Abstract
Action-potential-induced LTD (AP-LTD) is a form of synaptic plasticity that reduces synaptic strength in CA1 hippocampal neurons firing antidromically during sharp-wave ripples. This firing occurs during slow-wave sleep and quiet moments of wakefulness, which are periods of offline replay of neural sequences learned during encoding sensory information. Here we report that rapid and persistent down-regulation of different mRNA transcripts of the BDNF gene accompanies AP-LTD, and that AP-LTD is abolished in mice with the BDNF gene knocked out in CA1 hippocampal neurons. These findings increase understanding of the mechanism of APLTD and the cellular mechanisms of memory consolidation.
Keywords: LTD, BDNF, Slow wave sleep, antidromic action potentials, sharp wave ripple complexes
Pyramidal neurons in the CA1 region of hippocampus fire antidromically (from the axon into the cell body and dendrites) during brief bursts of high-frequency oscillations (100-300 Hz) termed sharp wave ripple complexes (SPW-R) [1, 2]. Antidromic action potential firing in these neurons in the absence of excitatory synaptic input induces a form of synaptic plasticity that reduces the strength of synapses in a cell-wide manner, termed action potential-induced long-term depression (AP-LTD) [3]. Mechanisms known to promote antidromic firing during SPWRs facilitate AP-LTD induction [3]. Although AP-LTD has not yet been studied in vivo, the phenomenon appears relevant to memory consolidation because SPW-Rs occur during slow-wave sleep (SWS) and quiet periods of wakefulness. These behavioral states are characterized by decreased sensory input and offline replay of neural sequences learned during encoding sensory information [4, 5], and disrupting SPW-Rs impairs memory retention [6, 7]. Compared to other forms of hippocampal synaptic plasticity, the mechanisms of AP-LTD are relatively unexplored. Here the possible involvement of brain-derived neurotrophic factor (BDNF), a protein involved in several forms of synaptic plasticity, is examined in relation to synaptic plasticity induced by antidromic action potentials.
AP-LTD is an unusual form of synaptic plasticity because synaptic activation is not necessary to initiate the depression in synaptic strength, and therefore the plasticity is not synapse-specific. Instead, the mechanism for AP-LTD induction is independent of glutamatergic receptor activation, but it is dependent on ectopic action potential firing via TTX-sensitive sodium channels in axons that are activated by action potential backpropagation into the soma and dendrites. Intracellular calcium signaling is necessary for AP-LTD, as shown by blocking AP-LTD in the presence an inhibitor of L-type voltage-dependent Ca2+ channels (L-VDCC) during antidromic action potential firing [3].
Our hypothesis was that BDNF mRNA levels would be reduced during AP-LTD in parallel with the reduction in synaptic strength; however, the involvement of BDNF in synaptic plasticity is complex. The BDNF gene contains at least nine differentially regulated promoters and multiple untranslated 5’ exons alternatively spliced to one protein-coding 3’ exon [8, 9]. Because multiple transcripts may be obtained from the BDNF gene, with potential subcellular differences and distinct temporal profiles in response to neuronal activity, we measured changes in abundance of the most prominently expressed BDNF transcripts containing exon I, exon II, exon IV and total (full-length) BDNF mRNA [9]. As background support for our hypothesis, previous research has shown that exogenous BDNF promotes long-term potentiation (LTP) in hippocampal slices [10], enhances basal synaptic strength in response to low-frequency stimulation [11], and lowers the threshold of stimulation required for LTP induction [12], while synaptically-induced LTD is prevented by BDNF application [13-15], and blockade of BDNF signaling turns chemically-induced LTP into LTD [16].
The results show that AP-LTD is accompanied by a rapid reduction in BDNF mRNA abundance, with different exons showing different levels of regulation and different temporal responses, and that AP-LTD is impaired in mice with deletion of BDNF restricted to the CA1 region. Together these results indicate that this form of synaptic plasticity, which is associated with SPW-Rs, requires BDNF downregulation.
