Abstract
Optic neuropathies are characterized by retinal ganglion cell (RGC) death, resulting in the loss of vision. In glaucoma, the most common optic neuropathy, RGC death is initiated by axonal damage, and can be modeled by inducing acute axonal trauma through procedures such as optic nerve crush (ONC) or optic nerve axotomy. One of the early events of RGC death is nuclear atrophy, and is comprised of RGC-specific gene silencing, histone deacetylation, heterochromatin formation, and nuclear shrinkage. These early events appear to be principally regulated by epigenetic mechanisms involving histone deacetylation. Class I histone deacetylases HDACs 1, 2, and 3 are known to play important roles in the process of early nuclear atrophy in RGCs, and studies using both inhibitors and genetic ablation of Hdacs also reveal a critical role in the cell death process. Select inhibitors, such as those being developed for cancer therapy, may also provide a viable secondary treatment option for optic neuropathies.
Keywords: Histone deacetylase (HDAC), neurodegeneration, retinal ganglion cell, nuclear atrophy, heterochromatin, apoptosis, optic nerve injury, glaucoma
1. Introduction
Optic neuropathies, such as glaucoma, result in retinal ganglion cell (RGC) death. In glaucoma, increased intraocular pressure culminating in increased strain at the optic nerve head is thought to induce axonal damage leading to further pathology in this disease. Axogenic neurodegeneration can be mimicked in rodent models of acute optic nerve injury. Optic nerve crush (ONC) in rodents has been used extensively to model the pathophysiology to RGCs [1]. The sequence of apoptotic events has been described in this model, and includes early onset nuclear atrophy [2–4]. Global and focal changes in the chromatin of RGCs occur shortly after acute injury of the optic nerve and before the cell has reached the committed step of intrinsic apoptosis, pro-apoptotic BAX oligomerization at the mitochondrial outer membrane [2]. Epigenetic modification of histones, principally their deacetylation, has been implicated in not only early nuclear changes in RGCs, but also in their apoptotic death.
Histone deacetylases (HDACs) remove acetyl groups from lysine residues. In the nucleus, deacetylation of histones is involved in chromatin condensation, guiding gene expression during development and differentiation, and neuronal death [5, 6]. HDACs are grouped into four classes based on phylogenetic analysis [6]. Class I includes HDACs 1, 2, 3, and 8; class II includes HDACs 4, 5, 6, 7, 9, and 10; Class III includes SIRTs 1, 2, 3, 4, 5, 6, and 7; and class IV includes HDAC11 [6]. There is growing evidence that HDAC activity is a critical component of neuronal death, and is implicated in animal models of Huntington’s disease, stroke and ischemic injury, spinocerabellar ataxia type 7, and memory loss in cocaine-seeking behavior [7–10]. More recently, HDAC activity has been associated with the loss of retinal ganglion cells (RGCs) in models of optic nerve damage.
The advent of new and selective inhibitors of these enzymes, suggests an intriguing opportunity to target HDACs as a therapeutic strategy for neurodegenerative conditions. Here, we discuss the current state of knowledge of the roles of HDACs in the process of neuronal, and specifically, retinal ganglion cell degeneration.
2. HDACs regulate chromatin remodeling during nuclear atrophy in RGCs
HDACs 1, 2, 3, 5, and 6 have all been identified in the murine retina previously [3, 11]. In healthy RGCs, HDACs 1 and 2 are localized to the nuclei while HDAC3 is localized mainly to the cytoplasm [3, 11]. The optic nerve crush injury model has been used extensively to study HDAC activity in the process of RGC death. In this model, intrinsic apoptosis is activated leading to a loss of neurons beginning around 7 days after injury. After acute optic nerve injury, mRNA accumulation of class I HDACs increases, with Hdac2 and Hdac3 expression being the highest at 3 days post ONC [3]. HDAC3 translocates from the cytoplasm to the nucleus of RGCs by 3–5 days following optic nerve damage [3, 4]. These observations are consistent with other studies where HDAC3 is cytoplasmic in healthy neurons, but localized to the nuclei of diseased cortical neurons of a mouse model of Huntington’s disease [9].
