Abstract
Background:
Acute aortic dissection is a life-threatening cardiovascular emergency. Pentraxin-3 (PTX3) is proposed as a prognostic marker and found to be related to worse clinical outcomes in various cardiovascular diseases. This study sought to investigate the association of circulating PTX3 levels with in-hospital mortality in patients with acute Type A aortic dissection (TAAD).
Methods:
A total of 98 patients with TAAD between January 2012 and December 2015 were enrolled in this study. Plasma concentrations of PTX3 were measured upon admission using a high-sensitivity enzyme-linked immunosorbent assay system. Patients were divided into two groups as patients died during hospitalization (Group 1) and those who survived (Group 2). The clinical, laboratory variables, and imaging findings were analyzed between the two groups, and predictors for in-hospital mortality were evaluated using multivariate analysis.
Results:
During the hospital stay, 32 (33%) patients died and 66 (67%) survived. The patients who died during hospitalization had significantly higher PTX3 levels on admission compared to those who survived. Pearson's correlation analysis demonstrated that PTX3 correlated positively with high-sensitivity C-reactive protein (hsCRP), maximum white blood cell count, and aortic diameter. Multivariate logistic regression analysis demonstrated that PTX3 levels, coronary involvement, cardiac tamponade, and a conservative treatment strategy are significant independent predictors of in-hospital mortality in patients with TAAD. The receiver operating characteristic curve analysis further illustrated that PTX3 levels on admission were strong predictors of mortality with an area under the curve of 0.89. A PTX3 level ≥5.46 ng/ml showed a sensitivity of 88% and a specificity of 79%, and an hsCRP concentration ≥9.5 mg/L had a sensitivity of 80% and a specificity of 69% for predicting in-hospital mortality.
Conclusion:
High PTX3 levels on admission are independently associated with the in-hospital mortality in patients with TAAD.
Keywords: Mortality, Pentraxin-3, Aortic Dissection
Introduction
Acute aortic dissection (AAD) is a life-threatening cardiovascular disease associated with severe morbidity and mortality. The Stanford Type A involves the ascending aorta, whereas Type B dissection involves the descending aorta only.[1] Stanford-A is the most frequent type of dissection, and the mortality rate has been reported to be 50%–68% within the first 48 h after an acute event, with a mean mortality of up to approximately 1–2%/h, and that nearly 90% of patients succumbing within 30 days without appropriate treatment.[1,2] Although two validated risk stratification models including pre- and intra-operative variables have been described by the International Registry of Acute Aortic Dissection (IRAD),[3] the predictors of mortality in acute Type A AD (TAAD) are not fully elucidated. Recent studies have identified that some routine clinical biomarkers would be a valuable tool in early stratification of patients with acute TAAD.
Pentraxin-3 (PTX3) is a newly identified member of the pentraxin superfamily. As an evolutionarily conserved acute-phase inflammatory reactant, PTX3 shares some similarities with C-reactive protein (CRP), but differs in terms of structural domain, gene organization, cellular and tissue sources, inducing stimuli, and ligands recognized.[4] PTX3 is produced and released in several types of cells other than liver cells, such as vascular endothelial cells, macrophages, and smooth muscle cells, in response to primary inflammatory signals.[4,5] PTX3 is locally synthesized at the site of inflammation and may reflect the inflammatory status of the vasculature more directly than CRP.[5] Recent study has demonstrated that elevated plasma PTX3 levels were associated with plaque vulnerability in coronary artery disease patients.[6] Furthermore, plasma PTX3 is also implicated as a predictor of adverse clinical outcomes in patients with acute coronary syndrome[7] and could potentially function as an independent predictor of prognosis in patients with chronic heart failure.[8] To date, however, the prognostic value of circulating PTX3 levels in patients with TAAD has not been directly explored. The goal of the present study was to investigate the association of plasma PTX3 levels with in-hospital mortality in patients with acute TAAD.
Methods
Patient selection
Consecutive patients admitted with a new-onset acute TAAD and admitted to our hospital from January 2012 to December 2015 were eligible for analysis. AAD was diagnosed at the department of emergency medicine at our institution and classified according to the guidelines for the diagnosis and management of thoracic aortic disease by the American Heart Association.[9] The diagnosis of AAD was confirmed in all patients on the basis of typical clinical symptoms, chest radiography, transthoracic echocardiography (TTE), and computed tomography angiography (CTA).[1] Depending on the site of rupture, any dissection that involved the ascending aorta was defined as TAAD. Acute dissection is defined as within 1 week of symptom onset.[1,10] Exclusion criteria included patients with a history of previous episodes of aortic dissection (AD), aortic surgery, and/or endovascular interventions; patients with previous open heart surgery; obstructive sleep apnea-hypopnea syndrome; active systematic inflammatory disease, active infection, chronic inflammatory status including inflammatory rheumatic disease or malignant disease; acute and chronic liver disease; renal insufficiency (serum creatinine >2.5 mg/dl); and patients accompanied by coronary heart disease, Marfan syndrome, and those treated with nonsteroidal anti-inflammatory drugs or steroids. Written informed consent was obtained from all patients. The investigation was performed in accordance with the ethical standards in the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of the Second Xiangya Hospital of Central South University (No. 2012-026).