Methods
Experimental animals
All experiments were conducted in accordance with animal study protocols approved by the National Institutes of Child Health and Human Development Animal Care and Use Committee. For RT-PCR experiments hippocampal slices were prepared from adult (7 to 10-week-old) male Sprague-Dawley rats. For electrophysiological recordings hippocampal slices were prepared from adult male BDNF knockout and wild-type mice (8 to 10-week-old). Floxed BDNF gene mice [17] were used to generate mice with a CA1-restricted deletion of BDNF by crossing with a line expressing Cre recombinase in the CA1 region under the control of the calcium/calmodulin kinase II promoter [18]. The presence of Cre and floxed BDNF alleles was determined as previously described [19].
Stimulation protocols and tissue collection for mRNA
Hippocampal slices (400 μm) were prepared from Sprague-Dawley rats, BDNF KO or BDNF WT mice as described [3]. Field excitatory postsynaptic potentials (fEPSPs) in the CA1 stratum radiatum were recorded as described [3, 20]. Each slice was examined for its capacity to generate field EPSPs of at least 1 mV at a stimulus intensity of 40-70 μA and slices not meeting this criterion were discarded. The stimulation strength was in the range of 40-70 μA to evoke orthodromic fEPSPs (referred to as test EPSPs) to an amplitude of 50% of the subthreshold maximum. Antidromic stimulation was delivered concomitantly by two stimulating electrodes placed in the alveus/stratum oriens in the CA1 region to stimulate the maximal number of cells. The stimulus intensity for the antidromic stimulation was 100-130 μA to evoke a population spike of about 1 mV recorded in stratum pyramidale. Repetitive theta-burst stimulation (TBS) was applied antidromically with the second stimulating electrode to the alveus/stratum oriens in the CA1 in the presence of glutamate receptor blockers: AMPA/kainate receptor antagonist kynurenic acid (3 mM), NMDA receptor antagonist, AP5(50 μM), and group I/group II metabotropic glutamate receptor antagonist (RS)-MCGP (250 μM) for 25-35 min. Stimulation of the alveus may also stimulate cholinergic axons [21] but kynurenic acid at the concentration used also acts as an antagonist of α7 nicotinic acetylcholine receptors [22]. After cessation of stimulation, ACSF containing the drug cocktail was washed away and test stimuli delivered to assess changes in EPSP amplitude. TBS consisted of 10 stimulus bursts delivered at 5 Hz. Each burst consisted of 4 pulses delivered at 100 Hz. The duration of each pulse was 0.2 ms. Three TBSs were applied every 30 s and repeated three times in 5 minute intervals.
For experiments presented in Fig.3, L-VDCC agonist BayK 8644 (10 μM) was bath applied 10 min after start of perfusion with glutamate antagonists to non-stimulated slices. We demonstrated previously induction of long-lasting synaptic depression after application of BayK8644 and blockade of AP-LTD by nifedipine, when applied at the same concentrations (Fig. 3 in [3]).
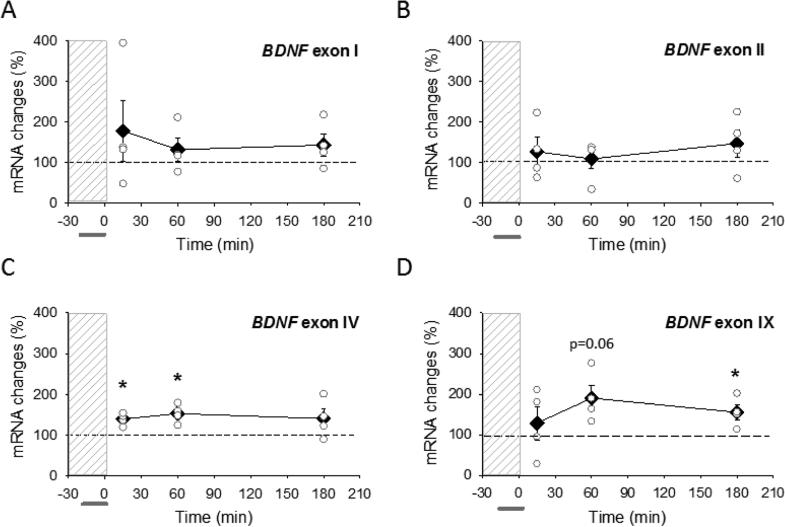
Fig. 3.