Heterochromatin formation is a signature feature of apoptosis [12, 13]. It initiates along the inner surface of the nuclear envelope and coalesces throughout the nucleus in a process defined as pyknosis. The nuclear envelope is also broken down and the condensed chromatin is fragmented and often detected in apoptotic bodies. DNA fragmentation assays, such as TUNEL [14], show that pyknosis is associated with DNA cleavage. The responsible endonuclease for this process is activated in a caspase-dependent manner [15]. Dying RGCs undergo pyknosis, but studies using Bax-deficient mice, in which the apoptotic pathway is blocked before caspase activation, suggest that chromatin remodeling is initiated well in advance of DNA cleavage [2]. We have documented several metrics of this process in RGCs, including heterochromatin formation and silencing of RGC-specific gene expression [2–4, 16]. All of these processes occur in Bax-deficient cells, and are related to HDAC activity.
Silencing of genes selectively expressed by RGCs occurs within 24 hours post optic nerve injury in rodents [3, 17]. Chromatin immunoprecipitation studies showed that the acetylation state of histone H4 associated with promoters of several RGC-specific genes was significantly reduced by 24 hours following ONC in the mouse [3]. Additionally, treatment of mice with the broad-spectrum inhibitor trichostatin A (TSA) was able to attenuate the silencing of many of these genes [3, 16]. Taken together, these data provide compelling evidence of a role of HDAC activity in this process. Which HDACs participate in this process is uncertain. Selective ablation of Hdacs in RGCs using conditional knockout mice has been used to address this question. Gene excision is accomplished by transducing RGCs with adeno-associated virus serotype 2, which has a high tropism for RGCs [18], carrying the CRE recombinase. Interestingly, genetic ablation of Hdac3 in mouse RGCs was unable to prevent silencing of the target genes [4], while double knockout of Hdac1 and Hdac2 had a similar lack of effect on the overall pattern of down-regulated genes when assessed by RNA sequencing [19]. Thus studies using inhibitors or genetic ablation do not reconcile. It is possible that genetic deletion experiments are complicated by other HDACs playing a compensatory role and it remains unclear how HDACs participate in the mechanism of gene silencing.
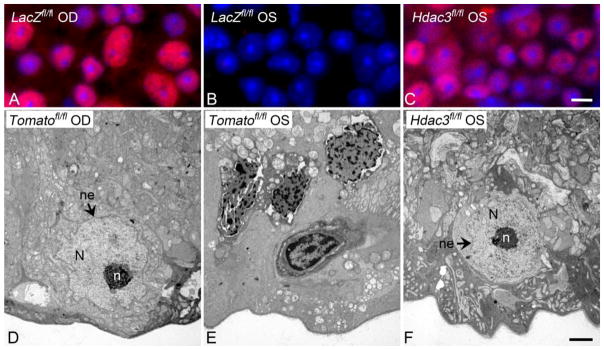
While gene silencing may involve focal changes in histone deacetylation, the process of global heterochromatin formation appears to be associated with widespread deacetylation. After optic nerve injury, RGC nuclei exhibit dramatic and widespread deacetylation of histones, a process that is initiated early and continues to a maximum decrease by 5 days (Figure 1) [3]. Unlike the process of gene silencing, global deacetylation is clearly attributable to the function of HDAC3. Mice lacking Hdac3 in RGCs exhibited nearly complete abrogation of this response, which was, in turn associated with a significant reduction in the formation of heterochromatin (Figure 1) [4].
Figure 1. Conditional knockout of Hdac3 prevents heterochromatin formation after optic nerve crush.
