Abstract
Purpose of review
Phospholipids are major constituents in the intestinal lumen after meal consumption. This article highlights current literature suggesting the contributory role of intestinal phospholipid metabolism toward cardiometabolic disease manifestation.
Recent findings
Group 1b phospholipase A2 (PLA2g1b) catalyzes phospholipid hydrolysis in the intestinal lumen. The digestive product lysophospholipid, particularly lysophosphatidylcholine (LPC), has a direct role in mediating chylomicron assembly and secretion. The LPC in the digestive tract is further catabolized into lysophosphatidic acid (LPA) and choline via autotaxin-mediated and autotaxin-independent mechanisms. The LPC and LPA absorbed through the digestive tract and transported to the plasma directly promote systemic inflammation and cell dysfunction, leading to increased risk of cardiovascular disease and obesity/diabetes. The choline moiety generated in the digestive tract can also be used by gut bacteria to generate trimethylamine, which is subsequently transported to the liver and oxidized into trimethylamine-N-oxide (TMAO) that also enhances atherosclerosis and cardiovascular abnormalities.
Summary
Products of phospholipid metabolism in the intestine through PLA2g1b- and autotaxin-mediated pathways directly contribute to cardiometabolic diseases through multiple mechanisms. The implication of these studies is that therapeutic inhibition of PLA2g1b and autotaxin in the digestive tract may be a viable approach for cardiovascular and metabolic disease intervention.
Keywords: Phospholipase A2, autotaxin, trimethylamine-N-oxide, lysophosphatidylcholine, lysophosphatidic acid, atherosclerosis, diabetes
INTRODUCTION
The incidence of obesity continues to escalate in recent years and has skyrocketed to near pandemic levels over the past 2 decades [1,2], leading to significant increased risk of metabolic diseases including diabetes, cardiovascular and fatty liver diseases [3]. A major causative factor for the increasing prevalence of obesity and obesity-related metabolic disorder is the chronic consumption of fat-rich meals. Hence, tremendous resources have been expended to reduce obesity and lower the risk of cardiometabolic diseases via limiting the amount of dietary lipids absorbed through the gastrointestinal tract.
Most of the efforts in reducing lipid absorption have focused on limiting triglyceride digestion in the intestinal lumen and the transport and absorption of fatty acids and cholesterol through the intestinal mucosa. It is important to note, however, that phospholipids are also abundantly present in the intestinal lumen during meal consumption. In fact, earlier studies have shown that phospholipid digestion is also required for intestinal lipid absorption [4]. The major enzyme responsible for phospholipid hydrolysis in the intestinal lumen is the group 1b phospholipase A2 (PLA2g1b) derived from the pancreas. However, compensatory enzyme(s) is/are also present to mediate intestinal phospholipid digestion and lipid absorption in the absence of PLA2g1b [5]. Interestingly, the Pla2g1b-null mice are resistant to diet-induced obesity, diabetes, hyperlipidemia, and atherosclerosis despite their near normal lipid absorption efficiency [6]. These observations suggested that the digested products generated from PLA2g1b hydrolysis of phospholipids may be different from those produced by the compensatory enzyme(s), and the PLA2g1b-derived metabolites may be bioactive mediators of cardiometabolic diseases. The physiological roles of PLA2g1b in metabolic disease promotion have been reviewed in this journal previously [6]. The current review is an update of the literature, focusing on PLA2g1b-derived metabolites in the digestive tract and their influence on cardiometabolic disease manifestation.
