Abstract
We report on a 15-year-old male with the 3q29 microdeletion syndrome and summarize the medical literature. He had intellectual disability, autism spectrum disorder, anxiety, obsessive compulsive tendencies, speech delay, delayed walking, a hypernasal voice, gait abnormalities, chronic constipation, gastroesophageal reflux disorder, urinary voiding dysfunction, abnormal skin pigmentation, and dysmorphic features. We present a review of the literature for the 3q29 microdeletion syndrome by comparing both the phenotype and the genetic defects in reported cases. Of the 38 previously reported cases with deletion size information, the most common chromosome deletion was 1.6 Mb in size including ~30 genes. This emerging microdeletion syndrome is characterized by intellectual disability, speech delay, behavioral problems, craniofacial dysmorphism, and musculoskeletal abnormalities.
Keywords: 3q29 microdeletion syndrome, microarray, phenotype, literature review
Introduction
The 3q29 microdeletion syndrome is emerging as a recognizable genetic disorder, now reported in 40 cases in the literature (Rossi et al., 2001; Koolen et al., 2004; Willatt et al., 2005; Baynam et al., 2006; Krepiski-Santos et al., 2006; Ballif et al., 2008; Shao et al., 2008; Digilio et al., 2009; Li et al., 2009; Tyshchenko et al., 2009; Clayton-Smith et al., 2010; Cobb et al., 2010; Quintero-Rivera et al., 2010; Dasouki et al., 2011; Petrin et al., 2011; Citta et al., 2013; Sagar et al., 2013). This microdeletion is typically about 1.6 Mb in size in reported cases with clinical features including mild to moderate learning disability, speech delay, ocular abnormalities, and craniofacial findings with a high nasal bridge. The presence of two homologous repeat regions on either side of common deletion breakpoints suggests that nonallelic homologous recombination may account for this recurring microdeletion syndrome that occurs more often than by chance (Quintero-Rivera et al., 2010). Here, we report on a 15-year-old white male with a 3q29 microdeletion identified by chromosomal microarray analysis, summarize the medical literature, and compare our patient’s phenotype, deletion size, and location with previously reported cases in an attempt to better define this microdeletion syndrome.
Case report
Our patient is a 15-year-old male who presented for genetic evaluation for autism spectrum disorder. He was born full term weighing 3.86 kg (75th centile). Elevated α-fetoprotein (AFP) levels were noted during the first and second trimester and a genetic amniocentesis showed a normal male karyotype. He had delayed speech, with less than 10 words noted at age 2 years, and gross motor delays, with sucking problems and delayed walking at 17 months. As a child, gastroesophageal reflux disorder, urinary voiding dysfunction, and constipation were noted and continue to present. At 2 years of age, he was diagnosed with an autism spectrum disorder. Cognitive testing showed a full-scale IQ of 64 requiring an individualized education plan in the school setting. In the ninth grade, math difficulties were noted, but he excelled in biology. He showed obsessive compulsive tendencies, severe anxiety, depression, and aggressive impulsive behaviors. He had no history of chronic infections.
During the physical exam at 15 years of age, he was a pleasant white male with dysmorphic features including a long narrow face, frontal bossing, a high nasal bridge, recessed eyes, a short philtrum, and thin upper lip. A high-arched palate, abnormal teeth, and micrognathia were noted, along with scoliosis and torsion of the upper body, long tapered fingers with a single palmar crease on the right hand, a crease between the second and third toes bilaterally, and dysplastic toenails. He also showed cutaneous pigmentary abnormalities including two freckles on the right hand, a café au lait spot on the right thigh, a pigmented hairy nevus on the right arm, and four freckles on the dorsum of the foot. He had a hypernasal voice with pressured speech and gait abnormalities, but with normal coordination. His reflexes were normal in the lower and upper extremities. No edema, joint laxity nor tenderness, leg length asymmetry, abdominal masses nor tenderness, nor cardiorespiratory distress were noted. His height was 179 cm (90th centile), weight was 62.5 kg (70th centile), BMI was 19.5 (40th centile), head circumference was 55 cm (50th centile), inner canthal distance was 3 cm (50th centile), outer canthal distance was 8.5 cm (30th centile), interpupillary distance was 5.9 cm (80th centile), ear length was 6.5 cm (75th centile), total hand length was 18 cm (60th centile), and middle finger length was 7.3 cm (30th centile). The family history was remarkable, with the father having a history of depression and anxiety along with unexplained foot length discrepancy. No further family history was noted.
