Abstract
The microvasculature is an extensive, heterogeneous, and complex system that plays a critical role in human physiology and disease. It nourishes almost all living human cells and maintains a local microenvironment that is vital for tissue and organ function. Operating under a state of continuous flow, with an intricate architecture despite its small caliber, and subject to a multitude of biophysical and biochemical stimuli, the microvasculature can be a complex subject to study in the laboratory setting. Engineered microvessels provide an ideal platform that recapitulates essential elements of in vivo physiology and allows study of the microvasculature in a precise and reproducible way. Here, we review relevant structural and functional vascular biology, discuss different methods to engineer microvessels, and explore the applications of this exciting tool for the study of human disease.
1. Introduction
The term microvasculature refers to vessels smaller than 100–150 μm that are generally located deep within the tissue of functioning organs. It is the interface between circulating blood and organ parenchyma (cells and tissues in the specific organ microenvironment), and functions to integrate systemic processes with changes in the local tissue microenvironment. As such, it plays an important role in homeostatic processes as diverse as organ perfusion/oxygenation, angiogenesis, thrombosis, fluid and solute balance, blood pressure, and inflammation [1]. Microvascular dysfunction can lead to dysregulation of these fundamental processes and is a central mediator in a variety of human diseases. Studies of the microvasculature and its role in human disease, however, have been hindered due to both a lack of accessibility in vivo and a lack of modeling tools in vitro. In the last decade, advances in microfabrication techniques have made it possible to recreate microvascular structure and function within a laboratory setting. This has made the study of the human microvasculature significantly more accessible, and research within engineered microvascular systems continues to enhance our understanding of fundamental biologic principles and human pathophysiology [2].
To successfully utilize engineered microvessels as a platform for the study of human disease and therapeutics, it is essential to understand the native biology that these laboratory systems seek to replicate, as well as the technical aspects involved in different approaches for microvessel formation. This review begins with a description of basic vascular biology including components of the microvasculature and related human diseases. Next, we provide a discussion of how vessels form naturally within the human body, as a prelude to discussing strategies used for the formation of microvessels in vitro. Finally, we discuss current and future applications of engineered microvessels for the study of human disease. The development of engineered microvessels for tissue or organ regeneration is an exciting related field that is beyond the scope of this review, and we direct the interested reader to contemporary articles on the topic for more information [2–4].
2. Background
2.1. Human Vascular Anatomy.
The human circulatory system can be divided into the pulmonary and systemic circulation. The systemic circulation carries oxygenated blood from the left side of the heart to the tissues of the body and, at the most basic level of function, provides oxygen and other nutrients to all tissues of the body while removing metabolites. Deoxygenated blood is then returned to the heart via a series of veins and passes through the pulmonary circulation where it is oxygenated and returned to the left heart. The blood vessels within the circulatory system (Fig. 1(a)) are classified based on their size and function into arteries (0.1 mm to >1 cm in size), arterioles (10–100 μm), capillaries (4–12 μm), venules (10–100 μm), and veins (0.1 mm to >1 cm). Large arteries and veins function as conducting vessels, and consistently have three layers within their walls: the tunica intima, tunica media, and tunica adventitia [5]. Microvessels comprise capillaries, arterioles, and postcapillary venules, and function as resistance and transport vessels to regulate hydrodynamics and the exchange of nutrients and waste products within tissues. Histologically, arterioles contain only one or two layers of smooth muscle cells and a thin, poorly defined, tunica adventitia. They control the regional perfusion of capillary beds through contraction of circumferentially arranged vascular smooth muscle cells (VSMCs) [5,6]. Capillaries lack either a tunica media or adventitia and are composed of a single layer of endothelial cells with an underlining basal lamina, surrounded by scattered pericytes which provide support and help maintain vascular integrity (Fig. 1(a)) [7,8].
Fig. 1.
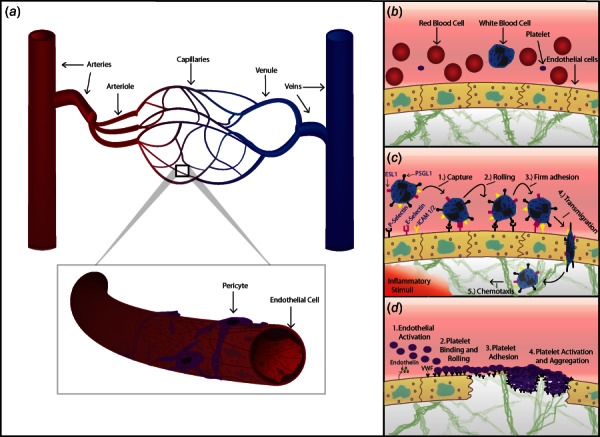
(a) Schematic of the systemic circulation. An artery is shown dividing into smaller arteries, arterioles, and capillaries. These drain into postcapillary venules, which collect into veins. A capillary is shown in magnification, consisting of a single layer of endothelial cells supported by scattered pericytes. (b) Circulating blood, including red blood cells, platelets, and a neutrophil are shown within a blood vessel bounded by endothelial cells. Vessels are supported by ECM components within the interstitium. (c) Leukocyte recruitment in response to inflammation. Cytokines released in response to inflammatory stimuli, such as infection, injury, allergen, or tumor, prompt endothelial cells to express leukocyte adhesion molecules including P-selectin and E-selectin. Initial capture occurs when these molecules bind ligands on circulating leukocytes such as P-selectin glycoprotein ligand-1 (PSGL-1) or E-selectin ligand-1 (ESL1). Leukocytes “roll” on these receptors and slow, allowing time for additional ligands on leukocytes to firmly adhere to the endothelium via receptors such as intercellular adhesion molecule (ICAM) 1 and 2. Leukocytes then transmigrate through the endothelium and toward inflammatory stimuli (chemotaxis). (d) Endothelial interactions and primary hemostasis. Upon vessel injury the endothelium rapidly adopts a procoagulant profile, secreting endothelin (which promotes local vasoconstriction), and releasing VWF which binds circulating platelets via their GP1B receptors. VWF is also expressed on subendothelial collagen and is exposed by injury. Platelets “carpet” the endothelium and roll forward until they slow and firmly adhere to exposed collagen. Platelet activation then occurs, which leads platelets to change shape, degranulate, and aggregate, forming the platelet plug of primary hemostasis.
2.2. Components of the Microvasculature and Their Role in Human Disease
2.2.1. The Endothelium.
The endothelium is the innermost lining of the vasculature, consisting of a single layer of elongated cells oriented in the direction of flow. It has a total surface area between 350 and 1000 m2, making it one of the largest “organs” in the body [1,9]. It interacts with blood in the lumen and perivascular cells on the vessel wall, and plays important roles in multiple homeostatic processes including regulation of vascular permeability, control of vasomotor tone, hemostasis, hormonal regulation, inflammation, and angiogenesis [10]. In normal physiologic states, the endothelium is quiescent and expresses an anticoagulant and anti-inflammatory phenotype. Endothelial cells contain specialized granules called Weibel–Palade bodies (WPBs), which are composed of proteins including von Willebrand factor (VWF) and P-selectin [11]. When exposed to circulating mediators or local stressors, endothelial cells can release their granules and rapidly adopt a procoagulant, pro-inflammatory phenotype, a process termed endothelial activation. The endothelium is remarkably heterogeneous, with morphology, protein expression, and gene expression varying between large and small vessels, artery and veins, between organs, and even within tissue of the same organ [9]. Such endothelial heterogeneity is both driven by, and contributes to, differences in the biochemical, cellular, and biophysical microenvironment, and results in marked differences in endothelial cell activation and dysfunction in response to stimuli [12].
Given that the endothelium forms a critical interface between blood and tissue, it is not surprising that endothelial activation and dysfunction is implicated in a diverse spectrum of human disease. For example, endothelial dysfunction occurs in processes such as hypertension, hypercholesterolemia, and atherosclerosis, which result in human pathology including coronary artery disease, stroke, and peripheral vascular disease [13–15]. Endothelial cell functions are also dysregulated in pulmonary hypertension, with the major available treatments for this disease focused on endothelial nitric oxide, endothelin, and prostacyclin signaling [16,17]. Endothelial activation is associated with many human vascular disorders, from inborn errors in coagulation to acquired disorders such as deep venous thrombosis, microangiopathic hemolytic disorders, and the vasoplegia and inflammation seen in sepsis [1,10,12,18–20]. Disruption of endothelial barrier function and enhanced leukocyte adhesion and transmigration across the endothelium can contribute to many diseases including sepsis [12], acute respiratory distress syndrome [21], and tumor progression [22].
Endothelial cells are joined by tight junctions and gap junctions, and are surrounded by a basement membrane. Whereas small lipid-soluble molecules can pass through endothelial cells via simple diffusion, other molecules are transported either paracellularly between endothelial cells, or transcellularly via either invagination of pinocytotic vesicles or via receptor-mediated endocytosis [5]. The structure of the endothelium varies by location to provide selective vascular permeability in different anatomic regions. Continuous endothelium is found in organs with desired low permeability, such as the central nervous system (CNS). Fenestrated endothelium allows easier diffusion of small hydrophilic molecules and water, and is found in organs such as the gastrointestinal tract and kidney glomeruli which are involved in secretion/filtration/absorption. Discontinuous endothelium allows solutes and macromolecules to diffuse and is found in the liver, bone marrow, and spleen, to allow for trafficking of proteins and cells [23]. In addition to morphologic differences, regional variation in permeability is manifested through varying levels of pinocytosis (infolding and budding-off of the cell membrane to encapsulate and transport materials) and differential expression of tight-junction proteins such as the claudins, junctional adhesion molecule (JAM-1), and occludin. The blood–brain barrier, for example, is formed from endothelium with extensive tight junction formation, and also exhibits low levels of pinocytosis [9].
Far from an inert conduit for blood, the endothelium has become recognized as a heterogeneous and dynamic system that plays a vital role in human physiology and disease [10,24]. Our understanding of the endothelium's role in these and other diseases has increased markedly over the several decades following the development of endothelial cell culture techniques in the 1970s [25]. The advent of engineered microvessels, which model the heterogeneous microvasculature seen in vivo, promises to further enhance our understanding of endothelial biology and its role in human disease.