Data evaluation
The protocol for the enrolled patients included the documentation of the patients’ characteristics. Clinical variables included the baseline characteristics such as age, gender, smoking status, cardiovascular risk factors and comorbidities, and family history; as the time point of onset of symptoms and physical examination on admission were all assessed on admission. Complications, such as myocardial infarction, pericardial effusion, cardiac tamponade, aortic rupture, acute heart failure, cardiogenic shock, and acute renal failure, were also noted. The electrocardiographic findings; image diagnostic findings including those from chest radiography, echocardiography, and CTA; and details of medical or surgical therapy and outcomes were all entered into a database. The measurement of the diameter of the ascending aorta was based on CTA. Left ventricular ejection fraction was evaluated with TTE.
Definition of cardiovascular risk factors
The diagnosis of essential hypertension was established by a clinical record of systolic blood pressure ≥140 mmHg, and/or diastolic blood pressure ≥90 mmHg, or the use of antihypertensive agents. Diabetes mellitus was defined by patients having been informed of this diagnosis by a physician before admission or was receiving hypoglycemic treatments (dietary, oral anti-diabetic agents, or insulin) or patients who presented to the catheterization laboratory with serum hemoglobin A1c levels ≥6.5%. Dyslipidemia was defined by medical history or the use of lipid-lowering medications to reduce lipid levels or fasting serum low-density lipoprotein levels >140 mg/dl.[11]
Laboratory assays
The venous blood samples were drawn from all patients upon admission before administration of any medication. Blood samples were collected using standardized sterile tubes and centrifuged at 3500 r/min for 10 min at 4°C, and the serum and plasma were immediately frozen and stored at −80°C until being assayed. Levels of PTX3 were measured using a high-sensitivity enzyme-linked immunosorbent assay system for human plasma (Perseus Proteomics, Tokyo, Japan). The normal physiological concentration of plasma PTX3 is lower than 2 ng/ml. High-sensitivity CRP (hsCRP) levels were measured using immunonephelometric method (IMMAGE Immunochemistry System; Beckman Coulter, California, USA). The neutrophil-to-lymphocyte ratio was calculated as total neutrophil counts divided by those of lymphocytes using the same blood samples drawn on admission. Other blood tests, including lipids, troponin I, and creatinine were assayed using routine laboratory methods. Glomerular filtration rate was estimated on the basis of serum creatinine, age, and gender, using the Cockcroft-Gault formula as previously described.[12]
Statistical analysis
The Kolmogorov–Smirnov test was used to test the normality of distribution of continuous variables. Continuous data were reported as mean ± standard deviation (SD) if normally distributed or median (Q1, Q3) if not normally distributed, and they were compared using Student's t-test and Mann–Whitney U-test, respectively. Categorical variables were expressed as percentages and compared by Chi-square test or Fisher's exact test. Pearson's correlation coefficient was used to disclose the association between two continuous variables. Receiver operating characteristic (ROC) analyses were used to detect the cutoff value of PTX3 levels in prediction of in-hospital mortality in patients with TAAD. Multivariate logistic regression analysis was used to identify independent predictors of in-hospital mortality. All variables with P < 0.10 by univariate analysis were included in this multiple regression model. All statistical tests were performed using SPSS software, version 17.0 (SPSS Inc, Chicago, Illinois, USA). P < 0.05 was regarded as statistically significant.
Results
Baseline clinical characteristics, laboratory variables, and imaging findings
Of the 146 consecutive patients who presented with TAAD admitted to our hospital during the study period, a total of 98 patients fit the inclusion criteria and were included in this study. The mean age of the study population was 53.6 ± 9.8 years (range, 36–71 years) and 70% (n = 69) were male. Among them, 32 (33%) patients died during their hospital stay and 66 (67%) patients survived. The mean time from hospital admission to death was 4.0 ± 2.3 days. Thus, the study comprised two groups of patients: death (Group 1) and survival (Group 2). The causes of death included pericardium tamponade (n = 3), low cardiac output after operation (n = 5), aortic rupture (n = 7), acute myocardial infarction/cardiogenic shock (n = 8), acute heart failure (n = 2), and multiple organ failure (n = 7). The median onset of symptoms before admission was 2.8 h (1.2, 7.5). Fifty-five patients (56%) underwent emergency surgical repair, and the others were treated conservatively.