Conditional knockout of Hdac3 in RGCs was achieved by injecting AAV2-Cre virus into the left (OS) eye of Hdac3fl/fl mice prior to ONC. AAV2-Cre injected into the OS eyes of Rosa26-Tomatofl/fl mice served as virus-injected controls. Contralateral right (OD) eyes served as uncrushed and non-injected controls. Retinas were harvested at 5 days following crush injury for evaluation. (A) Retinal whole mount showing staining for acetylated histone H4 in unaffected OS eyes. (B) Crush elicits wide spread deacetylation of histones, but this process is blocked in RGCs lacking Hdac3 (C). DAPI counterstain. (Scale bar = 10 μm). (D) Transmission electron micrograph (TEM) image of a healthy cell in the ganglion cell layer (GCL) of a Rosa26-Tomatofl/fl control OD eye. (E) TEM image of heterochromatic cells in the GCL of an AAV2-Cre/GFP injected and crushed Rosa26-Tomatofl/fl OS eye. (F) TEM image of a cell in the GCL of the Hdac3 conditional knockout crushed OS eye. Healthy nuclei (N) are euchromatic and have well-formed nucleoli (n) and intact nuclear envelopes (ne), while injured cells exhibit heterochromatic nuclei with degrading nuclear envelopes. (Scale bar = 2μm)
Given the evidence currently available, we posit that RGC apoptosis is associated with at least two phases of histone deacetylation. The first appears to be rapid and focally restricted to areas of active gene expression, while the second is more prolonged and widespread. This latter phase may specific to the later stages of nuclear atrophy as a prelude to the activation of catabolic pathways that lead to DNA fragmentation.
3. HDAC inhibition prevents RGC death
HDAC activity modulates changes in chromatin structure and influences the gene expression profile of damaged RGCs. It is not known if these changes directly precipitate downstream apoptotic events. The importance of HDAC activity in the process of RGC death was initially interrogated using broad-spectrum HDAC inhibitors, which exhibit neuroprotective effects in a wide range of models of neurodegeneration [20]. Rats treated with the class I and II HDAC inhibitor valproic acid (VPA), had significantly less RGC loss by 8 days after acute optic nerve injury than vehicle treated rats [21]. Similarly, treatment of purified rat RGCs with VPA or sodium butyrate (SB), another class I and class IIa HDAC inhibitor, prevented histone deacetylation and protected RGCs from death in vitro [22]. TSA was similarly protective in mouse models of acute optic nerve damage [3] and acute hypertension induced retinal ischemia [11]. More selective inhibition of HDACs 1, 2, and 3 with MS-275, protected ganglion cells as long as 6 weeks following optic nerve injury [23]. Inhibitors are also reportedly protective in rodent models of experimental glaucoma. Treatment with VPA in a rat model of ocular hypertension helped preserve retinal function as assessed by electroretinograms, and reduced the rate of RGC loss [24]. TSA attenuated progressive RGC soma degeneration in the DBA/2J mouse model of glaucoma, but had no significant effect on axonal degeneration [16]. Interpreting results obtained with inhibitors, particularly broad spectrum ones, may be complicated by the indiscriminate nature in which they act. Prevention of HDAC activity in the RGC nucleus, for example, may be protective, but preventing deacetylation of non-histone targets may be detrimental. The development of more selective inhibitors may greatly advance both the understanding of the roles of different HDACs, while providing a more granular therapeutic approach that selectively targets pathologic HDACs and preserves the vital activities of others.
4. Class I HDACs 1, 2, and 3 regulate apoptosis in differentiated neurons
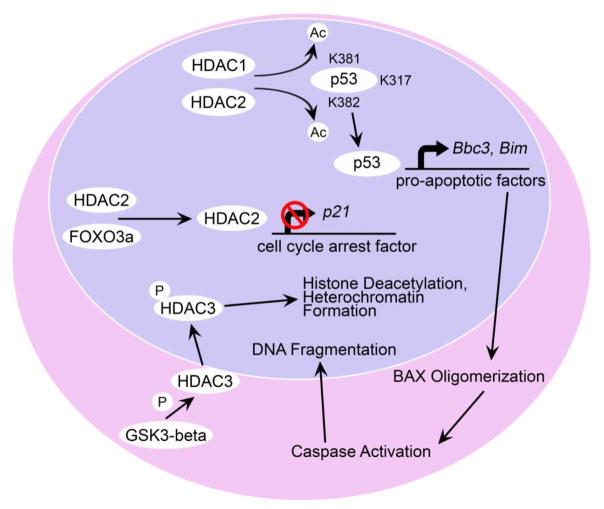
The experiments using HDAC inhibitors, reviewed above, indicate that blocking HDAC functions can have a profound protective effect on the loss of not only RGCs, but also differentiated neurons in general, in a variety of neurodegenerative conditions. While some of the consequences of HDAC activity may not be directly lethal to cells, RGCs, for example can tolerate heterochromatic chromatin for 18 months if the apoptotic program is blocked [25], their actions can clearly precipitate further downstream events that lead to cell death. A clue to these effects were demonstrated by experiments by Bardai and D’Mello [26], who showed that forced expression of HDAC3 promoted the death of hippocampus-derived HT22 cells, and rat cortical neurons, but not primary kidney fibroblasts, or HEK293 and HeLa cell lines. In neurons, HDAC3 is activated by phosphorylation from GSK3β serine/threonine kinase (Figure 2): a mechanism that is normally inhibited by growth factor-stimulation of the PI3K/Akt signaling pathway [26]. The selectivity of HDAC3 expression to induce the death of neurons is not well understood, but it may be mediated through the activation of the tumor suppressor protein p53. Deacetylation of p53 can activate its function as a transcription factor either by promoting nuclear accumulation [27] or enhancing binding to p53 response elements on target genes [28], or both. In neurons, deacetylation of lysine residues K381 and K382 (human p53), appear to be critical for activating pro-apoptotic gene expression [28], while other studies have identified K317 (mouse p53) as being important for the same function [29]. RGCs in mice exhibit significant deacetylation of residue K381 within a few days after optic nerve injury, a process that is attenuated in cells lacking both Hdac1 and Hdac2 [19].
