Abstract
Attentional selection requires the interplay of multiple brain areas. Theoretical accounts of selective attention predict different areas with different functional properties to support endogenous covert attention. To test these predictions, we devised a demanding attention task requiring motion discrimination and spatial selection and performed whole-brain imaging in macaque monkeys. Attention modulated the early visual cortex, motion-selective dorsal stream areas, the lateral intraparietal area, and the frontal eye fields. This pattern of activation supports early selection, feature-based, and biased-competition attention accounts, as well as the frontoparietal theory of attentional control. While high-level motion-selective dorsal stream areas did not exhibit strong attentional modulation, ventral stream areas V4d and the dorsal posterior inferotemporal cortex (PITd) did. The PITd in fact was, consistently across task variations, the most significantly and most strongly attention-modulated area, even though it did not exhibit signs of motion selectivity. Thus the recruitment of the PITd in attention tasks involving different kinds of motion analysis is not predicted by any theoretical account of attention. These functional data, together with known anatomical connections, suggest a general and possibly critical role of the PITd in attentional selection.
SIGNIFICANCE STATEMENT Attention is the key cognitive function that selects sensory information relevant to the current goals, relegating other information to the shadows of consciousness. To better understand the neural mechanisms of this interplay between sensory processing and internal cognitive state, we must learn more about the brain areas supporting attentional selection. Here, to test theoretical accounts of attentional selection, we used a novel task requiring sustained attention to motion. We found that, surprisingly, among the most strongly attention-modulated areas is one that is neither selective for the sensory feature relevant for current goals nor one hitherto thought to be involved in attentional control. This discovery suggests a need for an extension of current theoretical accounts of the brain circuits for attentional selection.
Keywords: attention, fMRI, inferotemporal cortex, macaque monkey, motion
Introduction
The visual system is not a passive analyzer of sensory information. Rather momentarily important information can be selected at the expense of task-irrelevant information (Chun and Wolfe, 2001). This active selection process, attention, constitutes a critical link between the processing of information about the outer world and internal cognitive set. Therefore, to understand the neural mechanisms of attention, identification of the brain areas supporting attentional selection and their functional specificities is critical (Kanwisher and Wojciulik, 2000; Kastner and Ungerleider, 2000).
Here, to determine the sets of areas supporting endogenous selective attention, we use functional magnetic resonance imaging (fMRI) in the rhesus monkey, taking advantage of the extensive knowledge of functional specializations within its visual system (Felleman and Van Essen, 1991) and of single unit effects of attention in various cortical areas (Colby and Goldberg, 1999; Maunsell and Treue, 2006; Reynolds and Heeger, 2009) in the macaque monkey. The main task (Fig. 1A; see Materials and Methods), in brief, required subjects to select one of two random dot surfaces (RDSs) as the target and covertly track rapid-motion sequences within the target surface over extended periods of time (Fig. 1B). The task required attentive motion discrimination. When motion directions ceased changing on the target surface, subjects had to report the direction of motion by generating a saccade in the same direction. The task design emphasizes endogenous top-down covert spatial attention because (1) the task-relevant event was not a feature change that could capture attention exogenously (Mühlenen et al., 2005), (2) the rapid serial visual presentation (RSVP) design put the visual system under a high perceptual load, (3) attention had to be continuously deployed over seconds, and (4) attentional deployment was dissociated from saccade preparation. Combining the core features of the paradigm, motion processing, endogenous sustained attention, and dissociation of attention from motor intent, with imaging activity across the entire brain, we addressed three fundamental questions about the neural mechanisms of attention.
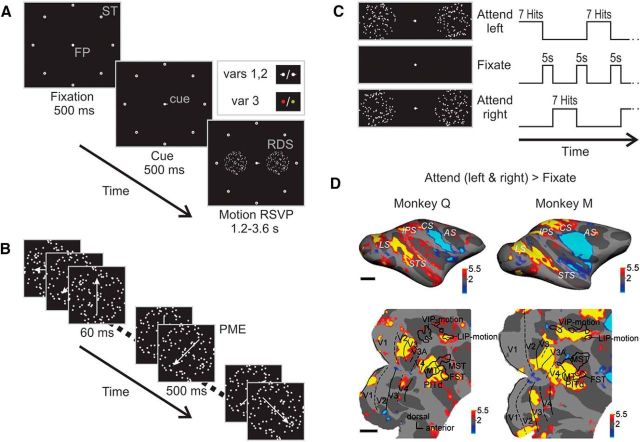
Figure 1.
Attentive motion-discrimination task: design and overall cortical activation pattern during active task performance. A, Stimulus sequence and configuration. Subjects initiated a trial by foveating the central fixation spot (FP), surrounded by eight saccade targets (ST). After a 500 ms delay, a cue appeared (shown is the spatial cue of task variants 1 and 2, extending from the fixation spot, pointing either left or right), indicating to which side attention had to be deployed. Inset shows the two cueing procedures: in task variants 1 and 2 (top), a spatial cue was used (bar to the left for attend left; bar to the right for attend right), in variant 3 (bottom), the target was cued symbolically by the color of the fixation point (monkey Q: red for left; green for right; monkey M: green for left; red for right). After 500 ms, two RDSs appeared to the left and the right of the fixation spot at equidistant positions. B, Event sequence of one of the RDSs. While both RDSs were changing their motion direction every 60 ms, the subject had to track the target stimulus for 20–60 direction changes until the translation direction ceased changing for 500 ms (PME) in monkey Q and 800 ms in monkey M, respectively, followed again by rapid direction changes. Monkeys were required to respond to the target surface PME by a saccade to the corresponding ST. C, Temporal sequence of behavioral conditions (attentive motion discrimination and passive fixation) during scanning. Monkeys alternated between paying attention to the left (L, top), passive fixation (F, center), and paying attention to the right (R, bottom) in sequence LFRFLFR. During the L and R blocks, subjects had to complete seven trials successfully (hits). Thus, block duration was variable, lasting on average ∼30 s. Passive fixation blocks lasting 5 s each separated the two attention conditions. D, Statistical parametric maps of contrast task versus fixation overlaid on the inflated (top) and flattened (bottom) right hemispheres of monkey Q (left) and monkey M (right). Yellow and red colored regions were significantly more activated by performance of the attentive motion-discrimination task than by passive fixation at p < 0.005, corrected for multiple comparisons, while cyan-blue regions were significantly less active during attention task performance. Activity in the somatosensory and primary motor cortex was reduced during active task performance. Scale bar, 1 cm. LS, Lunate sulcus; IPS, intraparietal sulcus; CS, central sulcus; AS, arcuate sulcus. Bottom, Same parametric maps overlaid on flattened posterior hemispheres. Dashed and solid black lines mark vertical and horizontal meridians, respectively. Motion-sensitive areas (black outlines) in the STS, MT, MST, and FST areas, and in the intraparietal sulcus, area VIP, and area LIP, mapped with a motion localizer (see Materials and Methods).
First, what is the locus of attentional selection? Selection has been proposed to occur early under high perceptual load (Tsal et al., 1994), and thus attentional modulation may occur as early as in area V1 even for spatially distant stimuli. On the other hand, competition between the neural ensembles representing target and distracter (Desimone and Duncan, 1995) or the match of stimulus and receptive field (RF) size (Luck and Hillyard, 2000; Hopf et al., 2006) have been proposed as the main determinants of attentional modulation. According to these theories, no competition in an area with small RFs, like V1, is expected, and attention effects are predicted to increase with increasing RF sizes along the visual processing hierarchy.