2.2.2. Pericytes.
Pericytes are perivascular cells which are intimately associated with the endothelium of the microvasculature. Once believed to play mainly a structural role in their associations with native vasculature, pericytes have recently drawn intense research interest, as understanding of their integral role in vascular biology has grown. Larger vessels are generally characterized by the presence of VSMCs, but may have pericytelike cells within their subendothelium [26]. Pericytes are fibroblastlike in appearance, with a large nucleus and cytoplasmic projections which travel longitudinally over the surface of capillaries [8,27,28]. These cells share a basement membrane with endothelial cells and can communicate both mechanically and chemically with them. They are able to contact multiple endothelial cells, even across different capillaries [29]. Pericytes can regulate capillary diameter via contraction or dilation in response to circulating vasoactive mediators [8]. They can also support endothelial integrity, and higher pericyte density around capillaries is associated with lower vascular permeability. For example, the ratio of pericytes to endothelial cells is estimated to vary from 1:1 in the brain/retina to 1:10 in skin and lungs, and 1:100 in smooth muscles [30]. Pericytes are multipotent cells and have been shown to have myogenic, adipogenic, osteogenic, and chondrogenic potential [31]. The differentiation of these cells has been implicated in human disease processes including diabetic retinopathy, tumor angiogenesis, and fibrotic disease in the lung and kidney [27,32,33].
2.2.3. Vascular Smooth Muscle Cells.
VSMCs are present in arteries, arterioles, certain larger venules (“muscular venules”), veins, and lymphatic vessels. They control perfusion by contracting or dilating to change vessel diameter [5]. VSMCs are heterogeneous due to their various developmental origins and are involved in a variety of physiologic functions [34]. These cells are responsible for depositing extracellular matrix (ECM). They can be contractile, or synthetic with increased proliferative and migratory tendencies [35]. In response to tissue injury, VSMCs tend to switch to a synthetic phenotype to promote wound healing, ECM maintenance, and vessel repair. The modulation of VSMC phenotypes is correlated with a number of human disease states. For example, VSMC proliferation is one hallmark for diseases such as pulmonary hypertension, systemic hypertension, and atherosclerosis [6,16].
2.2.4. The Extracellular Matrix.
The native vasculature consists of varied ECM components depending on vessel size and function. Large vessels contain considerable amounts of elastin and collagen to support high pressure, flow, and pulsation. Microvessels and capillaries have less structural proteins, but rather a specialized basement membrane between the endothelium and pericytes rich in proteins such as collagen IV, laminin, fibronectin, and proteoglycans. Proper ECM structure and function is important to support vascular integrity and facilitate signaling between the intracellular and extracellular environments [36,37]. A change in ECM structure is often correlated with the progression of many physiologic and pathophysiologic processes, including tumor angiogenesis, wound healing, inflammation, and aging [38]. New blood vessel formation requires degradation of the basement membrane by matrix metalloproteinases—an important event for angiogenesis [39]. Advances in the understanding of ECM biology continue to further our ability to replicate this environment in the laboratory setting. For example, the use of materials that can be remodeled by cultured cells, and coculturing endothelial cells with surrounding fibroblasts, are two techniques used to create a microenvironment that mimics the in vivo ECM [2].
2.3. Formation of Blood Vessels In Vivo.
Blood vessels are formed in vivo via two main processes: vasculogenesis and angiogenesis. Vasculogenesis involves the de novo formation of new vessels, as observed during embryogenesis, when angioblasts migrate to sites of future vascular growth and form “blood islands,” eventually giving rise to a new vascular network. Many factors, including vascular endothelial growth factor (VEGF), angiopoietin-1, and integrins, all play important roles in this process [40]. Angiogenesis is the formation of new blood vessels via the growth and expansion of existent vascular networks. Angiogenesis is a critical component of embryogenesis and organogenesis, and is also responsible for the majority of new blood vessel growth in the adult organism. This reflects an adaptive response following events such as tissue injury or ischemia, but can be maladaptive in diseases like cancer, pulmonary hypertension, or diabetic retinopathy. Angiogenesis begins when, in response to stimuli such as inflammation, hypoxia, or malignancy, mediators such as VEGF, angiopoietin-2, and fibroblast growth factor are released. These molecules promote pericyte detachment, degradation of the basement membrane by matrix metalloproteinases increased endothelial permeability, and ultimately lead to protein extravasation, endothelial cell migration, and formation of new vessels [37].
2.4. Techniques for Engineering Synthetic Microvessels.
Early in vitro studies of the human microvasculature were performed in two-dimensional flow chambers constructed with rigid materials such as plastic or glass. This allowed study of the endothelium under flow, but did not accurately replicate native ECM, vessel structure, or hemodynamics [19]. Advances in biomaterials and microfabrication techniques led to development of three-dimensional microfluidic chambers and tubular, branching synthetic vessels with complex geometries, perfused with media under continuous flow. Strategies for microvessel formation vary, though many have in common that a patterned chip is formed via microfabrication techniques, such as soft lithography, with vessels formed within a hydrogel matrix on this platform. Ideally, substrates used for creating the platform are clear, inert, nontoxic, implantable, and allow micropatterning with good fidelity at the submicrometer level. Poly(dimethylsiloxane) (PDMS) is one of the most commonly used materials for this purpose [41]. Biomaterials such as alginates [42], agarose [43], collagen and fibrin [44], poly (ethylene glycol) dimethacrylate (PEGDMA) [45], and methacrylated gelatin (GelMA) [46] have been used to form the hydrogel component of such systems, with high biocompatibility but varying transport properties and ability for cellular remodeling. The techniques available for microvessel formation can be divided into microvessel patterning and vasculogenesis/angiogenesis-based techniques (Fig. 2), similar to classification found elsewhere [2,47].
Fig. 2.
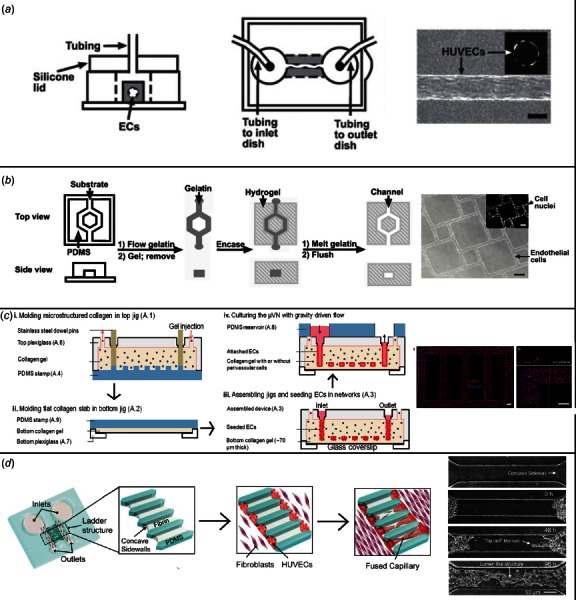
Methods for formation of engineered microvessels. (a) Needle-removal technique. A needle is embedded in collagen gel and removed to form a channel. This is then seeded with endothelial cells and perfused, as shown. An endothelialized microvessel is shown under magnification on the right side of the figure (scale bar is 100 μm). (Reproduced with permission from Lee et al. [47]. Copyright 2014 by Materials Research Society, based on original figure found in Ref. [48].) (b) Dissolvable matrix technique. A gelatin mesh is formed on a PDMS stamp and then embedded within ECM materials (here, Matrigel). Heating leads to dissolution of the gelatin, leaving behind channels which can be seeded with endothelial cells as shown in the rightward portion of the figure. (Reprinted with permission from Golden and Tien [49]. Copyright 2007 by The Royal Society of Chemistry.) (c) Layering method. A collagen slab is formed on top of a patterned PDMS (i). A separate top collagen is similarly formed (ii). The two collagen slabs are joined, and channels within the collagen are seeded with endothelial cells (iii). The microvascular network is then cultured under flow conditions (iv). At the far right, a fluorescent micrograph shows cultured/stained endothelial cells within engineered microvessels (scale bar 100 μm). Adapted from Ref. [50]. (d) Angiogenesis/vasculogenesis-based technique. HUVECs were placed at either end of a PDMS construct, next to a fibrin-filled channel, and cocultured with fibroblast cells. Over time, perfusable capillaries formed via angiogenesis, spanning this fibrin channel. (Reprinted with permission from Yeon et al. [51]. Copyright 2012 by The Royal Society of Chemistry.)
2.4.1. Microvessel Patterning Techniques.
Microvessel patterning techniques involve the initial formation of empty cylinders which are then seeded with cells. Early such techniques were developed by the Tien Laboratory [48,52]. They utilized lithographic techniques to mold a silicone stamp with 1 mm deep indentations, within which a 120 μm diameter stainless steel needle was embedded in collagen and later removed. Endothelial cells were then introduced and formed a confluent layer with good barrier function (Fig. 2(a)) [48]. Following that, the Khademhosseini Laboratory utilized the needle-removal technique to study the effects of fibroblast encapsulation within a hydrogel matrix [46]. Similarly, Yoshida et al. embedded silica tubes within a poly-γ-glutamic acid matrix, removing them to form synthetic microvessels which they lined with a bilayer of VSMCs and human umbilical vein endothelial cells (HUVECs) for assays of vascular permeability [53].
The above approaches are limited in the pattern and complexity of vessels that can be formed. To create a network of vessels with more complicated geometry, Zheng et al. used a patterned PDMS stamp to imprint channels onto a collagen hydrogel which was then bonded to a second flat collagen hydrogel layer (Fig. 2(c)). This created a small network of vessels in which detailed studies of angiogenesis and vessel–blood interactions could be performed [50]. Bischel et al. created branching synthetic vessels by forming a PDMS construct with hollow lumens, filling it with a liquid hydrogel polymer, and using flowing media to wash the center away. The remaining hollow lumens were then seeded with endothelial cells and used to study angiogenesis [54]. Other labs have had success in creating complex vessels using sacrificial molding techniques whereby a negative mold is created out of dissolvable materials, embedded within a hydrogel structure, and then dissolved to leave behind hollow microvessels. The Tien Laboratory pioneered this technique by embedding a gelatin mesh within a collagen/fibrin matrix, with the mesh then dissolved via temperature change (Fig. 2(b)) [49]. The Miller Laboratory utilized bioprinting to create a sacrificial carbohydrate lattice which was embedded in cell-laden hydrogel, and then dissolved by a flowing buffer [55].
Microvessel patterning techniques offer advantages in that they allow precise patterning of vessels which can immediately be perfused with media. Given the controlled geometry of resultant microvessels, the hemodynamic forces within these devices can be accurately predefined for the study of their effect on the cultured cells. Drawbacks include that creating endothelialized microvessel diameters <50 μm using these methods alone is difficult, due to problems with achieving confluence of cultured endothelial cells in smaller vessels [56]. Future protocols are likely to produce increasingly complex microvascular networks and continue to incorporate emerging technology such as bioprinting.