Figure 2. Potential pathways of HDAC activity in neuronal toxicity.
HDAC1 and HDAC2 reportedly activate p53 by deacetylating its K381 and K382 residues, where it leads to an increase in the expression of genes involved in apoptosis, including, Bbc3 (PUMA), and Bim [27–30]. These BH3-only activator proteins then facilitate the activation of BAX, leading to caspase activity and the activation of an endonuclease that digests DNA. HDAC2 may have an additional function in silencing other p53-target genes, such as p21. In this mechanism, HDAC2 is associated with FOXO3a, which recruits it to the p21 promoter site, where it deacetylates histones in this region and represses transcription [34]. HDAC3 plays a primary role in histone deacetylation, and heterochromatin formation [3, 4]. In neurons, HDAC3 is activated by phosphorylation from GSK3β serine/threonine kinase, which becomes active when the PI3K/Akt signaling pathway is attenuated by loss of trophic factor support [26].
Once activated, p53 directs the transcription of a variety of genes, including those involved in cell-cycle checkpoints and others that induce apoptosis. Importantly, in this latter category, p53 induces the expression of activator proteins containing the BH3 homology domain from the larger BCL2 gene family, including Bbc3 and Bim, which lead directly to the activation of latent BAX in cells (Figure 2). Why HDAC3 is selective toxicity to neurons is not well understood, but promoter targeting of p53 is regulated differentially, depending on whether or not the cell is actively dividing [30]. The p53 response elements are more highly conserved in cell cycle regulatory genes than in the promoters of pro-apoptotic genes [31]. In addition, p53 preferentially targets cell cycle regulatory genes in DNA binding assays, regardless of the stress conditions [32]. Neurons, however, may have a propensity to target p53 to pro-apoptotic genes such as Bbc3 and Bim. This process that may be influenced by early apoptotic changes in chromatin structure that suppress cell-cycle regulatory p53 targets [33]. Consistent with this, HDAC2 activity in cortical granular neurons was found to be associated with the inhibition of expression of potentially protective genes. HDAC2 associates with the transcription factor FOXO3a directing it to the promoter region of the cell cycle arrest gene p21, where it suppressed p21 expression by hypoacetylating the promoter (Figure 2) [34]. Knockdown of Hdac2 in these cells [34] or pulse inhibition with HDAC inhibitors [35] was protective after exposure to oxidative stress and tied directly to the up-regulation of p21 expression. Paradoxically, Hdac1/2 deletion in RGCs reduces p21 expression after optic nerve damage [19], suggesting a more complex interaction that still needs to be resolved.