Activation of L-type VDCC is not sufficient to decrease BDNF mRNA. (A-D) Abundance of BDNF mRNAs in CA1 region of hippocampus at 15 min, 60 min and 180 min after chemically induced LTD. BayK 8644 was applied (horizontal bar) to non-stimulated slices during perfusion with glutamatergic antagonists (dashed vertical bar). Abundance of BDNF transcripts containing exon I, exon II, exon IV and exon IX BDNF are expressed as percent of values from time-matched control, unstimulated slices and normalized to the housekeeping gene GAPDH. Data show means ± SEM (filled diamonds) and individual experimental data (open circles). *p < 0.05, t-test.
Hippocampal slices were collected 15, 60 and 180 min after stimulation and snap frozen. To minimize potential masking effects from other hippocampal regions, the CA1 region from each slice was quickly microdissected and stored at −80° C. Matched control slices, which had not been stimulated, were collected at the same time, from the same recording chamber.
Semi-quantitative real-time RT-PCR
RNA was extracted from hippocampal slices using TRIzol® reagent (Invitrogen, Carlsbad, CA). Total RNA (2 μg) was reverse transcribed with Superscript II, as previously described [23]. Semi-quantitative, real-time PCR was performed on a Roche LightCycler using the Faststart DNA Master SYBR Green 1 PCR reaction mix (Roche Diagnostics, Indianapolis, IN), essentially as described previously.
Data analysis was performed as described [23]. Data were analyzed by the 2(-delta detla C (T)) method of analysis of RT-PCR data with respect to an untreated control as described by Livak and Schmittgen [24].
The following primer sequences were used:
| BDNF exon 1 FP | AAGACACTGAGTCTCCAGGAC |
| BDNF exon 2c FP | TATCTCCAGGATCTAGCCACC |
| BDNF exon 4 FP | AGCAGCTGCCTTGATGTTTAC |
| BDNF exon 9 FP | AGCAAACGTCCACGGACAAG, |
| BDNF exon RP | TTGTCCGTGGACGTTTGCTTC, |
| GAPDH FP | AATGCATCCTGCACCACCAAC |
| GAPDH RP | TGGATGCAGGGATGATGTTCTG |
Statistical analysis
PCR data were analyzed statistically by the method of 2(-Delta Delta C(T)) [24], to compare mRNA expression levels relative to an unstimulated control. Control samples were unstimulated in the same recording chamber as slices that received electrical stimulation, and collected at the same time point. Student's two-tailed, one-sample t-test was used to assess statistical significance between the matched experimental and control slices at the same time point using Sigma Plot 10.0 software (SPSS, Chicago, IL, USA) and Minitab software (version 5, Minitab Inc., State College PA). Values were expressed as percent of control, with variance for the controls estimated from the variance of the experimentals. All data points are presented in the graphs and summary data and statistical results are provided in Table 1 as mean ± SEM (standard error of mean).
Table 1.
BDNF mRNA regulation
| stimulation | time | BDNF exon | mean±SEM | N | t value | P value | significance |
|---|---|---|---|---|---|---|---|
| Antidromic | 15’ | exon I | −43.5±7.1 | 4 | 6.28 | 0.008 | ** |
| exon II | −20.4±7.2 | 4 | 2.96 | 0.059 | * | ||
| exon IV | −8.2±6.1 | 4 | 1.50 | 0.230 | |||
| exon IX | −31.9±12.8 | 4 | 2.58 | 0.082 | |||
| 60’ | exon I | −62.7±6.6 | 4 | 9.63 | 0.002 | ** | |
| exon II | −46.4±10.7 | 4 | 4.41 | 0.022 | * | ||
| exon IV | −22.3±12.1 | 4 | 1.92 | 0.151 | |||
| exon IX | −2.0±22.1 | 4 | 0.14 | 0.9 | |||
| 180’ | exon I | −15.8±18.6 | 5 | 0.90 | 0.419 | ||
| exon II | −17.7±11.0 | 5 | 1.70 | 0.165 | |||
| exon IV | 4.4±5.0 | 5 | 0.67 | 0.541 | |||
| exon IX | 7.4±10.5 | 4 | 0.62 | 0.582 | |||
| BayK8644 | 15’ | exon I | 78.5±75.3 | 4 | 1.03 | 0.379 | |
| exon II | 26.3±35.4 | 4 | 0.71 | 0.526 | |||
| exon IV | 38.5±7.5 | 4 | 4.96 | 0.016 | ** | ||
| exon IX | 28.7±41.5 | 4 | 0.67 | 0.553 | |||
| 60’ | exon I | 31.3±28.5 | 4 | 1.06 | 0.365 | ||
| exon II | 8.6±25.0 | 4 | 0.30 | 0.782 | |||
| exon IV | 52.2±11.4 | 4 | 4.48 | 0.021 | ** | ||
| exon IX | 90.5±30.8 | 4 | 2.90 | 0.062 | |||
| 180’ | exon I | 42.8±28.0 | 4 | 1.49 | 0.233 | ||
| exon II | 46.8±34.8 | 4 | 1.32 | 0.279 | |||
| exon IV | 39.5±23.6 | 4 | 1.63 | 0.201 | |||
| exon IX | 55.2±18.2 | 4 | 2.97 | 0.059 | * |
p≤0.05
p≤0.01; t-test, comparing to corresponding control, unstimulated slices. N = 1 represents pooling of experiments performed on 8-10 hippocampal slices combined for analysis of BDNF mRNA and an equal number of paired control slices.
