Abstract
There is demand for non-dimethyl sulfoxide (DMSO) cryoprotective agents that maintain cell viability without causing poor postthaw function or systemic toxicity. The focus of this investigation involves expanding our understanding of multicomponent osmolyte solutions and their ability to preserve cell viability during freezing. Controlled cooling rate freezing, Raman microscopy, and differential scanning calorimetry (DSC) were utilized to evaluate the differences in recovery and ice crystal formation behavior for solutions containing multiple cryoprotectants, including sugars, sugar alcohols, and small molecule additives. Postthaw recovery of mesenchymal stem cells (MSCs) in solutions containing multiple osmolytes have been shown to be comparable or better than that of MSCs frozen in 10% DMSO at 1°C/min when the solution composition is optimized. Maximum postthaw recovery was observed in these multiple osmolyte solutions with incubation times of up to 2 h before freezing. Raman images demonstrate large ice crystal formation in cryopreserved cells incubated for shorter periods of time (∼30 min), suggesting that longer permeation times are needed for these solutions. Recovery was dependent upon the concentration of each component in solution, and was not strongly correlated with osmolarity. It is noteworthy that the postthaw recovery varied significantly with the composition of solutions containing the same three components and this variation exhibited an inverted U-shape behavior, indicating that there may be a “sweet spot” for different combinations of osmolytes. Raman images of freezing behavior in different solution compositions were consistent with the observed postthaw recovery. Phase change behavior (solidification patterns and glass-forming tendency) did not differ for solutions with similar osmolarity, but differences in postthaw recovery suggest that biological, not physical, methods of protection are at play. Lastly, molecular substitution of glucose (a monosaccharide) for sucrose (a disaccharide) resulted in a significant drop in recovery. Taken together, the information from these studies increases our understanding of non-DMSO multicomponent cryoprotective solutions and the manner by which they enhance postthaw recovery.
Keywords: : mesenchymal stromal cell, cryopreservation, osmolyte
Background
Mesenchymal stem cells (MSCs) have generated much interest in the field of cell therapies and the unique biological properties of this cell type have led to the development of MSC-based therapies for a wide range of diseases, see Sharma et al.1 for review. The ability to preserve cells enables transportation of the cells from the site of manufacture to the site of administration and coordination of the therapy with patient availability. Conventional methods of cell freezing typically use the cryoprotective agent dimethyl sulfoxide (DMSO), which is associated with poor postthaw behavior2,3 and with dangerous systemic side effects.4
There is demand for non-DMSO cryoprotective agents that maintain cell viability without causing systemic toxicity or poor postthaw function. Single molecule replacement of DMSO has been explored, but results have been suboptimal. Multicomponent solutions have also been explored and factorial experiments to identify favorable combinations and freezing protocols have shown increased success.5,6 Previously published work by this group7 describes algorithm optimization of multicomponent cryopreservative solutions, including molecules that target different aspects of cell protection during freezing.
Diverse biological systems (plants, insects, etc.) survive high salt environments, dehydration, drought, freezing temperatures, and other stresses through the use of osmolytes.8 It is common for multiple osmolytes to be present to stabilize the cells. For example, a mixture of five osmolytes has been used to stabilize the cells9 in the human kidney ex vivo. Sugars, specifically trehalose, have been studied extensively for the osmoprotective effects they exert and have shown moderate cryoprotectivity at appropriate concentrations.10–12 Polyols (such as glycerol) have been used as cryoprotectants for proteins, functionally stabilizing protein structure by maintaining hydrogen bonding in protein-bound water.13,14 Amino acids have been utilized for liposomal cryopreservation,15,16 and ampholytic amino acid polymers have demonstrated cryoprotection and result in cellular recoveries similar to DMSO.17,18 Evidence from several groups indicates that the use of multiple cryoprotectants from the different families above, such as trehalose and proline,16 or trehalose and glycerol,19 exhibit improved cellular survival compared with either cryopreservative alone, suggesting that additive13 or synergistic stabilizing effects are possible when multiple cryopreservatives are used.20 However, the concentrations of these cryoprotectants dictate whether they will be stabilizing or destabilizing21,22 and concentrations that result in stabilization optimums may differ when cryoprotectants are combined.23
In a previous study, we established our ability to optimize the composition of a multicomponent osmolyte solution7 using a computational algorithm. The focus of this investigation involves expanding our understanding of multicomponent osmolyte solutions and their ability to preserve cell viability during freezing. The study characterizes interactions between solutes using surface plots to describe how concentrations of multiple components contribute to recovery. Differences in single cell response to freezing using different experimental parameters (incubation time, composition) were captured using low-temperature Raman spectroscopy. These studies will help expand our understanding of these solutions and the manner by which they enhance postthaw recovery.
Materials and Methods
Cell culture
Human H9 ESC-derived MSCs were isolated and generously gifted for this research by Trivedi and Hematti.24 MSCs were cultured in alpha-MEM (Gibco) supplemented with nonessential amino acids (Gibco) and 10% FBS (qualified; Gibco) in a 37°C incubator at 5% CO2. Cell confluency was maintained between 20% and 80% and media were changed every 3–4 days. Cells were used for experiments only between passages 8 and 12.