2.4.2. Angiogenesis/Vasculogenesis-Based Techniques.
A different approach to microvessel formation involves attempting to recapitulate the natural processes of angiogenesis and/or vasculogenesis within a laboratory setting to drive synthetic microvessel formation. Vascular cells within the body respond to a variety of physical inputs and biochemical signals to guide their migration and development into organized vascular structures. In the absence of such factors, endothelial cells cultured in isolation will grow into disorganized networks of cells. By incorporating physical cues and cellular signaling, angiogenesis/vasculogenesis-based techniques for microvessel formation seek to coax cultured vascular cells to form organized microvascular structures [2,57].
Raghavan et al. demonstrated the use of physical cues to guide vasculogenesis. They formed PDMS channels filled with endothelial cells within a collagen matrix and stimulated vasculogenesis via addition of growth factors. They found that vessels would grow in organized fashion along provided conduits. Altering channel geometry or collagen concentration had effects on vessel growth pattern [58]. Similarly, by combining HUVECs with a GelMA prepolymer, Nikkhah et al. used photolithography to create linear constructs which were able to promote endothelial cells to form primitive vascular structures. They found that varying the height of these constructs affected the endothelial cell morphogenesis [59]. Providing interstitial flow via creation of pressure gradients is another physical cue that has been used to guide self-organization of vessels [60]. On a nanometer level, patterning of ECM components themselves has been shown to promote cellular differentiation, migration, and morphogenesis, and offers potential for future vasculogenesis-based models [57]. Recent exciting work by Kusuma et al. showed that human pluripotent stem cells could be induced to form microvascular networks consisting of both pericytes and endothelial cells within a hyaluronic acid matrix [61].
Angiogenesis-based approaches use coculture with other cell lines or the strategic addition of growth factors to drive the sprouting of new vessels from prevascularized larger vessels. One commonly used technique is to encourage the angiogenic formation of a capillary network between two larger endothelialized vessels, first created by microfabrication techniques. Yeon et al. used this strategy to successfully promote the angiogenic formation of perfusable synthetic capillaries between two prevascularized microvessels, utilizing coculture of HUVECs with fibroblast cells (Fig. 2(d)) [51]. Kim et al. developed a model to study both angiogenesis and vasculogenesis using fibroblast cell coculture within a prevascularized construct [62]. They prepared a PDMS microfluidic device with five distinct channels and filled the center channel with a collagen and fibrin matrix. By seeding the middle channel with HUVECs and the outermost channels with lung fibroblasts, they were able to observe spontaneous vasculogenesis which led to functional, perfusable, vessels that spanned the center channel. By placing HUVECs on only one side of the center channel and fibroblasts only in the outermost channel on the opposite side, they were able to observe angiogenesis take place, with sprouting vessels crossing the center channel. Rather than using cellular coculture, many investigators have employed growth factors such as VEGF, fibroblast growth factors, or platelet-derived growth factors to promote angiogenesis. The combination of growth factors, such as fibroblast growth factor with VEGF, promotes increased angiogenesis compared with the use of a single growth factor [63]. The addition of growth factor gradients also enhances angiogenesis [64]. Nguyen et al. used six growth factors and described varied effects on angiogenesis and anti-angiogenesis with different combinations of these growth factors [65].
Compared to microvessel patterning techniques, angiogenesis/vasculogenesis-based techniques allow for formation of smaller and more complex vascular patterns, branching down to the few micrometer level. This would be useful for tissue engineering applications [47]. These techniques cannot yet form larger vessels, however, and do not offer precise control of geometry as yielded by direct fabrication and patterning. Additionally, it can take many days to weeks for perfusable vessels to form using angiogenesis/vasculogenesis techniques [56]. Ultimately, the optimal method for synthetic microvessel formation depends on the intended use of the vessel and may involve combining different techniques.
3. Engineered Microvessels for the Study of Human Disease
One of the most promising applications of engineered microvessels is to study human diseases in vitro. Compared to current animal models, the engineered microvessels offer several advantages including the ability to work with human cells and samples, higher throughput, good reproducibility, small volume requirements for reagents, and lack of ethical concerns. They also allow the study of interactions of individual vascular and blood components in a stepwise fashion. Additionally, the transparency of most vascular constructs allows for real-time monitoring of ongoing biologic processes. Engineered microvessels have promise for the study of many areas of human vascular pathophysiology including studies of endothelial–blood interactions, endothelial–perivascular interactions, and the related phenomena of angiogenesis and tumor biology. One extension of this microvascular research is creating models to mimic organ-specific structure and functions.
3.1. Endothelial–Blood Interactions.
The primary function of blood vessels is to carry blood and provide a surface that prevents improper blood clotting or cellular activation. Circulating blood cells do not adhere to the vessel wall under normal conditions (Fig. 1(b)), but can interact actively with the vessel wall at sites of vascular injury, or following endothelial activation. Under these conditions, the activated endothelial cells release their granules and express adhesion molecules that initiate the binding and activation of platelets and leukocytes (Figs. 1(c) and 1(d)). Engineered microvessels have been successfully applied to explore these fundamental processes, particularly on the role of hemodynamic factors and biochemical factors during inflammation and initiation of thrombosis.
3.1.1. Microvascular Hemodynamics.
The hemodynamics of the human microcirculation are complicated and traditionally difficult to study in vitro due to factors including the lack of accessibility and visibility of small vessels, complex blood protein and cellular components, nonlinear blood rheology, and complex blood-vessel interactions. Advances in biomaterials and tissue engineering now provide the ability to form microfluidic structures out of pliant materials with good fidelity down to the scale of the human microvasculature [66]. Engineered microvessels allow study of how factors such as shear stress, vessel geometry, and flow rates modulate endothelial function and human disease states [67–69]. Synthetic microvessels have also been used to emulate the shear stress imposed on blood components by mechanical circulatory devices and explore related pathologic effects [70].
3.1.2. Vascular Permeability.
Disruption of microvessel permeability is seen in acute inflammation and has been associated with diseases including sepsis and malaria. Therefore, being able to assess changes in vessel wall permeability is very important for the study of many human diseases. Culture under flow has been shown to enhance endothelial cell barrier function, compared to culture under static conditions, making engineered microvessels an attractive platform for permeability studies [71]. In one study, two adjacent microchannels with intervening ECM hydrogel were created to study endothelial permeability and its modulation by surrounding tumor cells [72]. They monitored changes in the intensity of fluorescently labeled dextran in the hydrogel over time and found that vessel permeability increased in response to the presence of macrophages and increased exposure to the inflammatory cytokine tumor necrosis factor-α (TNF-α). Emulating interorgan differences in permeability is a related area of research and is an important component of developing different organ-on-a-chip systems. As one example, microfluidic devices have been developed which emulate the low permeability of the blood brain barrier [73]. These models may prove useful to examine disease states in which CNS permeability is disrupted, or evaluate the CNS penetration of pharmaceutical candidates [74].
3.1.3. Inflammation.
The endothelium is an important mediator of local and systemic inflammatory processes. In its resting state, the endothelium expresses few adhesion molecules for circulating blood cells. When activated by circulating pro-inflammatory cytokines, local damage, or changes in biomechanical forces, the endothelium rapidly changes to a pattern of expression that favors leukocyte adhesion and transmigration. WPBs merge with the surface of the endothelium to release their contents, and P-selectin is transferred to the surface of the endothelial cell [25]. This can occur as soon as 15 min after activation, and other adhesion molecules begin to be expressed over the ensuing hours [12]. These adhesion molecules interact with ligands on the surface of lymphocytes and lead to leukocyte rolling (slowing of leukocytes with recurrent attachment to the endothelium and release), initiating inflammation. If inflammation progresses, rolling leukocytes become activated, recruit additional leukocytes, firmly adhere, and transmigrate through endothelial tissue in a complex and coordinated fashion (Fig. 1(c)) [25,75].
Microfluidic devices are an attractive platform for the study of endothelium-mediated inflammatory processes. Leukocyte chemotaxis, rolling, adhesion, extravasation, and interstitial migration have all been demonstrated within microfluidic devices [76–78]. One representative study made use of TNF-α to induce endothelial expression of leukocyte adhesion molecules within engineered vessels and prompt neutrophil rolling and adherence to the activated endothelium. Exposure to a cytokine gradient external to the synthetic microvessel encouraged diapedesis and chemotaxis [77]. Microfluidic models have also led to increased understanding of specific patterns of T-cell and neutrophil chemotaxis at the level of single cells [79,80]. These models have been applied to patient samples in specific disease states such as the acute respiratory distress syndrome [81] and trauma/burns [82].
3.1.4. Thrombosis.
Under normal physiologic conditions, the endothelium provides a nonthrombogenic surface but, when activated, can switch to a procoagulant state [12,83]. As in inflammation, release of the contents of WPBs occurs rapidly as an early response to endothelial disruption. P-selectin and VWF then bind circulating platelets, and in small venules, the platelets can roll along the endothelium, similar to leukocyte adhesion and rolling during inflammation. If there is no further signaling, the platelets will leave the vessel wall [25]. In the presence of endothelial damage with exposed subendothelial collagen, however, platelets can adhere firmly and become activated, leading to primary hemostasis (Fig. 1(d)). Similarly, in inflamed but intact endothelium, platelets can adhere firmly to the endothelium, become activated, bind circulating leukocytes, and propagate the inflammatory process [1,84]. Such interrelationship of the inflammatory and coagulation pathways reaches its apotheosis in cases of overwhelming inflammation, such as severe sepsis or massive trauma, where widespread microvascular thrombi can result from dysregulation of inflammatory cascades [85].
The adhesion and aggregation of platelets, and initiation of thrombosis, largely depend on blood flow and shear stress at local sites [86]. Microfluidic devices offer an improvement over traditional planar culture to study the role of flow and three-dimensional vessel geometries on thrombotic progression [66]. Typical vessel wall shear stress is 500–1600 s−1 in arterioles and the microvascular bed. At wall shear stress greater than 500 s−1, the initial adhesion of platelets to the vessel wall is primarily mediated by the interaction of the platelet glycoprotein(GP)Ib-IX-V receptor complex to the VWF secreted from WPBs in the endothelial cells [86]. Increased shear stress (e.g., beyond 5000 s−1) can result in additional endothelial cell release of VWF and increased platelet adhesion. This was demonstrated by Westein et al. who developed a microfluidic device with stenotic areas built into flow channels and showed that platelets interacted with VWF to aggregate in poststenotic areas [68]. Zheng et al. engineered three-dimensional microvessels to study the initial events of thrombosis and highlighted the important roles of endothelial activation state and vessel geometry in blood-vessel interactions (Fig. 3(a)). They showed that activated, but not resting, endothelium displayed VWF fibers on their surface which bound platelets, with 3D networks of VWF fibers forming near vessel bifurcations and junctions [50]. These findings were refined in their subsequent study that showed the effects of vessel diameter, 3D vessel architecture, fluid shear stress, and flow acceleration on the 3D assembly of the shear-sensitive protein VWF [69]. VWF assembly was then observed to lead to complex interactions with blood cells, including platelets, leukocytes, and red blood cells [69].