Second, is attentional modulation in this task specific to the motion-processing pathway? Theories of feature-based attention posit that attention can selectively recruit cortical areas (Corbetta et al., 1990; O'Craven et al., 1997) or even specific cell groups (Uka and DeAngelis, 2004) whose feature selectivities are most informative about the task-relevant features. Thus areas with large fractions of direction-selective cells (Zeki, 1974; Baizer, 1982; Desimone and Ungerleider, 1986; Orban et al., 1986; Galletti et al., 1990; Colby et al., 1993) should be modulated in an attentive motion-discrimination task.
Third, what areas are most involved in attentional selection? A frontoparietal network of areas is thought to endogenously control the “spotlight of attention” (Kastner and Ungerleider, 2000). In the macaque, both the lateral intraparietal area (area LIP; Gottlieb et al., 1998; Bisley and Goldberg, 2003) and the frontal eye fields (FEFs; Thompson et al., 2005) have been suggested to contain salience maps and should thus belong to the network responsible for guiding attention to task-relevant regions.
Materials and Methods
All procedures were performed at the Center for Advanced Imaging of Bremen University. They conformed to the National Institutes of Health Guide for Use and Care of Laboratory Animals, regulations for the welfare of experimental animals issued by the federal government of Germany, and stipulations of local Bremen authorities.
Subjects and surgery
Two male rhesus monkeys (6–10 kg) were used in this study. Before training and MR scanning, each monkey was implanted with a MR-compatible plastic head post (Ultem, General Electric Plastics) attached to the skull by ceramic screws (zirconium oxide, Thomas Recording) and dental cement (Grip Cement, Caulk, Dentsply International). All procedures followed standard anesthetic, aseptic, and postoperative treatment protocols, described in detail by Tsao et al. (2003) and Wegener et al. (2004).
Visual stimulation and tasks
Attentive motion-discrimination tasks. The main task was a motion-discrimination and tracking task in which animals had to foveate a central fixation spot (0.25° of visual angle) and covertly pay attention to one of two RDSs positioned on the horizontal meridian to the left and right of the central fixation spot (Fig. 1A). RDSs were presented in a circular aperture 6° in diameter and positioned 5° from the fixation spot on the horizontal meridian. Dot density of each surface was five dots per square degree of visual angle, translation velocity 7°/s. Eye position was monitored by an infrared pupil-tracking system (ETL-200, ISCAN). A trial started with an initial fixation period (Fig. 1A), followed by a cue period that indicated the target RDS that had to be attended during the subsequent motion RSVP period. The trial ended with the response of the subject (see below), after which all stimuli were removed from the screen and a juice reward was delivered.
In the first two variants of the task, the target RDS was cued by a short bar (0.35 × 0.06°) to the left or right side of the fixation spot (Fig. 1A). RDSs randomly changed translation direction (Fig. 1B) every 60 or 50 ms (monkey Q and M, respectively) in random multiples of 20°, until the translation direction ceased changing for up to 500 ms [the prolonged motion event (PME)] in monkey Q and up to 800 ms in monkey M, respectively, to be followed, when no response had occurred yet, by further rapid direction changes. The PME occurred at a random point in time after ≥20 and at most ≤60 brief motion events, independently in target and distracter stimulus sequence. Monkeys had to pay attention to the target motion sequence to both detect the occurrence of the PME and to discriminate its direction (in one of eight motion directions: 0, 45, 90, 135, 180, 225, 270, or 315°). For example, when the target RDS PME was to the lower left, a saccade had to be generated to the ST on the lower left. And this response was required independently of whether the left or right RDS was the target. Note (1) that STs did not overlap with the RDSs, (2) that the saccade direction was unknown to the subject during the trial until the occurrence of the PME, and (3) that saccade directions were independent of target surface location. Thus in the attentive motion-discrimination tasks, the location of sustained covert attention was dissociated from saccade preparation and generation. Monkeys had to report the motion direction of the PME by a saccade to one of eight peripheral saccade target (ST) dots positioned 8.5° from the fixation spot (Fig. 1A). ST window sizes (invisible) around the STs were 5° by 5° in size. A trial was rated successful if the animal initiated a response within 800 ms after target PME onset, and if the saccade reached the correct target directly, i.e., without passage through other response windows, in <500 ms. Ninety-two percent of saccades (of a total of 15,129) to the correct surface were completed within 100 ms, 98% within 200 ms. When the eye left the central fixation window, the two RDSs were switched off immediately. When the eyes reached the ST, all stimuli on the screen were switched off. Thus only direct saccades to the correct STs led to successful trial completion. Successful completion of a trial was rewarded with a drop of water or juice. Blocks of active task performance were interleaved with blocks of fixation during which only a fixation spot was presented and the monkeys were rewarded for keeping fixation.
In task variant one, blocks of attention task trials were separated by brief periods of passive fixation (Fig. 1C). Attention blocks lasted until seven trials were successfully completed. Fixation blocks were 5 s long. In task variant one, the sequence of blocks alternated between the two attention conditions (attend left and attend right) and a fixation condition, i.e., multiples of attend left–fixate–attend right–fixate (LFRF), etc. In task variant two, a neutral (N) condition was used in which the same RDSs as in active task blocks were shown. The neutral condition was interspersed such that the sequence of blocks was now LFNFRFNF. The neutral condition lasted until 3–6 trials were completed successfully. The fixation period lasted 10.5–30 s (same duration for a given scanning day). In variant three of the motion-discrimination task (Fig. 1A, inset), the bar cue was replaced by a symbolic cue, the color of the fixation spot. Stimulus configurations were otherwise identical to those in variants one and two. During the attention condition, green and red (32 cd/m2 each) indicated the target location (red cued the left side in monkey Q, green the right, and vice versa in monkey M), during the passive condition. The fixation spot was white (10 cd/m2).
Attentive motion-detection task.
In the attentive motion-detection task (see Fig. 5), the brief motion events were incoherent, with all dots moving in random directions. The occurrence of a coherent motion event (CME) of low coherence (10%) for ≤500 ms for monkey Q and for ≤800 ms for monkey M had to be detected and reported by a saccade onto the target surface. Thus, in contrast to the discrimination tasks, the detection task allowed monkeys to plan the saccade to the target surface they were paying attention to. The temporal sequence of events and all other task requirements were identical to those of the attentive motion-discrimination task. Detection (E) and discrimination (I) tasks were alternated within each run, thus facilitating direct comparison of attentional modulation across tasks. The sequence of tasks followed the sequence IFEF.
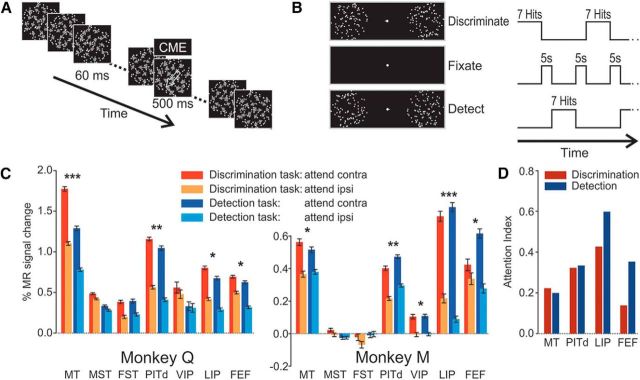
Figure 5.