Demographic data, baseline clinical characteristics, and laboratory variables of the study participants according to in-hospital mortality are listed in Table 1. The mean age was significantly older in Group 1 as compared to Group 2 (P = 0.014). There were no significant differences between the two groups in terms of gender, body mass index, prevalence of cardiovascular risk factors, and time from symptoms’ onset to hospital admission. Moreover, the left ventricular ejection fraction and medications before admission were also similar. With respect to laboratory findings, the plasma PTX3 levels and hsCRP levels were significantly higher in Group 1 than in Group 2 (6.84 [3.82, 15.46] ng/ml vs. 3.85 [2.44, 5.96] ng/ml, P < 0.001; 14.7 [10.5, 21.6] mg/L vs. 5.9 [2.6, 10.3] mg/L, P = 0.003, respectively) [Figure 1]. The mean white blood cell (WBC) count as well as neutrophil-to-lymphocyte ratio on admission were also significantly higher in Group 1 [Table 1]. The CT and echocardiographic findings were similar between the two groups. However, the incidence of coronary involvement and the occurrence of cardiac tamponade were significantly higher in Group 1 as compared to Group 2. The aortic diameters were significantly larger in Group 1 [Table 1]. There was a significant difference in the two groups for surgical treatment (11 [34%] vs. 44 [67%], P = 0.003). Stratified analysis for each treatment strategy showed that PTX3 concentrations were significantly higher in patients who died than in survivors in surgically as well as in medically treated patients [Table 2]. In patients who died during hospitalization, the subgroup analysis showed that the plasma PTX3 levels were significantly higher in patients surgically treated than in those under conservative treatment (7.11 [4.13, 16.94] ng/ml vs. 6.31 [3.35, 13.78] ng/ml, P = 0.033).
Table 1.
Baseline clinical characteristics of patients with TAAD
| Characteristics | Group 1 (death, n = 32) | Group 2 (survival, n = 66) | Statistics | P |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 55.9 ± 10.3 | 50.8 ± 9.2 | 2.474* | 0.014 |
| Male sex, n (%) | 21 (66) | 48 (73) | 0.522† | 0.470 |
| Body mass index (kg/m2) | 25.3 ± 2.5 | 24.1 ± 2.2 | 0.221* | 0.825 |
| Cardiovascular risk factors, n (%) | ||||
| Diabetes mellitus | 9 (28) | 14 (21) | 0.573† | 0.449 |
| Hypertension (>140/90 mmHg) | 23 (72) | 51 (77) | 0.340† | 0.560 |
| Hyperlipidemia | 14 (44) | 23 (35) | 0.727† | 0.394 |
| Current smoker | 19 (59) | 31 (47) | 1.327† | 0.249 |
| Physical findings on admission | ||||
| Admission systolic blood pressure (mmHg) | 164 ± 29 | 172 ± 24 | −1.444* | 0.152 |
| Admission diastolic blood pressure (mmHg) | 76 ± 18 | 80 ± 19 | −0.994* | 0.322 |
| Heart rate (beats/min) | 82 ± 23 | 83 ± 18 | −0.235* | 0.814 |
| Medications before admission, n (%) | ||||
| Beta-blockers | 5 (16) | 14 (21) | 0.430† | 0.512 |
| ACEI/ARB | 10 (31) | 30 (46) | 1.800† | 0.180 |
| Calcium channel blocker | 19 (59) | 45 (68) | 0.738† | 0.390 |
| Laboratory tests | ||||
| PTX3 (ng/ml) | 6.84 (3.82, 15.46) | 3.85 (2.44, 5.96) | −4.190‡ | <0.001 |
| hsCRP (mg/L) | 14.7 (10.5, 21.6) | 5.9 (2.6, 10.3) | −2.964‡ | 0.003 |
| Peak troponin I (ng/ml) | 72 (38, 119) | 69 (31, 101) | −1.056‡ | 0.287 |
| NT-proBNP (pg/ml) | 84.7 (25.3, 214.1) | 79.9 (19.6, 138.3) | −0.618‡ | 0.542 |
| D-dimer (μg/ml) | 4.6 (3.7, 5.7) | 3.9 (1.9, 5.4) | −0.894‡ | 0.368 |
| WBC (109/L) | 14.2 ± 5.5 | 12.7 ± 4.8 | 2.351* | 0.021 |
| Neutrophil-to-lymphocyte ratio | 12.3 ± 7.1 | 9.1 ± 5.9 | 2.923* | 0.004 |
| Serum creatinine (μmol/L) | 85.5 ± 20.1 | 83.2 ± 16.9 | 0.593* | 0.554 |
| Glomerular filtration rate (ml·min−1·1.73 m−2) | 89.6 ± 22.5 | 93.5 ± 20.8 | −0.847* | 0.398 |
| Total cholesterol (mg/dl) | 170.5 ± 34.7 | 166.4 ± 25.1 | 0.667* | 0.506 |
| LDL-C (mg/dl) | 99.1 ± 29.4 | 95.6 ± 21.2 | 0.673* | 0.503 |
| Time from symptoms’ onset to admission (h) | 3.7 (1.7, 10.2) | 2.2 (1.0, 6.1) | 0.894‡ | 0.368 |
| Time from admission to death (days) | 4.0 ± 2.3 | – | – | – |
| Surgical treatment, n (%) | 11 (34) | 44 (67) | 9.126† | 0.003 |
| CT, echocardiography characteristics | ||||
| Ascending aortic diameter, mm | 47.4 ± 8.3 | 40.2 ± 6.5 | 2.866* | 0.006 |
| Coronary involvement, n (%) | 15 (47) | 7 (11) | 16.284† | <0.001 |
| Cardiac tamponade, n (%) | 7 (22) | 4 (6) | Fisher | 0.036 |
| Pleural effusion, n (%) | 8 (25) | 14 (21) | 0.178† | 0.673 |
| Bicuspid aortic valve, n (%) | 4 (13) | 6 (9) | Fisher | 0.724 |
| Left ventricular ejection fraction (%) | 51.9 ± 12.4 | 54.1 ± 11.0 | −0.890* | 0.375 |
Data are expressed as mean ± SD, as percentages, or as median (Q1, Q3). *t value; †χ2 value; ‡Z value. –: Data not available; TAAD: Type A aortic dissection; PTX3: Pentraxin-3; CT: Computed tomography; LDL-C: Low-density lipoprotein-cholesterol; WBC: White blood cell; hsCRP: High-sensitivity C-reactive protein; SD: Standard deviation; ACEI: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin receptor blocker.