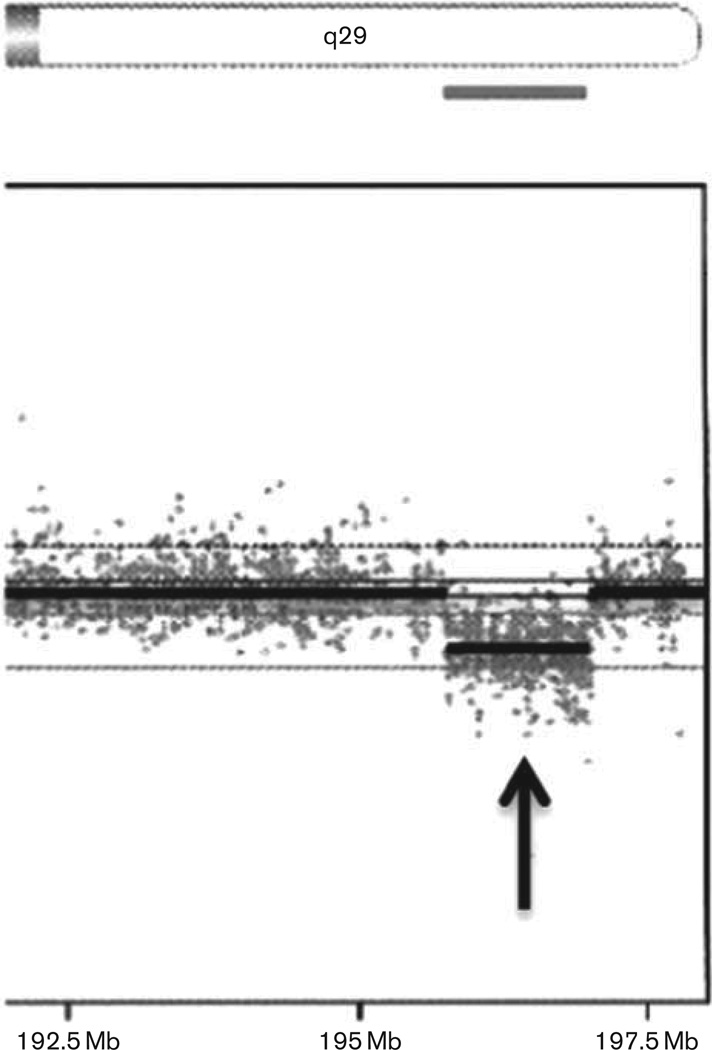
On the basis of his dysmorphic features together with a diagnosis of autism spectrum disorder, a genome-wide copy number analysis was carried out using an Illumina single-nucleotide polymorphism microarray containing more than 845 000 single-nucleotide polymorphism markers covering both coding and noncoding human genome sequences (CombiMatrix Diagnostics Laboratory, Irvine, California, USA). Genomic imbalances were reported using UCSC Human Genome Build 19 (NCBI build 37, February 2009) showing a 1.24 Mb deletion involving chromosome 3q29 (hg 19 coordinates 195 788 299–197 033 296). Genes within this region include TFRC, LINC00885, ZDHHC19, SLC51A, PCYT1A, TCTEX1D2, TM4SF19-TCTEX1D2, TM4SF19, UBXN7, RNF168, C3orf43, WDR53, FBXO45, NRROS, CEP19, PIGX, PAK2, SENP5, NCBP2, NCBP2-AS2, PIGZ, MFI2-AS1, MFI2, DLG1, MIR4797, and DLG1-AS1 (Fig. 1). Parental fluorescence in-situ hybridization (FISH) studies were carried out using homebrew BAC DNA probes specific to 3p11.1 (RP11-458D16, control) and 3q29 (RP11-481O2, deletion) for both of the patient’s parents. Ten metaphase cells and 200 interphase cells were examined in each parent. These tests showed that neither parent carried this deletion in blood cells, with no evidence of mosaicism indicating a de-novo process. Several of the deleted genes can play a role in his clinical presentation and will be discussed along with data summarized from the medical literature involving the 40 previously reported cases with a 3q29 deletion.
Fig. 1.
A 1.24 Mb deletion (arrow) was found within the chromosome 3q29 band at genomic coordinates 195 788 299–197 033 296 with microarray analysis (NCBI build 37, February 2009, hg 19). Genes included within this deletion are TFRC, LINC00885, ZDHHC19, SLC51A, PCYT1A, TCTEX1D2, TM4SF19-TCTEX1D2, TM4SF19, UBXN7, RNF168, C3orf43, WDR53, FBXO45, NRROS, CEP19, PIGX, PAK2, SENP5, NCBP2, NCBP2-AS2, PIGZ, MFI2-AS1, MFI2, DLG1, MIR4797, and DLG1-AS1.
Discussion
Chromosome 3q29 microdeletion syndrome has been described previously in the literature as a recurring cytogenetic disorder. On the basis of a review of the literature, the most prevalent features of this microdeletion syndrome included mild to moderate learning problems, speech delay, a high nasal bridge and ocular abnormalities (Table 1). Common features identified with review of 40 previous cases included autism, psychiatric disorders, hypernasal voice, delayed walking, low birth weight or failure to thrive, short stature, ataxia or gait abnormality, abnormal skull shape specifically brachycephaly, microcephaly, a long narrow face, low-set posteriorly rotated ears, a prominent or broad nose with a short philtrum, thin upper lip and high-arched palate, abnormal teeth, micrognathia, scoliosis with a chest cavity deformity, long or tapered fingers and curved toes, constipation and gastroesophageal reflux disorder, inguinal hernias, hypospadias, heart defects, and an abnormal brain MRI or computed tomography scan. Other features that were occasionally seen in this disorder included developmental regression, facial asymmetry, frontal bossing, large, protuberant ears, upslanting palpebral fissures, broad nostrils with long smooth philtrum, cleft lip/palate, joint contractures or ligamentous laxity, abnormal palmar creases, nail hypoplasia, fifth finger clinodactyly, toe syndactyly, abnormal skin pigmentation, hypertrophic pyloric stenosis, urinary voiding dysfunction, horseshoe kidney, and recurrent middle ear infections.
