Abstract
OBJECTIVE
To report a contemporary series of surgically treated patients with tumors involving kidneys with fusion anomalies.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of all 10 patients treated at a single tertiary care institution for tumors involving kidneys with fusion anomalies between the years 2000 and 2015. One patient, diagnosed with lymphoma, did not undergo surgical treatment and was therefore excluded. Data regarding patient, tumor, and treatment characteristics were collected and described.
RESULTS
The study cohort included 7 male and 2 female patients, at a median age of 52 years. Seven patients underwent open partial nephrectomy. Nephroureterectomy was performed on 2 patients; 1 open and 1 laparoscopic. All patients had localized disease at diagnosis. Tumor histologies were renal cell carcinoma in 5 patients, renal oncocytoma in 1 patient, urothelial carcinoma in 2 patients, and a well-differentiated liposarcoma involving the kidney in 1 patient. Accessory blood vessels were identified in 8 of 9 patients. Median estimated blood loss was 300 mL (interquartile range: 150–1000). Four patients had postoperative complications, including 3 major (Clavien grade ≥ 3) and 3 minor (Clavien grade ≤ 2) complications. During a median follow-up of 19.2 months (interquartile range: 3–34.8), 1 patient with urothelial carcinoma developed a bladder recurrence. None of the patients developed new-onset chronic kidney disease during the early postoperative period.
CONCLUSION
Localized renal cortical tumors in kidneys with fusion anomalies may be treated with partial nephrectomy; however, complication rates are relatively high. Preoperative imaging of the blood vessels is necessary, as most patients have an accessory blood supply.
Fusion anomalies of the kidney are rare congenital disorders that include horseshoe kidneys (HKs) and kidneys with crossed fused ectopia (CFE).1 The estimated incidence of HK in the general population is 0.15%–0.25%,2 and the incidence of CFE is substantially lower (0.01%–0.1%).1 Patients with an HK more often develop hydronephrosis, urinary tract infections, and urolithiasis when compared to the general population.3 Tumors involving an HK appear in 12% of patients with these anomalies.4 Although a significantly higher risk of developing nephroblastoma and urothelial carcinoma was reported by previous studies, the most common histological subtype is renal cell carcinoma, found in 50% of HK patients with a renal tumor.4,5
Surgical resection of tumors involving kidneys with fusion anomalies may be complicated by the presence of aberrant and accessory blood vessels, and involvement of the renal isthmus with possible growth from 1 hemi-kidney to the other. An abnormally low renal location may lead to late detection and treatment of advanced stage disease.1,4,6–10 Moreover, fusion of the lower poles of the two hemi-kidneys limits renal mobilization and increases surgical complexity especially when treating posterior renal masses.10 Initial reports described the use of radical nephrectomy and nephroureterectomy for the treatment of tumors involving fused kidneys. These procedures had a complicated course and were associated with substantial blood loss.4,6 Recent reports have demonstrated the feasibility of partial nephrectomy for the treatment of these tumors, whether using open, laparoscopic, or robot-assisted laparoscopic surgical approaches.10–14 In addition, preoperative superselective renal artery embolization may facilitate organ-preserving surgery.15,16 For patients who are not candidates for surgical resection, percutaneous computed tomography (CT)-guided radiofrequency ablation has evolved as a treatment option.17
Previous reports evaluating the role of partial nephrectomy in patients with HK or CFE included mostly single case studies. The present study aims to report on the surgical treatment of tumors involving kidneys with fusion anomalies in a contemporary series of patients.
MATERIALS AND METHODS
Between 2000 and 2015, a total of 10 patients were treated at our institution, a tertiary care center, for tumors involving kidneys with fusion anomalies. After obtaining institutional review board approval, we retrospectively reviewed the medical records of these patients. One patient, diagnosed with lymphoma, did not undergo surgical treatment and was therefore excluded from the study, leaving a total of 9 patients for analysis.