Design of attentive motion-detection task and comparison of activation patterns during attentive discrimination and detection tasks. A, Event sequence of one of the RDSs. The sequence of events was identical to that in the motion-discrimination task (Fig. 1B), but motion during the brief 60 ms periods was incoherent, while motion during the task-relevant prolonged motion event was 10% coherent (CME). The duration of the CME was ≤500 ms for monkey Q and ≤800 ms for monkey M. Monkeys were required to respond to the target surface CME by a saccade onto the target surface, regardless of motion direction. B, Temporal sequence of behavioral conditions (attentive motion discrimination, passive fixation, and attentive motion detection) during scanning. The attentive motion-detection task (E) employed a spatial cue, rapid serial visual stimulus presentation, and alternated with the attentive motion-discrimination task (I) and passive fixation periods (F) in a sequence IFEFIFE. During attentive discrimination and attentive detection, monkeys had to complete seven trials successfully (hits). Thus, duration of these blocks was variable, on average ∼30 s. Passive fixation blocks separated the two attention paradigms and lasted 5 s. C, β Values for four attention conditions are shown as bar graphs. Significant activation differences between the two conditions attend contralateral (attend contra) and attend ipsilateral (attend ipsi) are indicated, when they occurred in both subjects, as follows: *p < 0.01 (ANOVA, multiple-comparison corrected); ***, most significant differences; **, differences with at least half the F value of the most significant difference. Because the occipital lobe was not covered by slices in these scans, early cortical areas could not be included in the analysis. D, AIs for the four ROIs with significant activation and attention modulation across animals and conditions. Areas MT and PITd show the same degree of modulation in both attention tasks, while area LIP and the FEF show stronger attentional modulation during motion-detection tasks than during motion-discrimination tasks. AIs in area PITd are very similar to those in Figure 3.
Monkeys first performed variant one of the motion-discrimination task, then both this variant and the motion-detection task, and only afterward they performed variant two of the motion-discrimination task.
Both monkeys were also trained on a passive fixation task, in which they were rewarded for maintaining fixation within the central fixation window. During the fixation task, various stimuli could be presented. The fixation task was used, without any further visual stimuli, as a control for the attentive motion-discrimination task, variant 1, and the motion-detection task. It also served as the basis for the localizer scans detailed below.
In all tasks, monkeys had to keep fixation inside a central fixation window of width 1.50° of visual angle for monkey Q, and 1.75° for monkey M, and height 2.00° for monkey Q and 2.75° for monkey M. Monkeys had been trained with a fixation window width of 0.75° and height of 1.75°. These window sizes were chosen to constrain eye movements while minimizing trial abortions due to fixation errors during the trials, which lasted several seconds.
Localizer experiments
To define visual cortical areas and estimate the direct stimulus impact on a given cortical location, we conducted the following localizer experiments on separate days. In all localizer experiments monkeys were rewarded for keeping their gaze within a 1.5° fixation window in intervals of 2.5–5 s.
Meridian mapping.
A retinotopic mapping procedure to define early visual areas was composed of two alternating 30 s blocks (V and H), separated by 30 s fixation periods with a gray background only. During block type V, a vertical black-and-white checkerboard wedge (20° width) was shown with 1 Hz contrast reversals; during block type H, a horizontal wedge was shown. The data were analyzed by contrasting blocks of vertical checkerboard wedges with blocks of horizontal checkerboards.
Center periphery mapping.
To effectively separate activations resulting from foveal stimuli (fixation spot and bar cue) from activations resulting from the RDSs, we modified the meridian mapping stimulus to alternate not between blocks of horizontal and vertical wedges, but between a foveal and a peripheral aperture displaying the checkerboard pattern, contrast inverting at 1 Hz frequency. The diameter of the foveal aperture was 1° (to encompass the spatial extent of the bar cue in the attention task); the diameter of the peripheral aperture was 6.5° (to encompass the spatial extent of the RDS in the attention task).
Motion mapping.
To localize motion-responsive areas, we used a set of motion stimuli adopted from Nelissen et al. (2006). Stimuli subtended the entire projection screen with a diagonal corresponding to 37.2° of visual angle. Blocks of 30 s of blank screen were alternated with blocks of different motion types of random dot patterns (50% white 0.2° dots on a black background; dot density was ∼10 dots per square degree). The different types were as follows: (1) slow translation with a velocity of 1°/s, (2) fast translation with a velocity of 8°/s, (3) rotation, (4) expansion and contraction, (5) static presentation, (6) opponent motion with a stripe width of 4°, and (7) opponent motion with a stripe width of 1°. For the first four motion types, motion direction changed with a frequency of 1 Hz to avoid adaptation. We scanned 20 complete runs, each containing all aforementioned stimulus conditions, in monkey M and 23 in monkey Q. Motion selectivity was assessed by contrasting activations during all motion conditions with the static condition.
Saccade mapping.
To map saccade-related activity and localize the FEF, we had both monkeys perform a guided saccade task: in alternating 30 s blocks, monkeys were required to either maintain fixation on a central target, or make saccades every 1.5 s to a new random location within a grid of 3 × 3 possible STs (grid spacing, 8.5°).
Stimulus presentation and behavioral monitoring and synchronization to scanner were achieved by custom written software, run on a PC under Windows XP. During scanning, stimuli were displayed at 75 Hz with a resolution of 1280 × 1024 pixels, using a video beamer (DLA-G15E, JVC) on a back projection screen, 49 cm in front of the monkey's eyes.
Scanning procedures
After performance of the attentive motion-discrimination task reached asymptote (after 6–8 months training), each monkey was scanned in a horizontal 3T MR head-scanner (Allegra, Siemens). A radial surface coil was positioned immediately over the monkey's head.
Each experiment consisted of 10–15 functional scans of 6–9 min each. Functional time series consisted of gradient-echoplanar whole-brain images: repetition time (TR) = 1.5 s or 3.0 s; echo time (TE) = 30 ms; 1.56 × 1.56 × 2 mm3 or 1.5 × 1.5 × 1.5 mm3 voxel size (24 transversal slices or 32 coronal slices). The effective field of view consisted of the entire macaque brain or was covered sequentially in independent sessions by partially overlapping slice positioning. In addition, for each subject, a high-resolution anatomical volume (3D-MPRAGE, 256 × 256 matrix, 128 slices, 0.5 × 0.5 × 0.5 mm3 voxel size) was obtained in the ketamine–medetomidine-anesthetized monkey. These anatomical MR data were used to generate inflated and flattened cortical representations for each subject using Freesurfer software (http://surfer.nmr.mgh.harvard.edu).
For all functional imaging of monkey M, a contrast agent, ferumoxtran-10 (Fe; Sinerem, Guerbet; concentration: 21 mg of Fe/ml in saline; dosage: 8 mg of Fe/kg), was injected into the femoral vein before each scan session. Sinerem is the same contrast agent as MION (monocrystalline iron-oxide monoparticle), produced under a different name (dextran-coated iron oxide agent; Vanduffel et al., 2001). To confirm independence of the main result of the use of a contrast agent, we measured the BOLD response (without contrast agent) in monkey Q for scans of the attentive motion-discrimination task (Figs. 1, 2). All other functional scans in monkey Q used Sinerem.
Figure 2.