Cell freezing
Cells diluted in Normosol-R™ blank solution (Hospira) were transferred to freezing vials and an equal volume of cryoprotectant solutions at 2 × their final concentrations were added to the vials stepwise. Ten percent DMSO controls were also prepared, in which cells suspended in MSC media were added to vials and DMSO introduced in the same stepwise fashion. For incubation studies, these cell suspensions were incubated at room temperature for 0, 30 min, 1, 2, or 4 h before freezing according to the following protocol using a controlled rate freezer (Planer Series III Kryo 10):
1. Starting temp 20°C
2. −10°C/min to 0°C
3. Hold at 0°C for 15 min
4. −1°C/min to −8°C
5. −50°C/min to −45°C
6. +15°C/min to −12°C
7. −3°C/min to −100°C
The final 1× concentrations present with cells for each solution tested are listed in the Results section.
Thawing
Frozen vials were thawed in a 37°C water bath. Vials were submerged halfway and agitated until only a miniscule ice pellet remained. Cells were assessed for viability immediately postthaw.
Viability and functionality
Cells were assessed for viability at the conclusion of each experiment. Both prefreeze and postthaw vial counts were performed using Acridine Orange/Propidium Iodide (AO/PI). Briefly, cells were combined with AO/PI, loaded into a hemocytometer, and counted manually using a fluorescent microscope. A minimum of 200 total cells were counted for each sample.
Viability was calculated for all samples by dividing the number of live cells by the number of total cells. Recovery was calculated directly by dividing the number of live cells postthaw by the number of live cells prefreeze.
Cell surface phenotype of the cells was determined using flow cytometry both before and after freezing. Cells in all prefreeze and postthaw populations were >99% positive for MSC markers (CD73, CD90, CD105) and negative for hematopoietic marker CD45 (data not shown). These results are consistent with the MSC phenotype.25
Osmolarity
Osmolarity of solutions was measured using an OSMETTE ™ osmometer (Precision Systems) for each solution and all measurements were repeated in triplicate.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) was performed on a TA Differential Scanning Calorimeter Q1000. Experimental solutions without cells were frozen to −150°C using the following protocol:
1. Set starting temperature to 20°C
2. Cool to −150°C at 10°C/min
3. Hold for 3 min at −150°C
4. Warm to 20°C at 10°C/min
Confocal Raman system
Confocal Raman microspectroscopy measurements were conducted using a WITec Confocal Raman Microscope System Alpha 300R (WITec, Ulm, Germany) with a UHTS300 spectrometer and DV401 CCD detector with 600/mm grating. The WITec spectrometer was calibrated with a Mercury–Argon lamp. A wavelength of 532 nm Nd:YAG laser powered at 10 mw was used as an excitation source. The laser was transmitted to the microscopy in a single fiber. A 100× air objective (NA 0.90; Nikon Instruments, Melville, NY) was used for focusing the 532-nm excitation laser to the sample. Samples were frozen using a controlled temperature stage described in more detail elsewhere.26
Raman measurement of frozen MSC cells
MSC cells were trypsinized and washed with DPBS solution before being suspended in experimental solutions. Roughly 1 μL of cell suspension was placed on an aluminum sheet, covered with a piece of mica (Ted Pella, Redding, CA) and sealed with Kapton tape (DuPont, Wilmington, DE), to prevent evaporation/sublimation during each experiment. Cell suspensions were cooled to −6°C, at which point the sample was seeded by a nitrogen-cooled needle. Subsequently, the solution was cooled down at 3°C/min to −50°C. Ten Raman images of 30 × 30 μm were collected.
Raman image/spectral analysis
Spectrums at each pixel were analyzed using characteristic wave numbers of common intracellular and extracellular materials (Table 1), and were integrated with background subtraction to result in an image. Spectra for the osmolytes used in the investigation overlapped, so a broad peak centered at 850 cm−1 was used to generate Raman images for all osmolytes. Data analysis was performed by Windows-based Project FOUR software plus version 4.0.
Table 1.
Peak Assignments for Molecules of Interest Detected Using Raman Spectroscopy
Statistics
Averages plus or minus standard error of the mean are reported unless otherwise noted. Student's t-tests were performed to determine statistically significant differences (p < 0.05) between samples tested in the osmolarity and sugar substitution studies.
Results
Permeation/partitioning of solute
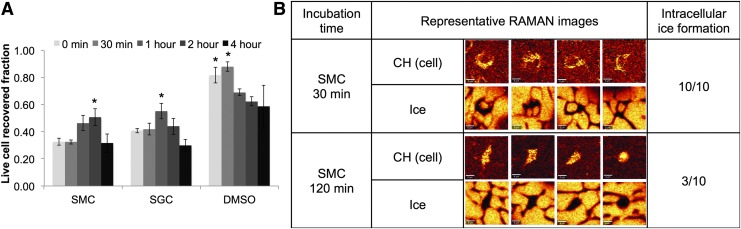
Several of the osmolytes studied here are larger (>∼300 Da) and, as a result, may take longer to enter the cell. Other large molecules, including trehalose are known to have very limited penetration.32 To determine the proper incubation time for the cells in the multicomponent osmolyte solutions, solutions of SMC (sucrose = 150 mM, mannitol = 125 mM, creatine = 12.5 mM) and SGC (sucrose = 150 mM, glycerol = 2.5%, creatine = 12.5 mM) were incubated with cells at room temperature in Normosol® for 0, 30 min, 1, 2, or 4 h before undergoing freezing at 3°C/min. Live cell recovery increased and experienced a maximum at 1 h for SGC samples (significantly greater than at least one other time point, p < 0.05), and 2 h for SMC samples (significantly greater than at least one other time point, p < 0.05). Longer incubation times (4 h) decreased the viability. As expected, incubation times of more than 30 min resulted in a decrease in viability for cells in DMSO.