Prior to surgery, patients with tumors incidentally detected on ultrasound undergo additional axial imaging to define the aberrant kidney anatomy and identify accessory blood vessels. Magnetic resonance imaging or CT angiography is performed when vascular anatomy is unclear despite initial imaging. At our institution, the indications to surgically treat tumors in patients with fusion anomalies of the kidney are the same as those in patients without kidney anomalies. When feasible, patients with a suspected renal cortical tumor undergo partial nephrectomy, whereas patients with urothelial tumor undergo nephroureterectomy. Partial nephrectomies are performed using an open surgical approach. Vertical midline or flank incisions are used based on tumor location and at the discretion of the treating physician. Due to the aberrant vasculature and anatomy of the fused kidney, partial nephrectomies are performed using regional ischemia when possible, thus retaining perfusion to the rest of the kidney and preserving renal function. Cold ischemia is used at the discretion of the treating physician. After the operation, patients are followed based on standard of care for their tumor histology and stage.
Patient data including gender, age at diagnosis, body mass index, Charlson comorbidity index score,18 and presenting symptoms (incidental, local, or systemic) were collected. R.E.N.A.L. nephrometry score19 was used to report the complexity of renal cortical tumors. Treatment characteristics including the surgical procedure performed, operative time, estimated blood loss, and the use of ischemia during the operation were noted. Intraoperative findings including the number of blood vessels supplying the operated side of the kidney, the type of isthmus (parenchymatous or fibrous), and tumor location were also noted. Surgical specimens were evaluated by genitourinary pathologists, and tumor diameter, histology, and stage were reported according to the 2010 American Joint Committee on Cancer/Union for International Cancer Control TNM classification system. Surgical margin status was defined as positive or negative. The postoperative course was reviewed and postoperative complications were graded according to the Clavien-Dindo classification system.20 If relevant, disease recurrence was defined as either local or distant based on imaging study findings. Estimated glomerular filtration rate (eGFR) at follow-up was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, and chronic kidney disease was defined as an eGFR < 60 mL/min/1.73 m2.21
Clinical, pathologic, and treatment-related data were summarized using descriptive statistics. The median and interquartile range (IQR), and mean and standard deviation (SD), were used for continuous variables.
RESULTS
The study cohort included 7 male patients and 2 female patients, with a median age at nephrectomy of 52 years (IQR: 41–58). Median body mass index was 27.1 (IQR: 24.9–32), and median Charlson comorbidity index score was 2 (IQR: 2–4). Six patients were diagnosed incidentally after undergoing imaging studies for other causes. Three patients were diagnosed after seeking treatment for local symptoms; 2 had macroscopic hematuria and 1 had a symptomatic abdominal mass. Figure 1 and Supplementary Figure S1 show incidentally discovered tumors involving the right hemi-kidney of an HK and upper moiety of a kidney with CFE, respectively. Median tumor size was 4.8 cm (IQR: 3.6–8). R.E.N.A.L. nephrometry scores were ≥9 in 4 of 5 patients with a renal cortical tumor. Tumor histology was renal cell carcinoma in 5 patients, renal oncocytoma in 1 patient, urothelial carcinoma in 2 patients, and a well-differentiated liposarcoma involving the kidney in 1 patient. All patients had localized disease without lymphovascular invasion. Surgical margins were negative for all patients except for the patient with the liposarcoma. Patient and tumor characteristics are reported in Table 1.
Figure 1.
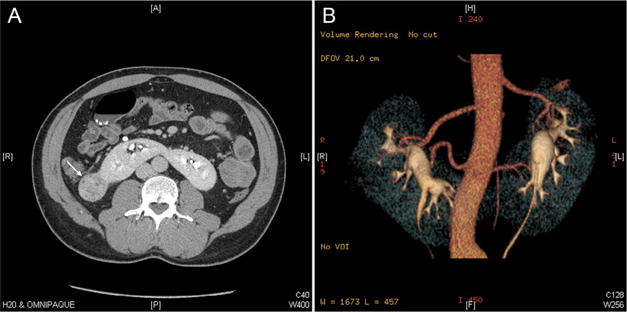
Computed tomography image of a patient with a horseshoe kidney. (A) Axial image demonstrating a tumor involving the right hemi-kidney of the horseshoe kidney (arrow), and a parenchymatous isthmus. (B) 3D reconstruction of the computed tomography image, demonstrating 2 accessory arteries supplying each hemi-kidney. (Color version available online.)
Table 1.