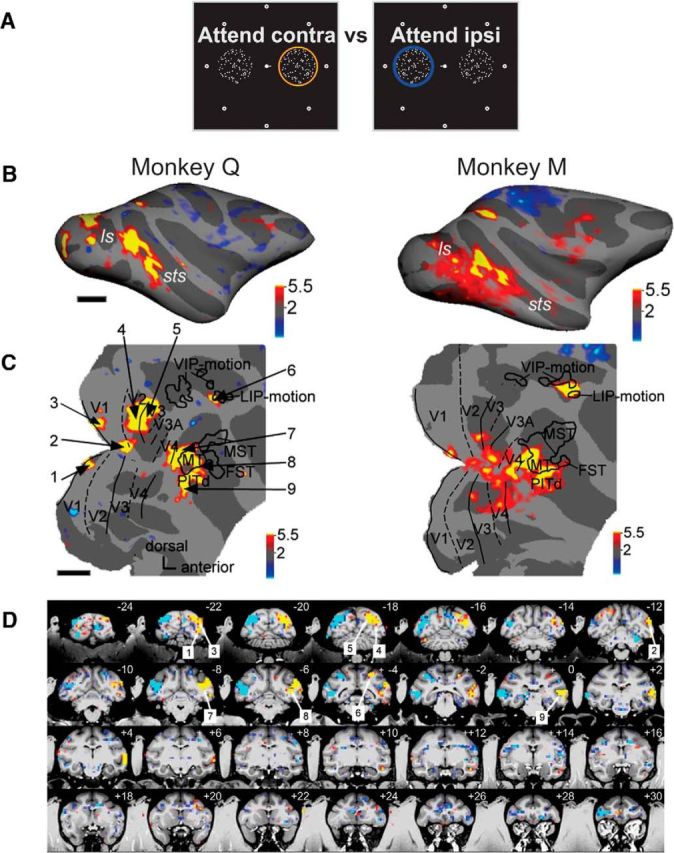
Spatial attention modulates activity in specific cortical areas of the occipital, temporal, parietal, and frontal lobes. A, Schematic of the two stimulus conditions contrasted in Figure 2: attention to the contralateral RDS (attend contra) versus attention to the ipsilateral RDS (attend ipsi). Attention location is indicated by a colored ring over the cued surface. Significant response enhancements for the attend contralateral versus the attend ipsilateral condition are shown in yellow and red; significant response enhancements to the opposite condition are in blue. B, Statistical parametric maps for the contrast attend contra vs attend ipsi; conventions as in Figure 1D, top. C, Same parametric maps overlaid on flattened posterior hemispheres; conventions as in Figure 1D, bottom; thresholds at p < 0.05, corrected. Left, Numbers point to regions of significant activation shown on coronal slices in D: 1, area V1 lower hemifield; 2, foveal representation; 3, area V1 upper hemifield; 4, area V2; 5, area V3; 6, posterior parietal area LIP; 7, area V4t; 8, area MT; 9, area PITd. D, Parametric maps on coronal slices of high-resolution anatomy, left hemisphere on the right. Cyan and blue indicate higher activity for contrast attend left > attend right; yellow and red indicate higher activity for contrast attend right > attend left.
Data analysis
Functional data were analyzed using a block design in SPM5. Freesurfer was used for registering functional to anatomical volumes and for surface flattening. Scans that showed unacceptable levels of movement artifacts or during which performance was low were discarded. Functional data were motion-corrected and spatially smoothed with a Gaussian kernel of 2 mm full-width-at-half-maximum. Realignment parameters were included as covariates of no interest in the general linear model (Friston et al., 1995).
For each functional contrast, significance was assessed by t scores, displayed as a statistical parametric map. Strength of activation was determined by the mean GLM β values (scaled to percentage signal change).
Boundaries of retinotopic visual areas were determined by meridian mapping (Sereno et al., 1995). Boundaries of areas inside the superior temporal and intraparietal sulci were determined by mapping with a motion localizer aided by anatomical landmarks obtained from an anatomical atlas (Saleem and Logothetis, 2007) for V4t, MT, medial superior temporal (MST), fundus of the STS (FST), LIP, and ventral intraparietal (VIP) areas. To identify the brain regions activated by RDSs, “peripheral activation zones” were defined by the contrast peripheral versus central stimulation of the center-periphery mapping data. The intersection of visual cortical area with the peripheral activation zone defined the ROIs for which attentional modulation was assessed for retinotopic areas V1–V4. FEFs were defined by the saccade versus no-saccade contrast of the guided saccade task.
Response magnitude and response difference across conditions were computed for each ROI by taking the mean of the β values for the attend contralateral and the attend ipsilateral condition and the difference, respectively. For this computation, insignificant response differences were set to zero. To compare the strength of attentional modulation across areas with different degrees of activation, an attention index (AI) was computed according to the formula (C − I)/(C + I), where C is the β value during the attend contralateral condition, and I is the β value during the attend ipsilateral condition.
Results
We conducted two main attention tasks: the attentive motion-discrimination task and the attentive motion-detection task. To define ROIs, we conducted five fMRI experiments. We charted retinotopic visual areas using meridian mapping with a checkerboard stimulus (Sereno et al., 1995; Vanduffel et al., 2002). We devised a second retinotopic localizer to differentiate brain regions representing the positions of the RDSs from regions responding to fixation spots and spatial cues in the attention task. Third, we identified motion-sensitive regions by comparing activity to moving versus static random dot displays, and a second motion localizer (Nelissen et al., 2006) to differentiate motion specializations. Fifth, we trained animals to perform a guided saccade task to identify regions involved in saccade generation. We used the resulting functional maps and anatomical criteria to identify visual cortical areas and subregions of interest (see Materials and Methods).
The attentive motion-discrimination task (Fig. 1A,B) was demanding for both monkeys. During scanning, they detected the prolonged target motion event in 70% (monkey Q) and 51% (monkey M) of the trials, respectively. After detection, they discriminated correctly and generated a saccade in the correct direction 97% and 86% of the time (chance level, 12.5%), respectively. Blocks of attention task trials were separated by periods of passive fixation and a neutral condition (Fig. 1C). In one variant, the neutral condition was devoid of the two RDSs. In the second variant the same RDSs as in active task blocks were displayed.
We observed small differences in fixation across attention conditions: analysis of 2589 trials for monkey Q and 3624 trials for monkey M during motion RSVPs in discrimination and motion-detection tasks showed that monkey Q's fixation positions exhibited a mean difference of 0.18° along the horizontal axis (p < 0.001, Mann–Whitney U test) and a mean difference of 0.07° (p < 0.001; Mann–Whitney U test) along the vertical axis, while monkey M's eye traces differed, on average, by 0.38° along the horizontal axis (p < 0.001; Mann–Whitney U test) and by 0.09° (p < 0.001; Mann–Whitney U test) along the vertical axis. Thus, significant differences in eye positions occurred in both monkeys. Can they have influenced our fMRI results? This is likely not the case, and quite certainly not to a sizeable extent, for a number of reasons. First, the actual differences were small. Differences in the vertical direction were particularly small and would not have any systematic effect on our results due to the vertical axis mirror symmetry of our stimulus array. Differences in horizontal eye position were smaller than the length of the central bar cue (±0.09° and ±0.19° fixation differences vs ±0.35° bar cue length). If these differences in eye position had any effect on neural activity, the biggest one should have been on foveal regions of early retinotopic cortical areas, as the central fixation spot was shifted to either side of the fovea and the high cortical magnification factor in this region should have amplified any such differences. Yet, activity differences close to the fovea were not included in the analysis and should, in any case, have had the opposite effect to the one we measured, since an eye movement toward the target surface moved the fixation spot to the side of the distracter surface. The second effect of eye-position differences was a slight shift of the attended surface (6° in diameter) closer (<0.2°) to the fovea or farther away in the attend contralateral versus the attend ipsilateral condition. This likely had no sizable effect either for three reasons. First, our ROIs were defined based on the center/periphery localizer that used a peripheral stimulus of 6.5° of visual angle. Thus, cortical regions identified by this localizer included both shifted positions of the attention task. Second, we found attention effects throughout this ROI, not just in the parts closest to the fovea as would be expected if the shifts induced activity differences. Third, the differences in fixation position were quite small. Thus, we do not think that differences in cortical activation across the attend contralateral versus attend ipsilateral condition were to any relevant extent due to differences in eye positions.
Contrasting activation during active versus passive conditions allowed identification of brain regions with task-related activation in the broadest sense, i.e., related to sensory processing, attention, response generation, and interactions between these components. Active attentive motion processing activated cortical regions in occipital, temporal, parietal and, to a lesser extent, frontal lobes (Fig. 1D), specifically retinopic visual areas V1, V2, V3, V3A, V4d, V4t; in superior temporal sulcus (STS) areas MT, FST, MST (weakly); in the dorsal part of posterior inferotemporal cortex (PITd; Hikosaka, 1997); in intraparietal areas VIP and LIP; and in prefrontal area FEF.