Figure 1.
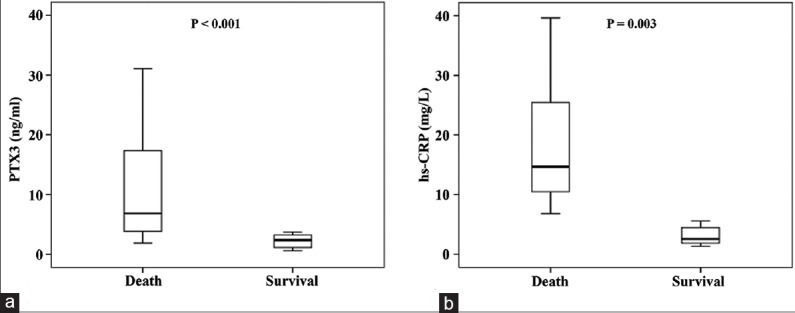
Comparison of (a) plasma pentraxin-3 (PTX3) and (b) high-sensitivity C-reactive protein (hs-CRP) levels between survived and dead patients admitted with acute Type A aortic dissection.
Table 2.
PTX3 concentrations (ng/ml) on admission in patients with acute TAAD
| Patient group | n/N | Group 1 (death) | Group 2 (survival) | Z | P |
|---|---|---|---|---|---|
| All patients | 32/98 | 6.84 (3.82, 15.46) | 3.85 (2.44, 5.96) | −4.190 | <0.001 |
| Surgically treated | 11/55 | 7.11 (4.13, 16.94) | 4.08 (2.53, 6.25) | −1.812 | 0.007 |
| Medically treated | 21/43 | 6.31 (3.35, 13.78) | 3.46 (2.15, 5.40) | −3.090 | 0.002 |
Data are presented as n/N or median (Q1, Q3). n/N: Dead patients/total patients; TAAD: Type A aortic dissection; PTX3: Pentraxin-3.
Relationships among pentraxin-3, high-sensitivity C-reactive protein, and imaging findings
Pearson's correlation analysis documented that the plasma PTX3 levels had a positive correlation with aortic diameter (r = 0.527, P < 0.001), while a negative correlation with the time from the onset of symptoms to hospital admission (r = −0.264, P = 0.005). Interestingly, plasma PTX3 levels were weakly correlated with CRP (r = 0.216, P = 0.015) and maximum WBC count (r = 0.327, P = 0.007).
Univariate and multiple logistic regression analysis for in-hospital mortality
The results of the univariate and multivariate analyses for the predictors of in-hospital mortality are listed in Table 3. The univariable analysis showed that significant predictors of in-hospital mortality were age >65 years, PTX3 levels, hsCRP levels on admission, neutrophil-to-lymphocyte ratio, aortic diameter, cardiac tamponade, coronary involvement, time from symptoms’ onset to hospital admission, and a conservative treatment strategy. In a multivariable logistic regression model by using in-hospital mortality as the dependent variable with adjustments for significant variables as identified from the univariable analysis, PTX3 and hsCRP levels on admission, conservative treatment strategy, and cardiac tamponade together with coronary involvement remained independent predictors of in-hospital mortality. The ROCs curves of PTX3 levels and hsCRP for predicting in-hospital mortality are shown in Figure 2. The ROC curve analysis further illustrated that PTX3 levels on admission were the strong predictors of mortality with an area under the curve (AUC) of 0.89 (95% confidence interval [CI]: 0.83–0.96, P < 0.001), while the AUC of CRP was 0.69 (95% CI: 0.51–0.88; P = 0.022). A PTX3 level ≥5.46 ng/ml showed a sensitivity of 88% and a specificity of 79% [Figure 2a], and an hsCRP level ≥9.5 mg/L had a sensitivity of 80% and a specificity of 69% [Figure 2b] for predicting in-hospital mortality.
Table 3.
