Abstract
Background:
Polypoidal choroidal vasculopathy (PCV) is characterized by the presence of polyps with or without a branching vascular network and more prevalent among Asians. The aim of this study was to compare the outcomes of conbercept therapy between two different angiographic subtypes of PCV.
Methods:
Fifty-eight patients of PCV were classified into two phenotypes according to indocyanine green angiography (ICGA). In Type 1, both feeder and draining vessels are visible on ICGA and network vessels are numerous. In Type 2, neither feeder nor draining vessels are detectable, and the number of network vessels is small. The patients were treated with intravitreal conbercept (IVC) for 3 months. Additional IVC was given at subsequent monthly visits, if needed. The patients were followed up for 12 months, and changes in mean best-corrected visual acuity (BCVA), central retinal thickness (CRT), subretinal fluid (SRF) thickness, pigmented epithelial detachment (PED), hemorrhage, and number of polypoidal lesions were evaluated.
Results:
The mean BCVA in Type 2 PCV (15.92 ± 9.76 letters) achieved a significantly greater improvement than that in the Type 1 (14.10 ± 9.07 letters) at month 12 (t = 2.37, P < 0.01). Moreover, the mean CRT decrease was numerically greater in Type 2 (120.44 ± 73.81 μm) compared with Type 1 (106.48 ± 72.33 μm) at month 6 (t = 4.31, P < 0.01), and greater in Type 2 (130.21 ± 76.28 μm) compared with Type 1 (111.67 ± 79.57 μm) at month 9 (t = 1.87, P < 0.01). There was no significant difference between the two types for the decrease in SRF thickness, PED height, and regression of polyps from month 3 to 12 (t = 2.97, P > 0.05).
Conclusion:
Classification systems for PCV will show differences in presentation, natural history, or response to anti-vascular endothelial growth factor treatment and might, therefore, provide a new key to the choice of treatment for the disease.
Keywords: Angiographic Subtypes, Anti-vascular Endothelial Growth Factor, Conbercept, Polypoidal Choroidal Vasculopathy
Introduction
Polypoidal choroidal vasculopathy (PCV) is a macular disease affecting the elderly that is more prevalent among Asians as compared with Caucasians and has been found to account for 25% to 50% of cases of presumed neovascular age-related macular degeneration in Asian patients.[1,2,3,4] The natural course of PCV is variable. The visual outcomes following PCV are generally better compared with choroidal neovascularization (CNV) following AMD. When the pathologic changes affect the subfoveal area, episodes of exudation will result in RPE and photoreceptor degeneration, scarring, and irreversible vision loss.[5,6,7,8]
There is no effective and widely accepted method for treating PCV. Photodynamic therapy (PDT) is one of the most common treatments, and several studies have reported improved vision and reduced polypoidal lesions after PDT treatment. However, the mean best-corrected visual acuity (BCVA) usually decreases below baseline, and the recurrence rate is 59–79% 3–5 years after the initiation of PDT monotherapy.[9,10,11,12] Furthermore, several serious complications after PDT were reported, such as massive subretinal hemorrhages, retinal pigment epithelium tears, and retinal atrophy.[13,14,15,16,17]
Intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents, such as bevacizumab or ranibizumab, can reduce subretinal fluid (SRF) and stabilize the vision of eyes with PCV.[18,19,20,21] However, both of these drugs are less effective at decreasing the abnormal choroidal vasculature characteristics of PCV compared to PDT. In addition, compared to typical AMD, PCV responds less favorably to anti-VEGF therapy, and polyp closure occurs incompletely in a majority of cases.[22,23,24]
Conbercept (KH902; Chengdu Kanghong Biotech Co., Ltd., Sichuan, China) is the most recently approved member of the anti-VEGF family of drugs. Conbercept was developed to provide a more potent and prolonged anti-VEGF effect and was approved by the China Food and Drug Administration in November 2013. Similar to aflibercept, conbercept consists of the VEGF-binding domains of human VEGFR-1 and VEGFR-2 combined with the Fc portion of human immunoglobulin G-1. In addition to a high affinity for all isoforms of VEGF-A, conbercept also binds to placental growth factor and VEGF-B. The structural difference between conbercept and aflibercept is that conbercept also contains the fourth binding domain of VEGFR-2, which is essential for receptor dimerization and enhances the association rate of VEGF to the receptor. Because this domain of VEGFR-2 has a lower isoelectric point, the addition of this domain to KH902 decreases the positive charge of the molecule and results in decreased adhesion to the extracellular matrix.[25,26,27]
Currently, there is no uniform classification system for PCV based on its angiographic characteristics. Classification systems for diseases should utilize differences in presentation, natural history, clinical outcomes, and treatment responses. Previous reports have discussed the PCV subtypes and categorized PCV as polypoidal CNV and PCV in the narrow sense (also referred to as typical PCV).[28,29,30] In our clinical practice, we classified PCV patients into two different types. In this study, we postulated that the PCV vascular subtypes observed during the initial clinical examinations are independently associated with the long-term clinical outcomes. We compared patient outcomes between two different angiographic PCV subtypes following conbercept therapy.