Our main interest was to isolate cortical areas modulated specifically by selective attention (Fig. 2A). We thus contrasted the two spatial attention conditions: attend contralateral and attend ipsilateral. These spatial attention conditions are dissociated from saccade planning, since saccades to any of the eight different targets were generated equally frequently in both conditions. The attended surface induced significantly larger activity than the distracter in several brain regions, starting with V1 (Figs. 2B–D, 3A) and continuing with occipital visual areas V2, V3, V3A, and V4d. Spatial effects of attention were similarly strong in motion-selective areas V4t and MT, but weak or absent in neighboring motion-selective areas MST and FST (Fig. 2D). We found strong effects of spatial attention in a region of inferotemporal cortex, area PITd (Figs. 2B--D, 3A). Area PITd was also the most significantly modulated area in both animals. Within the parietal lobe, we found attentional modulation of comparable magnitude and consistency across animals in area LIP only, and not in areas 7a and VIP. Within the frontal lobes, in which task performance led to only weak modulation (Fig. 1D, top), we found attentional modulations in the FEF. Thus covert spatial attention modulates activity in early retinotopic areas, a subset of motion-selective areas, frontoparietal attentional control areas LIP and FEF, and in area PITd.
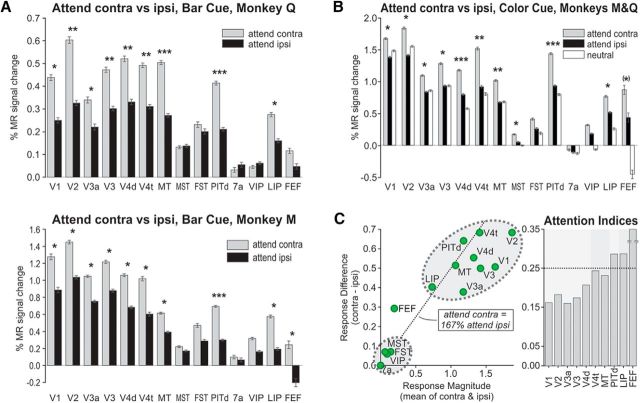
Figure 3.
Spatial attention modulates activity in cortical ROIs in the occipital, temporal, parietal, and frontal lobes. A, β Values of the GLM for monkey Q (BOLD, top) and monkey M (Sinerem, bottom) across ROIs (left to right: V1, V2, V3a, V3, V4d, V4t, MT, MST, FST, PITd, 7a, VIP, LIP, and FEF) in the attentive discrimination task with central bar cue (Fig. 1A, var1). Responses during the attend contralateral (Attend contra) condition are shown in gray; responses during the attend ipsilateral (Attend ipsi) condition in black. Significant activation differences in the two conditions: *p < 0.01 (ANOVA, multiple-comparison corrected); ***, most significant differences; **, differences with at least half the F value of the most significant difference. B, β Values for the motion-discrimination task with color cue (Fig. 1A, var3; averaged across monkeys Q and M, Sinerem both). Responses to the passive fixation task condition (neutral) are shown in white. Significant activation differences between the two attention conditions (Attend contra and Attend ipsi) are indicated by asterisks following the same convention as in A. FEF modulation was marginally significant (p = 0.03). C, Left, Scatter plot of activation differences (Attend contra vs Attend ipsi, vertical axis) for each ROI versus mean activation during the two attention conditions (horizontal axis) averaged across attentive motion-discrimination tasks variant 1 (vars1) and variant 3 (vars3; A and B). Areas MST, FST, VIP, and 7a show weak and insignificant activation and attention modulation across tasks and animals. The dotted line indicates conditions with response enhancement during the Attend contra condition that is enhanced by 67% (corresponding to an AI of 0.25) relative to the response during the Attend ipsi condition. Right, Bar graph of AIs of ROIs with both visual activation and attentional modulation. Areas V1, V2, V3a, and V3 show similar and lower levels of attentional modulation; motion-selective areas V4t and MT show more; and area PITd, area LIP, and the FEF show the strongest. The AI for the FEF, which typically exhibited little mean activation, was the largest of these three areas, and was cutoff at the top for display purposes.
It is important to consider whether these differences in response magnitudes might have arisen from systematic differences in visual stimulation due to systematic differences in eye positions between attention conditions (Treue and Maunsell, 1999; Silver et al., 2005), here between the attend left and the attend right conditions. In both monkeys, eye positions were slightly, but significantly drawn toward the target surface along the horizontal axis [±0.09° of visual angle difference in monkey Q (n = 2859 trials), and by ±0.19° in monkey M (n = 3624 trials), p < 0.001, Mann–Whitney U test] and the vertical axis (0.07° in monkey Q and 0.09° in monkey M, respectively, p < 0.001, Mann–Whitney U test). These differences in fixation position were small (approximately one-quarter and one-half the width of the central bar cue in monkeys M and Q, respectively, or 3 and 1.5% the horizontal width of the RDS) and unlikely to have affected results in a systematic fashion, because analyses were based on retinotopic ROIs (see Materials and Methods) with a diameter 0.5° larger than the RDSs and thus containing them completely. Furthermore activity modulation at more foveal regions should have yielded effects in the opposite direction from the one reported here, since deviant eye positions moved the central fixation spot to the hemifield opposite the target RDS.
In a second set of attention experiments in the same animals, the color of the fixation spot served as a symbolic cue of attention direction (Fig. 1A). We found a very similar pattern of results (Fig. 3B): attentional modulation in retinotopic visual areas from V1 to V4d, in motion selective areas V4t and MT (weak activation and small attention effects in MST and FST), in frontoparietal attentional control areas LIP and FEF, and in area PITd (strong activation and attention effects). The overall pattern of visual activation and attentional modulation across these two tasks (Fig. 3C) shows consistent activation of early retinotopic areas, motion selective areas V4t and MT, area PITd, and area LIP. Modulation by spatial-selective attention grew from early retinotopic areas through motion-selective areas V4t and MT to area PITd, area LIP, and the FEF.
Among the areas most strongly modulated by attention across tasks and task variants was area PITd. Area PITd was also the area most significantly modulated in both animals and task variants. This finding was surprising because, unlike all other occipitotemporal visual areas modulated, area PITd is not known to be motion selective. To directly test whether the attention-modulated part of area PITd might constitute a hitherto unrecognized motion glob, we used a second motion localizer (see Materials and Methods) that had been shown to be effective in localizing a wide range of motion-selective areas, including a joint motion-selective and shape-selective temporal lobe area, the lower superior temporal (LST) area, in the lower bank of the STS (Nelissen et al., 2006). We mapped motion-selective regions and determined their spatial relationship to area PITd (Fig. 4A). In three of four hemispheres, attention-modulated area PITd bordered, but did not contain, motion-selective regions. In one hemisphere, a partial overlap between motion-selective regions and area PITd existed (Fig. 4A, left). Areas V4d and PITd, in contrast to nearby area MT, did not show significant motion selectivity in either animal (Fig. 4A). This result and the more medial location of area LST within the STS (Nelissen et al., 2006), lets us conclude that area LST and attention-modulated area PITd are two different regions. Attention modulation of area PITd appears to be unlikely to result from a role in the analysis of motion information.