Fig. 3.
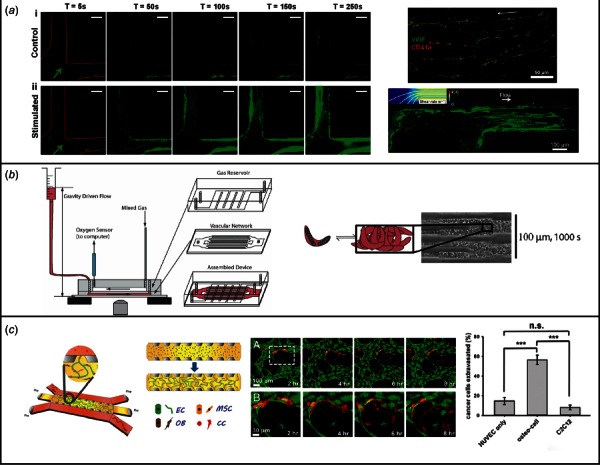
(a) Engineered microvessels for the study of thrombosis and microhemodynamics. Engineered microvessels were seeded with HUVEC cells and exposed to phorbol-12-myristate-13-acetate (PMA) to stimulate VWF secretion (left image). Platelet aggregation in these stimulated vessels was compared with control vessels which did not have PMA exposure. In the upper right image, VWF can be seen oriented along stimulated vessels in the direction of flow. VWF aggregates in narrow vessels with high shear stress, strong flow acceleration, or sharp turns, as demonstrated in the lower right image. Figure adapted from Refs. [50] and [69]. (b) Engineered microvessels for the modeling of human thrombotic disease. A branching microfluidic network was created using soft lithography, consisting of a gas channel network separated from a vascular network by a PDMS membrane. Perfusing the vascular network with blood from patients with sickle cell disease and varying the oxygen content of the gas channel network, the authors were able to provoke vascular occlusion events (detailed on the right side of the figure). (Reprinted with permission from Higgins et al. [87]. Copyright 2007 by The National Academy of Sciences of the U.S.A.) A similar construct was endothelialized in later work, for further examination of microangiopathic diseases (see Ref. [88]). (c) Engineered microvessels for the study of tumor metastasis. A microvessel model was created with microfluidic channels flanking either side of a larger chamber filled with fibrin gel matrix. A tricellular culture was created within the center chamber, with HUVECs, human bone human bone marrow-derived mesenchymal stem cells (MSCs), and bone marrow-derived mesenchymal stem cells with partial differentiation toward bone (OB). Growth factors were added and capillary formation occurred over 4 days, spanning the two center chamber and connecting the two flanking chambers. Breast cells were then introduced into these microvessels, and extravasation observed (middle image). The rate of extravasation in this “bone microenvironment” was compared with extravasation in a muscle environment (using myoblast cell line C2C12), or a control of acellular matrix material, and found to be highest in the bone microenvironment (see figure on the right). This is consistent with the clinical tendency of breast cancer to metastasize to bone tissue. (Reprinted with permission from Jeon et al. [89]. Copyright 2015 by The National Academy of Sciences of the U.S.A.)
Other groups have used engineered microvessels to study specific hematologic disorders by creating microfluidic models of sickle cell disease and other disorders of microvascular occlusion. Sickle cell disease is characterized by production of an abnormal hemoglobin (hemoglobin S) which polymerizes under conditions of low oxygen tension, leading to loss of red blood cell deformability and episodes of small vessel occlusion. Higgens et al. created a multilayered PDMS construct consisting of a 12 μm diameter microvascular network separated by a thin, gas-permeable membrane from a larger chamber with circulating gas. By perfusing the vascular construct with blood from patients with sickle cell disease and varying the oxygen content of the adjacent gas mixture, they were able to recreate vascular occlusion events and study the hemodynamic factors involved [87]. A later study in an endothelialized microvessel model used patient samples to examine sickle cell disease and the microangiopathic disorder of hemolytic uremic syndrome (Fig. 3(b)). They were able to show the beneficial effects of hydroxyurea in treating sickle cell disease in vitro in this model [88]. Engineered microvessels may be applied in the future to laboratory medicine coagulation assays and the design of antiplatelet or anticoagulant pharmaceuticals [90].
3.2. Endothelial–Perivascular Interactions.
Endothelial interaction with both pericytes and vascular smooth muscle cells is critical for the proper function of the microvasculature. Pericytes have increasingly been cocultured with endothelial cells for the formation and study of engineered microvessels [50,91–96]. Several engineering models have shown that pericytes promote sprouting angiogenesis in coculture with endothelial cells within engineered microvessel models [50,91], and contribute to ECM formation and vessel formation during vasculogenesis [92,93,95]. Similarly, VSMCs have been cocultured with endothelial cells for microvessel formation [50,53]. Despite these promising early studies, endothelial/perivascular cell coculture models remain in their infancy. Future studies on the role of endothelial–perivascular cell interactions in diseases such as hypertension, pulmonary hypertension, and atherosclerosis may be of particular interest.
3.3. Angiogenesis/Vasculogenesis and Tumor Biology.
In addition to being used for the creation of microvessels, our understanding of the processes of angiogenesis and vasculogenesis themselves has been enhanced through engineered microvessels. Some of the studies which have explored these processes in humans are discussed earlier in this paper [50,51,62–65]. One area of human disease intimately associated with angiogenesis is tumor biology. Cancer grows according to a series of complicated interactions between the tumor cell and various tissue microenvironments [97]. Tumors promote angiogenesis and drive the creation of tumor vessels. These vessels may be highly abnormal, with disorganized vasculature networks, dysfunctional or absent perivascular cells and lymphatics, and increased vascular permeability—all factors which are thought to potentially promote tumor intravasation and metastasis [97,98]. To metastasize, a tumor cell must detach from a primary tumor, navigate the ECM within the tumor environment, deform to enter through endothelial cell–cell junctions, interact with endothelium to slow and adhere at the site of metastasis, extravasate, and form a secondary tumor site. These events involve complicated physical interactions with endothelial cells, stromal cells, growth factor gradients, and the ECM [99]. Engineered microvessels are well suited to the study of both tumor angiogenesis and metastasis, and have helped advance understanding within the field of cancer biology.
Several microvessel models have been engineered for the observation of tumor angiogenesis and metastasis. In an angiogenesis/vasculogenesis-based model, Kim et al. found that coculture of endothelial cells with cancer cells within microvessel models led to aberrant angiogenesis when compared to coculture with fibroblasts [62]. To visualize the initial stages of metastasis, Wong and Searson utilized a rod to form microchannels within a collagen matrix that had been seeded with breast cancer cells, and cultured endothelial cells within these microchannels. Using real-time live-cell fluorescence microscopy, they observed cancer cell tunneling/migration with the ECM until a vessel wall was reached, and then observed cancer cell behavior including instances of cancer dormancy, vessel intravasation, and/or promotion of localized angiogenic sprouting [100]. Examining the role of endothelial permeability on intravasation events, Zervantonakis et al. found that addition of macrophages or TNF-α led to increased endothelial permeability and increased tumor intravasation in a model consisting of an endothelium-lined microchannel parallel to a channel filled with tumor cells and separated by a section of ECM [72]. Similarly, using a needle-based molding technique, Buchanan et al. showed that coculture with tumor cells increases vascular permeability. They also found that increasing shear stress is associated with decreased permeability and downregulation of tumor angiogenic genes, suggesting that the low-flow state seen in many tumor vessels may contribute to tumor angiogenesis [101]. Extravasation events were studied and photographed in an angiogenesis/vasculogenesis-based model created by Chen et al. [102]. In an elegant in vitro tumor model, Jeon et al. created a bone-specific microvessel model to study the well-known fact that specific tumors preferentially metastasize to specific organ systems, using the model of breast cancer metastasis to bone [89]. They cocultured HUVECs in microchannels surrounded by human bone marrow-derived mesenchymal stem cells (MSCs), differentiated toward either smooth muscle or osteoblast phenotypes, within a hydrogel matrix over 4 days. They then introduced the breast cancer cells into the endothelialized microchannels and compared the rates of cancer cell extravasation among a microenvironment mimicking bone, one mimicking smooth muscle, and an acellular collagen matrix. They found that the breast cancer cells extravasated at significantly higher rates within the bone-mimicking microenvironment (Fig. 3(c)).
3.4. Organ-Specific Applications.
There has been a recent tremendous interest in developing in vitro model systems to mimic the function of entire organs. These “organ-on-a-chip” models offer significant potential for the modeling of human diseases, the development of pharmaceuticals, and the practice of precision medicine [103]. Since the creation of any functioning organ tissue is dependent on a blood supply, and because the endothelium varies greatly between organs in structure and function, developing organ-specific microvessels is of primary importance in the formation of organ-on-a-chip systems. Here, we will discuss recent efforts to incorporate endothelialized microvasculature into these systems.
In one of the first examples of functional human organ-specific tissue supported by a microvessel network, Huh et al. created a “lung-on-a-chip” consisting of a synthetic alveolar capillary interface capable of simulating human breathing [104]. They microfabricated two microchannels separated by a 10 μm PDMS membrane and seeded human alveolar and human pulmonary microvascular endothelial cells on either side of this membrane, respectively. Once confluent, air was introduced into the microchannel on the alveolar side and hooked up to a vacuum to simulate the cyclical alveolar/endothelial stretch seen during human breathing. They observed surfactant production, good structural integrity, and normal epithelial barrier function within their model. They then studied TNF-α administration and were able to observe intercellular adhesion molecule (ICAM) expression and neutrophil adherence and diapedesis in response to inflammation, consistent with in vivo responses to inflammation. Using a more angiogenesis-based approach, Chiu et al. created a model of vascularized cardiac tissue [105]. They explanted arteries and veins from a mouse, placing them on either end of a micropatterned PDMS platform coated with a collagen–chitosan hydrogel with added Tβ4 (a pro-angiogenic peptide). Over 3 weeks of culture, a vessel bed with capillaries grew across the gel spanning from the artery and vein. This vascular bed enhanced the function of cardiomyocytes that were cultured over it.