Methods
Patients
We performed a retrospective institution-based study of 58 eyes of 58 consecutive treatment-naive patients who presented with symptomatic PCV between July 2013 and July 2014 at the Department of Ophthalmology in the Peking University People's Hospital.
All cases with PCV underwent fluorescein angiography (FA), optic coherence tomography (OCT), and indocyanine green angiograms with HRA2 (Heidelberg Engineering, Heidelberg, Germany). The diagnosis of PCV was based on indocyanine green angiography (ICGA) results, which showed a branching vascular network (BVN) that terminated in aneurysmal enlargements, that is, polypoidal lesions.[5,31] Exclusion criteria included patients with concomitant ocular disease, such as uveitis or proliferative diabetic retinopathy, or those receiving other treatments, such as PDT, laser, and intraocular surgery, within 3 months before the first intravitreal conbercept (IVC) treatment or during the 1-year treatment period. Patients were not offered IVC treatment if they had uncontrolled hypertension, recent myocardial infarction, or cerebral vascular accident.[20]
This study was approved by the Ethics Committee for Human Research of Peking University People's Hospital and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from patients before treatment.
Polypoidal choroidal vasculopathy classification
We devised a classification system based on previous research which divided PCV into two types.[29,30,32] In the first type, both feeder and draining vessels are visible on ICGA, and network vessels are numerous. In the second group, neither the feeder nor the draining vessels are detectable, and the number of network vessels is small [Figure 1a and 1b].
Figure 1.

Two different angiographic subtypes of PCV. (a) Type 1 PCV both feeder and draining vessels were observed in the early phase of indocyanine green angiography. (b) Type 2 PCV neither feeder nor draining vessels were visible in the early phase of indocyanine green angiography. PCV: Polypoidal choroidal vasculopathy.
Intervention
Patients received monthly intravitreal injections of 0.5 mg of conbercept for 3 months. After the initial treatment, repeat treatment was applied as needed for 1 year, followed the PrONTO study,[33] which included a 0.1-unit decrease of logMAR in the presence of fluid at the macula detected by OCT, >100-µm increase in central retinal thickness (CRT), new-onset classic CNV, new macular hemorrhage, persistent macular fluid detected by OCT, and active leakage on FA.
Main outcome measures
Each patient underwent a comprehensive ophthalmic examination, including BCVA letter score with the Early Treatment Diabetic Retinopathy Study (ETDRS) chart (4 m), slit-lamp biomicroscopy of the anterior segment, and dilated ophthalmoscopy examination of the posterior pole at each of the scheduled visits from the baseline until the final follow-up examination at 12 months. FA, OCT, and ICGA were performed at baseline and at 3, 6, 9, and 12 months. OCT and fundus examinations were performed at 1, 2, 4, 5, 7, 8, 10, and 11 months. All patients underwent OCT examination with vertical and horizontal cross-sections centered on the fovea. Efficacy assessments included polyp regression, OCT-measured CRT, SRF thickness, retinal pigmented epithelial detachment (PED) height, and visual acuity changes. The areas of individual polyps were calculated. The polyp area was measured by the investigator according to the guidance provided by the CRC in the CRC manual. The investigator measured the area of the best-fit circles around all individual polyps excluding the hypofluorescent halo. The areas of individual polyps were summed, thereby deriving the total polyp area. CRT was defined as the mean retinal thickness within the 1 mm diameter circle centered at fovea. CRT was also obtained from the computational software output.
Statistical analysis
All efficacy variables were analyzed using the full analysis dataset using the last observation carried forward method. The differences in the polyp areas between baseline and 3, 6, 9, and 12 months and the mean changes in BCVA from baseline to each visit were analyzed using the paired Student's t- test with 95% confidence intervals. The Chi-square test or Fisher's exact test was used for the proportion of patients who gained more than 15 letters and lost at least 15 letters. Other secondary end points, as well as demographic data at baseline, were evaluated using summary statistics. All statistical tests were two-sided. P < 0.05 was considered statistically significant. All the above analyses were performed using SPSS software version 22.0 (SPSS Inc., USA).
Results
All patients completed a 12-month treatment with IVC in our hospital and qualified for the study criteria. The details are listed in Table 1. The mean age of the 58 patients was 65.9 ± 9.4 years (range, 53.0–76.0 years). Gender distribution was 35 men (60.3%) and 23 women (39.7%). Furthermore, the average number of injections was 9.6 ± 3.0.
Table 1.