Table 1.
Clincal features of individuals with 3q29 microdeletion syndrome
| Clinical features | Fre- quency (%) |
Number of individuals reported |
Our patient |
Rossi et al. (2001) | Koolen et al. (2004) | Willatt et al. (2005) | Baynam et al. (2006) | Krepiski- Santos et al. (2006) |
Ballif et al. (2008) | Digilio et al. (2009) | Li et al. (2009) | Tyshchenko et al. (2009) | Quintero-Rivera et al. (2010) | Cobb et al. (2010) | Clayton-Smith et al. (2010) | Petrin et al. (2011) | Dasouki et al. (2011) | Sagar et al. (2013) | Citta et al. (2013) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth/neurodevelopment | |||||||||||||||||||
| Intellectual disability (IQ score ≤75) |
92 | 34/37 | 1 | 1/1 | 1/1 | 6/6 | 1/1 | 1/1 | 7/7 | 4/4 | 0/2 | 1/1 | 2/2 | 0/1 | 4/4 | – | – | 1/1 | 4/4 |
| Autism/autistic features | 32 | 9/28 | 1 | – | – | 2/6 | 1/1 | – | 1/7 | 0/4 | – | – | 2/2 | 1/1 | 1/4 | 0/1 | – | 1/1 | – |
| Psychiatric disordera | 50 | 11/22 | 1 | – | – | – | 1/1 | – | 1/7 | 1/4 | – | – | 2/2 | 1/1 | 2/4 | – | 1/1 | 1/1 | – |
| Developmental regression |
6 | 2/36 | – | – | 0/1 | 0/5 | 0/1 | – | 0/7 | 0/4 | 0/2 | 0/1 | 1/2 | 0/1 | 0/4 | – | 0/3 | – | 1/4 |
| Speech delay | 64 | 25/39 | 1 | – | 1/1 | 5/5 | 1/1 | – | 3/7 | 4/4 | 0/2 | 1/1 | 2/2 | 1/1 | 3/4 | – | 3/3 | – | 0/4 |
| Nasal voice | 32 | 8/25 | 1 | – | – | – | 1/1 | – | 1/7 | 0/4 | – | 0/1 | 1/2 | 0/1 | 3/4 | – | – | – | 1/4 |
| Delayed walking | 41 | 14/34 | 1 | – | 1/1 | 2/5 | 0/1 | – | 2/7 | 1/4 | 0/2 | 1/1 | 2/2 | 0/1 | 3/4 | – | 1/1 | – | 0/4 |
| Low birth weight (< 3rd percentile)/failure to thrive |
33 | 10/30 | – | – | 0/1 | 1/5 | 0/1 | 1/1 | – | 4/4 | 0/2 | 0/1 | 1/2 | 0/1 | 1/4 | – | 1/2 | 0/1 | 1/4 |
| Short stature | 24 | 7/29 | – | – | 0/1 | 1/6 | 0/1 | 1/1 | – | 2/4 | 0/1 | 0/1 | 0/2 | 0/1 | 2/4 | – | 1/2 | – | 0/4 |
| Ataxia gait/gait abnormality |
38 | 9/24 | 1 | – | – | 3/6 | 1/1 | – | 2/7 | – | – | – | 1/2 | 0/1 | 0/4 | 0/1 | – | 1/1 | – |
| Facial dysmorphism | – | – | – | 1/1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Abnormal skull shapeb | 18 | 4/22 | – | – | 1/1 | 2/6 | 0/1 | 0/1 | – | – | – | 0/1 | 1/2 | 0/1 | 0/4 | – | – | – | 0/4 |
| Brachycephaly | 23 | 6/26 | – | – | 0/1 | 0/6 | 0/1 | 0/1 | – | 3/4 | – | 0/1 | 1/2 | 0/1 | 0/4 | – | – | – | 2/4 |
| Microcephaly | 55 | 18/33 | – | – | 0/1 | 1/5 | 1/1 | 1/1 | 5/7 | 4/4 | – | 0/1 | 1/2 | 0/1 | 4/4 | – | 0/1 | – | 1/4 |
| Facial asymmetry | 17 | 4/24 | – | – | 0/1 | 0/6 | 0/1 | 0/1 | – | 0/4 | 0/1 | 1/1 | 2/2 | 0/1 | 0/4 | – | – | 1/1 | |
| Long narrow face | 34 | 12/35 | 1 | – | 0/1 | 2/6 | 1/1 | 1/1 | 0/7 | 0/4 | 0/1 | 0/1 | 1/2 | 1/1 | 2/4 | – | 1/1 | – | 2/4 |
| Frontal bossing | 17 | 4/23 | 1 | – | 1/1 | 1/6 | 0/1 | 0/1 | – | 0/4 | 1/1 | 0/1 | 0/2 | 0/1 | 0/4 | – | – | – | – |