One specialized vascular bed is the BBB, which is composed of endothelial cells with strong expression of tight junctions, lined by pericytes and astrocytes. The BBB is disrupted in many severe disease states, and modeling the BBB has important pharmaceutical applications, such as determining the degree of CNS penetration of a drug and the potential for neurotoxicity. As such, there have been numerous attempts to replicate the BBB in vitro. Microfluidic models are appropriate for the study of the BBB given that shear stress is important for maturing of tight junctions as is exposure to cell signaling molecules [103]. Booth et al. created a microfluidic chip with two PDMS layers separated by a polycarbonate membrane, with endothelial cells and astrocytes seeded on opposite sides of the membrane, respectively. This model displayed appropriate low vascular permeability [73]. A more complex model was developed by Brown et al. which contains all three BBB components (pericytes, astrocytes, and endothelial cells), along with neurons [106]. This model made use of brain and vascular compartments separated by a polycarbonate membrane, and the inclusion of neurons allows the researcher to assess neuronal response to permeability changes or CNS penetration of pharmaceuticals.
Another specialized vasculature is found in the kidney, which filters blood and contains a specialized, fenestrated endothelium to aid in this process. The functional unit of the kidney is the nephron, which can be divided into the glomerulus and the renal tubule. Both have functionally distinct parenchyma and vasculature. Glomeruli are the major site for filtration, whereas tubules are critical for reabsorption and secretion and demand a high amount of energy to support active transport. By isolating and purifying human kidney peritubular microvascular endothelial cells (HKMECs), Ligresti et al. [107] were able to create a perfusable 3D network of HKMECs using a method of fabrication similar to that used in Ref. [50]. They demonstrated a unique fenestrated morphology of HKMECs, which had not been shown previously. They compared the vascular networks endothelialized with these cells to the HUVECs and found the decreased angiogenic tendencies, an increased sensitivity to flow, and dependence on a high concentration of VEGF. They also showed that HKMECs differ significantly from HUVECs with respect to their transcriptional profile. This kidney-specific microvascular model will be useful for examining renal disease mechanisms and studying the renal toxicity of medications. Perhaps even more importantly, it serves to highlight the endothelial variation seen among organs and the importance of using organ-specific endothelial models for organ-on-a-chip applications.
Future organ-on-a-chip systems are likely to continue incorporating organ-specific endothelium within a finely tuned microenvironment, in hopes of better mimicking the in vivo organ-specific environment. The use of patient samples and/or cells may lead to the creation of further disease-specific microvascular models. Finally, combination of several different organ systems into a single “body-on-a-chip” may allow for pharmaceutical screening and disease modeling [108].
4. Conclusions and Future Directions
Research into engineered microvessels has advanced markedly in the decade following the development of early prevascularization techniques [48]. Newer microvessel platforms have been created which incorporate a variety of biomaterials and utilize novel techniques including sacrificial materials, functionalized biomaterials, 3D printing, and stem cells. In addition, new models show increasing sophistication with regard to local microenvironmental factors including vessel flow and geometry, endothelial heterogeneity, micropatterning, and cellular signaling.
We envision that these above technologic advances will lead to exciting future applications for engineered microvessels. One example is their potential use in the field of precision medicine. Medical science has increasingly recognized that patients suffering from the same illness may experience markedly varied disease courses and respond differently to treatment. Creating patient-specific microvessel models could lead to individualized treatment plans. In this way, for example, a patient with cancer could have cells from his tumor grown in a microvessel model and tested against various chemotherapies. Complex microvascular models can further be expanded to replicate the large-scale structure and function of tissues, or even entire organs, in “organs-on-chips.” These human organs-on-chips can be used for drug screening and toxicity testing of new medications before they are applied on patients. Engineered functional tissues and organs could also be used for the ex vivo replacement of organ function (e.g., temporary life support during liver or lung failure), and in the future may be implanted to replace organs in vivo.
In summary, engineered microvessels can be created at a scale similar to that of the native microvasculature while providing the ability to work entirely with human cellular constructs and patient samples and without the ethical concerns of animal research. These platforms are highly flexible such that biomaterials used, vessel architecture, flow rate, cell types, luminal flow and pressure, and biochemical factors can all be modified. Future exciting directions include further elucidation of endothelial–perivascular interactions, development of disease-specific models, refinement of organ-on-a-chip technologies, creation of larger vessels (including resistance vessels), improvements in scalability, development of high-throughput assays for pharmaceutics, and research into precision medicine applications.
Acknowledgment
We would like to acknowledge the support received from National Institutes of Health Grant No. DP2DK102258 (to Y.Z.) and American Heart Association Grant No. 12SDG9230006 (to Y.Z.).
Contributor Information
Samuel G. Rayner, Department of Pulmonary and , Critical Care Medicine, , University of Washington School of Medicine, , Campus Box 356522, , Seattle, WA 98195 , e-mail: srayner@uw.edu
Ying Zheng, Department of Bioengineering, , University of Washington, , 3720 15th Avenue NE, , Seattle, WA 98105;; Center for Cardiovascular Biology, , Institute for Stem Cell and , Regenerative Medicine, , University of Washington, , Seattle, WA 98109 , e-mail: yingzy@uw.edu
References
- [1]. Zheng, Y. , Chen, J. , and López, J. A. , 2014, “ Microvascular Platforms for the Study of Platelet-Vessel Wall Interactions,” Thromb. Res., 133(4), pp. 525–531. 10.1016/j.thromres.2013.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Hasan, A. , Paul, A. , Vrana, N. E. , Zhao, X. , Memic, A. , Hwang, Y.-S. , Dokmeci, M. R. , and Khademhosseini, A. , 2014, “ Microfluidic Techniques for Development of 3D Vascularized Tissue,” Biomaterials, 35(26), pp. 7308–7325. 10.1016/j.biomaterials.2014.04.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Bae, H. , Puranik, A. S. , Gauvin, R. , Edalat, F. , Carrillo-Conde, B. , Peppas, N. A. , and Khademhosseini, A. , 2012, “ Building Vascular Networks,” Sci. Transl. Med., 4(160), p. 160ps23. 10.1126/scitranslmed.3003688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Laschke, M. W. , and Menger, M. D. , 2015, “ Prevascularization in Tissue Engineering: Current Concepts and Future Directions,” Biotechnol. Adv., 34(2), pp. 112–121. 10.1016/j.biotechadv.2015.12.004 [DOI] [PubMed] [Google Scholar]
- [5]. Ross, M. H. , and Pawlina, W. , 2006, Histology: A Text and Atlas: With Correlated Cell and Molecular Biology, Lippincott Wiliams & Wilkins, Baltimore, MD. [Google Scholar]
- [6]. Feihl, F. , Liaudet, L. , Waeber, B. , and Levy, B. I. , 2006, “ Hypertension: A Disease of the Microcirculation?,” Hypertension, 48(6), pp. 1012–1017. 10.1161/01.HYP.0000249510.20326.72 [DOI] [PubMed] [Google Scholar]
- [7]. Gökçinar-Yagci, B. , Uçkan-Çetinkaya, D. , and Çelebi-Saltik, B. , 2015, “ Pericytes: Properties, Functions and Applications in Tissue Engineering,” Stem Cell Rev., 11(4), pp. 549–559. 10.1007/s12015-015-9590-z [DOI] [PubMed] [Google Scholar]
- [8]. Van Dijk, C. G. M. , Nieuweboer, F. E. , Pei, J. Y. , Xu, Y. J. , Burgisser, P. , Van Mulligen, E. , El Azzouzi, H. , Duncker, D. J. , Verhaar, M. C. , and Cheng, C. , 2015, “ The Complex Mural Cell: Pericyte Function in Health and Disease,” Int. J. Cardiol., 190(1), pp. 75–89. 10.1016/j.ijcard.2015.03.258 [DOI] [PubMed] [Google Scholar]
- [9]. Pries, A. , and Kuebler, W. , 2006, “ Normal Endothelium,” Handb. Exp. Pharmacol., 176(1), pp. 1–40. 10.1007/3-540-32967-6_1 [DOI] [PubMed] [Google Scholar]
- [10]. Aird, W. C. , 2015, “ Endothelium and Haemostasis,” Hamostaseologie, 35(1), pp. 11–16. 10.5482/HAMO-14-11-0075 [DOI] [PubMed] [Google Scholar]
- [11]. Weibel, E. R. , and Palade, G. E. , 1964, “ New Cytoplasmic Components in Arterial Endothelia,” J. Cell Biol., 23(1), pp. 101–112. 10.1083/jcb.23.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Hack, C. E. , and Zeerleder, S. , 2001, “ The Endothelium in Sepsis: Source of and a Target for Inflammation,” Crit. Care Med., 29(7 Suppl.), pp. S21–S27. 10.1097/00003246-200107001-00011 [DOI] [PubMed] [Google Scholar]