Comparison of basic characteristics between the two PCV subgroups
| Items | Type 1 | Type 2 | All cases | Statistics | P |
|---|---|---|---|---|---|
| Gender (n) | |||||
| Male | 22 | 13 | 35 | 2.61* | >0.05 |
| Female | 13 | 10 | 23 | ||
| Age (years), mean ± SD (range) | 66.5 ± 8.7 (58.0–76.0) | 63.9 ± 9.6 (53.0–70.0) | 65.9 ± 9.4 (53.0–76.0) | 3.62† | >0.05 |
| Eye (n) | |||||
| Right | 15 | 16 | 31 | 1.78* | >0.05 |
| Left | 12 | 15 | 27 |
*: χ2 value; †: t value. PCV: Polypoidal choroidal vasculopathy; SD: Standard deviation.
Polypoidal choroidal vasculopathy vascular subtypes
Using the classification system, 35 patients (56.5%) were Type 1 and 23 (43.5%) were Type 2. There were no significant differences between the two PCV subtypes in terms of gender or laterality [Table 1].
Evaluation of total clinical outcomes
Serial changes in mean BCVA, CRT, SRF, and PED changes over 12 months of treatment during the study are displayed in Table 2 and Figure 2.
Table 2.
Mean BCVA, CRT, SRF, and PED over 12 months of treatment with intravitreal conbercept for PCV (n = 58)
| Items | Baseline | Month 3 | Month 6 | Month 9 | Month 12 |
|---|---|---|---|---|---|
| BCVA (letters), mean ± SD | 50.18 ± 13.67 | 55.73 ± 14.81 | 62.86 ± 15.16 | 64.45 ± 17.93 | 64.41 ± 17.34 |
| CRT (µm), mean ± SD | 397.96 ± 157.25 | 324.28 ± 136.89 | 294.33 ± 119.44 | 285.09 ± 97.05 | 272.19 ± 87.78 |
| SRF (µm), mean ± SD | 123.38 ± 119.84 | 53.30 ± 88.58 | 23.91 ± 58.17 | 27.67 ± 64.72 | 19.30 ± 43.18 |
| PED (µm), mean ± SD | 187.09 ± 190.56 | 170.56 ± 183.58 | 164.53 ± 176.36 | 112.44 ± 200.47 | 93.60 ± 166.26 |
| Resolution of polyps (%) | 0 | 46.6 | 56.9 | 60.3 | 65.5 |
PCV: Polypoidal choroidal vasculopathy; SD: Standard deviation; BCVA: Best-corrected visual acuity; CRT: Central retinal thickness; SRF: Subretinal fluid; PED: Pigmented epithelial detachment.
Figure 2.

Mean BCVA (a), CRT (b), SRF (c), and PED (d) over 12 months of 58 eyes with PCV. Error bars: ± standard error of means. *P < 0.05 compared with baseline. PCV: Polypoidal choroidal vasculopathy; BCVA: Best-corrected visual acuity; CRT: Central retinal thickness; SRF: Subretinal fluid; PED: Pigmented epithelial detachment.
The mean BCVA of these 58 eyes was 50.18 ± 13.67 letters at baseline, 55.73 ± 14.81 letters (t = 3.27, P = 0.03) at month 3, 62.86 ± 14.81 letters (t = 6.71, P < 0.01) at 6, 64.45 ± 17.93 letters (t = 5.87, P < 0.01) at 9, and 64.41 ± 17.34 letters (t = 7.83, P < 0.01) at 12 months [Figure 2a]. CRT decreased from 397.96 ± 157.25 µm in the 58 eyes examined at baseline to 324.28 ± 136.89 µm (t = 9.33, P < 0.01) at month 3, 294.33 ± 119.44 µm (t = 9.31, P < 0.01) at 6, 285.09 ± 97.05 µm (t = 11.23, P < 0.01) at 9, and 272.19 ± 87.78 µm (t = 10.32, P < 0.01) at 12 months [Figure 2b]. SRF thickness involving the fovea decreased from 123.38 ± 119.84 µm in the 48 eyes examined at baseline to 53.30 ± 88.58 µm (t = 12.73, P < 0.01) at month 3, 23.91 ± 58.17 µm (t = 11.52, P < 0.01) at 6, 27.67 ± 64.72 µm (t = 6.63, P < 0.01) at 9, and 19.30 ± 43.18 (t = 8.15, P < 0.01) at month 12 [Figure 2c]. Four eyes showed SRF at every follow-up point despite additional IVC. Thus, there were only four nonresponders to conbercept in terms of SRD. RPED was observed in 33 eyes (56.9%) at baseline and 20 eyes (34.5%) at month 12. PED height involving the fovea decreased from 187.09 ± 190.56 µm in the 48 eyes examined at baseline to 170.56 ± 183.58 µm (t = 9.89, P = 0.67) at month 3, 164.53 ± 176.36 µm (t = 10.89, P = 0.56) at 6, 112.44 ± 200.47 µm (t = 11.23, P = 0.07) at 9, and 93.60 ± 166.26 (t = 13.23, P < 0.01) at 12 months [Figure 2d]. Specifically, PED disappeared in 13 eyes, was reduced in 8, and remained unchanged in 6, while showing enlargement in 6 eyes. Moreover, at month 12, among them, 30 (51.7%) patients had complete regression of polyps, 8 (13.8%) patients had partial regression, and 20 (34.5%) patients had increased (recurrent or new onset) polyps.