| Low-set, posteriorly rotated ears |
43 | 15/35 | – | – | 1/1 | 1/6 | 0/1 | 0/1 | 4/7 | 1/4 | 1/1 | 0/1 | 1/2 | 1/1 | 0/4 | 1/1 | – | – | 4/4 |
| Large protuberant ears | 9 | 2/23 | – | – | 0/1 | 0/6 | 1/1 | 1/1 | – | 0/4 | 0/1 | 0/1 | 0/2 | 0/1 | 0/4 | – | – | – | – |
| Cup-shaped ears | 13 | 3/24 | – | – | 0/1 | 0/6 | 0/1 | 0/1 | – | 0/4 | 0/1 | 0/1 | 0/2 | 0/1 | 2/4 | 1/1 | – | – | – |
| Down-slanting palpebral fissures |
17 | 4/24 | – | – | 1/1 | 1/6 | 0/1 | 0/1 | – | 1/4 | 0/1 | 0/1 | 0/2 | 1/1 | 0/4 | – | 0/1 | – | – |
| Upslanting palpebral fissures |
8 | 2/24 | – | – | 0/1 | 0/6 | 0/1 | 0/1 | – | 1/4 | 0/1 | 0/1 | 0/2 | 0/1 | 1/4 | – | 0/1 | – | – |
| High nasal bridge | 72 | 26/36 | 1 | – | 0/1 | 4/6 | 1/1 | 0/1 | 4/7 | 4/4 | 0/1 | 0/1 | 2/2 | 1/1 | 4/4 | 0/1 | – | 1/1 | 4/4 |
| Prominent or broad nasal tip/nose |
40 | 10/25 | – | – | 0/1 | 0/6 | 1/1 | 0/1 | – | 1/4 | 1/1 | 0/1 | 1/2 | 1/1 | 4/4 | 0/1 | 1/1 | – | – |
| Broad nostrils | 9 | 2/23 | – | – | 1/1 | 0/6 | 0/1 | 0/1 | – | 1/4 | 0/1 | 0/1 | 0/2 | 0/1 | 0/4 | – | – | – | – |
| Short philtrum | 51 | 18/35 | 1 | – | 0/1 | 6/6 | 0/1 | 0/1 | 0/7 | 3/4 | 0/1 | 0/1 | 0/2 | 1/1 | 4/4 | – | – | – | 3/4 |
| Long philtrum | 9 | 2/23 | – | – | 0/1 | 0/6 | 0/1 | 0/1 | – | 1/4 | 1/1 | 0/1 | 0/2 | 0/1 | 0/4 | – | – | – | – |
| Smooth philtrum | 17 | 4/23 | – | – | 1/1 | 1/6 | 0/1 | 0/1 | – | 1/4 | 1/1 | 0/1 | 0/2 | 0/1 | 0/4 | – | – | – | – |
| Thin upper lip | 30 | 7/23 | 1 | – | 0/1 | 0/6 | 0/1 | 0/1 | – | 4/4 | 1/1 | 1/1 | 0/2 | 0/1 | 0/4 | – | – | – | – |
| Cleft lip/palate/ submucous cleft |
9 | 3/32 | – | – | 0/1 | 1/6 | 0/1 | 0/1 | 0/7 | 0/4 | 0/1 | 0/1 | 0/2 | 0/1 | 0/4 | 1/1 | 1/1 | – | – |
| High-arched palate | 33 | 12/36 | 1 | – | 1/1 | 0/6 | 0/1 | 1/1 | 2/7 | 1/4 | 0/1 | 0/1 | 1/2 | 0/1 | 3/4 | 0/1 | 1/1 | – | 1/4 |
| Abnormal teethc | 20 | 7/35 | 1 | – | 0/1 | 0/6 | 1/1 | 0/1 | 2/7 | 0/4 | 0/1 | 0/1 | 2/2 | 0/1 | 0/4 | – | 0/1 | – | 1/4 |
| Micrognathia | 29 | 7/24 | 1 | – | 0/1 | 0/6 | 0/1 | 0/1 | – | 2/4 | 0/1 | 0/1 | 1/2 | 0/1 | 2/4 | – | 1/1 | – | |
| Ocular abnormalitiesd | 58 | 11/19 | – | – | – | – | – | 1/1 | – | 0/4 | – | 1/1 | 1/2 | 0/1 | 4/4 | – | 0/1 | – | 4/4 |
| Musculoskeletal abnormalities | |||||||||||||||||||
| Scoliosis | 28 | 5/18 | 1 | – | – | – | 0/1 | – | – | 0/4 | – | 0/1 | 1/2 | 0/1 | 1/4 | – | – | – | 2/4 |
| Chest cavity deformitye | 29 | 10/35 | – | – | 1/1 | 2/6 | 0/1 | – | 1/7 | 0/4 | – | 0/1 | 0/2 | 0/1 | 2/4 | 0/1 | 1/1 | 1/1 | 2/4 |
| Joint contractures | 19 | 4/21 | – | – | – | 0/6 | 1/1 | – | – | 0/4 | – | 0/1 | 1/2 | 0/1 | 2/4 | 0/1 | – | – | – |
| Ligamentous laxity | 11 | 4/35 | – | – | – | 1/6 | 0/1 | – | 0/7 | 0/4 | – | 0/1 | 0/2 | 1/1 | 0/4 | – | – | – | 2/4 |