Results
1. BDNF mRNA abundance after AP-LTD
To determine if stimulation that induces AP-LTD alters expression of BDNF mRNA, a semiquantitative RT-PCR assay was used to estimate the abundance of BDNF mRNA in isolated CA1 of stimulated and control unstimulated hippocampal slices. For this purpose, 5′-end forward primers were designed that are specific for BDNF exons I, IIc, IV, IX, and a 3′-end primer was generated that is complementary to sequences in exon IX, that is common to all BDNF transcripts, and used as reverse primer for all transcripts (Fig. 1A)
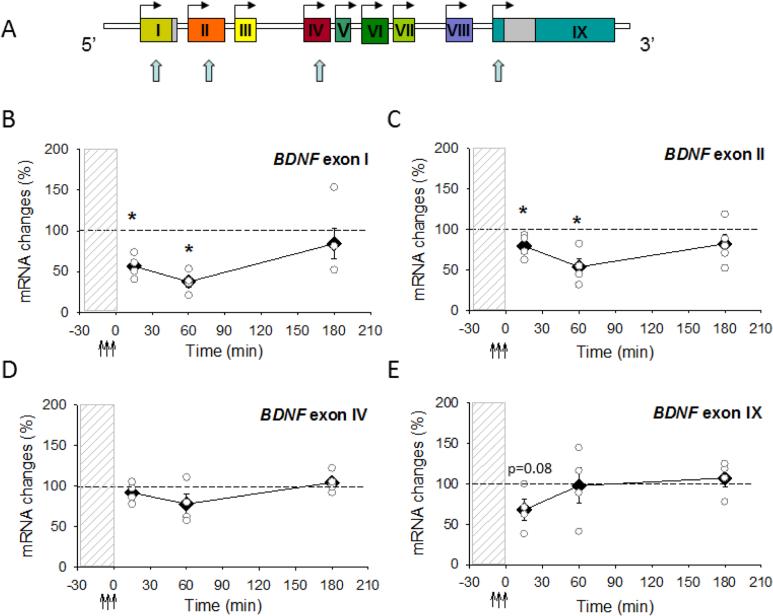
Fig. 1.
AP-LTD induction results in rapid changes in BDNF mRNA abundance. (A) Rat BDNF gene structure showing nine exons. In rodents BDNF transcripts containing exons I, II, IV and IX (blue arrows) are predominantly expressed. (B-E) Abundance of BDNF mRNAs at 15 min, 60 min and 180 min in CA1 region of hippocampus after induction of AP-LTD by antidromic stimulation (↑↑↑) in the presence of glutamatergic antagonists (dashed vertical bar). Abundance of BDNF transcripts containing exon I, exon II, exon IV and exon IX BDNF are expressed as percent of values from time-matched control, unstimulated slices and normalized to the housekeeping gene GAPDH. Data show means ± SEM (filled diamonds) and individual experiments data (open circles). *p < 0.05, t-test.
