Abstract
The efficacy and safety of Salvia officinalis combined with statin have not been evaluated in dyslipidemic diabetes mellitus type 2 (DDMT2) so far. The plant extract antioxidant activity was determined by the DPPH radical scavenging assay. The total flavonoid, total phenolic and quercetin contents of the capsules containing the plant extract were also measured. Moreover, the effects of 2-month extract intake (500 mg capsule three times a day) as add-on to daily use of 15 mg glyburide, 2000 mg metformin and 10 mg atorvastatin on the blood levels of fasting glucose (FG), 2 h postprandial glucose (2hPPG), glycosylated hemoglobin (HbA1c), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglyceride, high-density lipoprotein cholesterol (HDL-C), serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), creatinine and body mass index were studied in 50 patients and compared with the placebo group (n=50).The extract IC50 in the DPPH assay was 87.26±0.003 µg/mL (mean±SD), whereas the ascorbic acid IC50 was 5.626± 0.001 µg/mL (mean±SD). The total flavonoid, total phenolic and quercetin contents of the capsule containing the plant extract were 39.76±3.58 mg of rutin equivalents (mean±SD), 30.33±1.23 mg of gallic acid (mean±SD) and 0.13 mg, respectively. The extract lowered FG, 2hPPG, HbA1c, TC, LDL-C and triglyceride levels, but increased HDL-C level compared to the placebo at the endpoint (P<0.05). The extract did not affect the other parameters significantly and no adverse effect was reported. The extract has substantial antioxidant activity which may be beneficial for the prevention of the cardiovascular complications of DDMT2. Moreover, addition of the extract to statin therapy is apparently safe and further improves lipid profile.
Key Words: Salvia officinalis, statin, dyslipidemia, diabetes mellitus
Dyslipidemic diabetes mellitus type 2 (DDMT2) is common worldwide. It is characterized by blood lipid changes as raised total cholesterol, low- density- lipoprotein cholesterol (LDL-C) and triglyceride and decreased level of high- density- lipoprotein cholesterol (HDL-C) alongside hyperglycemia. Also, it is a major cause of atherosclerotic cardiovascular disease (CVD). Co-administration of several antihyperglycemic drugs is usually necessary for the management of the hyperglycemia. Nevertheless, standard antihyperglycemic therapies do not have sufficient efficacy and safety. Many patients do not respond adequately to the therapies. Therefore, novel antihyperglycemic agents with more efficacy and safety are needed (1-4). LDL-C is the most important target in therapy of dyslipidemia. The use of statins is the preferred treatment strategy for both primary and secondary prevention of CVD. However, some patients respond inadequately, about 15 % are intolerant, and other factors prevent achieving cholesterol goals in as many as 40 % of patients. Thus, there is great need for additional drugs to prevent and treat CVD, whether as monotherapy or in combination with statin drugs (2-7). The plant kingdom can be a source of new anti-diabetic and anti-dyslipidemic drugs (8).
Plants with agonistic action on peroxisome proliferator-activated receptor γ (PPAR γ) may be useful in the treatment of dyslipidemia and diabetes mellitus type 2 (9). Agonists of PPAR γ prevent atherosclerotic CVD (10). Salvia officinalis L. (sage) (Lamiaceae family) leaves are used widely as a food flavoring. Moreover, infusion of 4-6 g of dry sage leaves in two divided doses per day is taken in the traditional medicine as an antihyperglycemic agent to treat diabetes mellitus (11). Sage consists of volatile oils, tannins, diterpenes, triterpenes, steroids, flavones and flavonoids (12-14). Sage leaf extracts (constituents) have demonstrated a variety of pharmacological effects such as in vitro agonistic action on the PPAR γ (12, 13), in vitro α-glucosidase and pancreatic lipase inhibition, and lipid absorption inhibitory and antihypertriglyceridemic effects in mice (14, 15), antioxidant and lipid peroxidation inhibitory effects in rat brain and liver homogenates in vitro (16), anti-inflammatory effect in rat hind paw acute inflammation induced with oil of turpentine (17), antihyperglycemic effect in streptozocin-induced diabetic rats (18), insulin sensitizer and gluconeogenesis inhibitory effects in primary cultures of hepatocytes from healthy sage-tea-drinking rats (metformin-like effects) (19). There have been few clinical trials on the antihyperglycemic and antidyslipidemic effects of sage (20-23). Notably, the efficacy and safety of sage combined with statin therapy to improve lipid profile have not been evaluated so far. Hence, the present study was undertaken. Since antioxidant property is clinically important for the prevention of the cardiovascular complications of DDMT2 (24), the radical scavenging activity of the extract was also studied. Moreover, the extract was standard-ized by determination of the total flavonoid, total phenolic, and quercetin contents.
