PubMed] [Google Scholar]
- 24.Naguib M, el Gammal M, Elhattab YS, Seraj M. Midazolam for caudal analgesia in children: Comparison with caudal bupivacaine. Can J Anaesth. 1995;42:758–64. doi: 10.1007/BF03011172. [DOI] [PubMed] [Google Scholar]
- 25.Kumar P, Rudra A, Pan AK, Acharya A. Caudal additives in pediatrics: A comparison among midazolam, ketamine, and neostigmine coadministered with bupivacaine. Anesth Analg. 2005;101:69–73. doi: 10.1213/01.ANE.0000153862.95153.2E. [DOI] [PubMed] [Google Scholar]