The results showed that different BDNF transcripts responded differently to antidromc stimulation. In agreement with our preliminary report [20], we found that expression of mRNAs bearing BDNF exon I decreased significantly very rapidly after AP-LTD (Fig. 1). Levels of BDNF exon 1 were reduced by 40% 15 min after AP-LTD stimulation relative to expression in control slices incubated simultaneously in the same recording chamber but unstimulated (Fig. 1B). A prominent decrease in BDNF transcripts containing exon II was also detected at this time point (Fig. 1C). Similarly to exon I and exon II, a 32% decrease in total BDNF mRNA (exon IX) was observed 15 min after AP-LTD (Fig. 1E). In contrast to other exons, expression of exon IV was not affected significantly by antidromic firing (Fig. 1D). The downregulation of BDNF mRNA transcripts persisted for at least 60 min for exon I and exon II containing transcripts (Fig. 1B, C). However, exon IV- containing BDNF transcript and total BDNF mRNA were not affected 60 and 180 min after stimulation to induce AP-LTD (Fig. 1D, E).
Together these results summarized in Table 1 show that BDNF mRNA transcripts containing exons I and II decrease rapidly, within 15 min after AP-LTD stimulation, and the reduction persists for a long period of time (at least 1 hr). Secondly, different BDNF transcripts respond differently to antidromic firing of action potentials. The most prominent effects are on exon I and exon II. Transcripts containing exon IV were not regulated. The reduction in BDNF expression induced by AP-LTD correlates with the decrease in synaptic responses produced by this antidromic stimulation [3].
2. Dependence of AP-LTD on BDNF mRNA
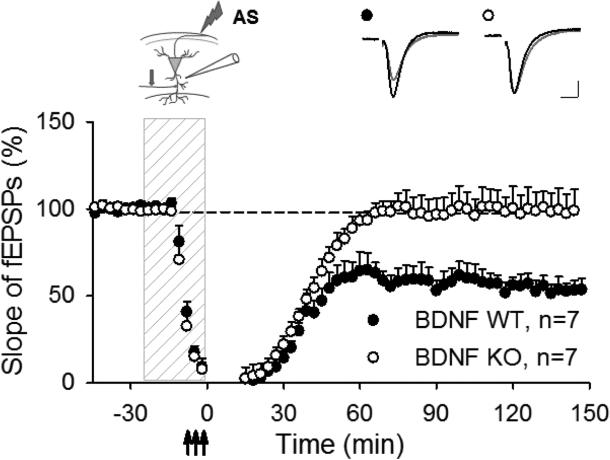
Our data showing that mRNA transcripts for several exons of the BDNF gene were decreased during AP-LTD suggests that down regulation of BDNF mRNA may be necessary for AP-LTD. To further explore the role of BDNF in synaptic changes induced by antidromic stimulation, we used mice in which the genetic deletion of BDNF was restricted only to CA1 neurons [10]. Repeated theta-burst stimulation (TBS) delivered antidromically to alveus in the presence of glutamatergic antagonists evoked robust AP-LTD in CA1 stratum radiatum of wild type mice (54.6±5.2 %, p < 0.001, t- test, comparing to pre-stimulated level, N=7, Fig. 2), consistent with our previous results in rat [3]. However AP-LTD was absent in hippocampal slices prepared from BDNF KO mice (99.3±11.1 %, p<0.01, t-test, comparing to BDNF WT mice, N=7; Fig. 2). These data demonstrate that BDNF gene expression in CA1 region is necessary for AP-LTD expression.
Fig. 2.
AP-LTD is dependent on BDNF gene expression in CA1. Antidromic stimulation delivered to the alveus during application of glutamatergic antagonists (dashed vertical bar) induced AP-LTD in stratum radiatum of wild type mice (WT), but absent in mice deficient in BDNF (KO). The inserts show electrode placement (arrow indicates position of the test stimulation electrode) and representative synaptic responses before (black) and after (grey) repetitive stimulation. Calibration: 0.25 mV, 5 ms.
3. BDNF regulation by activation of L-type VDCC
LTD can be induced chemically by incubation in the L-VDCC agonist Bay K 8644 [3]. Considering other studies showing Ca2+-dependent stimulation of BDNF transcription [25], together with our data indicating that AP-LTD also requires L-VDCC activation [3], we tested whether pharmacological activation of L-VDCC in the absence of antidromic stimulation decreased BDNF. BDNF mRNA levels after chemical induction of LTD showed the opposite response to that evoked by antidromic stimulation: a tendency for increased BDNF mRNA levels (Fig. 3). The increase in BDNF mRNA reached statistically significant levels at 15 and 60 min for exon IV (Fig. 3C), and at 180 min for total BDNF (Fig. 3D), but the increasing trends were not significant for exon 1 and II BDNF transcripts (Fig. 3A, B). In summary, these results show that different calcium-dependent signaling mechanisms operate to regulate BDNF and APLTD in response to pharmacological stimulation, orthodromic, and antidromic action potential firing.