- [13]. Flammer, A. J. , Anderson, T. , Celermajer, D. S. , Creager, M. A. , Deanfield, J. , Ganz, P. , Hamburg, N. M. , Lüscher, T. F. , Shechter, M. , Taddei, S. , Vita, J. A. , and Lerman, A. , 2012, “ The Assessment of Endothelial Function: From Research Into Clinical Practice,” Circulation, 126(6), pp. 753–767. 10.1161/CIRCULATIONAHA.112.093245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Gutterman, D. D. , Chabowski, D. S. , Kadlec, A. O. , Durand, M. J. , Freed, J. K. , Ait-Aissa, K. , and Beyer, A. M. , 2016, “ The Human Microcirculation,” Circ. Res., 118(1), pp. 157–172. 10.1161/CIRCRESAHA.115.305364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Levy, B. I. , Ambrosio, G. , Pries, A. R. , and Struijker-Boudier, H. A. , 2001, “ Microcirculation in Hypertension: A New Target for Treatment?,” Circulation, 104(6), pp. 735–740. 10.1161/hc3101.091158 [DOI] [PubMed] [Google Scholar]
- [16]. Archer, S. L. , Weir, E. K. , and Wilkins, M. R. , 2010, “ Basic Science of Pulmonary Arterial Hypertension for Clinicians: New Concepts and Experimental Therapies,” Circulation, 121(18), pp. 2045–2066. 10.1161/CIRCULATIONAHA.108.847707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Budhiraja, R. , Tuder, R. M. , and Hassoun, P. M. , 2004, “ Endothelial Dysfunction in Pulmonary Hypertension,” Circulation, 109(2), pp. 159–165. 10.1161/01.CIR.0000102381.57477.50 [DOI] [PubMed] [Google Scholar]
- [18]. Gross, P. L. , and Aird, W. C. , 2000, “ The Endothelium and Thrombosis,” Semin. Thromb. Hemostasis, 26(5), pp. 463–478. 10.1055/s-2000-13202 [DOI] [PubMed] [Google Scholar]
- [19]. López, J. A. , and Zheng, Y. , 2013, “ Synthetic Microvessels,” J. Thromb. Haemostasis, 11(Suppl. 1), pp. 67–74. 10.1111/jth.12245 [DOI] [PubMed] [Google Scholar]
- [20]. Schouten, M. , Wiersinga, W. J. , Levi, M. , and van der Poll, T. , 2008, “ Inflammation, Endothelium, and Coagulation in Sepsis,” J. Leukocyte Biol., 83(3), pp. 536–545. 10.1189/jlb.0607373 [DOI] [PubMed] [Google Scholar]
- [21]. Mehta, D. , Ravindran, K. , and Kuebler, W. M. , 2014, “ Novel Regulators of Endothelial Barrier Function,” Am. J. Physiol.: Lung Cell. Mol. Physiol., 307(12), pp. L924–935. 10.1152/ajplung.00318.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Kerbel, R. S. , 2008, “ Tumor Angiogenesis,” N. Engl. J. Med., 358(19), pp. 2039–2049. 10.1056/NEJMra0706596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Van Hinsbergh, V. W. , 1997, “ Endothelial Permeability for Macromolecules. Mechanistic Aspects of Pathophysiological Modulation,” Arterioscler., Thromb., Vasc. Biol., 17(6), pp. 1018–1023. 10.1161/01.ATV.17.6.1018 [DOI] [PubMed] [Google Scholar]
- [24]. Vane, J. R. , Anggård, E. E. , and Botting, R. M. , 1990, “ Regulatory Functions of the Vascular Endothelium,” N. Engl. J. Med., 323(1), pp. 27–36. 10.1056/NEJM199007053230106 [DOI] [PubMed] [Google Scholar]
- [25]. Wagner, D. D. , and Frenette, P. S. , 2008, “ The Vessel Wall and Its Interactions,” Blood, 111(11), pp. 5271–5281. 10.1182/blood-2008-01-078204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Andreeva, E. R. , Pugach, I. M. , Gordon, D. , and Orekhov, A. N. , 1998, “ Continuous Subendothelial Network Formed by Pericyte-Like Cells in Human Vascular Bed,” Tissue Cell, 30(1), pp. 127–135. 10.1016/S0040-8166(98)80014-1 [DOI] [PubMed] [Google Scholar]
- [27]. Bergers, G. , and Song, S. , 2005, “ The Role of Pericytes in Blood-Vessel Formation and Maintenance,” Neuro Oncol., 7(4), pp. 452–464. 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Crisan, M. , Corselli, M. , Chen, W. C. W. , and Péault, B. , 2012, “ Perivascular Cells for Regenerative Medicine,” J. Cell. Mol. Med., 16(12), pp. 2851–2860. 10.1111/j.1582-4934.2012.01617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Gerhardt, H. , and Betsholtz, C. , 2003, “ Endothelial-Pericyte Interactions in Angiogenesis,” Cell Tissue Res., 314(1), pp. 15–23. 10.1007/s00441-003-0745-x [DOI] [PubMed] [Google Scholar]
- [30]. Sims, D. E. , 2000, “ Diversity Within Pericytes,” Clin. Exp. Pharmacol. Physiol., 27(10), pp. 842–846. 10.1046/j.1440-1681.2000.03343.x [DOI] [PubMed] [Google Scholar]
- [31]. Crisan, M. , Yap, S. , Casteilla, L. , Chen, C. W. , Corselli, M. , Park, T. S. , Andriolo, G. , Sun, B. , Zheng, B. , Zhang, L. , Norotte, C. , Teng, P. N. , Traas, J. , Schugar, R. , Deasy, B. M. , Badylak, S. , Buhring, H. J. , Giacobino, J. P. , Lazzari, L. , Huard, J. , and Péault, B. , 2008, “ A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs,” Cell Stem Cell, 3(3), pp. 301–313. 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- [32]. Hung, C. , Linn, G. , Chow, Y. H. , Kobayashi, A. , Mittelsteadt, K. , Altemeier, W. A. , Gharib, S. A. , Schnapp, L. M. , and Duffield, J. S. , 2013, “ Role of Lung Pericytes and Resident Fibroblasts in the Pathogenesis of Pulmonary Fibrosis,” Am. J. Respir. Crit. Care Med., 188(7), pp. 820–830. 10.1164/rccm.201212-2297OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Lin, S.-L. , Kisseleva, T. , Brenner, D. A. , and Duffield, J. S. , 2008, “ Pericytes and Perivascular Fibroblasts are the Primary Source of Collagen-Producing Cells in Obstructive Fibrosis of the Kidney,” Am. J. Pathol., 173(6), pp. 1617–1627. 10.2353/ajpath.2008.080433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Majesky, M. W. , 2007, “ Developmental Basis of Vascular Smooth Muscle Diversity,” Arterioscler., Thromb., Vasc. Biol., 27(6), pp. 1248–1258. 10.1161/ATVBAHA.107.141069 [DOI] [PubMed] [Google Scholar]
- [35]. Rensen, S. S. M. , Doevendans, P. A. F. M. , and van Eys, G. J. J. M. , 2007, “ Regulation and Characteristics of Vascular Smooth Muscle Cell Phenotypic Diversity,” Neth. Heart J., 15(3), pp. 100–108. 10.1007/BF03085963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. LeBleu, V. S. , Macdonald, B. , and Kalluri, R. , 2007, “ Structure and Function of Basement Membranes,” Exp. Biol. Med., 232(9), pp. 1121–1129. 10.3181/0703-MR-72 [DOI] [PubMed] [Google Scholar]
- [37]. Carmeliet, P. , and Jain, R. K. , 2011, “ Molecular Mechanisms and Clinical Applications of Angiogenesis,” Nature, 473(7347), pp. 298–307. 10.1038/nature10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Frantz, C. , Stewart, K. M. , and Weaver, V. M. , 2010, “ The Extracellular Matrix at a Glance,” J. Cell Sci., 123(24), pp. 4195–4200. 10.1242/jcs.023820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Davis, G. E. , and Senger, D. R. , 2005, “ Endothelial Extracellular Matrix: Biosynthesis, Remodeling, and Functions During Vascular Morphogenesis and Neovessel Stabilization,” Circ. Res., 97(11), pp. 1093–1107. 10.1161/01.RES.0000191547.64391.e3 [DOI] [PubMed] [Google Scholar]
- [40]. Ribatti, D. , Vacca, A. , Nico, B. , Roncali, L. , and Dammacco, F. , 2001, “ Postnatal Vasculogenesis,” Mech. Dev., 100(2), pp. 157–163. 10.1016/S0925-4773(00)00522-0 [DOI] [PubMed] [Google Scholar]
- [41]. McDonald, J. C. , and Whitesides, G. M. , 2002, “ Poly(Dimethylsiloxane) as a Material for Fabricating Microfluidic Devices,” Acc. Chem. Res., 35(7), pp. 491–499. 10.1021/ar010110q [DOI] [PubMed] [Google Scholar]
- [42]. Augst, A. D. , Kong, H. J. , and Mooney, D. J. , 2006, “ Alginate Hydrogels as Biomaterials,” Macromol. Biosci., 6(8), pp. 623–633. 10.1002/mabi.200600069 [DOI] [PubMed] [Google Scholar]
- [43]. Ling, Y. , Rubin, J. , Deng, Y. , Huang, C. , Demirci, U. , Karp, J. M. , and Khademhosseini, A. , 2007, “ A Cell-Laden Microfluidic Hydrogel,” Lab Chip, 7(6), pp. 756–762. 10.1039/b615486g [DOI] [PubMed] [Google Scholar]
- [44]. Bayless, K. J. , and Davis, G. E. , 2003, “ Sphingosine-1-Phosphate Markedly Induces Matrix Metalloproteinase and Integrin-Dependent Human Endothelial Cell Invasion and Lumen Formation in Three-Dimensional Collagen and Fibrin Matrices,” Biochem. Biophys. Res. Commun., 312(4), pp. 903–913. 10.1016/j.bbrc.2003.11.017 [DOI] [PubMed] [Google Scholar]
- [45]. Hutson, C. B. , Nichol, J. W. , Aubin, H. , Bae, H. , Yamanlar, S. , Al-Haque, S. , Koshy, S. T. , and Khademhosseini, A. , 2011, “ Synthesis and Characterization of Tunable Poly(Ethylene Glycol): Gelatin Methacrylate Composite Hydrogels,” Tissue Eng., Part A, 17(13–14), pp. 1713–1723. 10.1089/ten.tea.2010.0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Nichol, J. W. , Koshy, S. T. , Bae, H. , Hwang, C. M. , Yamanlar, S. , and Khademhosseini, A. , 2010, “ Cell-Laden Microengineered Gelatin Methacrylate Hydrogels,” Biomaterials, 31(21), pp. 5536–5544. 10.1016/j.biomaterials.2010.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Lee, H. , Chung, M. , and Jeon, N. L. , 2014, “ Microvasculature: An Essential Component for Organ-on-Chip Systems,” MRS Bull., 39(01), pp. 51–59. 10.1557/mrs.2013.286 [DOI] [Google Scholar]
- [48]. Chrobak, K. M. , Potter, D. R. , and Tien, J. , 2006, “ Formation of Perfused, Functional Microvascular Tubes In Vitro,” Microvasc. Res., 71(3), pp. 185–196. 10.1016/j.mvr.2006.02.005 [DOI] [PubMed] [Google Scholar]