Materials and methods
Preparation of the extract and placebo capsules
Sage was collected and extracted, and the extract and placebo capsules were prepared as described previously (22). The sage capsules contained 500 mg of the extract powder. The placebo capsules contained toast powder. The sage and placebo capsules were identical in appearance.
The extract DPPH assay
Inhibition of diphenyl-2-picrylhydrazyl (DPPH) radicals by the extract was assayed according to a method described previously (25). A mixture consisting of an extract solution at different concentrations (1.5 mL) and the methanolic solution of the DPPH reagent (1.5 mL) was prepared in a volumetric flask. The mixture was left to stand for 30 min in a dark place, and then the absorption was measured at 517 nm using a spectrophotometer (Human, USA). The radical scavenging activity (RSA) was calculated as a percentage of DPPH discoloration using the equation: RSA (%)= (Ac-As/Ac)×100 where Ac is the absorbance of the negative control and As is the absorbance of the plant sample or ascorbic acid.
Ascorbic acid was used as reference standard.
The assay results were expressed as IC50 denoting the antioxidant concentration which reduces the DPPH radicals about 50%.
Determination of the extract total phenolic content
The total phenolic contents were determined through the Folin-Ciocalteu colorimetric method described previously (26). In brief, the plant extract solution (1mL) was mixed with 500 μL of the Folin-Ciocalteu reagent and 5 mL distilled water in a volumetric flask. After 5 min, 1 mL of 15% sodium carbonate solution was added to the mixture and then kept in the dark for 30 min, after which the absorbance was determined at 725 nm using a spectrophotometer (Human, USA). Gallic acid was used to generate the standard curve, and the reduction of the Folin-Ciocalteu reagent by the samples was expressed as mg of gallic acid equivalents per capsule.
Determination of the total flavonoid content
The total flavonoid content was determined by a modified method described previously (27). The extract (1 mg/mL) or standard was mixed with 4 mL of distilled water and 300 μL of a 5% sodium nitrite solution and after 5 min, 300 μL of 10% aluminum chloride solution was added to the mixture. After 6 min, 2 mL of 1 M sodium hydroxide and 3 mL of distilled water were added to the mixture. The solution was properly mixed and absorbance was measured at 510 nm using a spectrophotometer (Human, USA). Rutin (100 μg/mL up to 1200 μg/mL) was used to construct the standard curve and the results were expressed as mg of rutin equivalents per capsule.
Determination of the extract quercetin content
Quercetin was measured by the HPLC method described previously (28). The chromato-graphic separations were achieved using a YMC triart C18 column (250 mm×4.6 mm, 5 μm). A reverse phase HPLC assay was carried out using an isocratic elution with a flow rate of 1 mL/min, a mobile phase of 35: 65 (acetonitrile: 0.2 phosphoric acid) and a detection wavelength of 360 nm. The injection volume was 50 μL of each solution. The total run time was 16 minutes for each injection. The solvents and distilled water were filtered previously through a PTF membrane using a set of glass bottles with the aid of a vacuum pump. The result was expressed as mg of quercetin per capsule.