Evaluation of clinical outcomes of subtypes
The clinical parameters of Type 1 PCV and Type 2 PCV are listed in Table 3. The improvement in BCVA was more prominent at all months in the Type 2 PCV group, and the Type 2 PCV group achieved a significantly greater improvement in their mean BCVA than the Type 1 PCV group found from month 6 to month 12 after the initial injection [t = 2.37, P < 0.01; Figure 3a]. Moreover, improvements in BCVA with conbercept treatment were associated with a decrease in CRT measured with OCT imaging. The CRT decrease observed at month 3 continued to decrease through month 12 in two groups. The CRT decrease was numerically greater in Type 2 (120.44 ± 73.81 µm) compared with that in Type 1 (106.48 ± 72.33 µm) at month 6 (t = 4.31, P < 0.01), and greater in Type 2 (130.21 ± 76.28 µm) compared with that in Type 1 (111.67 ± 79.57 µm) at month 9 [t = 1.87, P < 0.01; Figure 3b]. There was no significant difference between the two groups for the decrease in SRF thickness and PED height from month 3 to 12 [t = 2.97, P > 0.05; Figure 3c and 3d]. Moreover, there was no significant difference between the two groups for regression of polyps.
Table 3.
Visual acuity, CRT, SRF, and PED changes in the two subtypes over 12 months of treatment with intravitreal conbercept for PCV, 35 eyes in Type 1 and 23 in Type 2
| Items | Type | Month 3 | Month 6 | Month 9 | Month 12 |
|---|---|---|---|---|---|
| BCVA (letters), mean ± SD | 1 | 4.24 ± 3.62 | 11.48 ± 7.31 | 13.34 ± 8.11 | 14.10 ± 9.07 |
| 2 | 5.13 ± 3.57 | 13.50 ± 8.42 | 16.08 ± 8.72 | 15.92 ± 9.76 | |
| CRT (µm), mean ± SD | 1 | 68.07 ± 55.43 | 106.48 ± 72.33 | 111.67 ± 79.57 | 122.52 ± 83.73 |
| 2 | 83.91 ± 64.55 | 120.44 ± 73.81 | 130.21 ± 76.28 | 121.23 ± 80.36 | |
| SRF (µm), mean ± SD | 1 | 75.12 ± 66.21 | 81.74 ± 67.72 | 71.62 ± 68.69 | 83.79 ± 68.33 |
| 2 | 68.27 ± 65.44 | 75.09 ± 64.17 | 71.79 ± 63.21 | 77.03 ± 65.08 | |
| PED (µm), mean ± SD | 1 | 50.50 ± 43.87 | 60.59 ± 48.25 | 74.50 ± 55.43 | 61.20 ± 56.53 |
| 2 | 59.81 ± 50.47 | 69.81 ± 54.88 | 84.57 ± 59.72 | 70.03 ± 58.49 | |
| Resolution of polyps (%) | 1 | 45.7 | 51.4 | 57.1 | 62.7 |
| 2 | 47.8 | 60.9 | 60.9 | 65.2 |
PCV: Polypoidal choroidal vasculopathy; SD: Standard deviation; BCVA: Best-corrected visual acuity; CRT: Central retinal thickness; SRF: Subretinal fluid; PED: Pigmented epithelial detachment.
Figure 3.
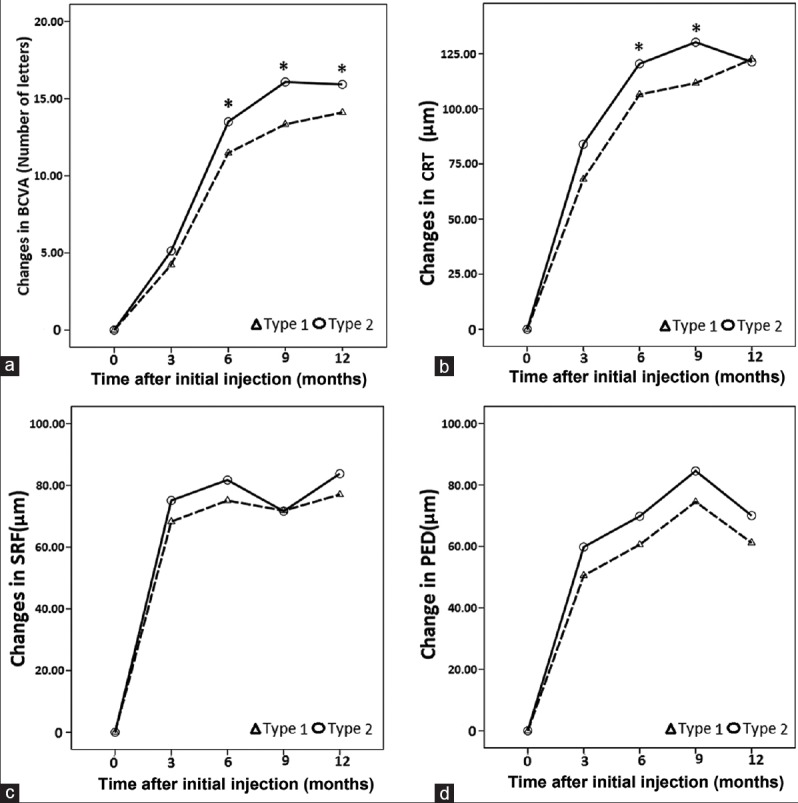
Mean BCVA (a), CRT (b), SRF (c), and PED (d) changes between two PCV subgroups over 12 months, *P < 0.05. PCV: Polypoidal choroidal vasculopathy; BCVA: Best-corrected visual acuity; CRT: Central retinal thickness; SRF: Subretinal fluid; PED: Pigmented epithelial detachment.