INTESTINAL LYSOPHOSPHATIDYLCHOLINE AND CHYLOMICRON BIOSYNTHESIS
Phospholipase A2 catalyzes the hydrolysis of the ester bond at the sn-2 position of phospholipids to yield free fatty acids and lysophospholipids. The absorption of lysophospholipids is dramatically reduced in the absence of PLA2g1b, thus indicating that the compensatory enzyme for phospholipid hydrolysis in the intestinal lumen is not a phospholipase A2, but most likely the phospholipase B that hydrolyzes phospholipids to phosphoglycerol in the distal intestine [7]. In the proximal intestine where normal lipid digestion and absorption occurs, the lysophospholipids produced by phospholipid hydrolysis, primarily lysophosphatidylcholine (LPC), are absorbed into enterocytes where they participate in intracellular lipid trafficking that is necessary for chylomicron assembly and secretion. In an elegant recent study, Siddiqi and Mansbach showed that the free fatty acids and LPC generated from phospholipid hydrolysis are transported intracellularly in association with caveolin-1 containing endocytic vesicles [*8]. The LPC on the surface of these vesicles activates protein kinase C-ζ (PKCζ), liberating it from the vesicles to allow the targeting of the vesicles to the endoplasmic reticulum where the free fatty acids are used for triglyceride biosynthesis. At the same time, the activated PKCζ released from caveolin-1 containing endocytic vesicles can bind and phosphorylate the secretion-associated Ras-related GTPase 1B (Sar1b), resulting in the disruption of the multiprotein complex that includes fatty acid binding protein-1 (FABP1). The FABP1 released from the multiprotein complex can bind to the ER membrane and organize the budding of the pre-chylomicron transport vesicles to transport pre-chylomicrons to the Golgi.
Despite the evidence indicating that LPC absorption is required for intracellular lipid trafficking and chylomicron biosynthesis in the intestine, two independent laboratories have provided indirect evidence implying that the re-esterification of LPC to PC is also necessary for normal lipid absorption and chylomicron assembly and transport. Both laboratories generated mouse models with defective expression of lysophosphatidylcholine acyltransferase-3 (LPCAT3), the enzyme responsible for resterification of LPC to phosphatidylcholine. The Jiang laboratory showed that the lack of LPCAT3 expression lower plasma levels of cholesterol, phospholipid, and triglyceride due to reduced lipid absorption [9]. Subsequent studies from the same laboratory documented that the dominant effect of LPCAT3 deficiency on plasma lipid levels is due to its defective expression in the intestine while liver LPCAT3 deficiency only impacts on plasma triglyceride levels [**10]. Studies from the Tontonoz laboratory yielded similar results, with additional observations that identified a mechanism related to reduced production of arachidonoyl phospholipids and the remodeling of enterocyte plasma membrane phospholipid composition being the gatekeeper important for passive lipid absorption and chylomicron production [**11,**12]. Although results from these studies have led to the suggestion that inhibiting intestinal LPCAT3 activity may be a novel approach for treatment of dietary lipid-associated hyperlipidemia and metabolic disorders [**10,**12], the desirability of this strategy requires extensive and detailed exploration considering that intestinal LPCAT3 activity appears to be required for survival of mice on lipid-rich diets and its inactivation causes the undesirable effects of food intake cessation and starvation [**12].
INTESTINAL LYSOPHOSPHOLIPID METABOLISM AND SYSTEMIC INFLAMMATION
In addition to its re-esterification by LPCAT3 into phosphatidylcholine, LPC may also be hydrolyzed by lysophospholipase D enzymes in the intestine to generate lysophosphatidic acid (LPA) and choline. Chronic feeding of LDL receptor-null mice with a high fat-cholesterol Western type diet increases the levels of unsaturated LPA but not saturated LPA in the intestine [13]. Subsequent study revealed that unsaturated LPA is produced from unsaturated LPC hydrolysis by autotaxin, also called ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (gene name ENPP2), but the enzyme responsible for converting saturated LPC to saturated LPA remains unknown [**14]. The contribution of unsaturated LPA toward cardiometabolic diseases was illustrated by studies showing that adding LPA with unsaturated fatty acyl moiety to a normal chow diet directly leads to dyslipidemia and aortic atherosclerosis in Ldlr−/− mice whereas adding LPA with saturated fatty acyl moiety has no effect [**14]. Intestinal-derived unsaturated LPA promotes dyslipidemia and atherosclerosis via several mechanisms including the induction of lipid and cholesterol biosynthesis genes, alteration of peroxisome proliferator-activated receptor signaling, mitochondria dysfunction, and oxidative stress [15]. Unsaturated LPA produced in the digestive tract and absorbed into plasma circulation also promotes inflammation as evident by increased serum amyloid A [15].