Effects of various variables on in-hospital mortality in univariate and multivariate logistic regression analyses
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age >65 years | 2.12 (0.81–4.92) | 0.02 | 1.79 (0.73–5.05) | 0.17 |
| Admission SBP (>140 mmHg) | 1.29 (0.95–2.09) | 0.19 | ||
| Admission DBP (>90 mmHg) | 1.67 (0.68–4.75) | 0.55 | ||
| Diabetes mellitus | 0.96 (0.90–1.53) | 0.27 | ||
| Hyperlipidemia | 0.79 (0.27–1.48) | 0.41 | ||
| Serum hsCRP levels | 3.53 (1.54–4.35) | 0.004 | 1.67 (1.24–2.63) | 0.02 |
| Plasma PTX3 levels | 4.93 (2.10–8.92) | <0.001 | 4.16 (1.72–10.21) | <0.001 |
| Neutrophil-to-lymphocyte ratio | 1.78 (0.84–3.58) | 0.05 | 1.26 (0.56–2.97) | 0.26 |
| Time from symptoms’ onset to admission (h) | 2.76 (1.12–7.11) | 0.04 | 2.15 (1.26–4.20) | 0.17 |
| Baseline LVEF (%) | 1.56 (0.77–3.44) | 0.27 | ||
| Aortic diameter (mm) | 1.24 (0.97–1.91) | 0.05 | 1.29 (1.10–2.92) | 0.19 |
| Coronary involvement | 3.48 (2.09–5.16) | <0.001 | 3.02 (1.86–4.79) | 0.02 |
| Conservative treatment | 2.37 (0.79–8.25) | 0.002 | 2.89 (0.92–9.96) | <0.001 |
| Cardiac tamponade | 3.45 (1.84–6.41) | <0.001 | 2.66 (1.52–5.07) | 0.004 |
PTX3: Pentraxin-3; hsCRP: High-sensitivity C-reactive protein; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; LVEF: Left ventricular ejection fraction; OR: Odds ratio; CI: Confidence interval.
Figure 2.
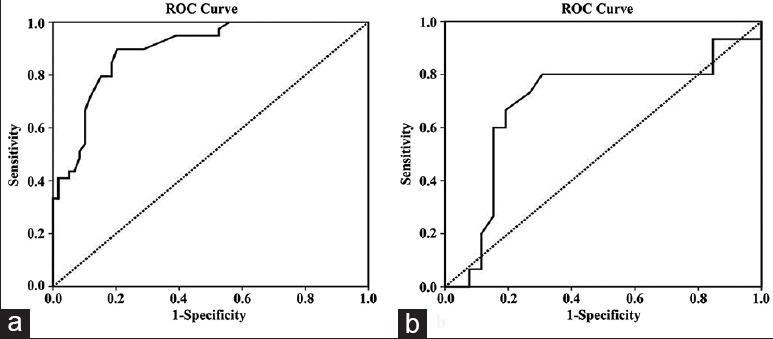
Receiver-operating characteristic curves of pentraxin-3 (a) and high-sensitivity C-reactive protein (b) levels for predicting in-hospital mortality.
Discussion
The major findings of this study are as follows: (1) plasma PTX3 levels were significantly elevated during the acute stage after AD, and the levels on admission were significantly higher in the patients who died during hospitalization than in the survivors. (2) PTX3 levels showed a good correlation with established predictors of in-hospital mortality such as maximum aortic diameter at the level of the dissection. (3) The in-hospital mortality rates in patients with acute TAAD remained high. PTX3 level on admission was found to be an independent predictor of in-hospital mortality. This preliminary study suggests that PTX3 might be a novel biomarker reflecting active tissue inflammation in the aortic wall following AD, and PTX3 might provide valuable information for risk stratification of patients with acute TAAD. These findings may lead the clinician to make clinical risk stratification by incorporating PTX3 into the current risk assessment tools for patients admitted with acute TAAD.