Complications
None of the patients experienced systemic complications related to IVC. Ocular complications, including uveitis, increased intraocular pressure, cataract progression, endophthalmitis, ocular toxicity, and retinal break or detachment, were not observed.
Discussion
IVC effectively improves macular exudative reactions and vision for PCV during 1-year period. This phenomenon has been found by several studies.[19,22,34,35,36,37,38,39,40,41] Lai et al.[34] reported the efficacy of intravitreal bevacizumab therapy for PCV. After intravitreal bevacizumab therapy for 3 months, vision and CRT significantly improved, but persistent polypoidal lesions were still present in ICGA of all the treated eyes. Yamashiro et al.[36] investigated ranibizumab monthly monotherapy, and the mean BCVA increased by 9.2 ± 12.4 letters during the 6 months follow-up. Using ranibizumab (as-needed) therapy, Marcus et al.[38] reported that the mean BCVA increased from baseline by 1.2 Snellen lines at 12 and 24 months. The mean CRT decreased by 53 and 67 μm from baseline at 12 months and 24 months, respectively.[37] In our study, we evaluated a novel anti-VEGF agent that is structurally similar to aflibercept but with a higher affinity for all VEGF-A isoforms. The mean improvement in BCVA from baseline was 12.3 ± 12.7 letters. This improvement corresponded with significant decreases in the mean CRT, SRF, and PED. Moreover, despite the reported significant visual improvement and reduction in foveal thickness after the intravitreal injection of ranibizumab or bevacizumab, only 0–40% of patients presented with complete polyp regression.[19,34,35,36,40] Ijiri et al.[42] evaluated the short-term efficacy of aflibercept monotherapy for patients with treatment-naïve PCV. The polyps disappeared completely in 48% of patients, and the polyp size decreased in 27% of patients by month 3. In our study, at month 3, the complete and partial polyp regression rates were 46.55% and 13.79%, respectively. By 12 months, Koh et al.[22] reported a polyp regression rate of 24.2% in the bevacizumab group and 23.3% in the ranibizumab group after receiving 3 monthly injections followed by as-needed injections of ranibizumab or bevacizumab. In our study, the complete and partial polyp regression rates were 51.72% and 13.79%, respectively. Therefore, conbercept is as or possibly more effective than bevacizumab or ranibizumab regarding polyp regression rates.
The reason for the discrepant responses between the macular exudative changes and polypoidal lesions to intravitreal anti-VEGF agents remains uncertain. In addition to the penetration extent of the anti-VEGF agents, the responses may be related to disease pathogenesis (macular exudative process compared to growth of the polypoidal lesions). Different clinical PCV subtypes with different clinical patterns and prognoses may also exist. As far as we know, a few authors have attempted to classify PCV lesions, but they generally did not compare the long-term clinical outcomes of different groups. Various studies have classified the polypoidal lesions based on the appearance of vascular patterns,[28,43] polyp configuration,[44,45,46] location[46] or size,[45] or clinical features.[11]
In this study, Type 1 and Type 2 PCV (classified by the presence or absence of a clear BVN with polypoidal lesions) significantly differed regarding long-term visual outcomes of conbercept therapy, which illustrates the usefulness of the classification system. The changes in the mean BCVA, CRT, SRF, and PED in patients with Type 2 PCV were more profound compared to patients with Type 1 PCV. These findings might be consistent with the results of Tan et al.[47] (Type 1 in our study may correspond to Types B and C, and Type 2 may correspond to Type A). Kawamura et al.,[30] according to spectral domain OCT, found that branching vessels network from feeder vessels may pass through ruptures in Bruch's membrane, spread between the RPE and Bruch's membrane, become deformed polypoidal vessels in Type 1 PCV, which represents the characteristics of CNV beneath the RPE. Costa et al.[48] thought that PCV lesions are a variety of CNV. Histopathological studies have found a choroidal arteriole and vein expanding through the break in Bruch's membrane as CNV associated with AMD.[49,50] In contrast, abnormally dilated vessels were found beneath Bruch's membrane in Type 2 (with no break in Bruch's membrane), which represents choroidal vasculature abnormalities. According to histopathological studies,[28,29,51] abnormally dilated vessels may push the RPE upward because of the increasing intravascular pressure for dilated vessels and exudation from these vessels within the choroid. Thus, the differential responses between the two types were due to different disease mechanisms, which show the usefulness of the classification system. Therefore, this classification system should be applied to future clinical studies. By classifying PCV, the patient can be counseled more accurately on his visual prognosis, and the presence of a more severe PCV subtype would alert the clinician to the need for a more stringent follow-up.