5. HDAC isoform targeting as a potential therapeutic for glaucoma
Since the treatment of glaucoma is currently limited to lowering intraocular pressure, there is a concerted effort to find adjunctive therapies that are directed specifically at the tissues, which are principally affected. To date, broad spectrum HDAC inhibitors have shown great promise as therapeutics in animal models of RGC death, however further interrogation of these and more selective inhibitors is necessary for eventual treatment of optic neuropathies such as glaucoma. RGC death in glaucoma occurs over time, and is often asynchronous and protracted. Given the chronic nature of glaucomatous disease, treatment administration would be necessary over months or years to prevent vision loss in patients who have been diagnosed. High doses and extended administration of broad spectrum HDAC inhibitors may have toxic effects on other cell types such as proliferative cells and immune cells [36, 37], and importantly, use of high doses of VPA, SB, or TSA is actually toxic to primary RGCs [22]. It has been suggested that use of HDAC isotype selective inhibitors may avoid potentially harmful side effects when attempting to specifically treat degenerating neurons [38]. An advantage in treating optic neuropathies, however, may be to locally administer HDAC isotype selective inhibitors using intravitreal injection or other adapted methods, which would reduce the potentially toxic effects of prolonged systemic administration of HDAC inhibitors. Protocols using monthly intravitreal injections of VEGF inhibitors are now standard practice for macular degeneration, so similar protocols to treat glaucoma may be feasible.
6. Conclusion
While the exact biological mechanisms of each individual HDAC are not fully elucidated in injured RGCs, investigations in these cells and other neurodegenerative disease models shed light on the varying pathways that HDACs regulate. Since inhibition of HDACs promotes both neuronal survival and cancer cell apoptosis, HDAC inhibitors are widely used for study and some are already in clinical trials [8, 10, 36, 39–42]. It is important to recognize that these inhibitors, especially broad-spectrum inhibitors, are toxic to rapidly dividing cells such as immune cells and intestinal cells when administered at high doses [36, 37]. The role of HDACs in nuclear atrophy and apoptosis of RGCs must be further investigated to determine which HDACs serve as appropriate targets for therapeutic inhibition in optic neuropathies such as glaucoma.
HIGHLIGHTS.
HDACs 1, 2, and 3 play major roles in retinal ganglion cell (RGC) death.
Nuclear atrophy is associated with increased HDAC activity in RGCs
HDAC3 is selectively toxic to neurons.
HDAC inhibition may yield new therapeutic options to treat optic neuropathies.
Acknowledgments
Supported by funding from the National Eye Institute, R01 EY012223, P30 EY016665, and unrestricted funding from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKinnon SJ, Schlamp CL, Nickells RW. Mouse models of retinal ganglion cell death and glaucoma. Exp Eye Res. 2009;88:816–824. doi: 10.1016/j.exer.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen KT, Mac Nair CE, Dietz JA, Schlamp CL, Nickells RW. Nuclear atrophy of retinal ganglion cells precedes the bax-dependent stage of apoptosis. Invest Ophthalmol Vis Sci. 2013;54:1805–1815. doi: 10.1167/iovs.11-9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelzel HR, Schlamp CL, Nickells RW. Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis. BMC Neurosci. 2010;11:62. doi: 10.1186/1471-2202-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt HM, Pelzel HR, Schlamp CL, Nickells RW. Histone deacetylase 3 (HDAC3) plays an important role in retinal ganglion cell death after acute optic nerve injury. Mol Neurodegener. 2014;9:39. doi: 10.1186/1750-1326-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiwari S, Dharmarajan S, Shivanna M, Otteson DC, Belecky-Adams TL. Histone deacetylase expression patterns in developing murine optic nerve. BMC Dev Biol. 2014;14:30. doi: 10.1186/1471-213X-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baltan S, Morrison RS, Murphy SP. Novel protective effects of histone deacetylase inhibition on stroke and white matter ischemic injury. Neurotherapeutics. 