Protocol of the clinical trial
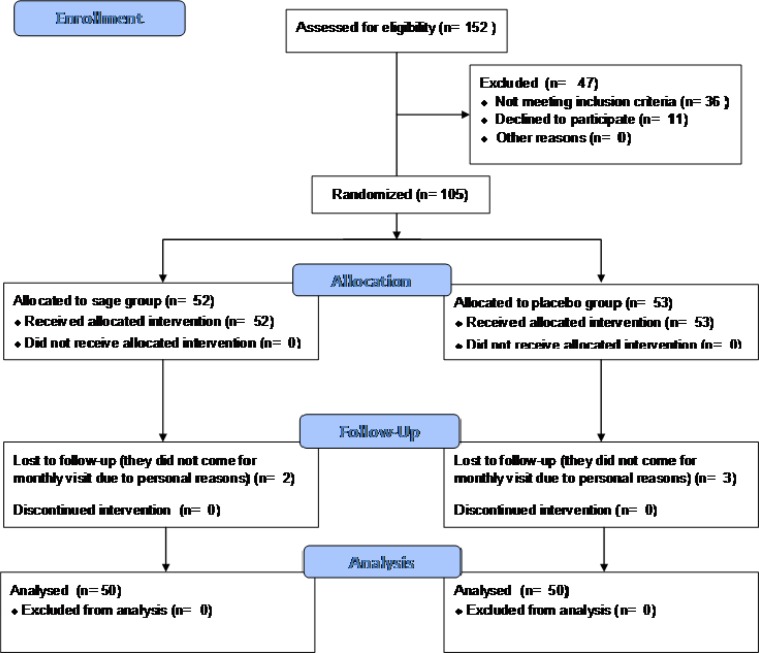
A 2-arm, randomized, double-blind, placebo- controlled parallel-group trial with the following eligibility criteria was performed in the Diabetes Clinic (Karaj City, Alborz Province of Iran). Recruitment was carried out from April 20, 2012 to October 30, 2015. Inclusion criteria consisted of Iranian male and female type 2 diabetic patients aged 40-60 years with levels of HbA1C below 8% and LDL-C between 100 and 130 mg/dL despite daily intake of 15 mg glyburide, 2000 mg metformin and 10 mg atorvastatin. Patients with cardiovascular, pulmonary, renal and hepatic diseases; pregnant and breast feeding women were excluded from the study. 152 patients were screened. The enrolled patients were equally randomized to the extract and placebo groups. Random allocation was performed by block randomization using random number table. The patients were instructed to take one extract or placebo capsule every 8 h for 2 months. The patients’ treatment, diet and physical activity remained unchanged throughout the trial. The patients were requested to report any adverse drug reaction. At the beginning and endpoint of the trial, the patients’ blood levels of fasting glucose, 2 h postprandial glucose, HbA1c (glycosylated hemoglobin), total cholesterol, triglyceride, LDL-C, HDL-C, serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT) and crea-tinine were determined using standard enzymatic kits produced by Pars Azmoon company (Tehran, Iran) and an auto- analyzer (Hitachi 902, Japan). The patients’ body mass index (BMI) was also measured at the baseline and endpoint. Fasting glucose, HbA1c and LDL- C were the primary outcome measures. The other blood variables and BMI were the secondary outcome measures. Compliance was measured by counting returned drugs and asking how many doses of the drugs were (or were not) taken. 50 patients in each group was the sample size calculated to detect 20 mg/dL difference of fasting glucose level between the groups, considering type I error= 0.05 and 80% power. The chi-squared and independent samples t tests were used for data analysis and p<0.05 was considered as significant. SPSS 20 was used for statistical analysis. The data were analyzed by the intention-to-treat approach. The Ethics Committee of the Endocrinology and Metabolism Research Institute affiliated to the Tehran University of Medical Sciences approved the protocol. The trial was performed in accordance with the revised declaration of Helsinki 2013. The patients signeda written informed consent form before enroll-ment. The trial was registered in the Iranian Registry of Clinical Trials with the number IRCT201506082288N7.