Increased incubation times have a profound influence on cell response to freezing as imaged using Raman spectroscopy (Fig. 1B). MSCs were incubated for 30 and 120 min in the same SMC composition described above, frozen to −50°C, and imaged using low-temperature Raman spectroscopy. Cells incubated for 30 min exhibited large internal ice crystals for 10/10 of the cells imaged. In contrast, cells incubated for 120 min exhibited ice in only 3/10 cells imaged. The formation of ice inside the cell is considered to be a damaging event and the ice formation observed here is consistent with the poorer postthaw viability observed in Figure 1A for cells incubated for only 30 min.
FIG. 1.
Recovery reaches a maximum with appropriate incubation time for slow penetrating components. (A) Fraction of live cells recovered for DMSO-free solutions, SMC and SGC, and DMSO solutions as a function of incubation time before freezing at 3°C/min; significance markers indicate significantly greater recovery (t-test) than at least one other time point for the same solution. Only 1-h SGC and 2-h SMC statistically outperformed other time points (p < 0.05). Both DMSO 0 h and DMSO 30 min outperformed other time points, but were statistically dissimilar from one another. (B) Raman images obtained of MSCs frozen at 3°C/min in SMC solution after 30 and 120 min of incubation before freezing. Raman images are rendered for both −CH and ice. DMSO, dimethyl sulfoxide; MSCs, mesenchymal stem cells. Color images available online at www.liebertpub.com/tec
Influence of osmolarity and composition
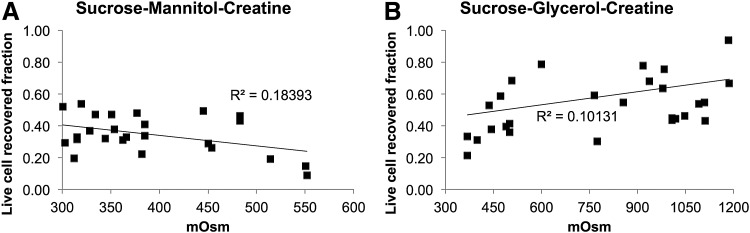
The range of solution compositions studied is important. It is common for cryopreservation solutions to contain high concentrations of cryoprotective agents and therefore exhibit high solution osmolarity. For example, a 10% DMSO solution has an osmolarity of ∼1400 mOsm. MSCs suspended in different combinations of sucrose, mannitol, and creatine (SMC), and sucrose, glycerol, and creatine (SGC) were frozen at 3°C/min, thawed, and postthaw recovery measured (information regarding solution composition and corresponding recovery and osmolarity is listed in the Appendix [Tables A1 and A2]). The postthaw recovery of SGC and SMC were plotted as a function of total osmolarity for a range of different tested compositions (Fig. 2). For SMC solutions, the range of solution osmolarities is low (<500 mOsm) and there is a weak negative correlation between osmolarity and postthaw recovery of MSCs (Fig. 2A, R2 = 0.18393). In contrast, SGC solutions were evaluated over a higher range of osmolarities (<1200 mOsm) and exhibited a weak positive correlation with the compositions tested (Fig. 2B, R2 = 0.10131). These weak correlations suggest that higher solution concentration does not necessarily correlate to higher levels of postthaw recovery.
FIG. 2.
Postthaw recovery of MSCs cryopreserved in SGC and SMC as a function of total solution osmolarity. Linear best fit is given with correlation coefficient. (A) Recovery of cells has slight negative correlation for different osmolarity sucrose–mannitol–creatine solutions. (B) Recovery of cells has slight positive correlation for different osmolarity sucrose–glycerol–creatine solutions.
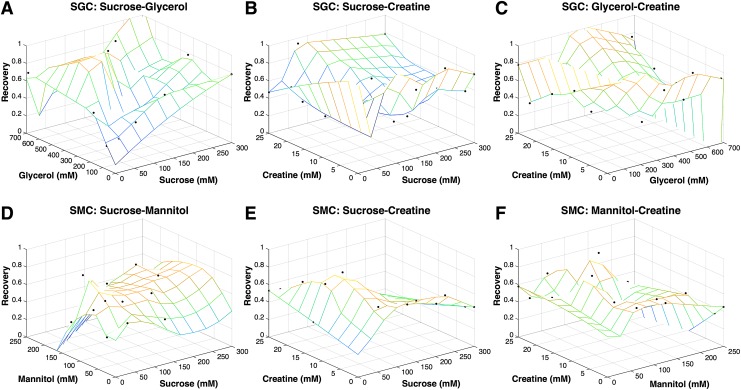
For the same three-component solution compositions used in Figure 2, MATLAB was used to generate scattered interpolant plots that mapped the postthaw recovery measured as a function of solution composition for two of the three components present in solution using 20–25 experimental points (recovery was averaged for vectors with same values for the two components being plotted). As described previously, the postthaw recovery varied significantly with composition for solutions containing the same three components (Fig. 3). It is noteworthy that the variation in the fraction of recovered cells postthaw exhibited an inverted U-shaped behavior. For example, the relationship between sucrose and creatine exhibits a maximum cell recovery for concentrations ∼150 mOsm of sucrose (Fig. 3B, E). The fraction of recovered cells diminishes for concentrations above and below that optimum. Something similar is observed in Figure 3D, where the optimum concentration of mannitol ∼150 mOsm and the fraction of recovered cells increases with increasing sucrose concentration to the highest concentration tested (300 mOsm).