This study was limited by the retrospective design, and the results should be confirmed with a prospective study with strict definitions for patient enrollment. Nonetheless, the angiographic PCV subtypes affected the outcome of conbercept therapy, and this information might be useful to establish personalized medicine for PCV patients in the future.
Financial support and sponsorship
This study was supported by a grant from the Capital Health Research and Development of Special (No. 2014-3-4086).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
References
- 1.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV).1990. Retina. 2012;32(Suppl 1):1–8. [PubMed] [Google Scholar]
- 2.Shechtman D, Tyler JA. Idiopathic polypoidal choroidal vasculopathy (IPCV) presenting with simultaneous choroidal neovascular membrane (CNM) Optom Vis Sci. 2004;81:491–8. doi: 10.1097/00006324-200407000-00009. doi: 10.1097/00006324-200407000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Giovannini A, Amato GP, D’Altobrando E, Giuliani M. Optical coherence tomography (OCT) in idiopathic polypoidal choroidal vasculopathy (IPCV) Doc Ophthalmol. 1999;97:367–71. doi: 10.1023/a:1002102630242. doi: 10.1023/A:1002102630242. [DOI] [PubMed] [Google Scholar]
- 4.Wong RL, Lai TY. Polypoidal choroidal vasculopathy: An update on therapeutic approaches. J Ophthalmic Vis Res. 2013;8:359–71. [PMC free article] [PubMed] [Google Scholar]
- 5.Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T, et al. Polypoidal choroidal vasculopathy: Incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003;121:1392–6. doi: 10.1001/archopht.121.10.1392. doi: 10.1001/archopht.121.10.1392. [DOI] [PubMed] [Google Scholar]
- 6.Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144:15–22. doi: 10.1016/j.ajo.2007.03.047. doi: 10.1016/j.ajo.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 7.Yamagishi T, Koizumi H, Yamazaki T, Kinoshita S. Changes in fundus autofluorescence after treatments for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2014;98:780–4. doi: 10.1136/bjophthalmol-2013-303739. doi: 10.1136/bjophthalmol-2013-303739. [DOI] [PubMed] [Google Scholar]
- 8.Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol. 2008;19:208–12. doi: 10.1097/ICU.0b013e3282fb7c33. doi: 10.1097/ICU.0b013e3282fb7c33. [DOI] [PubMed] [Google Scholar]
- 9.Mauget-Faÿsse M, Quaranta-El Maftouhi M, De La Marnièrre E, Leys A. Photodynamic therapy with verteporfin in the treatment of exudative idiopathic polypoidal choroidal vasculopathy. Eur J Ophthalmol. 2006;16:695–704. doi: 10.1177/112067210601600506. [DOI] [PubMed] [Google Scholar]
- 10.Spaide RF, Donsoff I, Lam DL, Yannuzzi LA, Jampol LM, Slakter J, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina. 2002;22:529–35. doi: 10.1097/00006982-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: One-year results of a prospective case series. Ophthalmology. 2004;111:1576–84. doi: 10.1016/j.ophtha.2003.12.056. doi: 10.1016/j.ophtha. 2003.12.056. [DOI] [PubMed] [Google Scholar]
- 12.Lee SC, Seong YS, Kim SS, Koh HJ, Kwon OW. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica. 2004;218:193–201. doi: 10.1159/000076844. doi: 10.1159/000076844. [DOI] [PubMed] [Google Scholar]
- 13.Fernández M, Gil M, Gomez-Ulla F, Charlón P. Verteporfin photodynamic therapy combined with intravitreal ranibizumab for polypoidal choroidal vasculopathy controversy concerning long-term followup. Case Rep Med 2012. 2012:897097. doi: 10.1155/2012/897097. doi: 10.1155/2012/897097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomi F, Oshima Y, Mori R, Kano M, Saito M, Yamashita A, et al. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: The Fujisan Study. Retina. 2015;35:1569–76. doi: 10.1097/IAE.0000000000000526. doi: 10.1097/IAE.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 15.Hata M, Mandai M, Kojima H, Kameda T, Miyamoto N, Kurimoto Y. Five-year visual outcomes of typical age-related macular degeneration and/or polypoidal choroidal vasculopathy patients who received photodynamic therapy (PDT) as initial treatment in comparison with patients prior to the PDT era. Nippon Ganka Gakkai Zasshi. 2012;116:937–45. [PubMed] [Google Scholar]