Despite significant advances in the treatment of many cardiac conditions, acute TAAD remains the most catastrophic condition affecting the thoracic aorta, with high in-hospital mortality due to potentially fatal complications such as coronary involvement, even in centers that have extensive expertise and interest in the treatment of high-risk patients.[13] The IRAD reported in-hospital mortality rate of 32.5% in 547 patients for acute TAAD.[14] Similarly, in this analysis of 98 patients who had TAAD during the study period, the overall in-hospital mortality rate was up to 32.7%, which was within the range (between 20% and 30%) obtained from other recent single-center studies.[15] Given the high mortality, predictive tools to identify patients at an increased risk of death are needed to assist clinicians in optimal treatment. According to the IRAD, the independent variables of mortality are advanced age (≥70 years), hypotension, shock, or tamponade at presentation, previous heart disease and postoperative renal failure, and mesenteric or myocardial ischemia.[14] However, there is a growing interest in bedside risk assessment for in-hospital death, and in many current research studies, several biomarkers of vascular damage, thrombosis, and inflammation have been evaluated as contributors in the diagnosis of AAD or as risk prediction tools.[16,17,18,19,20]
PTX3 is synthesized locally at the inflammatory sites by vascular endothelium and smooth muscle cells or by macrophages upon exposure to primary inflammatory signals such as interleukin-1 (IL-1), tumor necrosis factor-α, and oxidized low-density lipoprotein.[4,5,6] Furthermore, PTX3 is highly expressed in vascular cells and vascular inflammatory cells of human atherosclerotic lesions.[21] Importantly, PTX3 appears to be also a useful marker of cardiovascular risk, and high concentrations of circulating PTX3 have been shown to be consistently and strongly associated with adverse cardiovascular events in patients with non-ST-segment elevation myocardial infarction[7,22] as well as in those with chronic heart failure.[8,23] A recent study by Akgul et al.[24] suggests that high-admission PTX3 level was associated with increased in-hospital cardiovascular mortality and 2-year all-cause mortality in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Taken together, PTX3 may represent a useful marker for localized vascular inflammation and damage in the cardiovascular system. Arao et al.[11] have recently explored the association of plasma PTX3 levels with the amount of pleural fluid accumulation in Stanford Type B AD patients. Their results demonstrated that the peak PTX3 level in patients with AAD was associated with the amount of transient pleural fluid accumulation. However, this study included only the patients who suffered Type B AD. In addition, it did not show significant differences between the PTX3 levels in patients with and without composite events of all-cause death and aortic repair due to limited samples.
The present study preliminary demonstrated that plasma PTX3 levels are independently associated with in-hospital mortality in patients with TAAD. Why elevated PTX3 level is associated with increased in-hospital mortality in patients with TAAD? Currently, the exact pathophysiologic mechanisms have not been clearly elucidated; however, we propose that these potential mechanisms might be responsible for the association of PTX3 with in-hospital mortality after TAAD. First, PTX3 is a useful marker for evaluating local inflammatory response and vascular injury. It is produced mainly in vascular-related cells, and AAD occurs instantly with extensive macrovascular disease involving aortic medial tears. Therefore, a higher circulating PTX3 level might indicate patients who have a more intense vascular inflammatory reaction and vascular injury. Second, vessel wall inflammation in patients with AAD has been associated with a high risk for disease progression. It has been demonstrated that inflammation could destroy the medial layer of the aortic wall and eventually lead to dilation, dissection, and rupture of the aortic wall.[25] Recent studies showed that inflammatory changes in the aortic wall were observed during the whole course of AD.[26,27] Qin et al.[28] have recently dynamically monitored the systemic inflammatory level and platelet activation with a new acute TAAD canine model. Their study found that both tumor necrosis factor-α and IL-6 were significantly climbed and reached highest levels at 6 h after the AD model was established. Moreover, the same start points for systemic inflammation and platelet activation were identified, and bivariate analysis demonstrated that peak mean platelet volume/platelet count ratios were positively correlated with inflammatory cytokine. Kuehl et al.[29] also revealed that patients who had serious clinical symptoms and developed disease progression had higher inflammatory cell activity in the aortic wall than asymptomatic and clinically stable patients. More interestingly, there is now substantial evidence to suggest that increased circulating inflammatory markers such as CRP,[18] neutrophil-to-lymphocyte ratio,[19] and WBC count[20] could predict clinical events in patients with acute TAAD, especially death during hospitalization.
Of note, the present study demonstrated a weak association between PTX3 and hsCRP concentrations (r = 0.216, P = 0.015), which is consistent with previous reports of other population such as non-ST-segment elevation myocardial infarction,[22] chronic heart failure,[23] or Stanford Type B AD.[11] This is because these inflammatory markers are produced differently. CRP is a short pentraxin produced in the liver in response to IL-6, whereas PTX3 is a long pentraxin produced by inflammatory and immune cells in response to IL-1. In addition, PTX3 is also distinct from CRP in ligand recognitions and innate immunity function.[11] These differences between PTX3 and CRP may have resulted in the weak association of these markers. Therefore, PTX3 provides important prognostic information independently from CRP and it is a more sensitive and immediate inflammatory marker than CRP in patients with TAAD.
Our study identified several clinical variables to be important predictors of death in patients with acute TAAD that are similar to those reported in prior studies.[14,15] By multivariate analysis, the logistic model identified the following complications independently associated with death during in-hospital stay: PTX3 and hsCRP levels on admission and conservative treatment strategy together with coronary involvement. The IRAD revealed that complications of dissection are more common in patients with an aortic diameter of 5.5 cm or greater; these patients have about a four times greater in-hospital mortality than those with a diameter smaller than 5.5 cm. Interestingly, the present study showed that plasma PTX3 levels had a positive correlation with aortic diameter. This correlation might also explain part of the increased in-hospital mortality rate in patients with elevated PTX3.