FIG. 3.
Scattered interpolant meshgrid plots of recovery versus two of three components for SGC (A–C) and SMC (D–F) solutions. Color images available online at www.liebertpub.com/tec
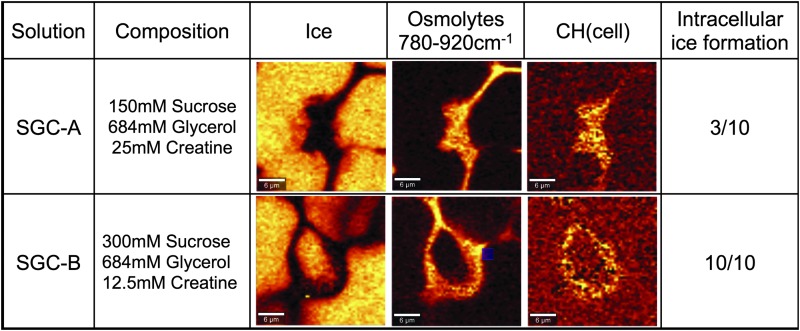
To understand differences in freezing response for different combinations of the same three osmolytes, freezing studies using two different compositions of SGC with similar total osmolarity (SGC-A: 150 mM Sucrose, 684 mM Glycerol, 25 mM Creatine and SGC-B: 300 mM Sucrose, 684 mM Glycerol, 12.5 mM Creatine) were repeated (Fig. 4). MSCs were frozen under the same conditions (1 h incubation and 3°C/min cooling rate) and imaged using low-temperature Raman confocal microscopy. Cells frozen in SGC-B exhibited ice inside the cells for 10/10 cells imaged and the formation of ice inside the cell is known to be damaging.33 In contrast, cells cryopreserved in SGC-A exhibited intracellular ice formation (3/10 cells imaged) implying that 7/10 cells survived freezing. Postthaw recovery trends (average ± SEM, n = 4) for cells frozen in SGC-A (0.82 ± 0.07) and SGC-B (0.71 ± 0.05) using conventional controlled rate freezing were consistent with the ice formation trends observed using Raman, in that SGC-A had higher recovery and fewer cells with ice crystal formation than SGC-B.
FIG. 4.
Low-temperature Raman microscopy of MSCs cryopreserved at 3 C/min in SGC with two different compositions. Images are rendered on ice, osmolyte mixture, and −CH. The fraction of cells with ice is described for 10 cells measured. Color images available online at www.liebertpub.com/tec
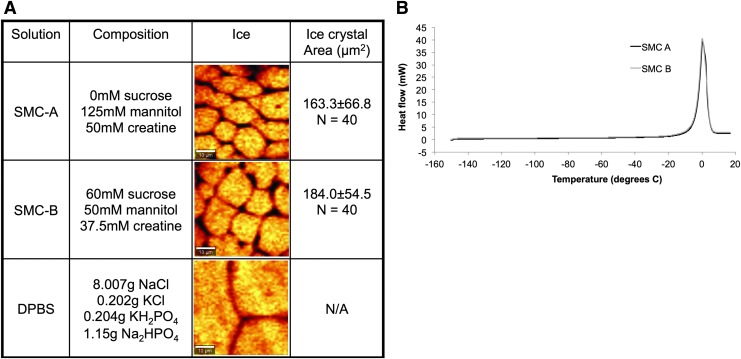
Solidification behavior
It is common to formulate cryopreservation solutions to result in full or partial vitrification (see Ref.34 for review). The next phase of the investigation involved characterizing the solidification behavior of the solutions tested. SMC solutions with similar osmolarity (SMC-A: 0 mM Sucrose, 125 mM Mannitol, 50 mM Creatine and SMC-B: 60 mM Sucrose, 50 mM Mannitol, 37.5 mM Creatine) and significantly different (p < 0.05) recovery (recovery ± SEM, n = 4; SMC-A = 0.73 ± 0.03, SMC-B = 0.62 ± 0.02) were seeded at −6°C, cryopreserved without cells (solution only) at 3°C/min, and imaged using Raman microscopy. Representative images are given in Figure 5A. There is little difference in the macroscopic solidification patterns observed between the two solutions imaged (in spite of the significant difference in postthaw viability observed between the two solutions). There is also no statistical difference in the area of ice crystals between the two solutions (p > 0.05). As a control, a DPBS solution was seeded at 0°C, cryopreserved at 3°C/min, and imaged using Raman microscopy. Only very small amount of solution was incorporated into ice and hydrohalite could be found in the narrow solution gap based on Raman spectra.
FIG. 5.
Physical behavior of multicomponent solutions associated with different recovery. (A) Low-temperature Raman spectroscopy of two SMC solutions at −50°C. Two different compositions with similar osmolarity were studied: one associated with high recovery (SMC-A) and the other associated with lower recovery (SMC-B). The average ice crystal size for the two solutions was not statistically significantly different (p > 0.05). (B) DSC thermograph for the same SMC solutions during warming. The melting curves are almost identical and display a large exotherm associated with melting of the solution, and no glass formation is observed for either solution. Color images available online at www.liebertpub.com/tec
Additional studies were performed using DSC to determine whether the different compositions exhibited differences in glass-forming tendency (Fig. 5B). No change in the melting curves was observed for either pair, and no signs of full or partial vitrification of the solutions were observed using DSC.