Patient and tumor characteristics of the study cohort (n = 9)
| Patient No. |
Age (Years) |
Kidney Anomaly |
Sex | Symptoms | Tumor Location | Tumor Size (cm) |
Tumor R.E.N.A.L. Score |
Tumor Histology | Tumor Stage |
Surgical Margins |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | HK | Male | Hematuria | Upper pole | 4.8 | NA | UC | TaN0M0 | Negative |
| 2 | 47 | HK | Female | Abdominal mass | Renal hilum and adjacent kidney | 30 | NA | Well-differentiated liposarcoma | NA | Positive |
| 3 | 52 | HK | Male | Incidental | Lower pole | 3.6 | 6× | Unclassified RCC | T1aNxM0 | Negative |
| 4 | 58 | HK | Male | Incidental | Lower pole abutting isthmus | 4.5 | 10a | Clear cell RCC | T1bNxM0 | Negative |
| 5 | 75 | HK | Female | Hematuria | Upper pole | 2 | NA | UC | T2NxM0 | Negative |
| 6 | 75 | CFE | Male | Incidental | Lower pole | 8.5 | 10p | Papillary type 1 RCC | T2aNxM0 | Negative |
| 7 | 41 | HK | Male | Incidental | Upper pole | 3 | NA* | Clear cell RCC | T1aNxM0 | Negative |
| 8 | 55 | HK | Male | Incidental | Lower pole | 8 | 10a | Clear cell RCC | T2aN0M0 | Negative |
| 9 | 56 | CFE | Male | Incidental | Upper pole | 6.9 | 9a | Oncocytoma | T1bNxM0 | Negative |
CFE, crossed fused ectopia; HK, horseshoe kidney; NA, not available; RCC, renal cell carcinoma; UC, urothelial carcinoma.
Preoperative axial imaging was not available for nephrometry score calculation.
Seven patients underwent open partial nephrectomy, 4 patients had a vertical midline incision, whereas the other 3 had a flank incision. Median operative time for partial nephrectomy was 157 minutes (IQR: 144–267) and median estimated blood loss was 400 mL (IQR: 200–1500). Two patients required treatment with packed red blood cells; 1 required 4 units due to an estimated blood loss of 4.2 liters, and the other required 2 units due to an estimated blood loss of 1 liter. Two patients underwent nephroureterectomy; 1 patient with an open vertical midline approach and the other laparoscopically. Operative times were 40 and 417 minutes and estimated blood loss 100 mL and 300 mL, respectively. Eight patients (89%) had at least 1 accessory blood vessel supplying the hemi-kidney involved by the tumor (Fig. 1B). Mean number of arteries and veins were 2.3 ± 0.9 and 1.4 ± 0.73, respectively. The isthmus type was parenchymatous in all patients except 1 (which was fibrous). Regional ischemia was used in 5 of 7 patients undergoing partial nephrectomy and cold ischemia was used in 2 of 7, with ischemia times of 23 and 29 minutes, respectively. Treatment characteristics are reported in Table 2.
Table 2.
Treatment characteristics of the study cohort (n = 9)
| Patient No. | Surgical Approach | Incision Type | Operative Time (min) | Cold Ischemia Time (min) | Estimated Blood Loss (mL) | Kidney Blood Supply (No.) | Isthmus Type | Complications (Clavien Grade) |
|---|---|---|---|---|---|---|---|---|
| 1 | Open NU | Vertical midline | 40 | None | 100 | 2A/1V | Parenchymatous | Paralytic ileus (1) |
| 2 | Open PN | Vertical midline | 400 | None | 1000 | 3A/1V | Parenchymatous | Pneumothorax (3b) |
| 3 | Open PN | Flank | 138 | 29 | 150 | 3A/3V | Parenchymatous | None |
| 4 | Open PN | Vertical midline | 289 | None | 4200 | 1A/2V | Parenchymatous | Bilateral atelectasis (1) |
| 5 | Laparoscopic NU | NA | 417 | None | 300 | 1A/1V | Parenchymatous | None |
| 6 | Open PN | Vertical midline | 157 | None | 250 | 2A/1V | NA | None |
| 7 | Open PN | Flank | 100 | 23 | 100 | 3A/1V | Thin parenchymatous | None |
| 8 | Open PN | Vertical midline | 149 | None | 400 | 3A/2V | Thin fibrous | None |
| 9 | Open PN | Flank | 244 | None | 2000 | 3A/1V | NA | Urinoma (3a), wound infection (3a), UTI (2) |
A, artery; NU, nephroureterectomy; PN, partial nephrectomy; UTI, urinary tract infection; V, vein.