| Long/tapered fingers | 37 | 11/30 | 1 | – | 1/1 | 3/6 | 1/1 | 0/1 | 0/7 | 0/4 | 0/1 | 0/1 | 2/2 | 1/1 | 2/4 | – | – | – | – |
| Abnormal palmar crease |
17 | 4/24 | 1 | – | 0/1 | 0/6 | 0/1 | 1/1 | – | 0/4 | 1/1 | 0/1 | 0/2 | 0/1 | 0/4 | – | 1/1 | – | – |
| Nail hypoplasia | 13 | 3/23 | – | – | 0/1 | 1/6 | 0/1 | 0/1 | – | 1/4 | 0/1 | 0/1 | 1/2 | 0/1 | 0/4 | – | – | – | – |
| 5th finger clinodactyly | 19 | 5/27 | – | – | 1/1 | 1/6 | 0/1 | 0/1 | – | 0/4 | 0/1 | 0/1 | 0/2 | 1/1 | 0/4 | – | – | – | 2/4 |
| Clinodactylous toes | 32 | 7/22 | – | – | 1/1 | 1/6 | 0/1 | – | – | 3/4 | 0/1 | 0/1 | 1/2 | 0/1 | 1/4 | – | – | – | – |
| Toe syndactyly | 9 | 2/23 | – | – | 0/1 | 0/6 | 0/1 | – | – | 0/4 | 0/1 | 0/1 | 0/2 | 0/1 | 1/4 | 1/1 | – | – | – |
| Gastrointestinal abnormalities | |||||||||||||||||||
| Constipation | 38 | 3/8 | 1 | – | – | – | – | – | – | – | – | – | 1/2 | 1/1 | 0/4 | – | – | – | – |
| Hypertrophic pyloric stenosis |
11 | 1/9 | – | – | – | – | – | – | – | – | – | 1/1 | 0/2 | 0/1 | 0/4 | – | – | – | – |
| GERD | 42 | 5/12 | 1 | – | – | – | – | – | – | – | 1/2 | – | 0/2 | 1/1 | 0/4 | – | 1/1 | 1/1 | – |
| Genitourinary defects | |||||||||||||||||||
| Urinary voiding dysfunction |
13 | 2/15 | 1 | 0/1 | – | – | – | – | 0/7 | – | – | – | 0/2 | 0/1 | 0/2 | – | 1/1 | – | – |
| Hypospadias | 21 | 3/14 | – | 1/1 | – | – | – | – | 1/7 | – | – | – | 0/2 | 0/1 | 0/2 | – | – | – | 1/2 |
| Horseshoe kidney | 10 | 2/21 | NA | 1/1 | – | – | – | – | 0/7 | 0/4 | – | 0/1 | 0/2 | 0/1 | 0/1 | – | – | 1/4 | |
| Other | |||||||||||||||||||
| Abnormal skin pigmentation |
14 | 3/22 | 1 | – | – | 1/6 | – | – | 0/7 | 0/4 | – | 0/1 | 1/2 | 0/1 | – | – | – | – | – |
| Heart defects (PDA, ASD) |
22 | 6/27 | – | – | – | – | 0/1 | – | 1/7 | 1/4 | 2/2 | 0/1 | 0/2 | 0/1 | – | 0/1 | 1/3 | – | 1/4 |
| Recurrent middle ear infections |
18 | 5/28 | – | – | – | 1/6 | – | – | 1/7 | 1/4 | – | 0/1 | 0/2 | 0/1 | 0/4 | – | 1/1 | 1/1 | – |
| Abnormal brain MRI/CT |
27 | 3/11 | NA | – | 1/1 | – | 0/1 | – | – | 1/2 | – | 0/1 | 0/1 | – | – | – | – | 0/1 | 1/4 |
| Inguinal hernia | 20 | 3/15 | – | – | – | – | – | – | – | – | 1/2 | – | 0/2 | 0/1 | 0/4 | – | 1/1 | – | 1/4 |
| De-novo | 69 | 22/32 | 1 | 1/1 | 1/1 | 5/5 | 1/1 | 1/1 | 5/8 | 0/2 | 0/1 | 1/1 | 2/2 | 1/1 | 0/3 | 0/1 | 1/1 | 1/1 | 1/1 |
| Inherited | 31 | 10/32 | – | 0/1 | 0/1 | 0/5 | 0/1 | 0/1 | 3/8 | 2/2 | 1/1 | 0/1 | 0/2 | 0/1 | 3/3 | 1/1 | 0/1 | 0/1 | 0/1 |
Bold indicates features with a frequency of 50% or greater.
ASD, atrial septal defect; CT, computed tomography; GERD, gastroesophageal reflux disorder; NA, not available; PDA, patent ductus arteriosis.
Psychosis, anxiety, self-injurious behavior, depression, hyperactivity, aggression, obsessive compulsive disorder, impulsive behavior.