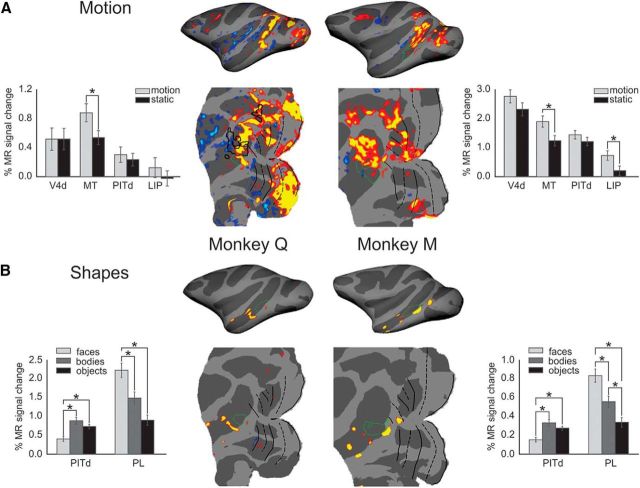
Figure 4.
Attentionally modulated parts of area PITd are located adjacent to motion-selective and face-selective areas. A, Areas activated by motion stimuli are shown in red/yellow (p < 0.05) overlaid onto inflated and flatmap representations of the right hemispheres for both monkeys. Green outlines depict the location of attentionally modulated area PITd. There is some overlap in one of monkey Q's hemispheres between areas involved in motion processing and area PITd. Asterisks in bar graphs mark significant differences (Wilcoxon signed-rank test at p < 0.01). There is little overlap between area PITd and motion-selective areas. B, Face-selective areas shown in red/yellow (p < 0.05). There is little if any overlap between area PITd and the face-selective cortex. In fact area PITd responds less to faces than to other objects. Asterisks in bar graphs mark significant differences (Wilcoxon signed-rank test at p < 0.01).
Faces and gaze draw and direct attention (Deaner and Platt, 2003; Hershler and Hochstein, 2005). Because the posterior temporal face areas (Tsao et al., 2008), the posterior lateral (PL) area and the middle lateral (ML) area, are located at similar anteroposterior locations as area PITd, we mapped face areas using the original image set that defined these areas and compared their location to that of area PITd. We found the posterior face patch to be located adjacent to, but not overlapping with area PITd (Fig. 4B). Despite its proximity to face-selective areas, area PITd did not show a preference for face stimuli. In fact, it was consistently and significantly more strongly activated by nonface objects. Thus area PITd abuts motion-selective and face-selective areas, but does not exhibit selectivity for either motion or facial shape.
Our attention task emphasized feature discrimination. This function has been proposed to be the core operation of the ventral stream (Goodale and Milner, 1992; Mirabella et al., 2007). To test whether it was the discrimination requirement that caused the recruitment of area PITd or, more generally, attentional demand, we devised a second difficult attentional motion-processing task, but one without a motion-discrimination requirement: monkeys had to detect the occurrence of a low CME that interrupted an incoherent motion sequence (Fig. 5A) and report the CME by saccading onto the target surface (see Materials and Methods). While monkeys were scanned, they performed blocks of trials of the new motion-detection task interleaved with blocks of the motion-discrimination task (Fig. 5B), and both responded with very similar reaction times across tasks (monkey Q: 417 ms in the motion-discrimination task and 415 ms in the motion-detection task; monkey M: 366 ms in the motion-discrimination task and 372 ms in the motion-detection task). The two attention tasks differed in three major ways: feature discrimination versus detection, feature strength (coherent vs incoherent motion), and covert attention–overt attention (saccade) contingency (dissociated in discrimination task, congruent in detection task).
We found area MT to be activated and modulated by spatial attention in both tasks, while motion-selective areas MST, FST, and VIP were much more weakly, if at all, activated and modulated by selective attention (Fig. 5C). In contrast, area PITd, once more, was robustly activated and modulated by spatial attention. Modulation was equally strong in both tasks. Thus recruitment of area PITd does not depend on discrimination, but selective attention. Areas LIP and the FEF were activated to a similar degree in both tasks, but attentional modulation was stronger in the FEF. Modulation in the motion-detection task was larger than in the motion-discrimination task in area LIP and the FEF (Fig. 5D), areas in which attention and saccade signals are often closely coupled (Juan et al., 2004). Greater similarity of effects in area LIP underscores the notion that this area can mediate spatial attentional control without movement intention (Colby and Goldberg, 1999). Attentional modulation of area PITd was very similar in both tasks (AIDet, 0.33; AIDis, 0.32) and also very similar to the degree of attentional modulation in the two independent motion-discrimination tasks (Fig. 3; AI, 0.29).
Discussion
The composition of the attention network we found had four main characteristics. First, attention modulated cortical activity as early as the primary visual cortex. This finding agrees with earlier results in humans (Huk and Heeger, 2000), ruling out the possibility that the sites of attentional modulation in man and macaque differ systematically (Heeger et al., 2001). High perceptual load, a key characteristic of our tasks, but not of others that did not find early attentional modulation (Wardak et al., 2010), might thus be critical for early attentional selection (Lavie and Tsal, 1994).
Second, we found areas known to contain speed-selective and direction-selective cells—V1, V2, V3, V3A, and V4d (Cheng et al., 1994; An et al., 2012; Li et al., 2013)—and motion areas V4t and MT, to be modulated by attentive motion processing. This is predicted by feature-based accounts of attention (Corbetta et al., 1990; Beauchamp et al., 1997; O'Craven et al., 1997). Yet not all motion-sensitive areas showed strong attention effects. Contrary to the expectation of increasing attention effects in the dorsal stream with hierarchical rank (Treue and Maunsell, 1996; Cook and Maunsell, 2002), motion-sensitive areas MST, FST, and VIP were inconsistently, if at all, modulated across tasks. These areas were also much less activated by task performance than area PITd, which occupies a comparable hierarchical rank (Felleman and Van Essen, 1991). Possibly preponderance of complex motion selectivity in areas MST, FST, VIP, irrelevant for task demands, might have caused weaker attentional effects at the level of these areas. The site of attentional selection appeared to be sharply focused, even separating areas with close anatomical links like areas MT and MST (Ungerleider and Desimone, 1986; Boussaoud et al., 1990).
Third, we did find attentional modulation in high-level dorsal areas LIP and the FEF. Because both areas were engaged in a task requiring endogenous top-down attention deployed over extended periods of time and dissociated from saccade planning, our results provide new evidence for the idea that these areas, though closely associated with oculomotor circuits, can mediate attentional control without movement intention (Colby and Goldberg, 1999; Kastner and Ungerleider, 2000).
Fourth, we found one other high-level cortical area to be modulated by attention. This area, area PITd, has until now been unsuspected to be involved in attentive motion processing. This is certainly not the first time the inferotemporal cortex has been found to be involved in attention (Moran and Desimone, 1985; Caspari et al., 2015; Patel et al., 2015). In those previous cases attention was drawn to classical ventral stream properties, such as shape and contrast. Area PITd contains shape-selective (Hikosaka et al., 1988) and color-selective (Conway and Tsao, 2009) neurons. Thus, involvement of this area in attentive shape-processing tasks is compatible with feature-based accounts of attention. To the best of our knowledge, this is the first time an inferotemporal cortex area has been found to be involved in an attention task requiring motion processing, and especially motion discrimination. We show this for two different qualities of motion processing, while we find no evidence for motion selectivity within area PITd. However, we also did not find evidence for motion selectivity in area V4d, in which motion-selective cells have been found (Cheng et al., 1994; An et al., 2012; Li et al., 2013). Thus electrophysiological recordings from area PITd will need to decide the possibility of a feature-selection account for PITd recruitment. Our results do not rule out this possibility, but render it implausible, especially given lack of recruitment of high-level motion-selective areas of similar hierarchical rank. Our work extends a finding of extraretinal modulation of activity in area V4 during an active motion task (Ferrera et al., 1994), and supports the conceptual proposal that motion within a static stimulus, such as our RDSs, constitutes an object attribute very much like color or shape (Ferrera et al., 1994). An alternative account that proposes ventral-stream recruitment during conscious fine-feature discrimination (Goodale and Milner, 1992; Mirabella et al., 2007) does explain V4d and PITd recruitment in the motion-discrimination task. An explanation for the recruitment of area PITd in the motion-detection task is more challenging. Interpreting motion coherence as a feature, the detection of a low-coherence signal might be considered similar enough to fine-feature discrimination, and would plausibly require a similar degree of awareness as the discrimination of motion directions (Goodale and Milner, 1992).