Median hospital length of stay following the procedure was 2.5 days (IQR: 2–3.25). Three patients who underwent partial nephrectomy developed postoperative complications: 1 had postoperative pneumothorax treated with continuous chest tube drainage (grade 3b); 1 had bilateral atelectasis treated with supportive measures (grade 1); and 1 patient had a delayed urinoma that required percutaneous drainage (grade 3a), a wound infection requiring local incision, drainage, and wound care (grade 3a), and a urinary tract infection requiring antibiotic treatment (grade 2). One patient who underwent open nephroureterectomy had a paralytic ileus treated with nasogastric tube insertion (grade 1). Median follow-up time was 19.2 months (IQR: 3–34.8). None of the patients who underwent partial nephrectomy had a disease recurrence at a median follow-up of 19.2 months (IQR: 2.5–33.2). One patient with urothelial carcinoma had a disease recurrence within the bladder 2.4 months after the operation. The same patient died 3.5 months after the operation; however, the cause of death was unknown.
Prior to the operation, 1 patient had an eGFR < 60 mL/min/1.73 m2 and was diagnosed with chronic kidney disease. None of the remaining 8 patients developed new-onset chronic kidney disease at a median follow-up of 2.4 months (IQR: 0.9–26.3) during which eGFR data were available. Median change in eGFR for patients treated with partial nephrectomy was −7 mL/min/1.73 m2 (IQR: −13, 10.7) at a median follow-up of 1.4 months (IQR: 0.7, 22.7). Changes in eGFR for the 2 patients who underwent nephroureterectomy were −14.1 mL/min/1.73 m2 and −16 mL/min/1.73 m2 at 2.4 and 34.8 months of follow-up, respectively.
COMMENT
In the current study we described the surgical treatment of 9 patients with tumors involving kidneys with fusion anomalies. In this contemporary cohort, all patients with renal cortical tumors were treated with open partial nephrectomy. Whereas median operative time and estimated blood loss were comparable to previous series of partial nephrectomy, overall and major complication rates were higher. Vascular anomalies were present in nearly 90% of patients, emphasizing the importance of preoperative planning with CT angiograms to avoid major bleeding complications.
Tumors involving kidneys with fusion anomalies are identified in 5% to 13% of patients with these anomalies.1,4 Compared to normal kidneys, kidneys with fusion anomalies have a higher risk of developing nephroblastomas, and the risk of developing urothelial carcinoma is 3–4 times higher, possibly due to embryopathogenic mechanisms or urinary stasis.4 However, the most common tumor reported in kidney fusion anomalies is renal cell carcinoma, which is apparent in 40% to 50% of cases.4–6 Similarly, in the current series 56% of patients had renal cell carcinoma. The majority of patients in earlier series presented with local symptoms including lumbar and abdominal pain, hematuria, and a palpable abdominal mass.4,6 In the current series, all renal cell carcinoma cases were identified incidentally and were confined to the kidney at the time of diagnosis. Patients with upper tract urothelial carcinoma presented with hematuria.
Accessory vessels with an anomalous origin are common among patients with fusion anomalies of the kidney. In a systematic study by Graves, 6 basic patterns of arterial blood supply to the HK were described.7 Many variations exist to these classic configurations, however, and the arterial distribution may be asymmetrical. Furthermore, the isthmus may be supplied by cranial or caudal vessels on 1 or both sides.8 In a study by Boatman et al, only 36% of patients with an HK had an arterial supply that fit the classic configurations described by Graves.8 In a recent study by Glodny et al, only 5% of patients with HK or CFE had 1 artery on each side. The average numbers of arteries supplying the right and left hemi-kidney were 2.4 and 1.9, respectively, and the numbers of veins were 2.4 and 1.7, respectively. Moreover, the vascular supply could not be classified due to its variability.1 In the current study, accessory blood vessels were observed in 8 of 9 patients (89%), with an average number of arteries and veins comparable to previous reports (2.3 and 1.4. respectively), emphasizing the importance of identifying variations in blood supply prior to the operation. Most patients with an HK (96%) and all patients with a CFE have an isthmus that consists of renal parenchyma. Only 4% of patients with HK have an isthmus consisting of connective (fibrous) tissue.1 All but 1 of the isthmi in the current study were parenchymatous. One patient underwent surgery for a lower pole tumor abutting the isthmus.