Some limitations of the present study have to be acknowledged: (1) the major limitation of this study is a single-center study limited to a specific study population and the sample size was relatively small. Therefore, the selection bias cannot be completely avoided although strict inclusion and exclusion criteria of participants were followed. (2) Allowing for the surgical intervention itself is thought to have a great effect on the PTX3 and CRP levels, thus the information on dynamic changes of PTX3 was not investigated, while serial measurements of PTX3 might be useful for evaluating changes in inflammatory status, estimating risk during the hospitalization. These issues should be evaluated in a future trial with a large-scale study population. (3) Multiple factors have shown to contribute to the outcomes of acute TAAD; thus, although a strong outcome predictor, plasma PTX3, may not be used as a “stand-alone” test in clinical routine, it will substantially improve outcome prediction in combination with the existing risk indices. Despite these limitations, our study provided the evidence for the prognostic significance of PTX3 in TAAD.
In conclusion, this study suggested that high PTX3 levels on admission were independently associated with the in-hospital mortality in patients with TAAD. Plasma PTX3 levels could provide useful information for identifying patients at a high risk among patients with TAAD. Further large-scale investigations and comparisons with other clinical parameters are required to confirm the predictive ability of PTX3 in the prognosis of TAAD.
Financial support and sponsorship
This work was supported in part by a grant from the 2015 Yu-Ying Plan of the Central South University (No. 502034007).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet. 2015;385:800–11. doi: 10.1016/S0140-6736(14)61005-9. doi: 10.1016/S0140-6736(14)61005-9. [DOI] [PubMed] [Google Scholar]
- 2.Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): New insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 3.Rampoldi V, Trimarchi S, Eagle KA, Nienaber CA, Oh JK, Bossone E, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: The International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83:55–61. doi: 10.1016/j.athoracsur.2006.08.007. doi: 10.1016/j.athoracsur.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11:328–34. doi: 10.1038/ni.1854. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K, Sugiyama A, Reid PC, Ito Y, Miyauchi K, Mukai S, et al. Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arterioscler Thromb Vasc Biol. 2007;27:161–7. doi: 10.1161/01.ATV.0000252126.48375.d5. doi: 10.1161/01.ATV.0000252126.48375.d5. [DOI] [PubMed] [Google Scholar]
- 6.Tazaki R, Tanigawa J, Fujisaka T, Shibata K, Takeda Y, Ishihara T, et al. Plasma pentraxin3 level is associated with plaque vulnerability assessed by optical coherence tomography in patients with coronary artery disease. Int Heart J. 2016;57:18–24. doi: 10.1536/ihj.15-248. doi: 10.1536/ihj.15-248. [DOI] [PubMed] [Google Scholar]
- 7.Guo R, Li Y, Wen J, Li W, Xu Y. Elevated plasma level of pentraxin-3 predicts in-hospital and 30-day clinical outcomes in patients with non-ST-segment elevation myocardial infarction who have undergone percutaneous coronary intervention. Cardiology. 2014;129:178–88. doi: 10.1159/000364996. doi: 10.1159/000364996. [DOI] [PubMed] [Google Scholar]
- 8.Latini R, Gullestad L, Masson S, Nymo SH, Ueland T, Cuccovillo I, et al. Pentraxin-3 in chronic heart failure: The CORONA and GISSI-HF trials. Eur J Heart Fail. 2012;14:992–9. doi: 10.1093/eurjhf/hfs092. doi: 10.1093/eurjhf/hfs092. [DOI] [PubMed] [Google Scholar]
- 9.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–369. doi: 10.1161/CIR.0b013e3181d4739e. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 10.Booher AM, Isselbacher EM, Nienaber CA, Trimarchi S, Evangelista A, Montgomery DG, et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med. 2013;126:730.e19–24. doi: 10.1016/j.amjmed.2013.01.020. doi: 10.1016/j.amjmed.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Arao K, Fujiwara T, Taniguchi Y, Jinnouchi H, Sasai H, Matsumoto M, et al. Implications of pentraxin 3 levels in patients with acute aortic dissection. Heart Vessels. 2015;30:211–7. doi: 10.1007/s00380-014-0470-2. doi: 10.1007/s00380-014-0470-2. [DOI] [PubMed] [Google Scholar]
- 12.Vervoort G, Klein Gunnewiek JM, Willems HL, Wetzels JF. Effect of creatinine assay standardization on the performance of Cockcroft-Gault and MDRD formula in predicting GFR. Nephrol Dial Transplant. 2006;21:2998–9. doi: 10.1093/ndt/gfl276. doi: 10.1093/ndt/gfl276. [DOI] [PubMed] [Google Scholar]