PLASMA LPC AND LPA IN INFLAMMATORY AND CARDIOMETABOLIC DISEASES
Lysophosphatidylcholine is present in plasma circulation at relatively high levels and includes species with both saturated and unsaturated fatty acids. The source of plasma LPCs includes intestinal derived LPC generated through PLA2g1b digestion as well as those produced by other phospholipase A2 enzymes present in plasma and in other tissues. The LPC in circulation can also be hydrolyzed by autotaxin present in the plasma that is derived from lymphatic high endothelial venules, adipose tissues, ovary, lung, and the liver. Both saturated and unsaturated LPCs as well as saturated and unsaturated LPAs have direct roles in eliciting inflammatory response, capable of activating neutrophils, various classes of T lymphocytes, monocytes/macrophages, and vascular endothelial and smooth muscle cells. Most of the effects of LPC in inflammatory response is mediated through activation of NFκB signaling pathway via binding and activation of the G-protein coupled receptor G2A, whereas the LPA effects are mediated through binding and activation of six other G-protein coupled receptors LPAR1-LPAR6 as well as the orphan receptors GPR87 and P2Y10. The roles of LPC and LPA in inflammatory disorders and atherosclerosis have been reviewed recently [16,17]. Studies exploring the influence of autotaxin in diet-induced obesity and diabetes are discussed below.
ROLE OF AUTOTAXIN IN OBESITY AND DIABETES
In addition to its expression in the digestive tract where it catalyzes the conversion of unsaturated LPC to unsaturated LPA, autotaxin is also highly expressed and secreted by adipocytes to activate adipocyte differentiation [18]. Moreover, autotaxin expression in adipocytes is elevated in obese and diabetic mice and humans [18,19], thus leading to the suggestion that adipocyte-derived autotaxin and its reaction products may also play a role in mediating obesity and diabetes. This hypothesis gained further support by experiments showing that acute elevation of plasma LPA levels impairs glucose tolerance and glucose-induced insulin secretion in high fat diet fed mice [20]. Several laboratories have independently generated autotaxin gene modified mice to test this hypothesis. While homozygous autotaxin deficient (Enpp2−/−) mice die in utero with vascular defects, Nishimura et al. found that heterozygous Enpp2+/− mice are healthy and their plasma LPA levels are ~50% of that in wild type mice [21]. Importantly, these investigators found that, in comparison to control wild type mice, the heterozygous Enpp2+/− mice gained less weight and displayed better glucose tolerance and insulin sensitivity in response to high fat diet feeding. Adipocyte-specific autotaxin inactivation was also found to reduce adiposity and improve glucose tolerance and insulin sensitivity by these investigators [21]. Conversely, transgenic mice with increasing autotaxin levels in the circulation via liver-specific over-expression or locally in adipose tissues through transgenic overexpression displayed increased adiposity in response to high fat diet [21,22]. Curiously and in contrast to these studies, Dusaulcy et al. found that adipocyte-specific autotaxin knockout mice have higher fat mass and larger adipocyte size after high fat feeding despite the lower plasma LPA levels and improved glucose tolerance [23]. Thus, whereas the contribution of autotaxin and its metabolites toward glucose intolerance and insulin resistance is undisputed, inconsistencies regarding body weight gain have not been resolved. Potential differences in autotaxin expression levels in other tissues as well as differences in the composition of the diets or genetic background of the animals used in the different experiments may explain some of the discrepant results. For example, the levels of saturated and unsaturated LPA were not reported. Whether saturated and unsaturated LPA have similar effects on adiposity, glucose tolerance, and insulin resistance have not been assessed. It would also be interesting to determine autotaxin expression levels in the digestive tract of these animals, as well as the contribution of intestinal-derived autotaxin in modulating diet-induced body weight gain.
INTESTINAL-DERIVED CHOLINE IN CARDIOMETABOLIC DISEASE
A second product of autotaxin-mediated LPC to LPA conversion is choline. Choline can be metabolized into trimethylamine (TMA) by bacteria residing in the gastrointestinal tract. In particular, the anaerobic bacteria in the Firmicutes and Proteobacteria phyla are the most active in choline consumption and TMA generation while another prominent phyla in the digestive tract, the Bacteroidetes, do not produce TMA from choline [*24]. The TMA produced by gut microbes is transported to the liver whereupon oxidization by flavin monooxygenase-3 (FMO3) converts the TMA to trimethylamine-N-oxide (TMAO). Interestingly, humans and mice consuming a low fat diet have low levels of Firmicutes and high levels of Bacteroidetes, while high fat diet reduces the number of Bacterioidetes and increases the number of Firmicutes and Proteobacteria [25–27]. These diet-induced changes in gut bacterial composition and the corresponding increase in TMAO may be one factor responsible for cardiometabolic disease risk associated with high fat feeding [28]. In fact, a large scale metabolomics screening study has identified TMAO as a biomarker for cardiovascular disease [28]. A causative relationship between TMAO and atherosclerosis was demonstrated in mice via fecal transplantation of gut microbes from atherosclerosis-prone and atherosclerosis-resistant strains of mice with high and low TMAO producing gut microbes, respectively. The results showed that recipients of high TMAO producing bacteria displayed increased atherosclerosis [**29].