Component substitution
There has been considerable interest in the use of sugars (specifically trehalose) for the preservation of cells.32 The next phase of the investigation involved determining whether the structure of the sugar has a significant influence on postthaw recovery. Specifically, a monosaccharide, glucose, was substituted for a disaccharide, sucrose, at the same concentration for both SMC and SGC compositions (Table 2).
Table 2.
| Solution name | Sugar (conc.) | Sugar alcohol (conc.) | Additive (conc.) |
|---|---|---|---|
| SMC | Sucrose (150 mM) | Mannitol (125 mM) | Creatine (12.5 mM) |
| GMC | Glucose (150 mM) | Mannitol (125 mM) | Creatine (12.5 mM) |
| SGC | Sucrose (150 mM) | Glycerol (2.5%) | Creatine (12.5 mM) |
| GGC | Glucose (150 mM) | Glycerol (2.5%) | Creatine (12.5 mM) |
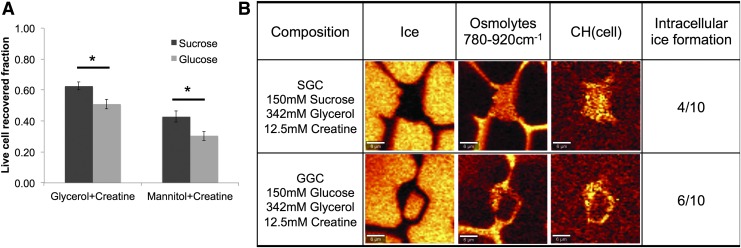
When a monosaccharide is substituted for a disaccharide for both of the solution compositions tested, the postthaw recovery of MSCs is reduced and those differences are statistically significant (Fig. 6A, p < 0.05). Cells in SGC and GGC were cryopreserved and imaged using Raman spectroscopy. For cells cryopreserved in SGC, Raman images demonstrated that a small fraction of the cells exhibited ice during freezing (4/10) when compared with cells cryopreserved in a solution containing GGC exhibiting ice inside the cell during freezing (6/10) (Fig. 6B). Images of the cell freezing response are consistent with the controlled rate freezing experiments described in Figure 6A.
FIG. 6.
Sugar substitution results in different recovery and ice formation behavior (A) postthaw recovery of MSCs cryopreserved in glycerol+creatine and mannitol+creatine solutions with either sucrose or glucose [n = 9, error bars represent standard error of the mean, sucrose solutions had significantly higher recovery than glucose solutions (p < 0.05)] (B) Low-temperature Raman microscopy of MSCs cryopreserved at 3°C/min in SGC and GGC concentrations listed. Images are rendered on ice, osmolyte mixture, and −CH. The fraction of cells with ice is consistent with cell recovery described in (A). Color images available online at www.liebertpub.com/tec
Discussion
Permeation/partitioning of solutes
Conventional cryoprotective agents (DMSO and glycerol) have small molecular weights (78 and 92 Da, respectively) and permeate the cell in a matter of minutes (see Ref. 35 for review). Little has been done to characterize the uptake of osmolytes with the exception of trehalose,36 which appears to penetrate poorly into the cell. The results of this investigation suggest that longer incubation times may be needed for cells frozen with non-DMSO cryoprotectants to allow for penetration of larger components and elicit maximum cell recovery. Raman measurement of mannitol concentrations inside and outside the cell indicated that permeation for this molecule takes ∼90 min (data not shown), which is consistent with the results of this study. The observation that postthaw recovery increases and the prevalence of ice inside the cell decreases with increasing incubation time also suggests that the presence of osmolytes inside the cell is needed for protection against intracellular ice formation during freezing. This observation is consistent with previous studies of the protective effect of trehalose,37 which demonstrated a similar outcome.
Influence of osmolarity and composition
It has been postulated that one mechanism of action for cryoprotective agents results from a colligative effect.38 Specifically, higher concentrations of cryoprotectants reduce concentrations of damaging solutes and thereby protect the cell. In contrast, the outcome of this investigation suggests that there is little relationship between total osmolarity of the solution and postthaw recovery for multiosmolyte solutions (Fig. 2). Similarly, the solidification behavior of the solutions (crystallization patterns and DSC thermograms) for the different compositions tested does not exhibit significant differences from one SGC or SMC composition to the other. The results of this investigation (in particular Fig. 2) suggest that higher levels of cryoprotective agents are not necessarily better and that the colligative effect does not play a role in improving recovery with these solutions. However, combinations of additives are effective (Fig. 3), and optimized compositions may result in higher recovery. We are in the process of completing additional studies to prove that supposition.
Additionally, as shown in Figure 5, there was no change in the physical solution behavior at these low osmolarity solution concentrations below −30°C. There is no eutectic peak indicating that a glass transition occurred in any of the samples at these concentrations (it would be expected around −80°C if it existed). Because this physical change is absent, we hypothesize that the mechanism of protection for these solutions is biological. This suggests that there is a biological “sweet spot” solution composition that results in maximum cell recovery.