Prominent metopic suture, scaphocephaly.
Widely spaced, crowded, enamel dysplasia.
Microphthalmia, cataract, esotropia, nystagmus, myopia.
Pectus carinatum, pectus excavatum.
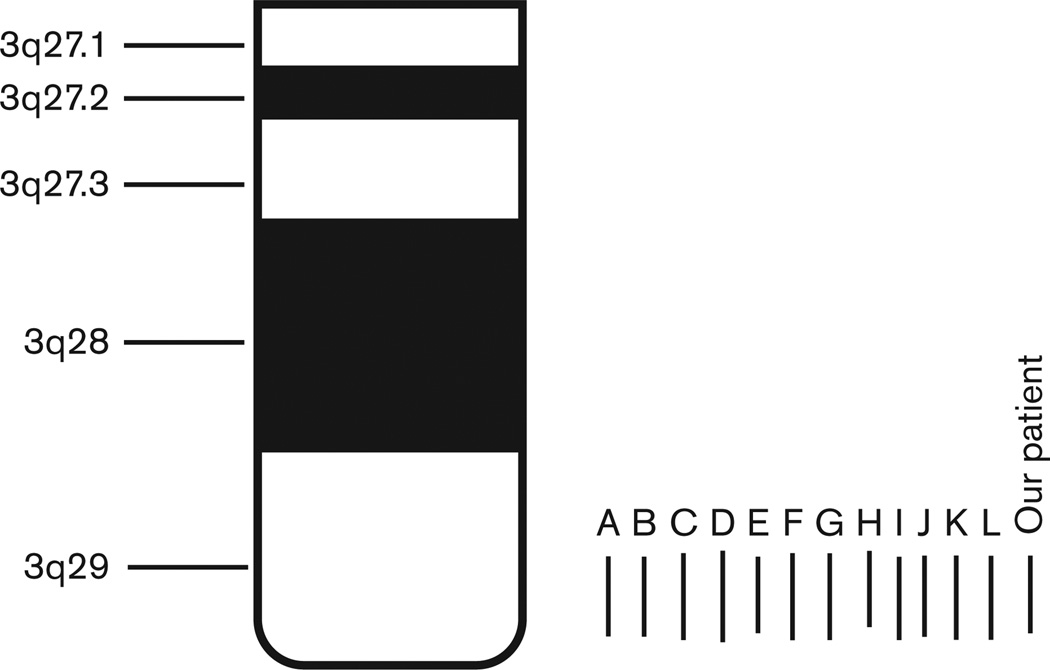
Our literature summary showed varied clinical findings, but with a relatively consistent deletion size of about 1.6 Mb. Deletions of this size are not usually detectable on a routine karyotype and chromosomal microarray analysis or targeted FISH analysis is required for detection. The average genomic coordinates generally occur from about 197–198.6 Mb from the p terminus of chromosome 3 based on NCBI 36 hg 18 build (2006) (Fig. 2 and Table 2). Mild to moderate learning disability appeared to be the most common recurrent feature and was reported in 92% of individuals. Although familial cases of 3q29 microdeletion syndrome are reported, 69% were classified as having a de-novo deletion. More research is needed to clarify the frequency of several features identified in a subset of individuals with 3q29 microdeletion syndrome and the involvement of specific deleted genes to further characterize the phenotype–genotype correlations. Of interest is the RNF168 gene found within our patient’s deletion and associated with RIDDLE syndrome (OMIM #611943). This syndrome is characterized by increased radiosensitivity, immunodeficiency, mild motor control and learning difficulties, facial dysmorphism and short stature, ataxia, microcephaly, and increased AFP levels (Stewart et al., 2009). It shares overlapping features with ataxia telangiectasia, an autosomal recessive chromosome breakage disorder. Our patient had several of these features including increased AFP levels. There were no reported cases of malignancies or leukemia in reported cases with the 3q29 deletion. Further research is needed to delineate the phenotype of the 3q29 microdeletion and RIDDLE syndrome with associated findings and specific gene involvement.
Fig. 2.
Location of interstitial chromosome 3q29 deletions, genomic coordinates, and size for our patient and others reported in the literature. Deletions of previously reported patients are as follows: A, patient 1 del(3)(q29q29)(197 262 362–198 794 848, hg 18) 1.5 Mb deletion (Digilio et al., 2009); B, patient 2 del(3)(q29q29) (197 262 362–198 794 848, hg 18) 1.5 Mb deletion (Digilio et al., 2009); C, patient 1 del(3)(q29q29)(197 194 509–198 823 098, hg 18) 1.6 Mb deletion (Quintero-Rivera et al., 2010); D, patient 2 del(3) (q29q29)(196 941 615–199 043 290, hg 18) 2.1 Mb deletion (Quintero-Rivera et al., 2010); E, only patient del(3)(q29q29) (197 256 140–198 570 020, hg 18) 1.3 Mb deletion (Cobb et al., 2010); F, patient 1 del(3)(q29q29)(197 174 369–198 842 531, hg 18) 1.66 Mb deletion (Dasouki et al., 2011); G, patient 2 del(3)(q29q29) (197 174 769–198 842 531, hg 18) 1.66 Mb deletion (Dasouki et al., 2011); H, patient 3 del(3)(q29q29)(197 085 422–198 217 248, hg 18) 1.13 Mb deletion (Dasouki et al., 2011); I, only patient del(3)(q29q29) (197 224 799–198 804 500, hg 18) 1.58 Mb deletion (Sagar et al., 2013); J, patient 1 del(3)(q29q29) (197 224 754–198 794 789, hg 18) 1.57 Mb deletion (Citta et al., 2013); K, patient 2 del(3)(q29q29) (197 216 353–198 823 667, hg 18) 1.607 Mb deletion (Citta et al., 2013); L, patient 4 del(3)(q29q29) (197 232 253–198 823 667, hg 18) 1.59 Mb deletion (Citta et al., 2013); Our patient, del(3)(q29q29) (197 272 696–198 517 693, converted to hg 18) 1.24 Mb deletion.