Most previous reports describing the surgical treatment of tumors involving HKs were case reports of single individuals. Rubio Briones et al reported the largest series of patients surgically treated for a tumor involving an HK. The authors described 10 patients treated by open radical nephrectomy and nephroureterectomy between the years 1967 and 1996. Vascular anomalies were apparent in 70% of the patients, complicating the surgery and leading to an average blood loss of 2195 mL. Prognosis was related to the tumor grade and stage, and 60% of patients were free of disease at an average follow-up period of 55 months. Renal function was reported to be normal during follow-up.4 Another series, by Stimac et al, included 4 patients with HK and 1 with CFE who were operated on between 1993 and 2002. All but 1 patient underwent radical heminephrectomy of the involved moiety, with isthmus resection and lymphadenectomy. One patient, with borderline renal function, underwent resection of the tumor involving the isthmus with preservation of both renal moieties. All operations were performed with a transperitoneal approach using a median laparotomy or chevron incision. During an average follow-up of 30 months, 4 of 5 patients were alive and disease free, with normal renal function. One patient with urothelial carcinoma died of metastatic disease 6 months after the operation.6
The feasibility of partial nephrectomy for the treatment of tumors involving HKs was initially reported in several case studies.11,13 Recently, Yecies et al reported a series of 8 patients with tumors involving HKs, who were treated with either heminephrectomy or partial nephrectomy. All operations but 1 were performed with an open surgical approach. Two patients required blood transfusion during the perioperative period; however, data regarding intraoperative blood loss was lacking. One patient developed a urine leak that required invasive treatment. Despite the increased surgical complexity, negative surgical margins were obtained in all procedures. Data regarding changes in renal function were limited to the immediate perioperative period; median change in eGFR was −6 mL/min/1.73m2. At a median follow-up of 38.5 months, cancer-specific survival was 87.5%.10 These findings suggest that partial nephrectomy for the treatment of tumors involving HKs may lead to good oncologic and functional results. In the current study, all 5 patients with renal cell carcinoma, as well as 1 patient with an oncocytoma and 1 patient with a well-differentiated liposarcoma involving the kidney, were treated with partial nephrectomy. Median estimated blood loss was 400 mL; however, 3 patients had an estimated blood loss of more than 1 liter, 2 of which required infusions of packed red blood cells. Overall and major complication rates of patients treated with partial nephrectomy in the current series were 43% and 29%, respectively (Table 3). In a previous report from our institution, partial nephrectomy for kidneys without an anomaly was associated with a median estimated blood loss of 350 mL and low overall and major complication rates of 19% and 4%, respectively.22 The complication rates of partial nephrectomy in the current series were substantially higher, likely the result of the surgical complexity associated with the treatment of tumors involving kidneys with fusion anomalies as represented by the relatively high nephrometry scores of these tumors. Two patients with urothelial carcinoma underwent radical heminephrectomy and ureterectomy with excision of the bladder cuff as recommended for upper tract urothelial carcinoma.23 One patient with urothelial carcinoma had a recurrence of the tumor in his bladder. None of the patients had new-onset chronic kidney disease.
Table 3.