One reason these solution compositions may be successful could be the result of their interactions to stabilize proteins within the cell. Osmolytes are well known for stabilizing protein folding by providing a thermodynamically unfavorable environment for denatured proteins,39 including during cryopreservation.40 Additionally, mixtures of two different osmolyte monomers have been found to have better stabilization effects than that of a single type of monomer at the same molar concentrations.41 Thus, literature supports the possibility that different osmolytes could stabilize different proteins and different parts of the cell to result in increased viability. Within mixtures of osmolytes, each osmolyte exerts independent protein stabilization effects.42 This effect is due to the low binding affinity between osmolytes and proteins, resulting in neither competition nor cooperation between osmolytes.42 Therefore, the activity of each osmolyte should be considered individually without cooperation between them.
Different combinations of osmolytes may also stabilize the cell membrane. Both surface and internal membranes are main areas of injury regardless of cooling rate.43 Normal cells have a layer of water surrounding the cell surface, which helps maintain surface protein folding.44 During freezing, the concentration of liquid water is decreased, which can lead to destabilization of these proteins.44 Combined with observations that membrane proteins are denatured and lost postthaw,44 stabilization of proteins in the membrane is very important to the success of cryopreservation. Meryman proposed that conformational stability of surface macromolecules depends on the interactions of solute and water at the cell surface,44 and osmolytes themselves have been postulated to replace water in the cell membrane during freezing.45 Osmolytes also play a role in preferential exclusion, which has been associated with protein stabilization caused by increases in surface tension due to sugars and amino acids present in solution.46 In the solutions used in this study, each component could differ in location (intracellular or extracellular), the macromolecules it stabilizes, and the surface area it stabilizes. These differences could help to protect multiple critical structures, thus decreasing the cumulative damage to the cell.
It is well established in literature that larger sugar osmolytes provide better stabilization.22,41,47 due to their increased polar contact area.41 Sugars can provide stabilization by replacing water surrounding membranes during dehydration.40,41 The trend observed for improved disaccharide behavior may be due to insertion between the phospholipid heads of the lipid membrane, creating more space than monosaccharides for additional binding.47 Binding of sugars to membranes can increase the rigidity of the membrane, which provides greater resistance to disruption.47 Cells dehydrate at slow cooling rates, making stabilization with osmolytes important. During thawing, changes in the protein environment could induce denaturing, but osmolytes, including sugars, can help with stabilization. This can reduce damage to membrane proteins, as well as internal proteins.
In a study that supplemented cryopreservation media with monosaccharides or disaccharides during freezing of boar sperm, glucose was found to be the most effective of the monosaccharides in maintaining postthaw sperm quality.48 Gómez-Fernández et al. found that disaccharides containing the same monomers with different links had similar results.48 Additionally, disaccharides are known for being better stabilizers than monosaccharides,41,48 and this is supported by the results presented in Figure 6.
This work assesses postthaw recovery of cells frozen in non-DMSO solutions. Another pending publication from this group will address the postthaw functionality of MSCs in more depth, and includes data confirming that MSCs frozen in non-DMSO solutions maintain both multilineage differentiation ability, and appropriate surface marker expression (greater than 99% positive for CD73, CD90, and CD105 surface markers, and less than 1% positive for CD45 for both SMC and SGC frozen cells).
As the field of cryobiology expands to include new applications that require nontoxic alternatives to DMSO, it is important to understand the role of multicomponent solutions in preserving cell function. In this study, we demonstrate that incubation time must be sufficient for penetration of components, concentration of components (not total osmolarity) determines recovery, and molecular substitution results in changes in recovery. We hypothesize that these differences are biological, as we have found no evidence of physical changes in ice crystal formation between these solutions.
Ultimately, these observations increase our understanding of multicomponent solution behavior, and move the field closer to clinically acceptable DMSO alternatives for cryopreservation of MSCs and other cryosensitive cell types.
Appendix
Table A1.
| SMC Concentrations | ||||
|---|---|---|---|---|
| Sucrose (mM) | Mannitol (mM) | Creatine (mM) | Live cell recovered fraction | Osmolarity |
| 75 | 63 | 13 | 0.47 | 351 |
| 225 | 188 | 3 | 0.43 | 483 |
| 225 | 125 | 0 | 0.29 | 451 |
| 0 | 63 | 25 | 0.54 | 319 |
| 75 | 125 | 3 | 0.48 | 377 |
| 30 | 25 | 25 | 0.31 | 316 |
| 225 | 188 | 3 | 0.46 | 483 |
| 150 | 63 | 6 | 0.41 | 385 |
| 0 | 25 | 25 | 0.52 | 301 |
| 75 | 63 | 19 | 0.38 | 354 |
| 30 | 0 | 25 | 0.29 | 303 |
| 30 | 63 | 13 | 0.37 | 328 |
| 150 | 63 | 0 | 0.22 | 382 |
| 30 | 25 | 25 | 0.33 | 316 |
| 300 | 250 | 3 | 0.09 | 552 |
| 150 | 188 | 3 | 0.49 | 446 |
| 30 | 25 | 19 | 0.20 | 312 |
| 150 | 25 | 6 | 0.33 | 366 |
| 0 | 125 | 13 | 0.32 | 344 |
| 150 | 63 | 6 | 0.34 | 385 |
| 30 | 63 | 25 | 0.47 | 334 |
| 30 | 125 | 19 | 0.31 | 362 |
| 300 | 250 | 0 | 0.15 | 551 |
| 150 | 188 | 19 | 0.26 | 454 |
| 225 | 250 | 3 | 0.19 | 514 |
Table A2.