Results
Phytochemical analysis of the extract
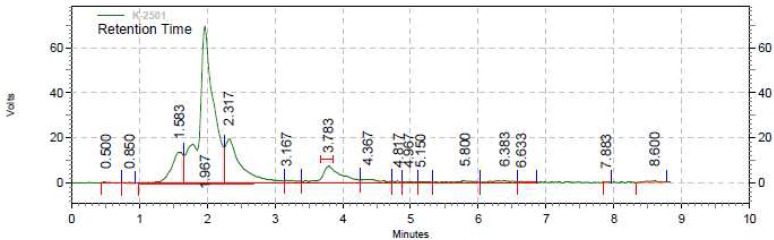
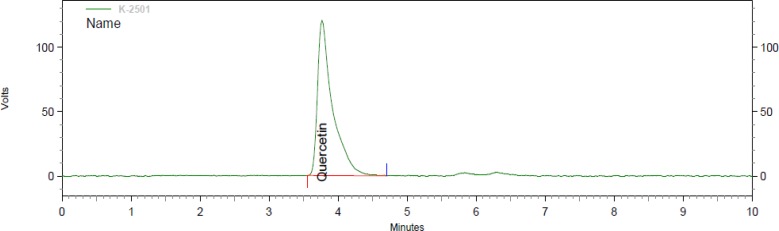
The IC50 of the extract was 87.26± 0.003 µg/mL (mean±SD), whereas the IC50 of ascorbic acid was 5.626 ± 0.001 µg/mL (mean ± SD). The total flavonoid content as milligram rutin equivalent per capsule was 39.76± 3.58 (mean± SD). The total phenolic content of the extract as milligrams of gallic acid per capsule was 30.53± 1.23 (mean± SD). The aforementioned values are means of 3 measurements. Moreover, the amount of quercetin in each capsule containing the plant extract was 0.13 mg. The figures 1 and 2 demonstrate the HPLC chromatograms of the test sample and standard quercetin.
Clinical trial
Fig. 1.
HPLC chromatogram of the test sample
Fig. 2.
HPLC chromatogram of the standard quercetin
Fig. 3.
The CONSORT diagram showing the flow of participants through the triel
105 patients entered the study. 50 patients in each of the sage and placebo groups completed the trial. The CONSORT flow diagram of the trial is shown in the figure 3. The patients fully complied with the protocol. Demographic data of the patients in the sage and placebo groups did not differ significantly (Table 1). There was no significant difference between the sage and placebo groups in terms of the blood parameters levels and BMI at the baseline. The extract significantly lowered the fasting glucose, 2 h postprandial glucose, HbA1c, total cholesterol, triglyceride and LDL-C (P< 0.001, P< 0.001, P= 0.007, P= 0.042, P= 0.020 and P= 0.012, respectively) but raised the HDL-C (P= 0.028) compared with the placebo group at the endpoint. Moreover, the extract did not affect the BMI significantly compared to the placebo group at the endpoint (Table 2). The patients did not report any adverse drug reaction.
Table 1.
Demographic characteristics of the study participants
| Parameter | Sage group | Placebo group |
|---|---|---|
| Gender | 21 males, 29 females | 22 males, 28 females |
| Age (years) | 54.77± 4.82 | 52.57± 5.69 |
| Duration of diabetes mellitus type 2 (years) | 10.6± 3.6 | 9.8± 4.2 |
| Where appropriate, values are given as mean± standard deviation. | ||
Table 2.