Patient and treatment characteristics in series of patients with tumors involving kidneys with fusion anomalies
| Series | Kidney Anomaly |
Presenting Symptoms |
Operation Type |
Median Operative Time (min) |
Patients With Vascular Anomalies |
Estimated Blood Loss (mL) |
Tumor Pathology | Patients With Postoperative Complications |
Functional Outcome |
Survival Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Rubio Briones et al4 | 10 HK | 9 local (90%); 1 incidental (10%) | 8 RN (80%); 2 NU (20%) | NA | 7 (70%) | 2195 (mean); 500–7000 (range) | 5 RCC (50%); 4 UC (40%); 1 Wilms’ tumor (10%) | NA | Patients alive at follow-up have normal renal function | Median FU—36.5 months, CSS—70%, OS—60% |
| Stimac et al6 | 4 HK; 1 CFE | 5 local (100%) | 3 RN (60%); 1 NU (20%); 1 PN (20%) | NA | NA | NA | 2 RCC (40%); 2 UC (40%); 1 oncocytoma (20%) | NA | Patients alive at follow-up have normal renal function | Median FU—12 months, CSS and OS—80% |
| Yecies et al10 | 8 HK | NA | 8 RN or PN* | 102 (IQR, 92–357) | NA | NA; 2 patients (25%) required blood transfusion | 7 RCC (88%); 1 carcinoid tumor (12%) | 1 overall (13%), 1 major (13%) | Median change in eGFR −6 mL/min/1.73 m2, none required renal replacement† | Median FU—38.5 months, CSS—87.5%, OS—62.5% |
| Current series | 7 HK; 2 CFE | 3 local (33%); 6 incidental (67%) | 7 PN (78%); 2 NU (22%) | 157 (IQR, 144–267) for PN; 229 (range 40–417) for NU | 8 (89%) | 400 (IQR, 200–1500) for PN; 200 (range 100–300) for NU | 5 RCC (56%); 2 UC (22%); 1 oncocytoma (11%); 1 liposarcoma (11%) | 3 overall (43%), 2 major (29%) for PN; 1 overall (50%), 0 major for NU | Median change in eGFR −7 mL/min/1.73 m2 for PN and −15.1 mL/min/1.73 m2 for NU; no new onset of CKD | Median FU—19.2 months CSS—100%, OS—89% |
CKD, chronic kidney disease; CSS, cancer-specific survival; eGFR, estimated glomerular filtration rate; FU, follow-up; IQR, interquartile range; OS, overall survival; RN, radical nephrectomy. Categorical variables are presented as number and percent, and continuous variables as median and interquartile range or mean and standard deviation
All patients underwent either partial nephrectomy or heminephrectomy. Exact number of patients undergoing each type of procedure was not mentioned.
There were 3 of 7 patients (43%) who had a preoperative eGFR > 60 mL/min/1.73 m2 that had a decline in eGFR at discharge to less than 60 mL/min/1.73 m2.
The current study serves to further establish the role of partial nephrectomy for the treatment of renal cortical tumors involving kidneys with fusion anomalies. In addition, the findings emphasize the importance of reviewing preoperative imaging studies to identify accessory blood vessels and minimize blood loss. The limitations of the study include its retrospective nature and the small cohort size. Due to the rare incidence of tumors involving kidneys with fusion anomalies, it is unlikely that any single institution will be able to report a large cohort of similar patients. Therefore, multicenter studies are required to better define the optimal treatment and outcome of these patients.
CONCLUSION
Tumors involving kidneys with fusion anomalies are a rare occurrence. Patients with presumed renal cortical tumors may be treated with partial nephrectomy, when feasible, with limited blood loss and preservation of renal function. However, overall (43%) and major (29%) complication rates were relatively high. Prior to the operation, it is important to review imaging studies, as most cases (89%) have aberrant and accessory blood vessels. Furthermore, identification of tumors involving the isthmus and appreciation of the aberrant renal anatomy are important for planning the surgical approach.
Supplementary Material
Acknowledgments
Funding Support: This study is supported by the Sidney Kimmel Center for Prostate and Urologic Cancers. Grant support is from the Stephen P. Hanson Family Fund Fellowship in Kidney Cancer. This research was also funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
APPENDIX: SUPPLEMENTARY DATA
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.urology.2016.07.034.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Glodny B, Petersen J, Hofmann KJ, et al. Kidney fusion anomalies revisited: clinical and radiological analysis of 209 cases of crossed fused ectopia and horseshoe kidney. BJU Int. 2009;103:224–235. doi: 10.1111/j.1464-410X.2008.07912.x. [DOI] [PubMed] [Google Scholar]