| SGC concentrations | ||||
|---|---|---|---|---|
| Sucrose (mM) | Glycerol (mM) | Creatine (mM) | Live cell recovered fraction | Osmolarity |
| 225 | 684 | 0 | 0.94 | 1184 |
| 300 | 342 | 0 | 0.78 | 917 |
| 30 | 342 | 25 | 1.02 | 672 |
| 300 | 0 | 25 | 0.78 | 600 |
| 300 | 171 | 19 | 0.59 | 765 |
| 0 | 684 | 19 | 0.64 | 978 |
| 75 | 68 | 19 | 0.53 | 437 |
| 30 | 171 | 25 | 0.36 | 501 |
| 75 | 68 | 25 | 0.38 | 443 |
| 225 | 684 | 3 | 0.67 | 1187 |
| 0 | 684 | 25 | 0.76 | 984 |
| 150 | 513 | 0 | 0.68 | 938 |
| 30 | 68 | 25 | 0.31 | 398 |
| 225 | 513 | 6 | 0.44 | 1019 |
| 75 | 684 | 13 | 0.46 | 1047 |
| 75 | 342 | 25 | 1.13 | 717 |
| 150 | 513 | 0 | 1.10 | 938 |
| 225 | 342 | 13 | 0.55 | 855 |
| 300 | 513 | 3 | 0.54 | 1091 |
| 150 | 684 | 3 | 0.43 | 1112 |
| 0 | 171 | 25 | 0.58 | 471 |
| 150 | 684 | 0 | 0.54 | 1109 |
| 0 | 68 | 25 | 0.33 | 368 |
| 150 | 342 | 6 | 0.30 | 773 |
| 30 | 684 | 19 | 0.45 | 1008 |
| 0 | 68 | 25 | 0.21 | 368 |
| 30 | 171 | 13 | 0.39 | 489 |
| 150 | 68 | 13 | 0.68 | 506 |
| 30 | 684 | 19 | 0.43 | 1008 |
| 30 | 171 | 25 | 0.41 | 501 |
Acknowledgments
The authors would like to thank Sriharsha Mushnoori, Joe Budenske, and Elizabeth Moy for their assistance in performing experiments. This work was funded by grant R21 EB016247-01A1. Parts of this work were carried out in the Characterization Facility, University of Minnesota, which receives partial support from NSF through the MRSEC program.
Disclosure Statement
No competing financial interests exist.
References
- 1.Sharma R.R., Pollock K., Hubel A., and McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion 54, 1418, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moll G., Alm J.J., Davies L.C., Von Bahr L., Heldring N., Stenbeck-Funke L., et al. . Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 32, 2430, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinnadurai R., Garcia M.A., Sakurai Y., Lam W.A., Kirk A.D., Galipeau J., et al. . Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Reports 3, 60, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windrum P., Morris T.C.M., Drake M.B., Niederwieser D., and Ruutu T. Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centres. Bone Marrow Transplant 36, 601, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Dong Q., Hill D., and VandeVoort C.A. Interactions among pre-cooling, cryoprotectant, cooling, and thawing for sperm cryopreservation in rhesus monkeys. Cryobiology 59, 268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freimark D., Sehl C., Weber C., Hudel K., Czermak P., Hofmann N., et al. . Systematic parameter optimization of a Me2SO- and serum-free cryopreservation protocol for human mesenchymal stem cells. Cryobiology 63, 67, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Pollock K., Budenske J.W., McKenna D.H., Dosa P.I., and Hubel A. Algorithm-driven optimization of cryopreservation protocols for transfusion model cell types including Jurkat cells and mesenchymal stem cells. J Tissue Eng Regen Med 2016. DOI: 10.1002/term-2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yancey P.H., Clark M.E., Hand S.C., Bowlus R.D., and Somero G.N. Living with water stress: evolution of osmolyte systems. Science 217, 1214, 1982 [DOI] [PubMed] [Google Scholar]
- 9.Yancey P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208(Pt 15), 2819, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Buchanan S.S., Pyatt D.W., and Carpenter J.F. Preservation of differentiation and clonogenic potential of human hematopoietic stem and progenitor cells during lyophilization and ambient storage. PLoS One 5, 1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawangwan T., Goedl C., and Nidetzky B. Glucosylglycerol and glucosylglycerate as enzyme stabilizers. Biotechnol J 5, 187, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Lynch A.L., Chen R., Dominowski P.J., Shalaev E.Y., Yancey R.J., and Slater N.K.H. Biopolymer mediated trehalose uptake for enhanced erythrocyte cryosurvival. Biomaterials 31, 6096, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Guo F., and Friedman J.M. Osmolyte-induced perturbations of hydrogen bonding between hydration layer waters: correlation with protein conformational changes. J Phys Chem B 113, 16632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson M.E., Malardier-Jugroot C., and Head-Gordon T. Effects of co-solvents on peptide hydration water structure and dynamics. Phys Chem Chem Phys 12, 393, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Mohammed A.R., Coombes A.G.A., and Perrie Y. Amino acids as cryoprotectants for liposomal delivery systems. Eur J Pharm Sci 30, 406, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Rudolph A.S., and Crowe J.H. Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology 22, 367, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Matsumura K., and Hyon S.H. Polyampholytes as low toxic efficient cryoprotective agents with antifreeze protein properties. Biomaterials 30, 4842, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Matsumura K., Bae J.Y., and Hyon S.H. Polyampholytes as cryoprotective agents for mammalian cell cryopreservation. Cell Transplant 19, 691, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Cicerone M.T., and Soles C.L. Fast dynamics and stabilization of proteins: binary glasses of trehalose and glycerol. Biophys J 86, 3836, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasnoor L.M., Kale V.P., and Limaye L.S. Prevention of apoptosis as a possible mechanism behind improved cryoprotection of hematopoietic cells by catalase and trehalose. Transplantation 80, 1251, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Plank C., Zauner W., and Wagner E. Application of membrane-active peptides for drug and gene delivery across cellular membranes. Adv Drug Deliv Rev 34, 21, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Singh L.R., Poddar N.K., Dar T.A., Kumar R., and Ahmad F. Protein and DNA destabilization by osmolytes: the other side of the coin. Life Sci 88, 117, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Rösgen J. Synergy in protein—osmolyte mixtures. J Phys Chem B 119, 150, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trivedi P., and Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol 36, 350, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D.S., et al. . Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Dong J., Malsam J., Bischof J.C., Hubel A., and Aksan A. Spatial distribution of the state of water in frozen mammalian cells. Biophys J 99, 2453, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harkness L., Novikov S.M., Beermann J., Bozhevolnyi S.I., and Kassem M. Identification of abnormal stem cells using Raman spectroscopy. Stem Cells Dev 21, 2152, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Mendelovici E., Frost R.L., and Kloprogge T. Cryogenic Raman spectroscopy of glycerol. J. Raman Spectrosc 31, 1121, 2000 [Google Scholar]
- 29.De Veij M., Vandenabeele P., De Beer T., Remon J.P., and Moens L. Reference database of Raman spectra of pharmaceutical excipients. J Raman Spectrosc 40, 297, 2009 [Google Scholar]
- 30.Mathlouthi M., and Vinh Luu D. Laser-raman spectra of d-glucose and sucrose in aqueous solution. Carbohydr Res 81, 203, 1980 [Google Scholar]
- 31.Gangopadhyay D., Sharma P., and Singh R.K. Temperature dependent Raman and DFT study of creatine. Spectrochim Acta A Mol Biomol Spectrosc 150, 9, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Eroglu A., Russo M.J., Bieganski R., Fowler A., Cheley S., Bayley H., et al. . Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat Biotechnol 18, 163, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Toner M., Cravalho E.G., and Karel M. Thermodynamics and kinetics of intracellular ice formation during freezing of biological cells. J Appl Phys 67, 1582, 1990 [Google Scholar]
- 34.Fahy G.M., and Wowk B. Chapter 2 principles of cryopreservation by vitrification. Methods Mol Biol 1257, 978, 1007 [DOI] [PubMed] [Google Scholar]
- 35.McGrath J. Low Temperature Biotechnology: Emerging Applications and Engineering Contributions. New York, NY: ASME, 1988 [Google Scholar]
- 36.Abazari A., Meimetis L.G., Budin G., Bale S.S., Weissleder R., Toner M., et al. . Engineered trehalose permeable to mammalian cells. PLoS One 10, e0130323, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acker J.P., Lu X., Young V., Cheley S., Bayley H., Fowler A., et al. . Measurement of trehalose loading of mammalian cells porated with a metal-actuated switchable pore. Biotechnol Bioeng 82, 525, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Santarius K.A., and Giersch C. Cryopreservation of spinach chloroplast membranes by low-molecular-weight carbohydrates. Cryobiology 20, 90, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Arakawa T., and Timasheff S.N. The stabilization of proteins by osmolytes. Biophys J 47, 411, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowe J.H., Crowe L.M., Carpenter J.F., Rudolph A.S., Wistrom C.A., Spargo B.J., et al. . Interactions of sugars with membranes. Biochim Biophys Acta 947, 367, 1988 [DOI] [PubMed] [Google Scholar]
- 41.Poddar N.K., Ansari Z.A., Singh RKB, Moosavi-Movahedi A.A., and Ahmad F. Effect of monomeric and oligomeric sugar osmolytes on ΔGD, the Gibbs energy of stabilization of the protein at different pH values: is the sum effect of monosaccharide individually additive in a mixture? Biophys Chem 138, 120, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Holthauzen L.M.F., and Bolen D.W. Mixed osmolytes: the degree to which one osmolyte affects the protein stabilizing ability of another. Protein Sci 16, 293, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol Cell Physiol 247, C125, 1984 [DOI] [PubMed] [Google Scholar]
- 44.Meryman H.T. Freezing injury and its prevention in living cells. Annu Rev Biophys Bioeng 3, 341, 1974 [DOI] [PubMed] [Google Scholar]
- 45.Goodrich R.P., Crowe J.H., Crowe L.M., and Baldeschwieler J.D. Alterations in membrane surfaces induced by attachment of carbohydrates. Biochemistry 30, 5313, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Arakawa T., Carpenter J.F., Kita Y.A., and Crowe J.H. The basis for toxicity of certain cryoprotectants: a hypothesis. Cryobiology 27, 401, 1990 [Google Scholar]
- 47.Strauss G., and Hauser H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc Natl Acad Sci. U S A 83, 2422, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gómez-Fernández J., Gómez-Izquierdo E., Tomás C., Mocé E., and de Mercado E. Effect of different monosaccharides and disaccharides on boar sperm quality after cryopreservation. Anim Reprod Sci 133, 109, 2012 [DOI] [PubMed] [Google Scholar]