The blood parameter levels and body mass index before and after intervention
| P-value |
Mean (SD)
after intervention |
P-value |
Mean (SD)
before intervention |
Parameter |
|---|---|---|---|---|
| 0.618 | 26.6 (7) S 27.5 (6) P |
0.782 | 27.7 (4.9) S 28.1 (6.5) P |
Body mass index (kg/m2) |
| < 0.001 | 158.3 (26.6) S 197.5 (46.4) P |
0.171 | 177.8 (32.4) S 188.8 (38.9) P |
Fasting glucose (mg/dL) |
| < 0.001 | 158.3 (26.6) S 206.8 (43.9) P |
0.609 | 207. 0 (43.9) S 202. 0 (43.2) P |
2 hours postprandial glucose (mg/dL) |
| 0.007 | 6.7 (0.9) S 7.3 (1.1) P |
0.320 | 7.2 (1.1) S 7.0 (1.1) p |
HbA1c (%) |
| 0.042 | 182.6 (36.8) S 203.6 (52.9) P |
0.273 | 228.2 (42.9) S 216.6 (50.4) P |
Total Cholesterol (mg/dL) |
| 0.020 | 179. 1 (52.8) S 220 (98.1) P |
0.176 | 274.1 (83.5) S 245 (105.7) P |
Triglyceride (mg/dL) |
| 0.012 | 109.3 (29.7) S 123. 5 (18. 4) P |
0.776 | 117.2 (9.1) S 116.7 (8.1) P |
LDL-C (mg/dL) |
| 0.028 | 50.5 (9.6) S 45.7 (9.3) P |
0.823 | 45.4 (11.1) S 44.8 (9.7) P |
HDL-C (mg/dL) |
| 0.714 | 22. 7 (13) S 23.8 (13.2) P |
0.699 | 21.2 (6.2) S 21.7 (5.9) P |
AST (U/L) |
| 0.717 | 24.9 (6.4) S 25.5 (8.2) P |
0.534 | 23.6 (9.2) S 24.8 (7.5) P |
ALT (U/L) |
| 0.209 | 1 (0.2) S 0. 9 (0.1) P |
0.169 | 0.9 (0.1) S 0. 9 (0.1) P |
Creatinine (mg/dL) |
P< 0.05 is significant (independent samples t test). S: sage group; P: placebo group; SD: standard deviation.
Discussion
The main findings of the present clinical trial were that sage combined with glyburide, metformin and atorvastatin further lowered fasting and 2 h postprandial glucose, HbA1c, total cholesterol, triglyceride and LDL-C and increased HDL- C in DDMT2 patients. Specifically, the current study demonstrated the benefit of sage added to the background statin therapy. Sage as add-on to statin therapy was apparently safe and further improved lipid profile in DDMT2 patients. The sage effects on the glycemic control and lipid profile may enhance the effects of the standard therapy as regards the prevention of the cardiovascular complications of the DDMT2.
The antioxidant activity of the extract was moderate compared to ascorbic acid. As oxidative stress has an important contributory role in the development of cardiovascular complications of DDMT2 (24), the sage antioxidant effect may be useful for the prevention of cardiovascular complications of DDMT2. The bioactive compounds that were identified and quantified in the extract used in the present trial were total flavonoids, total phenolics and quercetin. These compounds may be responsible for the antioxidant effect of sage. The sage effects concur with the previous clinical trials (20-23). In a crossover trial with 6 healthy female volunteers (aged 40-50), 4-week sage tea drinking (infusion prepared by pouring 300 ml of boiling water onto 4 g dried leaves of sage, twice a day) lowered blood LDL-C, total cholesterol and LDL-C/HDL-C but increased blood HDL-C without hepatotoxic or other adverse effects. However, the effects of sage on the blood triglyceride and VLDL levels were not evaluated in the trial. Moreover, the subjects were normo-lipidemic, the trial had a small sample size, and was not randomized, double-blind and placebo-controlled (20). In a 2-month randomized double-blind placebo-controlled trial, sage leaf (80%) ethanol aqueous extract (500 mg t.i.d.) (t.i.d. stands for “ter in die” which in Latin means three times a day) lowered blood total cholesterol, triglyceride and VLDL levels, but increased blood HDL-C in 34 hyperlpidemic (hypercholesterolemic and /or hyp-ertriglyceridemic) patients not receiving any other antihyperlipidemic agent (21). In another 3-month randomized double-blind placebo-controlled trial, sage leaf 80% ethanol aqueous extract (500 mg t.i.d.) reduced blood fasting glucose, HbA1c, total cholesterol, LDL-C and triglyceride, but increased blood HDL-C in 40 hyperlipidemic (hyperchole-sterolemic and/or hypertriglyceridemic) type 2 diabetic patients taking 10 mg glyburide and 1000 mg metformin daily, not receiving any other antihyperlipidemic agent (22). Besides, according to a randomized double-blind trial, intake of 150 mg sage extract tablet t.i.d. combined with standard antidiabetic treatment by 40 type 2 diabetic patients reduced 2 h postprandial glucose and total cholesterol levels without affecting fasting glucose, HbA1c and other lipid parameters compared to the placebo group (n= 40) after 3 months (23). Inadequate sage extract dose and small sample size are the main limitations of the just mentioned study. Comparison of the inclusion criteria and baseline glycemia and lipid levels of the present trial with those of the previous trials (21-23) indicates that the participants of this study had more severe hyperglycemia and hyperlipidemia (hypercholest-erolemia and hypertriglyceridemia). The present study validates the traditional use of sage for the treatment of diabetes mellitus. There is little data on the constituents and mechanisms mediating the antihyperglycemic and antidyslipidemic effects of sage. Liposoluble compounds including the phenols carnosic acid and carnosol together with unknown hydrosoluble ingredients as well as PPARγ agonistic and metformin-like actions and α-glucosidase, pancreatic lipase and lipid absorption inhibition have been implicated in the sage effects (12, 13, 15, 19, 21, 22).