2013;10:798–807. doi: 10.1007/s13311-013-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan CE, An MC, Papanikolaou T, Rugani C, Vitelli C, Ellerby LM. Histone deacetylase-3 interacts with ataxin-7 and is altered in a spinocerebellar ataxia type 7 mouse model. Mol Neurodegener. 2013;8:42. doi: 10.1186/1750-1326-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia H, Pallos J, Jacques V, Lau A, Tang B, Cooper A, Syed A, Purcell J, Chen Y, Sharma S, Sangrey GR, Darnell SB, Plasterer H, Sadri-Vakili G, Gottesfeld JM, Thompson LM, Rusche JR, Marsh JL, Thomas EA. Histone deacetylase (HDAC) inhibitors targeting HDAC3 and HDAC1 ameliorate polyglutamine-elicited phenotypes in model systems of Huntington’s disease. Neurobiol Dis. 2012;46:351–361. doi: 10.1016/j.nbd.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, Rusche JR, Wood MA. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A. 2013;110:2647–2652. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan J, Alsarraf O, Dahrouj M, Platt KA, Chou CJ, Rice DS, Crosson CE. Inhibition of HDAC2 protects the retina from ischemic injury. Invest Ophthalmol Vis Sci. 2013;54:4072–4080. doi: 10.1167/iovs.12-11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dillon N, Festenstein R. Unravelling heterochromatin: competition between positive and negative factors regulates accessibility. Trends Genet. 2002;18:252–258. doi: 10.1016/s0168-9525(02)02648-3. [DOI] [PubMed] [Google Scholar]
- 13.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 16.Pelzel HR, Schlamp CL, Waclawski M, Shaw MK, Nickells RW. Silencing of Fem1cR3 gene expression in the DBA/2J mouse precedes retinal ganglion cell death and is associated with histone deacetylase activity. Invest Ophthalmol Vis Sci. 2012;53:1428–1435. doi: 10.1167/iovs.11-8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Quigley HA, Pease ME, Yang Y, Qian J, Valenta D, Zack DJ. Changes in gene expression in experimental glaucoma and optic nerve transection: the equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci. 2007;48:5539–5548. doi: 10.1167/iovs.07-0542. [DOI] [PubMed] [Google Scholar]
- 18.Hellstrom M, Ruitenberg MJ, Pollett MA, Ehlert EM, Twisk J, Verhaagen J, Harvey AR. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Ther. 2009;16:521–532. doi: 10.1038/gt.2008.178. [DOI] [PubMed] [Google Scholar]
- 19.Lebrun-Julien F, Suter U. Combined HDAC1 and HDAC2 Depletion Promotes Retinal Ganglion Cell Survival After Injury Through Reduction of p53 Target Gene Expression. ASN Neuro. 2015;7 doi: 10.1177/1759091415593066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 21.Biermann J, Grieshaber P, Goebel U, Martin G, Thanos S, Di Giovanni S, Lagreze WA. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010;51:526–534. doi: 10.1167/iovs.09-3903. [DOI] [PubMed] [Google Scholar]
- 22.Biermann J, Boyle J, Pielen A, Lagreze WA. Histone deacetylase inhibitors sodium butyrate and valproic acid delay spontaneous cell death in purified rat retinal ganglion cells. Mol Vis. 2011;17:395–403. [PMC free article] [PubMed] [Google Scholar]
- 23.Chindasub P, Lindsey JD, Duong-Polk K, Leung CK, Weinreb RN. Inhibition of histone deacetylases 1 and 3 protects injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2013;54:96–102. doi: 10.1167/iovs.12-10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsarraf O, Fan J, Dahrouj M, Chou CJ, Yates PW, Crosson CE. Acetylation preserves retinal ganglion cell structure and function in a chronic model of ocular hypertension. Invest Ophthalmol Vis Sci. 2014;55:7486–7493. doi: 10.1167/iovs.14-14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semaan SJ, Li Y, Nickells RW. A single nucleotide polymorphism in the Bax gene promoter affects transcription and influences retinal ganglion cell death. ASN Neuro. 2010;2:e00032. doi: 10.1042/AN20100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardai FH, D’Mello SR. Selective toxicity by HDAC3 in neurons: regulation by Akt and GSK3beta. J Neurosci. 