The mechanism by which choline and TMAO promotes cardiovascular disease appears to be multifactorial. Firstly, oral supply of choline or TMAO have been shown to increase expression of CD36 and SR-A1 scavenger receptors in macrophages to promote lipid accumulation and foam cell formation [30]. TMAO also inhibiting reverse cholesterol transport to increase foam cell deposition in the vessel wall [31]. Secondly, TMAO also promotes vascular smooth muscle and endothelial cell inflammation by activating mitogen-activated protein kinase pathways and NFκB signaling cascade [*32]; and thirdly, TMAO may also directly contribute to left ventricular diastolic dysfunction and portend adverse outcomes in patients with chronic systolic heart failure [33].
The diet-induced increase of plasma TMAO requires supply of choline, typically as phosphatidylcholine, to the digestive tract as intravenous injection of phosphatidylcholine or choline did not show similar increase of plasma TMAO [30]. These latter observations highlighted the contributory role of PLA2g1b, intestinal autotaxin, and metabolites derived from their enzymatic reactions, in diet-induced cardiovascular disease. Several recent studies suggested that TMAO may also contribute to diabetes. In one study, dietary TMAO supplementation was shown to exacerbate hepatic insulin signaling dysfunction and gluconeogenesis and adipose tissue inflammation, leading to increased insulin resistance, glucose intolerance, and hyperinsulinemia in high fat diet-fed wild type mice [34]. Another study showed that reducing TMAO levels via FMO3 inactivation improves plasma glucose and insulin levels while increasing TMAO via FMO3 overexpression enhances hepatic gluconeogenesis in LDL receptor-null mice [*35]. However, interpretation of results from the latter study should take into consideration that modulating FMO3 expression may have effects independent of TMAO, including a direct role of FMO3 in modulating peroxisome proliferator-activated receptor-α and Kruppel-like factor 15 pathways [*35] as well as FMO3 regulation of the forkhead box O1 transcription factor [*36]. Therefore, pharmacologic inhibition of FMO3 to reduce TMAO levels for cardiometabolic disease intervention should be approached cautiously with considerations given to other potential side effects. The inhibition of PLA2g1b and autotaxin in the digestive tract may be more promising because inhibitors that act exclusively in the digestive tract are likely to have minimal adverse effects [37].
CONCLUSION
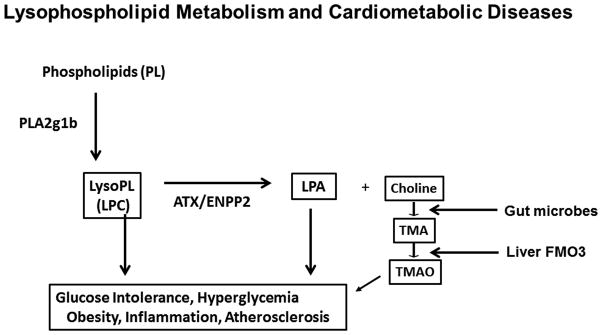
Phospholipid and lysosphospholipid metabolism in the digestive tract contributes to high fat diet-induced cardiovascular and metabolic disease risk through multiple mechanisms (summarized in Figure 1). Firstly, phospholipid hydrolysis by PLA2g1b, while not required for lipid absorption, generates lysophospholipids such as LPC to promote hyperlipidemia, systemic inflammation and cell dysfunction. Secondly, autotaxin-mediated catabolism of unsaturated LPC to unsaturated LPA generates additional bioactive metabolites with pro-inflammatory properties to promote cell dysfunction. Thirdly, the choline moiety generated from the autotaxin enzymatic reaction provides the substrate for microbial metabolism to generate TMA, which is ultimately delivered to the liver to produce TMAO to further enhance atherosclerosis. These mechanisms indicate that inhibition of phospholipase-autotaxin pathway in the digestive tract may be a viable strategy for cardiometabolic disease management.