- [49]. Golden, A. P. , and Tien, J. , 2007, “ Fabrication of Microfluidic Hydrogels Using Molded Gelatin as a Sacrificial Element,” Lab Chip, 7(6), pp. 720–725. 10.1039/b618409j [DOI] [PubMed] [Google Scholar]
- [50]. Zheng, Y. , Chen, J. , Craven, M. , Choi, N. W. , Totorica, S. , Diaz-Santana, A. , Kermani, P. , Hempstead, B. , Fischbach-Teschl, C. , López, J. A. , and Stroock, A. D. , 2012, “ In Vitro Microvessels for the Study of Angiogenesis and Thrombosis,” Proc. Natl. Acad. Sci. U.S.A., 109(24), pp. 9342–9347. 10.1073/pnas.1201240109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Yeon, J. H. , Ryu, H. R. , Chung, M. , Hu, Q. P. , and Jeon, N. L. , 2012, “ In Vitro Formation and Characterization of a Perfusable Three-Dimensional Tubular Capillary Network in Microfluidic Devices,” Lab Chip, 12(16), pp. 2815–2822. 10.1039/c2lc40131b [DOI] [PubMed] [Google Scholar]
- [52]. Price, G. M. , Wong, K. H. K. , Truslow, J. G. , Leung, A. D. , Acharya, C. , and Tien, J. , 2010, “ Effect of Mechanical Factors on the Function of Engineered Human Blood Microvessels in Microfluidic Collagen Gels,” Biomaterials, 31(24), pp. 6182–6189. 10.1016/j.biomaterials.2010.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Yoshida, H. , Matsusaki, M. , and Akashi, M. , 2013, “ Multilayered Blood Capillary Analogs in Biodegradable Hydrogels for In Vitro Drug Permeability Assays,” Adv. Funct. Mater., 23(14), pp. 1736–1742. 10.1002/adfm.201201905 [DOI] [Google Scholar]
- [54]. Bischel, L. L. , Young, E. W. K. , Mader, B. R. , and Beebe, D. J. , 2013, “ Tubeless Microfluidic Angiogenesis Assay With Three-Dimensional Endothelial-Lined Microvessels,” Biomaterials, 34(5), pp. 1471–1477. 10.1016/j.biomaterials.2012.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Miller, J. S. , Stevens, K. R. , Yang, M. T. , Baker, B. M. , Nguyen, D.-H. T. , Cohen, D. M. , Toro, E. , Chen, A. A. , Galie, P. A. , Yu, X. , Chaturvedi, R. , Bhatia, S. N. , and Chen, C. S. , 2012, “ Rapid Casting of Patterned Vascular Networks for Perfusable Engineered Three-Dimensional Tissues,” Nat. Mater., 11(7), pp. 768–774. 10.1038/nmat3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Bogorad, M. I. , DeStefano, J. , Karlsson, J. , Wong, A. D. , Gerecht, S. , and Searson, P. C. , 2015, “ Review: In Vitro Microvessel Models,” Lab Chip, 15(22), pp. 4242–4255. 10.1039/C5LC00832H [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Nikkhah, M. , Edalat, F. , Manoucheri, S. , and Khademhosseini, A. , 2012, “ Engineering Microscale Topographies to Control the Cell-Substrate Interface,” Biomaterials, 33(21), pp. 5230–5246. 10.1016/j.biomaterials.2012.03.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Raghavan, S. , Nelson, C. M. , Baranski, J. D. , Lim, E. , and Chen, C. S. , 2010, “ Geometrically Controlled Endothelial Tubulogenesis in Micropatterned Gels,” Tissue Eng., Part A, 16(7), pp. 2255–2263. 10.1089/ten.tea.2009.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Nikkhah, M. , Eshak, N. , Zorlutuna, P. , Annabi, N. , Castello, M. , Kim, K. , Dolatshahi-Pirouz, A. , Edalat, F. , Bae, H. , Yang, Y. , and Khademhosseini, A. , 2012, “ Directed Endothelial Cell Morphogenesis in Micropatterned Gelatin Methacrylate Hydrogels,” Biomaterials, 33(35), pp. 9009–9018. 10.1016/j.biomaterials.2012.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Hsu, Y.-H. , Moya, M. L. , Hughes, C. C. W. , George, S. C. , and Lee, A. P. , 2013, “ A Microfluidic Platform for Generating Large-Scale Nearly Identical Human Microphysiological Vascularized Tissue Arrays,” Lab Chip, 13(15), pp. 2990–2998. 10.1039/c3lc50424g [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Kusuma, S. , Shen, Y.-I. , Hanjaya-Putra, D. , Mali, P. , Cheng, L. , and Gerecht, S. , 2013, “ Self-Organized Vascular Networks From Human Pluripotent Stem Cells in a Synthetic Matrix,” Proc. Natl. Acad. Sci. U.S.A., 110(31), pp. 12601–12606. 10.1073/pnas.1306562110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Kim, S. , Lee, H. , Chung, M. , and Jeon, N. L. , 2013, “ Engineering of Functional, Perfusable 3D Microvascular Networks on a Chip,” Lab Chip, 13(8), pp. 1489–1500. 10.1039/c3lc41320a [DOI] [PubMed] [Google Scholar]
- [63]. Nillesen, S. T. M. , Geutjes, P. J. , Wismans, R. , Schalkwijk, J. , Daamen, W. F. , and van Kuppevelt, T. H. , 2007, “ Increased Angiogenesis and Blood Vessel Maturation in Acellular Collagen-Heparin Scaffolds Containing Both FGF2 and VEGF,” Biomaterials, 28(6), pp. 1123–1131. 10.1016/j.biomaterials.2006.10.029 [DOI] [PubMed] [Google Scholar]
- [64]. Barkefors, I. , Le Jan, S. , Jakobsson, L. , Hejll, E. , Carlson, G. , Johansson, H. , Jarvius, J. , Jeong, W. P. , Noo, L. J. , and Kreuger, J. , 2008, “ Endothelial Cell Migration in Stable Gradients of Vascular Endothelial Growth Factor A and Fibroblast Growth Factor 2: Effects on Chemotaxis and Chemokinesis,” J. Biol. Chem., 283(20), pp. 13905–13912. 10.1074/jbc.M704917200 [DOI] [PubMed] [Google Scholar]
- [65]. Nguyen, D.-H. T. , Stapleton, S. C. , Yang, M. T. , Cha, S. S. , Choi, C. K. , Galie, P. A. , and Chen, C. S. , 2013, “ Biomimetic Model to Reconstitute Angiogenic Sprouting Morphogenesis In Vitro,” Proc. Natl. Acad. Sci. U.S.A., 110(17), pp. 6712–6717. 10.1073/pnas.1221526110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Wong, K. H. K. , Chan, J. M. , Kamm, R. D. , and Tien, J. , 2012, “ Microfluidic Models of Vascular Functions,” Annu. Rev. Biomed. Eng., 14(1), pp. 205–230. 10.1146/annurev-bioeng-071811-150052 [DOI] [PubMed] [Google Scholar]
- [67]. Estrada, R. , Giridharan, G. A. , Nguyen, M. D. , Prabhu, S. D. , and Sethu, P. , 2011, “ Microfluidic Endothelial Cell Culture Model to Replicate Disturbed Flow Conditions Seen in Atherosclerosis Susceptible Regions,” Biomicrofluidics, 5(3), pp. 1–11. 10.1063/1.3608137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Westein, E. , van der Meer, A. D. , Kuijpers, M. J. E. , Frimat, J.-P. , van den Berg, A. , and Heemskerk, J. W. M. , 2013, “ Atherosclerotic Geometries Exacerbate Pathological Thrombus Formation Poststenosis in a Von Willebrand Factor-Dependent Manner,” Proc. Natl. Acad. Sci. U.S.A., 110(4), pp. 1357–1362. 10.1073/pnas.1209905110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Zheng, Y. , Chen, J. , and López, J. A. , 2015, “ Flow-Driven Assembly of VWF Fibres and Webs in In Vitro Microvessels,” Nat. Commun., 6(7858), p. 7858. 10.1038/ncomms8858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Dimasi, A. , Rasponi, M. , Sheriff, J. , Chiu, W. C. , Bluestein, D. , Tran, P. L. , Slepian, M. J. , and Redaelli, A. , 2015, “ Microfluidic Emulation of Mechanical Circulatory Support Device Shear-Mediated Platelet Activation,” Biomed. Microdevices, 17(6), pp. 1–11. 10.1007/s10544-015-0015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Young, E. W. K. , Watson, M. W. L. , Srigunapalan, S. , Wheeler, A. R. , and Simmons, C. A. , 2010, “ Technique for Real-Time Measurements of Endothelial Permeability in a Microfluidic Membrane Chip Using Laser-Induced Fluorescence Detection,” Anal. Chem., 82(3), pp. 808–816. 10.1021/ac901560w [DOI] [PubMed] [Google Scholar]
- [72]. Zervantonakis, I. K. , Hughes-Alford, S. K. , Charest, J. L. , Condeelis, J. S. , Gertler, F. B. , and Kamm, R. D. , 2012, “ Three-Dimensional Microfluidic Model for Tumor Cell Intravasation and Endothelial Barrier Function,” Proc. Natl. Acad. Sci. U.S.A., 109(34), pp. 13515–13520. 10.1073/pnas.1210182109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Booth, R. , and Kim, H. , 2012, “ Characterization of a Microfluidic In Vitro Model of the Blood–Brain Barrier (μBBB),” Lab Chip, 12(10), pp. 1784–1792. 10.1039/c2lc40094d [DOI] [PubMed] [Google Scholar]
- [74]. Rusanov, A. L. , Luzgina, N. G. , Barreto, G. E. , and Aliev, G. , 2016, “ Role of Microfluidics in Blood–Brain Barrier Permeability Cell Culture Modeling: Relevance to CNS Disorders,” CNS Neurol. Disord.: Drug Targets, 15(3), pp. 301–309. 10.2174/1871527315666160202125304 [DOI] [PubMed] [Google Scholar]
- [75]. Butcher, E. C. , 1991, “ Leukocyte-Endothelial Cell Recognition: Three (or More) Steps to Specificity and Diversity,” Cell, 67(6), pp. 1033–1036. 10.1016/0092-8674(91)90279-8 [DOI] [PubMed] [Google Scholar]
- [76]. Kim, E. , Schueller, O. , and Sweetnam, P. M. , 2012, “ Targeting the Leukocyte Activation Cascade: Getting to the Site of Inflammation Using Microfabricated Assays,” Lab Chip, 12(12), pp. 2255–2264. 10.1039/c2lc21078a [DOI] [PubMed] [Google Scholar]
- [77]. Molteni, R. , Bianchi, E. , Patete, P. , Fabbri, M. , Baroni, G. , Dubini, G. , and Pardi, R. , 2014, “ A Novel Device to Concurrently Assess Leukocyte Extravasation and Interstitial Migration Within a Defined 3D Environment,” Lab Chip, 15(1), pp. 195–207. 10.1039/c4lc00741g [DOI] [PubMed] [Google Scholar]
- [78]. Han, S. , Yan, J.-J. , Shin, Y. , Jeon, J. J. , Won, J. , Jeong, H. E. , Kamm, R. D. , Kim, Y.-J. , and Chung, S. , 2012, “ A Versatile Assay for Monitoring In Vivo-Like Transendothelial Migration of Neutrophils,” Lab Chip, 12(20), pp. 3861–3865. 10.1039/c2lc40445a [DOI] [PubMed] [Google Scholar]