Discussion
The results show that down regulation of BDNF mRNA abundance accompanies AP-LTD, and that expression of BDNF in CA1 of hippocampus is required for this form of synaptic plasticity, which is induced by antidromic firing in the absence of excitatory synaptic input. Given the well-established role of this neurotrophin in increasing synaptic strength, in counteracting LTD, and in promoting synaptogenesis and dendritic complexity, the rapid reduction of BDNF mRNA during AP-LTD could contribute to reducing synaptic strength and promote synaptic remodeling. Also, induction of LTP is impaired during SWS [26-27], and both LTD and loss of dendritic spines are thought to contribute to memory consolidation during sleep [28, 29]. Our findings are consistent with the proposal that AP-LTD acts as a mechanism to counteract LTP that develops in hippocampal circuits by synaptic activity during the wakeful state. In this way, AP-LTD would rescale synaptic weights downward in a cell-wide manner and thus prevent saturation of synaptic strength. In so doing, the down-scaling of synaptic strength following AP-LTD would then facilitate assimilation of new information after sleep. The loss of weaker synaptic inputs by AP-LTD would also promote formation of new functional assemblies of neurons that are more sharply tuned to preferred stimuli. The reduction in BDNF mRNA observed following AP-LTD is consistent with regulation of BDNF mRNA during sleep, which has been reported previously [30, 31].
The levels and activity of BDNF are modulated by several mechanisms, accounting for the multifaceted effects of this neurotrophin on synaptic plasticity. These mechanisms include activity-dependent effects on BDNF mRNA abundance and protein synthesis [32], secretion of BDNF from neurons [33], BDNF transcript and protein breakdown [32], post-translational modification from pro- to mature forms of BDNF [34], gene polymorphisms [35], epigenetic modifications [36] subcellular compartmentalization [37], interactions between BDNF signaling and other signaling pathways [38, 39]. Another mechanism can now be added to this list: down-regulation of BDNF mRNA by antidromic firing. Backpropagation of action potentials has been shown to stimulate secretion of BDNF from dendrites in a calcium-dependent manner, and spontaneous activity alone is not sufficient to do so [40]. The physiological and morphological changes in neurons mediated by dendritic BDNF secretion would be reduced by the reduction in BDNF transcripts shown in these experiments following AP-LTD.
In other studies, as was confirmed here, calcium influx into neurons stimulates transcription of BDNF, but in AP-LTD, the abundance of BDNF mRNA is reduced despite a rise in cytoplasmic calcium that accompanies action potential firing. Although this may appear contradictory, the apparent paradox can be resolved in several ways. The reduction in BDNF mRNA occurs rapidly, within 15 minutes, suggesting that mechanisms regulating mRNA stability and degradation are responsible for the reduction of BDNF transcripts during AP-LTD. Such mechanisms are likely exon-specific, accounting for the differences in reduction of exon I, II, III, and IV transcripts observed in AP-LTD. For example, miRNAs cause mRNA deadenylation, which promotes de-capping and more rapid degradation of transcripts [41]. It has been demonstrated that miRNA controlled degradation of mRNA transcripts accounts for most (>84%) of the decreased protein production. Although Ca2+ signaling activates BDNF transcription [25], Ca2+ signaling also influences miRNA and mRNA degradation. Thus, the abundance of BDNF mRNA depends upon both transcriptional regulation and mRNA degradation, and different signaling pathways are likely to be activated by orthodromic neuronal activity and antidromic activity.
Homeostatic controls are required to prevent a neural network from becoming incapacitated, and sleep is thought to be an important period for LTD and dendritic remodeling [29]. The present findings demonstrate that downregulation of BDNF mRNA accompanies APLTD. Considering the variety of pathological conditions and physiological processes influenced by BDNF mRNA levels, the findings could have broad neurological and pathophysiological significance, and advance understanding of the molecular mechanisms depressing synaptic strength as occurs in memory consolidation during SWS.
Highlights.