FIGURE 1.
Schematic diagram depicting the multiple roles of intestinal phospholipid digestion and metabolism in cardiometabolic diseases. PLA2g1b, group 1b phospholipase A2; LPC, lysophosphatidylcholine; LPA, lysophosphatidic acid; ATX/ENPP2, autotaxin/ectonucleotide pyrophosphatase/phosphodiesterase family member 2; TMA, trimethylamine; TMAO, trimethylamine-N-oxide; FMO3, flavin monooxygenase-3.
KEY POINTS.
Lysophospholipids generated in the intestinal lumen from phospholipase A2 group 1b digestion of phospholipids modulate intestinal chylomicron assembly and systemic inflammation to promote hyperlipidemia, atherosclerosis, and metabolic diseases.
Autotaxin-mediated conversion of unsaturated lysophospholipids to unsaturated lysophosphatidic acids in the intestinal lumen also contribute directly to systemic inflammation and cardiometabolic diseases.
The choline moiety liberated from lysophosphatidylcholine-to-lysophosphatidic acid conversion is metabolized by gut bacteria to form trimethylamine that is ultimately oxidized to trimethylamine-N-oxide that directly enhances cardiovascular disease development.
Phospholipid remodeling and metabolism in the intestinal lumen directly promotes cardiometabolic disease and inhibition of this pathway may be an option for intervention.
Acknowledgments
Financial support and sponsorship
No financial support was received for the preparation of this manuscript. Research conducted in the author’s laboratory is supported by grants DK069967, DK074932, HL118001, and HL131028 from the National Institutes of Health.
Footnotes
Conflicts of Interest
None
REFERENCES AND RECOMMENDED READING
- 1.Ogden CI, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kakkar AK, Dahiya N. Drug treatment of obesity: current status and future prospects. Eur J Intern Med. 2015;26:89–94. doi: 10.1016/j.ejim.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beil FU, Grundy SM. Studies on plasma lipoproteins during absorption of exogenous lecithin in man. J Lipid Res. 1980;21:525–536. [PubMed] [Google Scholar]
- 5.Richmond BL, Boileau AC, Zheng S, et al. Compensatory phospholipid digestion is required for cholesterol absorption in pancreatic phospholipase A2 deficient mice. Gastroenterology. 2001;120:1193–1202. doi: 10.1053/gast.2001.23254. [DOI] [PubMed] [Google Scholar]
- 6.Hui DY. Phospholipase A2 enzymes in metabolic and cardiovascular diseases. Curr Opin Lipidol. 2012;23:235–240. doi: 10.1097/MOL.0b013e328351b439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takemori H, Zolotaryov FN, Ting L, et al. Identification of functional domains of rat intestinal phospholipase B/lipase. Its cDNA cloning, expression, and tissue distribution. J Biol Chem. 1998;273:2222–2231. doi: 10.1074/jbc.273.4.2222. [DOI] [PubMed] [Google Scholar]
- 8*.Siddiqi S, Mansbach CM. Dietary and biliary phosphatidylcholine activates PKCz in rat intestine. J Lipid Res. 2015;56:859–870. doi: 10.1194/jlr.M056051. This study shows that lysophospholipids generated in the intestinal lumen and absorbed into enterocytes are required for appropriate intracellular lipid trafficking that is necessary for chylomicron assembly and secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Jiang H, Ding T, et al. Deficiency in lysophosphatidylcholine acyltransferase 3 reduces plasma levels of lipids by reducing lipid absorption in mice. Gastroenterology. 2015;149:1519–1529. doi: 10.1053/j.gastro.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Kabir I, Li Z, Bui HH, et al. Small intestine but not liver lysophosphatidylcholine acyltransferase 3 (Lpcat3) deficiency has a dominant effect on plasma lipid metabolism. J Biol Chem. 2016;291:7651–7660. doi: 10.1074/jbc.M115.697011. This study reveals the importance of LPCAT3 expression in intestine in mediating dietary lipid absorption and plasma lipoprotein homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Rong X, Wang B, Dunham MM, et al. LPCAT3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. eLIFE. 2015;4:e06557. doi: 10.7554/eLife.06557. This paper shows that LPCAT3 mediated reesterification of LPC in the intestine remodels phospholipid composition and is required for appropriate chylomicron secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Wang B, Rong X, Duerr MA, et al. Intestinal phospholipid remodeling is required for dietary-lipid uptake and survival on a high fat diet. Cell Metab. 