Table 2.
Chromosome 3q29 findings of reported individuals with 3q29 microdeletion syndrome
| Patients | Genetic testing and/or genomic coordinates | Deletion size (Mb) |
|---|---|---|
| Rossi et al. (2001) | ||
| Pt 1 | Karyotype and FISH | <2 |
| Koolen et al. (2004) | ||
| Pt 1 | MLPA and FISH | NA |
| Willatt et al. (2005) | ||
| Pt 1 | Karyotype and FISH | ~1.5 |
| Pt 2 | Karyotype and FISH | ~1.5 |
| Pt 3 | Karyotype and FISH | ~1.5 |
| Pt 4 | Karyotype and FISH | ~1.5 |
| Pt 5 | Karyotype and FISH | ~1.5 |
| Pt 6 | Karyotype and FISH | ~1.5 |
| Baynam et al. (2006) | ||
| Pt 1 | Karyotype and FISH | NA |
| Krepiski-Santos et al. (2006) | ||
| Pt 1 | Karyotype and FISH | 1.0 |
| Ballif et al. (2008) | ||
| Pt 1 | Karyotype and FISH | 1.6 |
| Pt 2 | Karyotype and FISH | 1.6 |
| Pt 3 | Karyotype and FISH | 1.6 |
| Pt 4 | Karyotype and FISH | 1.6 |
| Pt 5 | Karyotype and FISH | 1.6 |
| Pt 6 | Karyotype and FISH | 1.6 |
| Pt 7 | Karyotype and FISH | 1.6 |
| Digilio et al. (2009) | ||
| Pt 1 | 3:197 262 362–198 794 848 | ~1.5 |
| Pt 2 | 3:197 262 362–198 794 848 | ~1.5 |
| Li et al. (2009) | ||
| Pt 1 | Karyotype and FISH | 1.3–1.4 |
| Pt 2 | Karyotype and FISH | 1.3–1.4 |
| Tyshchenko et al. (2009) | ||
| Pt 1 | Karyotype and FISH | 1.6 |
| Quintero-Rivera et al. (2010) | ||
| Pt 1 | 3:197 194 059–198 823 098 | 1.6 |
| Pt 2 | 3:196 941 615–199 043 290 | 2.1 |
| Cobb et al. (2010) | ||
| Pt 1 | 3:197 256 140–198 570 020 | 1.3 |
| Clayton-Smith et al. (2010) | ||
| Pt 1 | Microarray | 1.6 |
| Pt 2 | Microarray | 1.6 |
| Pt 3 | Microarray | 1.6 |
| Pt 4 | Microarray | 1.6 |
| Petrin et al. (2011) | ||
| Pt 1 | Microarray | 1.5 |
| Dasouki et al. (2011) | ||
| Pt 1 | 3:197 174 369–198 842 531 | 1.66 |
| Pt 2 | 3:197 174 769–198 842 531 | 1.66 |
| Pt 3 | 3:197 085 422–198 217 248 | 1.13 |
| Sagar et al. (2013) | ||
| Pt 1 | 3:197 224 799–198 801 500 | 1.58 |
| Citta et al. (2013) | ||
| Pt 1 | 3:197 224 754–198 794 789 | 1.57 |
| Pt 2 | 3:197 216 353–198 823 667 | 1.607 |
| Pt 3 | Karyotype and FISH | ~1.5 |
| Pt 4 | 3:197 232 253–198 823 667 | 1.59 |
| Our patient | ||
| Pt 1 | 3:197 272 696–198 517 693 | 1.24 |
All genomic coordinates are based on NCBI 36 hg 18 build (2006).
FISH, fluorescence in-situ hybridization; Pt, patient.