- 2.Weizer AZ, Silverstein AD, Auge BK, et al. Determining the incidence of horseshoe kidney from radiographic data at a single institution. J Urol. 2003;170:1722–1726. doi: 10.1097/01.ju.0000092537.96414.4a. [DOI] [PubMed] [Google Scholar]
- 3.Kolln CP, Boatman DL, Schmidt JD, Flocks RH. Horseshoe kidney: a review of 105 patients. J Urol. 1972;107:203–204. doi: 10.1016/s0022-5347(17)60983-2. [DOI] [PubMed] [Google Scholar]
- 4.Rubio Briones J, Regalado Pareja R, Sanchez Martin F, Chechile Toniolo G, Huguet Perez J, Villavicencio Mavrich H. Incidence of tumoural pathology in horseshoe kidneys. Eur Urol. 1998;33:175–179. doi: 10.1159/000019551. [DOI] [PubMed] [Google Scholar]
- 5.Buntley D. Malignancy associated with horseshoe kidney. Urology. 1976;8:146–148. doi: 10.1016/0090-4295(76)90344-7. [DOI] [PubMed] [Google Scholar]
- 6.Stimac G, Dimanovski J, Ruzic B, Spajic B, Kraus O. Tumors in kidney fusion anomalies—report of five cases and review of the literature. Scand J Urol Nephrol. 2004;38:485–489. doi: 10.1080/00365590410018684. [DOI] [PubMed] [Google Scholar]
- 7.Graves FT. The arterial anatomy of the congenitally abnormal kidney. Br J Surg. 1969;56:533–541. doi: 10.1002/bjs.1800560717. [DOI] [PubMed] [Google Scholar]
- 8.Boatman DL, Cornell SH, Kolln CP. The arterial supply of horseshoe kidneys. Am J Roentgenol Radium Ther Nucl Med. 1971;113:447–451. doi: 10.2214/ajr.113.3.447. [DOI] [PubMed] [Google Scholar]
- 9.Lee CT, Hilton S, Russo P. Renal mass within a horseshoe kidney: preoperative evaluation with three-dimensional helical computed tomography. Urology. 2001;57:168, vi–ix. doi: 10.1016/s0090-4295(00)00857-8. [DOI] [PubMed] [Google Scholar]
- 10.Yecies T, Turner RM, Ii, Ferroni MC, Jacobs BL, Davies BJ. Partial and hemi-nephrectomy for renal malignancy in patients with horseshoe kidney. Can J Urol. 2016;23:8156–8159. [PubMed] [Google Scholar]
- 11.Tsivian A, Shtricker A, Benjamin S, Sidi AA. Laparoscopic partial nephrectomy for tumour excision in a horseshoe kidney. Eur Urol. 2007;51:1132–1133. doi: 10.1016/j.eururo.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 12.Bhayani SB, Andriole GL. Pure laparoscopic radical heminephrectomy and partial isthmusectomy for renal cell carcinoma in a horseshoe kidney: case report and technical considerations. Urology. 2005;66:880, e5–e6. doi: 10.1016/j.urology.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 13.Lee YS, Yu HS, Kim MU, et al. Retroperitoneoscopic partial nephrectomy in a horseshoe kidney. Korean J Urol. 2011;52:795–797. doi: 10.4111/kju.2011.52.11.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer BW, Strom K, Wong C. Hand-assisted laparoscopic nephroureterectomy with cystoscopic en-bloc excision of the distal ureter and bladder cuff and isthmusectomy in a horseshoe kidney for invasive urothelial carcinoma of the renal pelvis. JSLS. 2011;15:412–414. doi: 10.4293/108680811X13125733357197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim TH. Renal cell carcinoma in a horseshoe kidney and preoperative superselective renal artery embolization: a case report. Korean J Radiol. 2005;6:200–203. doi: 10.3348/kjr.2005.6.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilhelmsen S, Janitzky A, Porsch M, Liehr UB, Dudeck O. Value of preoperative superselective embolization of the isthmus in a patient with upper urinary tract urothelial carcinoma and horseshoe kidney. Cardiovasc Intervent Radiol. 2011;34(suppl 2):S98–S101. doi: 10.1007/s00270-009-9789-y. [DOI] [PubMed] [Google Scholar]
- 17.Husillos-Alonso A, Bueno-Chomon G, Lledo-Garcia E, Subira-Rios D, Ramon-Botella E, Hernandez-Fernandez C. First percutaneous computed tomography-guided radiofrequency ablation of renal tumor in horseshoe kidney. Urology. 2011;78:466–468. doi: 10.1016/j.urology.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephenson AJ, Hakimi AA, Snyder ME, Russo P. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol. 2004;171:130–134. doi: 10.1097/01.ju.0000101281.04634.13. [DOI] [PubMed] [Google Scholar]
- 23.Roupret M, Babjuk M, Comperat E, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