Finally, sage has promising pharmacological effects which justify more research. Conduction of more clinical trials regarding the effects of sage in the treatment of dyslipidemias and diabetes mellitus as well as identification of the compounds and mechanisms involved in the sage effects are warranted.
Acknowledgements
The Institute of Medicinal Plants affiliated with the Iranian Academic Center for Education, Culture and Research (ACECR) (Tehran, Iran) funded this study.
Conflicts of Interest
The authors declared no conflict of interest.
References
- 1.Brietzke SA. Oral antihyperglycemic treatment options for type 2 diabetes mellitus. Med Clin North Am. 2015;99:87–106. doi: 10.1016/j.mcna.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Taskinen MR, Boren J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239:483–95. doi: 10.1016/j.atherosclerosis.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Frank ML, Gerhardt AM. Treating dyslipidemia in patients with type 2 diabetes mellitus. Nurse Pract. 2015;40:18–22. doi: 10.1097/01.NPR.0000469253.13367.2c. quiz -3. [DOI] [PubMed] [Google Scholar]
- 4.Halcox J, Misra A. Type 2 diabetes mellitus, metabolic syndrome, and mixed dyslipidemia: how similar, how different, and how to treat? Metab Syndr Relat Disord. 2015;13:1–21. doi: 10.1089/met.2014.0049. [DOI] [PubMed] [Google Scholar]
- 5.Kones R, Rumana U. Current Treatment of Dyslipidemia: A New Paradigm for Statin Drug Use and the Need for Additional Therapies. Drugs. 2015;75:1187–99. doi: 10.1007/s40265-015-0428-4. [DOI] [PubMed] [Google Scholar]
- 6.Kones R, Rumana U. Current Treatment of Dyslipidemia: Evolving Roles of Non-Statin and Newer Drugs. Drugs. 2015;75:1201–28. doi: 10.1007/s40265-015-0429-3. [DOI] [PubMed] [Google Scholar]
- 7.Sando KR, Knight M. Nonstatin therapies for management of dyslipidemia: a review. Clin Ther. 2015;37:2153–79. doi: 10.1016/j.clinthera.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Sikand G, Kris-Etherton P, Boulos NM. Impact of functional foods on prevention of cardiovascular disease and diabetes. Curr Cardiol Rep. 2015;17:39. doi: 10.1007/s11886-015-0593-9. [DOI] [PubMed] [Google Scholar]
- 9.Liu HJ, Zhang CY, Song F, et al. A novel partial agonist of peroxisome proliferator-activated receptor gamma with excellent effect on insulin resistance and type 2 diabetes. J Pharmacol Exp Ther. 2015;353:573–81. doi: 10.1124/jpet.115.223107. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda K, Matsumura T, Senokuchi T, et al. Statins meditate anti-atherosclerotic action in smooth muscle cells by peroxisome proliferator-activated receptor-gamma activation. Biochem Biophys Res Commun. 2015;457:23–30. doi: 10.1016/j.bbrc.2014.12.063. [DOI] [PubMed] [Google Scholar]
- 11.Fleming T. PDR for Herbal Medicines. 4th ed. . New York: Medical Economics Company; 2015. [Google Scholar]
- 12.Rau O, Wurglics M, Paulke A, et al. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med. 2006;72:881–7. doi: 10.1055/s-2006-946680. [DOI] [PubMed] [Google Scholar]