Given that area PITd contains large RFs, some straddling the vertical meridian (Hikosaka, 1998), a biased competition account (Desimone and Duncan, 1995) for its recruitment is plausible, though this account does not explain lack of recruitment of several other high-level visual areas in the dorsal stream, such as areas MST, FST, VIP, and 7a. In that regard, area PITd appears more related to area LIP and the FEF, which were, though less activated than area PITd, similarly modulated across attention tasks and are thought to be generally involved in attentional processing and not specifically in attentive motion processing. This relationship might not exist by coincidence, since area PITd is directly connected to both area LIP (Blatt et al., 1990; Distler et al., 1993; Lewis and Van Essen, 2000; Gattass et al., 2005) and the FEF (Felleman and Van Essen, 1991; Schall et al., 1995; Bullier et al., 1996).
A key argument for the oculomotor network as a suitable place for a salience map (Fecteau and Munoz, 2006) is its connectivity with the ventral stream, which would provide necessary information about object properties along multiple feature dimensions. The same argument, then, holds for area PITd, which we found to be located right next to motion-selective and shape-selective areas, and which contains color-selective globs (Conway et al., 2007). Combined with its connectivity to dorsal stream areas MT and FST (Distler et al., 1993; Gattass et al., 2005), the ventral stream (V4, TEO, anterior TE; Felleman and Van Essen, 1991; Distler et al., 1993; Suzuki et al., 2000; Gattass et al., 2005), and more anterior STS areas (IPa, TEa, and TEm) (Distler et al., 1993; Gattass et al., 2005), area PITd is as well positioned as any part of the oculomotor system to gain access to relevant object properties. Since area PITd is not closely tied to oculomotor function, it might get engaged, as we have found, more strongly than area LIP and the FEF, when attention needs to be sustained, covertly, to one object over time, independently of ensuing eye movements. Consistent with this scenario, the strength of attentional modulation in area PITd did not increase when the direction of sustained covert attention coincided with the direction of planned and executed saccades, while it did in area LIP and especially in the FEF.
Two attentional control systems have been proposed in humans, a dorsal frontoparietal one regulating endogenous attention, and a ventral network, including the temporoparietal junction area (area TPJ), scanning the environment for potentially behaviorally relevant stimuli currently outside the focus of attention (Corbetta et al., 2008). During top-down attention, like in our attentive motion-tracking tasks, the dorsal attention network is supposed to be activated and the ventral one suppressed. Area PITd was similarly regulated as frontoparietal areas FEF and LIP. Thus area PITd, though a ventral area, is functionally different from area TPJ and not a component of the ventral attention network. Our results are consistent with a recent study that did not find an area TPJ homolog in the macaque monkey (Patel et al., 2015). Human resting-state studies have described a more ventral region to covary with the dorsal attention network (Fox et al., 2005, 2006), implying connectivity. This area has been ascribed to, based on Talairach coordinates, the MT+ complex, which human area PITd is proximal to. Thus human area PITd might be part of the “dorsal” attention network. The functional data provided in this study and anatomical data of PITd–LIP and PITd–FEF connectivity (Blatt et al., 1990; Felleman and Van Essen, 1991; Distler et al., 1993; Schall et al., 1995; Bullier et al., 1996; Lewis and Van Essen, 2000; Gattass et al., 2005) suggest the possibility, though a speculation at this time, that the network for endogenous control of attention is not confined to frontoparietal structures closely associated with the oculomotor system, but might encompass an additional, ventral component at a strategic location to gather information about object properties.
In this view, the location of area PITd in close proximity to face areas is of particular interest. A recent fMRI study in rhesus monkeys found head gaze following to activate a region in the posterior STS, directly posterior to face area ML (Marciniak et al., 2014). This area was suggested to subserve a visual function like extraction of gaze information from the face or the coordinate transformation of perceived gaze direction to calculate the goal for an attention shift. By location, this “gaze following patch” is likely part of or directly abuts attention-area PITd, which we have found here using stimuli and tasks devoid of a social-perceptual or social-attentive component. Thus, a parsimonious, but more radical explanation for both datasets would suggest that activation of the gaze following patch actually constituted an attention signal. Together, these two studies, using entirely unrelated stimuli and different tasks, suggest a role for area PITd in a potentially wide range of attention-related functions.
Footnotes
This work was supported by the German Ministry of Science (Grant 01GO0506, Bremen Center for Advanced Imaging) and the National Science Foundation (NSF Career, BCS-1057006). W.A.F. is a New York Stem Cell Foundation–Robertson Investigator. We thank Michael Borisov and Dennis Trenner for stimulus programming; Manfred Fahle, Hauke Kolster, Pablo Polosecki, and Torsten Stemmler for discussion; Sebastian Moeller and Doris Tsao for help with data analysis; Aleksandra Nadolski, Nicole Schweers, Katrin Thoss, and Ramazani Hakizimana for technical support and animal care; Bevil Conway for luminance measurements; and Guerbet for the contrast agent Sinerem. We thank Oldenburg University's Bibliotheks- und Informationssystem for providing space for work on this manuscript.
The authors declare no competing financial interests.