BDNF gene expression in hippocampal CA1 neurons is necessary for LTD induced by antidromic stimulation (AP-LTD).
BDNF mRNA transcripts are rapidly downregulated during AP-LTD.
Different BDNF mRNA transcripts (containing exons 1, II, IV, or IX) respond with distinct temporal profiles to antidromic stimulation.
In contrast to antidromic stimulation, BDNF mRNA transcripts increase in abundance after LTD is induced chemically by activation of L-VDCC.
Acknowledgements
We thank Jonathan Cohen for BDNF primer design and Alexei Morozov for providing BDNF floxed mice. This research was supported by funds for intramural research at NICHD, HD000713.
Abbreviations
- ACSF
Artificial cerebral spinal fluid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP-LTD
Antidromic action potential-induced LTD
- (DL)-AP5
2-Amino-5-phosphonopentanoic acid
- BDNF
Brain-derived neurotrophic factor
- CA1
Cornus ammonis region 1
- CaMKII
Calcium/calmodulin kinase II
- fEPSP
field excitatory postsynaptic potentials
- LTD
Long-term depression
- LTP
Long-term potentiation
- L-VDCC
L-type voltage-dependent calcium channel
- (RS)-MCPG
α-Methyl-4-carboxyphenylglycine
- NMDA
N-methyl-D-aspartate receptor
- RT-PCR
reverse transcription polymerase chain reaction
- TBS
theta burst stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bähner F, Weiss EK, Birke G, Maier N, Schmitz D, Rudolph U, Frotscher M, Traub RD, Both M, Draguhn A. Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc. Natl. Acad. Sci. U. S. A. 2011;108:607–616. doi: 10.1073/pnas.1103546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papatheodoropoulos C. A possible role of ectopic action potentials in the in vitro hippocampal sharp wave-ripple complexes. Neuroscience. 2008;157:495–501. doi: 10.1016/j.neuroscience.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Bukalo O, Campanac E, Hoffman DA, Fields RD. Synaptic plasticity by antidromic firing during hippocampal network oscillations. Proc. Natl. Acad. Sci. U.S.A. 2013;110:5175–5180. doi: 10.1073/pnas.1210735110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louie K, Wilson MA MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 6.Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 7.Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmusk T, Lendahl U, Funakoshi H, Arenas E, Persson H, Metsis M. Identification of brain-derived neurotrophic factor promoter regions mediating tissue-specific, axotomy-, and neuronal activity-induced expression in transgenic mice. J. Cell Biol. 1995;128:185–199. doi: 10.1083/jcb.128.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figurov A, D Pozzo-Miller L, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 11.Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 12.Huber KM, Sawtell NB, Bear MF. Brain-derived neurotrophic factor alters the synaptic modification threshold in visual cortex. Neuropharmacology. 1998;37:571–9. doi: 10.1016/s0028-3908(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 13.Akaneya Y, Tsumoto T, Hatanaka H. Brain-derived neurotrophic factor blocks long-term depression in rat visual cortex. J. Neurophysiology. 1996;76:4198–201. doi: 10.1152/jn.1996.76.6.4198. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita S, Yasuda H, Taniguchi N, Katoh-Semba R, Hatanaka H, Tsumoto T. Brain-derived neurotrophic factor prevents low-frequency inputs from inducing long-term depression in the developing visual cortex. J. Neurosci. 1999;19:2122–2130. doi: 10.1523/JNEUROSCI.19-06-02122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumura E, Kimura F, Taniguchi N, Tsumoto T. (2000) Brain-derived neurotrophic factor blocks long-term depression in solitary neurons cultured from rat visual cortex. J. Physiol. 2000;524:195–204. doi: 10.1111/j.1469-7793.2000.t01-2-00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montalbano A, Baj G, Papadia D, Tongiorgi E, Sciancalepore M. Blockade of BDNF signaling turns chemically-induced long-term potentiation into long-term depression. Hippocampus. 2013;10:879–889. doi: 10.1002/hipo.22144. [DOI] [PubMed] [Google Scholar]