- [79]. Hamza, B. , and Irimia, D. , 2015, “ Whole Blood Human Neutrophil Trafficking in a Microfluidic Model of Infection and Inflammation,” Lab Chip, 15(12), pp. 2625–2633. 10.1039/C5LC00245A [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Jain, N. G. , Wong, E. A. , Aranyosi, A. J. , Boneschansker, L. , Markmann, J. F. , Briscoe, D. M. , and Irimia, D. , 2015, “ Microfluidic Mazes to Characterize T-Cell Exploration Patterns Following Activation In Vitro,” Integr. Biol., 7(11), pp. 1423–1431. 10.1039/C5IB00146C [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Preira, P. , Forel, J.-M. , Robert, P. , Nègre, P. , Biarnes-Pelicot, M. , Xeridat, F. , Bongrand, P. , Papazian, L. , Theodoly, O. , Negre, P. , Biarnes-Pelicot, M. , Xeridat, F. , Bongrand, P. , Papazian, L. , and Theodoly, O. , 2016, “ The Leukocyte-Stiffening Property of Plasma in Early Acute Respiratory Distress Syndrome (ARDS) Revealed by a Microfluidic Single-Cell Study: The Role of Cytokines and Protection With Antibodies,” Crit. Care, 20(1), p. 8. 10.1186/s13054-015-1157-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Kotz, K. T. , Xiao, W. , Miller-Graziano, C. , Qian, W. , Russom, A. , Warner, E. A. , Moldawer, L. L. , De, A. , Bankey, P. E. , Petritis, B. O. , Camp, D. G. , Rosenbach, A. E. , Goverman, J. , Fagan, S. P. , Brownstein, B. H. , Irimia, D. , Xu, W. , Wilhelmy, J. , Mindrinos, M. N. , Smith, R. D. , Davis, R. W. , Tompkins, R. G. , and Toner, M. , Inflammation and the Host Response to Injury Collaborative Research Program, 2010, “ Clinical Microfluidics for Neutrophil Genomics and Proteomics,” Nat. Med., 16(9), pp. 1042–1047. 10.1038/nm.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Rosenberg, R. D. , and Aird, W. C. , 1999, “ Vascular-Bed Specific Hemostasis and Hypercoagulable States,” N. Engl. J. Med., 340(20), pp. 1555–1564. 10.1056/NEJM199905203402007 [DOI] [PubMed] [Google Scholar]
- [84]. Rumbaut, R. E. , Slaff, D. W. , and Burns, A. R. , 2005, “ Microvascular Thrombosis Models in Venules and Arterioles In Vivo,” Microcirculation, 12(3), pp. 259–274. 10.1080/10739680590925664 [DOI] [PubMed] [Google Scholar]
- [85]. Levi, M. , Keller, T. T. , van Gorp, E. , and ten Cate, H. , 2003, “ Infection and Inflammation and the Coagulation System,” Cardiovasc. Res., 60(1), pp. 26–39. 10.1016/S0008-6363(02)00857-X [DOI] [PubMed] [Google Scholar]
- [86]. Ruggeri, Z. M. , 2009, “ Platelet Adhesion Under Flow,” Microcirculation, 16(1), pp. 58–83. 10.1080/10739680802651477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Higgins, J. M. , Eddington, D. T. , Bhatia, S. N. , and Mahadevan, L. , 2007, “ Sickle Cell Vasoocclusion and Rescue in a Microfluidic Device,” Proc. Natl. Acad. Sci. U.S.A., 104(51), pp. 20496–20500. 10.1073/pnas.0707122105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Tsai, M. , Kita, A. , Leach, J. , Rounsevell, R. , Huang, J. N. , Moake, J. , Ware, R. E. , Fletcher, D. A. , and Lam, W. A. , 2012, “ In Vitro Modeling of the Microvascular Occlusion and Thrombosis That Occur in Hematologic Diseases Using Microfluidic Technology,” J. Clin. Invest., 122(1), pp. 408–418. 10.1172/JCI58753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89]. Jeon, J. S. , Bersini, S. , Gilardi, M. , Dubini, G. , Charest, J. L. , Moretti, M. , and Kamm, R. D. , 2015, “ Human 3D Vascularized Organotypic Microfluidic Assays to Study Breast Cancer Cell Extravasation,” Proc. Natl. Acad. Sci. U.S.A., 112(1), pp. 214–219. 10.1073/pnas.1417115112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Skommer, J. , and Wlodkowic, D. , 2015, “ Successes and Future Outlook for Microfluidics-Based Cardiovascular Drug Discovery,” Expert Opin. Drug Discovery, 10(3), pp. 231–244. 10.1517/17460441.2015.1001736 [DOI] [PubMed] [Google Scholar]
- [91]. Chang, W. G. , Andrejecsk, J. W. , Kluger, M. S. , Saltzman, W. M. , and Pober, J. S. , 2013, “ Pericytes Modulate Endothelial Sprouting,” Cardiovasc. Res., 100(3), pp. 492–500. 10.1093/cvr/cvt215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Stratman, A. N. , Schwindt, A. E. , Malotte, K. M. , and Davis, G. E. , 2010, “ Endothelial-Derived PDGF-BB and HB-EGF Coordinately Regulate Pericyte Recruitment During Vasculogenic Tube Assembly and Stabilization,” Blood, 116(22), pp. 4720–4730. 10.1182/blood-2010-05-286872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Stratman, A. N. , Malotte, K. M. , Mahan, R. D. , Davis, M. J. , and Davis, G. E. , 2009, “ Pericyte Recruitment During Vasculogenic Tube Assembly Stimulates Endothelial Basement Membrane Matrix Formation,” Blood, 114(24), pp. 5091–5101. 10.1182/blood-2009-05-222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. Waters, J. P. , Kluger, M. S. , Graham, M. , Chang, W. G. , Bradley, J. R. , and Pober, J. S. , 2013, “ In Vitro Self-Assembly of Human Pericyte-Supported Endothelial Microvessels in Three-Dimensional Coculture: A Simple Model for Interrogating Endothelial-Pericyte Interactions,” J. Vasc. Res., 50(4), pp. 324–331. 10.1159/000353303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Kim, J. , Chung, M. , Kim, S. , Jo, D. H. , Kim, J. H. , and Jeon, N. L. , 2015, “ Engineering of a Biomimetic Pericyte-Covered 3D Microvascular Network,” PLoS One, 10(7), pp. 1–15. 10.1371/journal.pone.0133880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Van der Meer, A. D. , Orlova, V. V. , ten Dijke, P. , van den Berg, A. , and Mummery, C. L. , 2013, “ Three-Dimensional Co-Cultures of Human Endothelial Cells and Embryonic Stem Cell-Derived Pericytes Inside a Microfluidic Device,” Lab Chip, 13(18), pp. 3562–3568. 10.1039/c3lc50435b [DOI] [PubMed] [Google Scholar]
- [97]. Katt, M. E. , Placone, A. L. , Wong, A. D. , Xu, Z. S. , and Searson, P. C. , 2016, “ In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform,” Front. Bioeng. Biotechnol., 4(12), pp. 1–14. 10.3389/fbioe.2016.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Carmeliet, P. , and Jain, R. K. , 2000, “ Angiogenesis in Cancer and Other Diseases,” Nature, 407(6801), pp. 249–257. 10.1038/35025220 [DOI] [PubMed] [Google Scholar]
- [99]. Wirtz, D. , Konstantopoulos, K. , and Searson, P. C. , 2011, “ The Physics of Cancer: The Role of Physical Interactions and Mechanical Forces in Metastasis,” Nat. Rev. Cancer, 11(7), pp. 512–522. 10.1038/nrc3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Wong, A. D. , and Searson, P. C. , 2014, “ Live-Cell Imaging of Invasion and Intravasation in an Artificial Microvessel Platform,” Cancer Res., 74(17), pp. 4937–4945. 10.1158/0008-5472.CAN-14-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Buchanan, C. F. , Verbridge, S. S. , Vlachos, P. P. , and Rylander, M. N. , 2014, “ Flow Shear Stress Regulates Endothelial Barrier Function and Expression of Angiogenic Factors in a 3D Microfluidic Tumor Vascular Model,” Cell Adhes. Migr., 8(5), pp. 517–524. 10.4161/19336918.2014.970001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Chen, M. B. , Whisler, J. A. , Jeon, J. S. , and Kamm, R. D. , 2013, “ Mechanisms of Tumor Cell Extravasation in an In Vitro Microvascular Network Platform,” Integr. Biol. (Cambridge), 5(10), pp. 1262–1271. 10.1039/c3ib40149a [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103]. Zheng, F. , Fu, F. , Cheng, Y. , Wang, C. , Zhao, Y. , and Gu, Z. , 2016, “ Organ-on-a-Chip Systems: Microengineering to Biomimic Living Systems,” Small, 12(17), pp. 2253–2282. 10.1002/smll.201503208 [DOI] [PubMed] [Google Scholar]
- [104]. Huh, D. , Matthews, B. D. , Mammoto, A. , Montoya-Zavala, M. , Hsin, H. Y. , and Ingber, D. E. , 2010, “ Reconstituting Organ-Level Lung Functions on a Chip,” Science, 328(5986), pp. 1662–1668. 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Chiu, L. L. Y. , Montgomery, M. , Liang, Y. , Liu, H. , and Radisic, M. , 2012, “ Perfusable Branching Microvessel Bed for Vascularization of Engineered Tissues,” Proc. Natl. Acad. Sci. U.S.A., 109(50), pp. E3414–3423. 10.1073/pnas.1210580109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Brown, J. A. , Pensabene, V. , Markov, D. A. , Allwardt, V. , Neely, M. D. , Shi, M. , Britt, C. M. , Hoilett, O. S. , Yang, Q. , Brewer, B. M. , Samson, P. C. , McCawley, L. J. , May, J. M. , Webb, D. J. , Li, D. , Bowman, A. B. , Reiserer, R. S. , and Wikswo, J. P. , 2015, “ Recreating Blood-Brain Barrier Physiology and Structure on Chip: A Novel Neurovascular Microfluidic Bioreactor,” Biomicrofluidics, 9(5), pp. 1–15. 10.1063/1.4934713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Ligresti, G. , Nagao, R. J. , Xue, J. , Choi, Y. J. , Xu, J. , Ren, S. , Aburatani, T. , Anderson, S. K. , MacDonald, J. W. , Bammler, T. K. , Schwartz, S. M. , Muczynski, K. A. , Duffield, J. S. , Himmelfarb, J. , and Zheng, Y. , 2015, “ A Novel Three-Dimensional Human Peritubular Microvascular System,” J. Am. Soc. Nephrol., 27(8), pp. 2370–2381. 10.1681/ASN.2015070747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. Williamson, A. , Singh, S. , Fernekorn, U. , and Schober, A. , 2013, “ The Future of the Patient-Specific Body-on-a-Chip,” Lab Chip, 13(18), pp. 3471–3480. 10.1039/c3lc50237f [DOI] [PubMed] [Google Scholar]