- 13.Christensen KB, Jorgensen M, Kotowska D, et al. Activation of the nuclear receptor PPARgamma by metabolites isolated from sage (Salvia officinalis L.) J Ethnopharmacol. 2010;132:127–33. doi: 10.1016/j.jep.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 14.Ninomiya K, Matsuda H, Shimoda H, et al. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorg Med Chem Lett. 2004;14:1943–6. doi: 10.1016/j.bmcl.2004.01.091. [DOI] [PubMed] [Google Scholar]
- 15.Moradabadi L, Montasser Kouhsari S, Fehresti Sani M. Hypoglycemic effects of three medicinal plants in experimental diabetes: inhibition of rat intestinal alpha-glucosidase and enhanced pancreatic insulin and cardiac Glut-4 mRNAs expression. Iran J Pharm Res. 2013;12:387–97. [PMC free article] [PubMed] [Google Scholar]
- 16.Oboh G, Henle T. Antioxidant and inhibitory effects of aqueous extracts of Salvia officinalis leaves on pro-oxidant-induced lipid peroxidation in brain and liver in vitro. J Med Food. 2009;12:77–84. doi: 10.1089/jmf.2008.0007. [DOI] [PubMed] [Google Scholar]
- 17.Oniga I, Parvu AE, Toiu A, et al. Effects of Salvia officinalis L. extract on experimental acute inflammation. Rev Med Chir Soc Med Nat Iasi. 2007;111:290–4. [PubMed] [Google Scholar]
- 18.Eidi M, Eidi A, Zamanizadeh H. Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;100:310–3. doi: 10.1016/j.jep.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Lima CF, Azevedo MF, Araujo R, et al. Metformin-like effect of Salvia officinalis (common sage): is it useful in diabetes prevention? Br J Nutr. 2006;96:326–33. doi: 10.1079/bjn20061832. [DOI] [PubMed] [Google Scholar]
- 20.Sa CM, Ramos AA, Azevedo MF, et al. Sage tea drinking improves lipid profile and antioxidant defences in humans. Int J Mol Sci. 2009;10:3937–50. doi: 10.3390/ijms10093937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kianbakht S, Abasi B, Perham M, et al. Antihyperlipidemic effects of Salvia officinalis L. leaf extract in patients with hyperlipidemia: a randomized double-blind placebo-controlled clinical trial. Phytother Res. 2011;25:1849–53. doi: 10.1002/ptr.3506. [DOI] [PubMed] [Google Scholar]
- 22.Kianbakht S, Dabaghian FH. Improved glycemic control and lipid profile in hyperlipidemic type 2 diabetic patients consuming Salvia officinalis L leaf extract: a randomized placebo Controlled clinical trial. Complement Ther Med. 2013;21:441–6. doi: 10.1016/j.ctim.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Behradmanesh S, Derees F, Rafieian-Kopaei M. Effect of Salvia officinalis on diabetic patients. J Renal Inj Prev. 2013;2:51–4. doi: 10.12861/jrip.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–80. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han J, Weng X, Bi K. Antioxidants from a Chinese medicinal herb – Lithospermum erythrorhizon. Food Chemistry. 2008;106:2–10. [Google Scholar]
- 26.Gutfinger T. Polyphenols in olive oils. Journal of the American Oil Chemists Society. 1981;58:966–8. [Google Scholar]
- 27.Yoo KM, Lee CH, Lee H, et al. Relative antioxidant and cytoprotective activities of common herbs. Food Chemistry. 2008;106:929–36. [Google Scholar]
- 28.Verma N, Trehan N. HPLC analysis of methanolic extract of herbs for quercetin content. J Pharmacog Phytochem. 2013;2:159–62. [Google Scholar]