- 16.Inoue M, Arakawa A, Yamane S, Kadonosono K. Long-term outcome of intravitreal ranibizumab treatment, compared with photodynamic therapy, in patients with polypoidal choroidal vasculopathy. Eye (Lond) 2013;27:1013–20. doi: 10.1038/eye.2013.179. doi: 10.1038/eye.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MW, Yeo I, Wong D, Ang CL. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy. Eye (Lond) 2009;23:1417–22. doi: 10.1038/eye.2008.265. doi: 10.1038/eye.2008.265. [DOI] [PubMed] [Google Scholar]
- 18.Mori R, Yuzawa M, Akaza E, Haruyama M. Treatment results at 1 year of ranibizumab therapy for polypoidal choroidal vasculopathy in eyes with good visual acuity. Jpn J Ophthalmol. 2013;57:365–71. doi: 10.1007/s10384-013-0245-9. doi: 10.1007/s10384-013-0245-9. [DOI] [PubMed] [Google Scholar]
- 19.Cho HJ, Kim JW, Lee DW, Cho SW, Kim CG. Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy. Eye (Lond) 2012;26:426–33. doi: 10.1038/eye.2011.324. doi: 10.1038/eye.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng CK, Peng CH, Chang CK, Hu CC, Chen LJ. One-year outcomes of intravitreal bevacizumab (avastin) therapy for polypoidal choroidal vasculopathy. Retina. 2011;31:846–56. doi: 10.1097/IAE.0b013e3181f84fdf. doi: 10.1097/IAE.0b013e3181f84fdf. [DOI] [PubMed] [Google Scholar]
- 21.Hikichi T. Individualized ranibizumab therapy strategies in year 3 after as-needed treatment for polypoidal choroidal vasculopathy. BMC Ophthalmol. 2015;15:37. doi: 10.1186/s12886-015-0026-y. doi: 10.1186/s12886-015-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, et al. EVEREST study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–64. doi: 10.1097/IAE.0b013e31824f91e8. doi: 10.1097/IAE.0b013e31824f91e8. [DOI] [PubMed] [Google Scholar]
- 23.Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009;148:70–8. doi: 10.1016/j.ajo.2009.02.012. doi: 10.1016/j.ajo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Lai TY, Lee GK, Luk FO, Lam DS. Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina. 2011;31:1581–8. doi: 10.1097/IAE.0b013e31820d3f3f. doi: 10.1097/IAE.0b013e31820d3f3f. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Sun X. Profile of conbercept in the treatment of neovascular age-related macular degeneration. Drug Des Devel Ther. 2015;9:2311–20. doi: 10.2147/DDDT.S67536. doi: 10.2147/DDDT.S67536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Xu G, Wang Y, Xu X, Liu X, Tang S, et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: Results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 2014;121:1740–7. doi: 10.1016/j.ophtha.2014.03.026. doi: 10.1016/j.ophtha. 2014.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Zhang J, Yan M, Luo D, Zhu W, Kaiser PK, et al. A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology. 2011;118:672–8. doi: 10.1016/j.ophtha.2010.08.008. doi: 10.1016/j.ophtha.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2005;89:602–7. doi: 10.1136/bjo.2004.049296. doi: 10.1136/bjo. 2004.049296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakashizuka H, Mitsumata M, Okisaka S, Shimada H, Kawamura A, Mori R, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49:4729–37. doi: 10.1167/iovs.08-2134. doi: 10.1167/iovs.08-2134. [DOI] [PubMed] [Google Scholar]
- 30.Kawamura A, Yuzawa M, Mori R, Haruyama M, Tanaka K. Indocyanine green angiographic and optical coherence tomographic findings support classification of polypoidal choroidal vasculopathy into two types. Acta Ophthalmol. 2013;91:e474–81. doi: 10.1111/aos.12110. doi: 10.1111/aos. 12110. [DOI] [PubMed] [Google Scholar]
- 31.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV) Retina. 1990;10:1–8. [PubMed] [Google Scholar]
- 32.Tanaka K, Nakayama T, Mori R, Sato N, Kawamura A, Yuzawa M. Associations of complement factor B and complement component 2 genotypes with subtypes of polypoidal choroidal vasculopathy. BMC Ophthalmol. 2014;14:83. doi: 10.1186/1471-2415-14-83. doi: 10.1186/1471-2415-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–83. doi: 10.1016/j.ajo.2007.01.028. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 34.Lai TY, Chan WM, Liu DT, Luk FO, Lam DS. Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008;92:661–6. doi: 10.1136/bjo.2007.135103. doi: 10.1136/bjo.2007.135103. [DOI] [PubMed] [Google Scholar]
- 35.Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, Kamei M, et al. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008;92:70–3. doi: 10.1136/bjo.2007.122283. doi: 10.1136/bjo. 2007.122283. [DOI] [PubMed] [Google Scholar]