2016;23:492–504. doi: 10.1016/j.cmet.2016.01.001. This paper shows that intestinal LPCAT3 deficiency leads to poor survival of mice on high fat diet. The paper also reveals that LPCAT3 activity in the intestine is required for maintaining appropriate membrane phospholipid composition and chylomicron secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chattopadhyay A, Navab M, Hough G, et al. A novel approach to oral apoA-I mimetic therapy. J Lipid Res. 2013;54:995–1010. doi: 10.1194/jlr.M033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Navab M, Chattopadhyay A, Hough G, et al. Source and role of intestinally derived lysophosphatidic acid in dyslipidemia and atherosclerosis. J Lipid Res. 2015;56:871–887. doi: 10.1194/jlr.M056614. This paper shows that unsaturated LPA in the intestine is derived from autotaxin-mediated hydrolysis of unsaturated LPC whereas saturated LPC to saturated LPA conversion is autotaxin independent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navab M, Hough G, Buga GM, et al. Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid. J Lipid Res. 2013;54:3403–3418. doi: 10.1194/jlr.M042051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevastou I, Kaffe E, Mouratis M-A, Aidinis V. Lysoglycerophospholipids in chronic inflammatory disorders: The PLA2/LPC and ATX/LPA axes. Biochim Biophys Acta. 2013;1831:42–60. doi: 10.1016/j.bbalip.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Smyth SS, Mueller P, Yang F, et al. Arguing the case for the autotaxin-lysophosphatidic acid-lipid phosphate phosphatase 3-signaling nexus in the development and complications of atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:479–486. doi: 10.1161/ATVBAHA.113.302737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferry G, Tellier E, Try A, et al. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem. 2003;278:18162–18169. doi: 10.1074/jbc.M301158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boucher J, Quilliot D, Praderes JP, et al. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia. 2005;48:569–577. doi: 10.1007/s00125-004-1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rancoule C, Attane C, Gres S, et al. Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high fat diet obese mice. Diabetologia. 2013;56:1394–1402. doi: 10.1007/s00125-013-2891-3. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura S, Nagasaki M, Okudaira S, et al. ENPP2 contributes to adipose tissue expansion and insulin resistance in diet-induced obesity. Diabetes. 2014;63:4154–4164. doi: 10.2337/db13-1694. [DOI] [PubMed] [Google Scholar]
- 22.Federico L, Ren H, Mueller PA, et al. Autotaxin and its product lysophosphatidic acid suppress brown adipose differentiation and promote diet-induced obesity in mice. Mol Endocrinol. 2012;26:786–797. doi: 10.1210/me.2011-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dusaulcy R, Rancoule C, Gres S, et al. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res. 2011;52:1247–1255. doi: 10.1194/jlr.M014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. 2015;6:e02481. doi: 10.1128/mBio.02481-14. This paper shows that choline metabolism to TMA in the gastrointestinal tract is dependent on the type and composition of the gut microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. e1711–1712. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang WHW, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Gregory JC, Buffa JA, Org E, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–5660. doi: 10.1074/jbc.M114.618249. This paper used fecal transplantation approach to document the role of gut microbiota composition in determining atherosclerosis susceptibility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Seldin MM, Meng Y, Qi H, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kB. J Am Heart Assoc. 2016;5:e002767. doi: 10.1161/JAHA.115.002767. This paper identifies one mechanism by which TMAO promotes vascular inflammation and increased atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang WHW, Wang Z, Shrestha K, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Cardiac Fail. 2015;21:91–96. doi: 10.1016/j.cardfail.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao X, Liu X, Xu J, et al. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–481. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 35*.Shih DM, Wang Z, Lee R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. This paper shows that FMO3 inhibition lowers plasma glucose and lipid levels to improve atherosclerosis, but the FMO3 effects are multi-factorial and not necessarily dependent on TMAO production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Miao J, Ling AV, Manthena PV, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. This paper shows that FMO3 regulates forkhead box O1 transcription factor in modulating atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charmot D. Non-systemic drugs: A critical review. Curr Pharm Des. 2012;18:1434–1445. doi: 10.2174/138161212799504858. [DOI] [PMC free article] [PubMed] [Google Scholar]