Acknowledgments
The authors thank the family and study participant as well as the NICHD HD02528 grant for partial support.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- Ballif BC, Theisen A, Coppinger J, Gowans GC, Hersh JH, Madan-Khetarpal S, et al. Expanding the clinical phenotype of the 3q29 microdeletion syndrome and characterization of the reciprocal microduplication. Mol Cytogenet. 2008;1:8. doi: 10.1186/1755-8166-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynam G, Goldblatt J, Townshend S. A case of 3q29 microdeletion with novel features and a review of cytogenetically visible terminal 3q deletions. Clin Dysmorphol. 2006;15:145–148. doi: 10.1097/01.mcd.0000198934.55071.ee. [DOI] [PubMed] [Google Scholar]
- Città S, Buono S, Greco D, Barone C, Alfei E, Bulgheroni S, et al. 3q29 microdeletion syndrome: cognitive and behavioral phenotype in four patients. Am J Med Genet A. 2013;161A:3018–3022. doi: 10.1002/ajmg.a.36142. [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J, Giblin C, Smith RA, Dunn C, Willatt L. Familial 3q29 microdeletion syndrome providing further evidence of involvement of the 3q29 region in bipolar disorder. Clin Dysmorphol. 2010;19:128–132. doi: 10.1097/MCD.0b013e32833a1e3c. [DOI] [PubMed] [Google Scholar]
- Cobb W, Anderson A, Turner C, Hoffman RD, Schonberg S, Levin SW. 1.3 Mb de novo deletion in chromosome band 3q29 associated with normal intelligence in a child. Eur J Med Genet. 2010;53:415–418. doi: 10.1016/j.ejmg.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Dasouki MJ, Lushington GH, Hovanes K, Casey J, Gorre M. The 3q29 microdeletion syndrome: report of three new unrelated patients and in silico ‘RNA binding’ analysis of the 3q29 region. Am J Med Genet A. 2011;155A:1654–1660. doi: 10.1002/ajmg.a.34080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digilio MC, Bernardini L, Mingarelli R, Capolino R, Capalbo A, Giuffrida MG, et al. 3q29 Microdeletion: a mental retardation disorder unassociated with a recognizable phenotype in two mother–daughter pairs. Am J Med Genet A. 2009;149A:1777–1781. doi: 10.1002/ajmg.a.32965. [DOI] [PubMed] [Google Scholar]
- Koolen DA, Nillesen WM, Versteeg MH, Merkx GF, Knoers NV, Kets M, et al. Screening for subtelomeric rearrangements in 210 patients with unexplained mental retardation using multiplex ligation dependent probe amplification (MLPA) J Med Genet. 2004;41:892–899. doi: 10.1136/jmg.2004.023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krepischi-Santos AC, Vianna-Morgante AM, Jehee FS, Passos-Bueno MR, Knijnenburg J, Szuhai K, et al. Whole-genome array-CGH screening in undiagnosed syndromic patients: old syndromes revisited and new alterations. Cytogenet Genome Res. 2006;115:254–261. doi: 10.1159/000095922. [DOI] [PubMed] [Google Scholar]
- Li F, Lisi EC, Wohler ES, Hamosh A, Batista DA. 3q29 interstitial micro-deletion syndrome: an inherited case associated with cardiac defect and normal cognition. Eur J Med Genet. 2009;52:349–352. doi: 10.1016/j.ejmg.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Petrin AL, Daack-Hirsch S, L’Heureux J, Murray JC. A case of 3q29 microdeletion syndrome involving oral cleft inherited from a nonaffected mosaic parent: molecular analysis and ethical implications. Cleft Palate Craniofac J. 2011;48:222–230. doi: 10.1597/09-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero-Rivera F, Sharifi-Hannauer P, Martinez-Agosto JA. Autistic and psychiatric findings associated with the 3q29 microdeletion syndrome: case report and review. Am J Med Genet A. 2010;152A:2459–2467. doi: 10.1002/ajmg.a.33573. [DOI] [PubMed] [Google Scholar]
- Rossi E, Piccini F, Zollino M, Neri G, Caselli D, Tenconi R, et al. Cryptic telomeric rearrangements in subjects with mental retardation associated with dysmorphism and congenital malformations. J Med Genet. 2001;38:417–420. doi: 10.1136/jmg.38.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar A, Bishop JR, Tessman DC, Guter S, Martin CL, Cook EH. Co-occurrence of autism, childhood psychosis, and intellectual disability associated with a de novo 3q29 microdeletion. Am J Med Genet A. 2013;161A:845–849. doi: 10.1002/ajmg.a.35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Shaw CA, Lu X-Y, Sahoo T, Bacino CA, Lalani SR, et al. Identification of chromosome abnormalities in subtelomeric regions by microarray analysis: a study of 5,830 cases. Am J Med Genet Part A. 2008;146A:2242–2251. doi: 10.1002/ajmg.a.32399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GS, Panier S, Townsend K, Al-Hakim AK, Kolas NK, Miller ES, et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell. 2009;136:420–434. doi: 10.1016/j.cell.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Tyshchenko N, Hackmann K, Gerlach EM, Neuhann T, Schrock E, Tinschert S. 1.6 Mb deletion in chromosome band 3q29 associated with eye abnormalities. Eur J Med Genet. 2009;52:128–130. doi: 10.1016/j.ejmg.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Willatt L, Cox J, Barber J, Cabanas ED, Collins A, Donnai D, et al. 3q29 microdeletion syndrome: clinical and molecular characterization of a new syndrome. Am J Hum Genet. 2005;77:154–160. doi: 10.1086/431653. [DOI] [PMC free article] [PubMed] [Google Scholar]