- 36.Yamashiro K, Tomita K, Tsujikawa A, Nakata I, Akagi-Kurashige Y, Miyake M, et al. Factors associated with the response of age-related macular degeneration to intravitreal ranibizumab treatment. Am J Ophthalmol. 2012;154:125–36. doi: 10.1016/j.ajo.2012.01.010. doi: 10.1016/j.ajo.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, et al. Results of 2 years of treatment with as-needed ranibizumab reinjection for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2013;97:617–21. doi: 10.1136/bjophthalmol-2012-302652. doi: 10.1136/bjophthalmol-2012-302652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcus DM, Singh H, Lott MN, Singh J, Marcus MD. Intravitreal ranibizumab for polypoidal choroidal vasculopathy in non-Asian patients. Retina. 2013;33:35–47. doi: 10.1097/IAE.0b013e3182618be0. doi: 10.1097/IAE.0b013e3182618be0. [DOI] [PubMed] [Google Scholar]
- 39.Chhablani JK, Narula R, Narayanan R. Intravitreal bevacizumab monotherapy for treatment-naïve polypoidal choroidal vasculopathy. Indian J Ophthalmol. 2013;61:136–8. doi: 10.4103/0301-4738.109390. doi: 10.4103/0301-4738.109390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita M, Nishi T, Hasegawa T, Ogata N. Response of serous retinal pigment epithelial detachments to intravitreal aflibercept in polypoidal choroidal vasculopathy refractory to ranibizumab. Clin Ophthalmol. 2014;8:343–6. doi: 10.2147/OPTH.S56539. doi: 10.2147/OPTH.S56539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang HM, Koh HJ. Long-term visual outcome and prognostic factors after intravitreal ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;156:652–60. doi: 10.1016/j.ajo.2013.05.038. doi: 10.1016/j.ajo. 2013.05.038. [DOI] [PubMed] [Google Scholar]
- 42.Ijiri S, Sugiyama K. Short-term efficacy of intravitreal aflibercept for patients with treatment-naïve polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253:351–7. doi: 10.1007/s00417-014-2707-2. doi: 10.1007/s00417-014-2707-2. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka K, Nakayama T, Mori R, Sato N, Kawamura A, Mizutani Y, et al. Associations of complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2) genotypes with subtypes of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2011;52:7441–4. doi: 10.1167/iovs.11-7546. doi: 10.1167/iovs.11-7546. [DOI] [PubMed] [Google Scholar]
- 44.Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I, et al. Polypoidal choroidal vasculopathy: Natural history. Am J Ophthalmol. 2002;133:639–48. doi: 10.1016/s0002-9394(02)01404-6. doi: 10.1016/S0002-9394(02)01404-6. [DOI] [PubMed] [Google Scholar]
- 45.Byeon SH, Lee SC, Oh HS, Kim SS, Koh HJ, Kwon OW. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol. 2008;52:57–62. doi: 10.1007/s10384-007-0498-2. doi: 10.1007/s10384-007-0498-2. [DOI] [PubMed] [Google Scholar]
- 46.Cackett P, Wong D, Yeo I. A classification system for polypoidal choroidal vasculopathy. Retina. 2009;29:187–91. doi: 10.1097/IAE.0b013e318188c839. doi: 10.1097/IAE.0b013e318188c839. [DOI] [PubMed] [Google Scholar]
- 47.Tan CS, Ngo WK, Lim LW, Lim TH. A novel classification of the vascular patterns of polypoidal choroidal vasculopathy and its relation to clinical outcomes. Br J Ophthalmol. 2014;98:1528–33. doi: 10.1136/bjophthalmol-2014-305059. doi: 10.1136/bjophthalmol-2014-305059. [DOI] [PubMed] [Google Scholar]
- 48.Costa RA, Navajas EV, Farah ME, Calucci D, Cardillo JA, Scott IU. Polypoidal choroidal vasculopathy: Angiographic characterization of the network vascular elements and a new treatment paradigm. Prog Retin Eye Res. 2005;24:560–86. doi: 10.1016/j.preteyeres.2005.01.001. doi: 10.1016/j.preteyeres. 2005.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Bressler SB, Silva JC, Bressler NM, Alexander J, Green WR. Clinicopathologic correlation of occult choroidal neovascularization in age-related macular degeneration. Arch Ophthalmol. 1992;110:827–32. doi: 10.1001/archopht.1992.01080180099035. [DOI] [PubMed] [Google Scholar]
- 50.Chang TS, Freund KB, de la Cruz Z, Yannuzzi LA, Green WR. Clinicopathologic correlation of choroidal neovascularization demonstrated by indocyanine green angiography in a patient with retention of good vision for almost four years. Retina. 1994;14:114–24. doi: 10.1097/00006982-199414020-00004. [DOI] [PubMed] [Google Scholar]
- 51.Okubo A, Sameshima M, Uemura A, Kanda S, Ohba N. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol. 2002;86:1093–8. doi: 10.1136/bjo.86.10.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]