- 13.Tang YF, Zhang GX, Liao ZL, Han L, Xu ZY. Surgical treatment of coronary malperfusion with acute type A aortic dissection. Chin Med J. 2016;129:1000–2. doi: 10.4103/0366-6999.179797. doi: 10.4103/0366-6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta RH, Suzuki T, Hagan PG, Bossone E, Gilon D, Llovet A, et al. Predicting death in patients with acute type A aortic dissection. Circulation. 2002;105:200–6. doi: 10.1161/hc0202.102246. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Jiang Y, Gao C, Feng J, Wang A. Risk factors for hospital death in patients with acute aortic dissection. Heart Lung Circ. 2015;24:348–53. doi: 10.1016/j.hlc.2014.10.009. doi: 10.1016/j.hlc.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Ranasinghe AM, Bonser RS. Biomarkers in acute aortic dissection and other aortic syndromes. J Am Coll Cardiol. 2010;56:1535–41. doi: 10.1016/j.jacc.2010.01.076. doi: 10.1016/j.jacc.2010.01.076. [DOI] [PubMed] [Google Scholar]
- 17.Wen D, Du X, Dong JZ, Zhou XL, Ma CS. Value of D-dimer and C reactive protein in predicting inhospital death in acute aortic dissection. Heart. 2013;99:1192–7. doi: 10.1136/heartjnl-2013-304158. doi: 10.1136/heartjnl-2013-304158. [DOI] [PubMed] [Google Scholar]
- 18.Okina N, Ohuchida M, Takeuchi T, Fujiyama T, Satoh A, Sakamoto T, et al. Utility of measuring C-reactive protein for prediction of in-hospital events in patients with acute aortic dissection. Heart Vessels. 2013;28:330–5. doi: 10.1007/s00380-012-0257-2. doi: 10.1007/s00380-012-0257-2. [DOI] [PubMed] [Google Scholar]
- 19.Kalkan ME, Kalkan AK, Gündes A, Yanartas M, Oztürk S, Gurbuz AS, et al. Neutrophil to lymphocyte ratio: A novel marker for predicting hospital mortality of patients with acute type A aortic dissection. Perfusion. 2015 doi: 10.1177/0267659115590625. pii: 0267659115590625. [Epub ahead of print] doi: 10.1177/0267659115590625. [DOI] [PubMed] [Google Scholar]
- 20.Wen D, Wu HY, Jiang XJ, Zhang HM, Zhou XL, Li JJ, et al. Role of plasma C-reactive protein and white blood cell count in predicting in-hospital clinical events of acute type A aortic dissection. Chin Med J. 2011;124:2678–82. [PubMed] [Google Scholar]
- 21.Koga S, Ikeda S, Yoshida T, Nakata T, Takeno M, Masuda N, et al. Elevated levels of systemic pentraxin 3 are associated with thin-cap fibroatheroma in coronary culprit lesions: Assessment by optical coherence tomography and intravascular ultrasound. JACC Cardiovasc Interv. 2013;6:945–54. doi: 10.1016/j.jcin.2013.04.024. doi: 10.1016/j.jcin.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Matsui S, Ishii J, Kitagawa F, Kuno A, Hattori K, Ishikawa M, et al. Pentraxin 3 in unstable angina and non-ST-segment elevation myocardial infarction. Atherosclerosis. 2010;210:220–5. doi: 10.1016/j.atherosclerosis.2009.10.033. doi: 10.1016/j.atherosclerosis.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki S, Takeishi Y, Niizeki T, Koyama Y, Kitahara T, Sasaki T, et al. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am Heart J. 2008;155:75–81. doi: 10.1016/j.ahj.2007.08.013. doi: 10.1016/j.ahj.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Akgul O, Baycan OF, Bulut U, Somuncu MU, Pusuroglu H, Ozyilmaz S, et al. Long-term prognostic value of elevated pentraxin 3 in patients undergoing primary angioplasty for ST-elevation myocardial infarction. Coron Artery Dis. 2015;26:592–7. doi: 10.1097/MCA.0000000000000280. doi: 10.1097/MCA.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 25.del Porto F, Proietta M, Tritapepe L, Miraldi F, Koverech A, Cardelli P, et al. Inflammation and immune response in acute aortic dissection. Ann Med. 2010;42:622–9. doi: 10.3109/07853890.2010.518156. doi: 10.3109/07853890.2010.518156. [DOI] [PubMed] [Google Scholar]
- 26.Luo F, Zhou XL, Li JJ, Hui RT. Inflammatory response is associated with aortic dissection. Ageing Res Rev. 2009;8:31–5. doi: 10.1016/j.arr.2008.08.001. doi: 10.1016/j.arr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 27.He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131:671–8. doi: 10.1016/j.jtcvs.2005.09.018. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Qin C, Zhang H, Gu J, Xiao Z, Yang Q, Meng W. Dynamic monitoring of platelet activation and its role in post-dissection inflammation in a canine model of acute type A aortic dissection. J Cardiothorac Surg. 2016;11:86. doi: 10.1186/s13019-016-0472-5. doi: 10.1186/s13019-016-0472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuehl H, Eggebrecht H, Boes T, Antoch G, Rosenbaum S, Ladd S, et al. Detection of inflammation in patients with acute aortic syndrome: Comparison of FDG-PET/CT imaging and serological markers of inflammation. Heart. 2008;94:1472–7. doi: 10.1136/hrt.2007.127282. doi: 10.1136/hrt.2007.127282. [DOI] [PubMed] [Google Scholar]