References
- An X, Gong H, Qian L, Wang X, Pan Y, Zhang X, Yang Y, Wang W. Distinct functional organizations for processing different motion signals in V1, V2, and V4 of macaque. J Neurosci. 2012;32:13363–13379. doi: 10.1523/JNEUROSCI.1900-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baizer JS. Receptive field properties of V3 neurons in monkey. Invest Ophthalmol Vis Sci. 1982;23:87–95. [PubMed] [Google Scholar]
- Beauchamp MS, Cox RW, DeYoe EA. Graded effects of spatial and featural attention on human area MT and associated motion processing areas. J Neurophysiol. 1997;78:516–520. doi: 10.1152/jn.1997.78.1.516. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296:462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- Bullier J, Schall JD, Morel A. Functional streams in occipito-frontal connections in the monkey. Behav Brain Res. 1996;76:89–97. doi: 10.1016/0166-4328(95)00182-4. [DOI] [PubMed] [Google Scholar]
- Caspari N, Janssens T, Mantini D, Vandenberghe R, Vanduffel W. Covert shifts of spatial attention in the macaque monkey. J Neurosci. 2015;35:7695–7714. doi: 10.1523/JNEUROSCI.4383-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Hasegawa T, Saleem KS, Tanaka K. Comparison of neuronal selectivity for stimulus speed, length, and contrast in the prestriate visual cortical areas V4 and MT in the macaque monkey. J Neurophysiol. 1994;71:2269–2280. doi: 10.1152/jn.1994.71.6.2269. [DOI] [PubMed] [Google Scholar]
- Chun MM, Wolfe JM. Visual attention. In: Goldstein B, editor. Blackwell handbook of perception. Oxford: Blackwell; 2001. pp. 272–310. [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Ann Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Ventral intraparietal area of the macaque: anatomic location and visual response properties. J Neurophysiol. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Conway BR, Tsao DY. Color-tuned neurons are spatially clustered according to color preference within alert macaque posterior inferior temporal cortex. Proc Natl Acad Sci U S A. 2009;106:18034–18039. doi: 10.1073/pnas.0810943106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BR, Moeller S, Tsao DY. Specialized color modules in macaque extrastriate cortex. Neuron. 2007;56:560–573. doi: 10.1016/j.neuron.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EP, Maunsell JH. Attentional modulation of behavioral performance and neuronal responses in middle temporal and ventral intraparietal areas of macaque monkey. J Neurosci. 2002;22:1994–2004. doi: 10.1523/JNEUROSCI.22-05-01994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Attentional modulation of neural processing of shape, color, and velocity in humans. Science. 1990;248:1556–1559. doi: 10.1126/science.2360050. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Platt ML. Reflexive social attention in monkeys and humans. Curr Biol. 2003;13:1609–1613. doi: 10.1016/j.cub.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Ann Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Desimone R, Ungerleider LG. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J Comp Neurol. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler C, Boussaoud D, Desimone R, Ungerleider LG. Cortical connections of inferior temporal area TEO in macaque monkeys. J Comp Neurol. 1993;334:125–150. doi: 10.1002/cne.903340111. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci. 2006;10:382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Rudolph KK, Maunsell JH. Responses of neurons in parietal and temporal visual pathways during a motion task. J Neurosci. 1994;14:6171–6186. doi: 10.1523/JNEUROSCI.14-10-06171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Galletti C, Battaglini PP, Fattori P. ‘Real-motion’ cells in area V3A of macaque visual cortex. Exp Brain Res. 1990;82:67–76. doi: 10.1016/S0079-6123(08)62591-1. [DOI] [PubMed] [Google Scholar]
- Gattass R, Nascimento-Silva S, Soares JG, Lima B, Jansen AK, Diogo AC, Farias MF, Marcondes M, Botelho MM, Mariani OS, Azzi J, Fiorani M. Cortical visual areas in monkeys: location, topography, connections, columns, plasticity and cortical dynamics. Philos Trans R Soc Lond B Biol Sci. 2005;360:709–731. doi: 10.1098/rstb.2005.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Heeger DJ, Gandhi SP, Huk AC, Boynton GM. Neuronal correlates of attention in human visual cortex. In: Braun J, Koch C, Davis JL, editors. Visual attention and cortical circuits. Cambridge, MA: MIT; 2001. pp. 25–47. [Google Scholar]
- Hershler O, Hochstein S. At first sight: a high-level pop out effect for faces. Vision Res. 2005;45:1707–1724. doi: 10.1016/j.visres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. Responsiveness of neurons in the posterior inferotemporal cortex to visual patterns in the macaque monkey. Behav Brain Res. 1997;89:275–283. doi: 10.1016/S0166-4328(97)00073-9. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. Representation of foveal visual fields in the ventral bank of the superior temporal sulcus in the posterior inferotemporal cortex of the macaque monkey. Behav Brain Res. 1998;96:101–113. doi: 10.1016/S0166-4328(97)00202-7. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Iwai E, Saito H, Tanaka K. Polysensory properties of neurons in the anterior bank of the caudal superior temporal sulcus of the macaque monkey. J Neurophysiol. 1988;60:1615–1637. doi: 10.1152/jn.1988.60.5.1615. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Luck SJ, Boelmans K, Schoenfeld MA, Boehler CN, Rieger J, Heinze HJ. The neural site of attention matches the spatial scale of perception. J Neurosci. 2006;26:3532–3540. doi: 10.1523/JNEUROSCI.4510-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huk AC, Heeger DJ. Task-related modulation of visual cortex. J Neurophysiol. 2000;83:3525–3536. doi: 10.1152/jn.2000.83.6.3525. [DOI] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci U S A. 2004;101:15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nat Rev Neurosci. 2000;1:91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Ann Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Lavie N, Tsal Y. Perceptual load as a major determinant of the locus of visual selection in visual attention. Percept Psychophys. 1994;56:183–197. doi: 10.3758/BF03213897. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1%3C112::AID-CNE8%3E3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Li P, Zhu S, Chen M, Han C, Xu H, Hu J, Fang Y, Lu HD. A motion direction preference map in monkey V4. Neuron. 2013;78:376–388. doi: 10.1016/j.neuron.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. The operation of selective attention at multiple stages of processing: evidence from human and monkey electrophysiology. In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: MIT; 2000. pp. 687–700. [Google Scholar]
- Marciniak K, Atabaki A, Dicke PW, Thier P. Disparate substrates for head gaze following and face perception in the monkey superior temporal sulcus. eLife. 2014:3. doi: 10.7554/eLife.03222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, Treue S. Feature-based attention in visual cortex. Trends Neurosci. 2006;29:317–322. doi: 10.1016/j.tins.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Mirabella G, Bertini G, Samengo I, Kilavik BE, Frilli D, Della Libera C, Chelazzi L. Neurons in area V4 of the macaque translate attended visual features into behaviorally relevant categories. Neuron. 2007;54:303–318. doi: 10.1016/j.neuron.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Mühlenen A, Rempel MI, Enns JT. Unique temporal change is the key to attentional capture. Psychol Sci. 2005;16:979–986. doi: 10.1111/j.1467-9280.2005.01647.x. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Vanduffel W, Orban GA. Charting the lower superior temporal region, a new motion-sensitive region in monkey superior temporal sulcus. J Neurosci. 2006;26:5929–5947. doi: 10.1523/JNEUROSCI.0824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Craven KM, Rosen BR, Kwong KK, Treisman A, Savoy RL. Voluntary attention modulates fMRI activity in human MT-MST. Neuron. 1997;18:591–598. doi: 10.1016/S0896-6273(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Orban GA, Kennedy H, Bullier J. Velocity sensitivity and direction selectivity of neurons in areas V1 and V2 of the monkey: influence of eccentricity. J Neurophysiol. 1986;56:462–480. doi: 10.1152/jn.1986.56.2.462. [DOI] [PubMed] [Google Scholar]
- Patel GH, Yang D, Jamerson EC, Snyder LH, Corbetta M, Ferrera VP. Functional evolution of new and expanded attention networks in humans. Proc Natl Acad Sci U S A. 2015;112:9454–9459. doi: 10.1073/pnas.1420395112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. London: Academic; 2007. [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki W, Saleem KS, Tanaka K. Divergent backward projections from the anterior part of the inferotemporal cortex (area TE) in the macaque. J Comp Neurol. 2000;422:206–228. doi: 10.1002/(SICI)1096-9861(20000626)422:2%3C206::AID-CNE5%3E3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. J Neurophysiol. 2005;93:337–351. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J Neurosci. 1999;19:7591–7602. doi: 10.1523/JNEUROSCI.19-17-07591.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsal Y, Meiran N, Lavie N. The role of attention in illusory conjunctions. Percept Psychophys. 1994;55:350–358. doi: 10.3758/BF03207605. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci U S A. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uka T, DeAngelis GC. Contribution of area MT to stereoscopic depth perception: choice-related response modulations reflect task strategy. Neuron. 2004;42:297–310. doi: 10.1016/S0896-6273(04)00186-2. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. J Comp Neurol. 1986;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RB, Orban GA. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/S0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Peuskens H, Denys K, Sunaert S, Todd JT, Orban GA. Extracting 3D from motion: differences in human and monkey intraparietal cortex. Science. 2002;298:413–415. doi: 10.1126/science.1073574. [DOI] [PubMed] [Google Scholar]
- Wardak C, Vanduffel W, Orban GA. Searching for a salient target involves frontal regions. Cereb Cortex. 2010;20:2464–2477. doi: 10.1093/cercor/bhp315. [DOI] [PubMed] [Google Scholar]
- Wegener D, Freiwald WA, Kreiter AK. The influence of sustained selective attention on stimulus selectivity in macaque area MT. J Neurosci. 2004;24:6106–6114. doi: 10.1523/JNEUROSCI.1459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the resus monkey. J Physiol. 1974;236:549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]