- 17.Ito W, Ghehab M, Thakur S, Li J, Morozov A. BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. 2011;10:365–74. doi: 10.1111/j.1601-183X.2010.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsien JA, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–26. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 19.Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- 20.Lee PR, Cohen JE, Becker KG, Fields RD. Gene Expression in the Conversion of Early-Phase to Late-Phase Long-Term Potentiation. Annals of the New York Acad. Sci. 2005;1048:259–271. doi: 10.1196/annals.1342.023. [DOI] [PubMed] [Google Scholar]
- 21.Araque A, Martin ED, Perea G, Arellano JI, Buno W. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci. 2002;22:2443–50. doi: 10.1523/JNEUROSCI.22-07-02443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albuquerque EX, Schwarcz R. Kynurenic acid as an antagonist of alpha7 nicotinic acetylcholine receptors in the brain: facts and challenges. Biochem Pharm. 2013;85:1027–32. doi: 10.1016/j.bcp.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee PR, Cohen JE, Tendi EA, Farrer R, DE Vries GH, Becker KG, Fields RD. Transcriptional profiling in an MPNST-derived cell line and normal human Schwann cells. Neuron Glia Biol. 2004;1:135–147. doi: 10.1017/s1740925x04000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;4:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 26.Leonard BJ, McNaughton BL, Barnes CA. Suppression of hippocampal synaptic plasaticity during slow-wave sleep. Brain Res. 1987;425:174–7. doi: 10.1016/0006-8993(87)90496-3. [DOI] [PubMed] [Google Scholar]
- 27.Bramham CR, Srebro B. Induction of long-term depression and potentiation by low-and high-frequency stimulation in the dentate area of the anesthetized rat: magnitude, time course and EEG. Brain Res. 1987;405:100–107. doi: 10.1016/0006-8993(87)90994-2. [DOI] [PubMed] [Google Scholar]
- 28.Maret W, Faraguna U, Nelson AB, Cirelli C, Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci. 2011;14:1418–20. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–21. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 31.Martinowich K, Schloesser RJ, Jimenez DV, Weinberger DR, Lu B. Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol Brain. 2011;11:11. doi: 10.1186/1756-6606-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panja D, Bramham CR. (2014) BDNF mechanisms in late LTP formation: A synthesis and breakdown. Neuropharmacology. 2014;76:664–76. doi: 10.1016/j.neuropharm.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15788–15792. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q, Jing D, Lafrancois JJ, Bath KG, Mark W, Ballon D, Lee FS, Scharfman HE, Hempstead BL. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 2014;7:796–806. doi: 10.1016/j.celrep.2014.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizui T, Ishikawa Y, Kumanogoh H, Lume M, Matsumoto T, Hara T, Yamawaki S, Takahashi M, Shiosaka S, Itami C, Uegaki K, Saarma M, Kojima M. BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proc. Natl. Acad. Sci. U.S.A. 2015;112:3067–3074. doi: 10.1073/pnas.1422336112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ventskovska O, Porkka-Heiskanen T, Karpofa NN. Spontaneous sleepw-wake cycle and sleep deprivation differentially induce Bdnf1, Bdnf4, Bdnf9a DNA methylation and transcripts levels in the basal forebrain and frontal cortex in rats. J. Sleep. Res. 2015;24:124–30. doi: 10.1111/jsr.12242. [DOI] [PubMed] [Google Scholar]
- 37.Heise C, Gardoni F, Culotta L, di Luca M, Verpelli C, Sala C. (2014) Elongation factor-2 phosphorylation in dendrites and the regulation of dendritic mRNA translation in neurons. Front. Cell. Neurosci. 2014;8:35. doi: 10.3389/fncel.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues TM, Jeronimo-Santos A, Sebastiano AM, Diogenes MJ. Adenosine A(2A) receptors as novel upstream regulators of BDNF-mediated attenuation of hippocampal long-term depression (LTD). Neuropharmacology. 2014;79:389–98. doi: 10.1016/j.neuropharm.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Briz V, Liu Y, Zhu G, Bi X, Baudry M. A novel form of synaptic plasticity in field CA3 of hippocampus requires GPER1 activation and BDNF release. J. Cell Biol. 2015;210:1225–1237. doi: 10.1083/jcb.201504092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuczewski N, Porcher C, Ferrand N, Fiorentino H, Pellegrino C, Kolarow R, Lessmann V, Medina I, Gaiarsa JL. Backpropagating action potentials trigger dendritic release of BDNF during spontaneous network activity. J Neurosci. 2008;28:7013–23. doi: 10.1523/JNEUROSCI.1673-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]