2011;31:1746–1751. doi: 10.1523/JNEUROSCI.5704-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uo T, Veenstra TD, Morrison RS. Histone deacetylase inhibitors prevent p53-dependent and p53-independent Bax-mediated neuronal apoptosis through two distinct mechanisms. J Neurosci. 2009;29:2824–2832. doi: 10.1523/JNEUROSCI.6186-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brochier C, Dennis G, Rivieccio MA, McLaughlin K, Coppola G, Ratan RR, Langley B. Specific acetylation of p53 by HDAC inhibition prevents DNA damage-induced apoptosis in neurons. J Neurosci. 2013;33:8621–8632. doi: 10.1523/JNEUROSCI.5214-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao C, Wu Z, Mazur SJ, Borges H, Rossi M, Lin T, Wang JY, Anderson CW, Appella E, Xu Y. Acetylation of mouse p53 at lysine 317 negatively regulates p53 apoptotic activities after DNA damage. Mol Cell Biol. 2006;26:6859–6869. doi: 10.1128/MCB.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 31.Horvath MM, Wang X, Resnick MA, Bell DA. Divergent evolution of human p53 binding sites: cell cycle versus apoptosis. PLoS Genet. 2007;3:e127. doi: 10.1371/journal.pgen.0030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian H, Wang T, Naumovski L, Lopez CD, Brachmann RK. Groups of p53 target genes involved in specific p53 downstream effects cluster into different classes of DNA binding sites. Oncogene. 2002;21:7901–7911. doi: 10.1038/sj.onc.1205974. [DOI] [PubMed] [Google Scholar]
- 33.Millau JF, Bandele OJ, Perron J, Bastien N, Bouchard EF, Gaudreau L, Bell DA, Drouin R. Formation of stress-specific p53 binding patterns is influenced by chromatin but not by modulation of p53 binding affinity to response elements. Nucleic Acids Res. 2011;39:3053–3063. doi: 10.1093/nar/gkq1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng S, Zhao S, Yan F, Cheng J, Huang L, Chen H, Liu Q, Ji X, Yuan Z. HDAC2 selectively regulates FOXO3a-mediated gene transcription during oxidative stress-induced neuronal cell death. J Neurosci. 2015;35:1250–1259. doi: 10.1523/JNEUROSCI.2444-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langley B, D’Annibale MA, Suh K, Ayoub I, Tolhurst A, Bastan B, Yang L, Ko B, Fisher M, Cho S, Beal MF, Ratan RR. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21(waf1/cip1) in cell cycle-independent neuroprotection. J Neurosci. 2008;28:163–176. doi: 10.1523/JNEUROSCI.3200-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bratland A, Dueland S, Hollywood D, Flatmark K, Ree AH. Gastrointestinal toxicity of vorinostat: reanalysis of phase 1 study results with emphasis on dose-volume effects of pelvic radiotherapy. Radiat Oncol. 2011;6:33. doi: 10.1186/1748-717X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, Lu RB, Gean PW, Chuang DM, Hong JS. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007;149:203–212. doi: 10.1016/j.neuroscience.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dietz KC, Casaccia P. HDAC inhibitors and neurodegeneration: at the edge between protection and damage. Pharmacol Res. 2010;62:11–17. doi: 10.1016/j.phrs.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cudkowicz ME, Andres PL, Macdonald SA, Bedlack RS, Choudry R, Brown RH, Jr, Zhang H, Schoenfeld DA, Shefner J, Matson S, Matson WR, Ferrante RJ A. L. S Northeast, V.A.A.L.S.R.C. National. Phase 2 study of sodium phenylbutyrate in ALS. Amyotroph Lateral Scler. 2009;10:99–106. doi: 10.1080/17482960802320487. [DOI] [PubMed] [Google Scholar]
- 40.Darras BT, Kang PB. Clinical trials in spinal muscular atrophy. Curr Opin Pediatr. 2007;19:675–679. doi: 10.1097/MOP.0b013e3282f1884c. [DOI] [PubMed] [Google Scholar]
- 41.Piepers S, Veldink JH, de Jong SW, van der Tweel I, van der Pol WL, Uijtendaal EV, Schelhaas HJ, Scheffer H, de Visser M, de Jong JM, Wokke JH, Groeneveld GJ, van den Berg LH. Randomized sequential trial of valproic acid in amyotrophic lateral sclerosis. Ann Neurol. 2009;66:227–234. doi: 10.1002/ana.21620. [DOI] [PubMed] [Google Scholar]
- 42.Soragni E, Miao W, Iudicello M, Jacoby D, De Mercanti S, Clerico M, Longo F, Piga A, Ku S, Campau E, Du J, Penalver P, Rai M, Madara JC, Nazor K, O’Connor M, Maximov A, Loring JF, Pandolfo M, Durelli L, Gottesfeld JM, Rusche JR. Epigenetic therapy for Friedreich ataxia. Ann Neurol. 2014;76:489–508. doi: 10.1002/ana.24260. [DOI] [PMC free article] [PubMed] [Google Scholar]