Abstract
Background and purpose
Loco-regionally recurrent head and neck cancer (HNC) in the setting of prior radiotherapy carries significant morbidity and mortality. The role of re-irradiation (re-RT) remains unclear due to toxicity. We determined prognostic factors for loco-regional control (LRC) and formulated a nomogram to help clinicians select re-RT candidates.
Material and methods
From July 1996 to April 2011, 257 patients with recurrent HNC underwent fractionated re-RT. Median prior dose was 65 Gy and median time between RT was 32.4 months. One hundred fifteen patients (44%) had salvage surgery and 172 (67%) received concurrent chemotherapy. Median re-RT dose was 59.4 Gy and 201 (78%) patients received IMRT. Multivariate Cox proportional hazards were used to identify independent predictors of LRC and a nomogram for 2-year LRC was constructed.
Results
Median follow-up was 32.6 months. Two-year LRC and overall survival (OS) were 47% and 43%, respectively. Recurrent stage (P = 0.005), non-oral cavity subsite (P < 0.001), absent organ dysfunction (P < 0.001), salvage surgery (P < 0.001), and dose >50 Gy (P = 0.006) were independently associated with improved LRC. We generated a nomogram with concordance index of 0.68.
Conclusion
Re-RT can be curative, and our nomogram can help determine a priori which patients may benefit.
Keywords: Re-irradiation, Head and neck cancer, Intensity modulated radiation therapy, Recurrent head and neck cancer
Loco-regional recurrent head and neck cancer (HNC) occurs in 8–30% of patients with squamous cell cancer of the head and neck despite aggressive multi-modality definitive treatment consisting of surgery, radiotherapy (RT), and chemotherapy [1–5]. Patients who have previously received RT presenting with unresectable recurrence are offered chemotherapy as standard of care, resulting in median overall survival (OS) of 5–9 months and a 2-year OS of only 10% in patients with solely loco-regionally recurrent disease [6]. In patients with resectable disease, surgery is the standard of care, but patients are still at high risk for loco-regional recurrence [7]. Because uncontrolled loco-regional progression is often the cause of death for these patients, investigators have incorporated re-irradiation (re-RT) in resectable and unresectable recurrent HNC management.
Haraf et al. demonstrated the feasibility of re-RT with concurrent chemotherapy in the recurrent setting, albeit with significant toxicity [8]. Subsequently, two single-arm prospective studies of concurrent chemotherapy and re-RT demonstrated 2-year OS of 15–26%, an improvement from historical cohorts treated with chemotherapy alone [9,10]. A randomized French trial demonstrated improved loco-regional control (LRC) and disease-free survival (DFS) in post-operative patients who received adjuvant re-RT with chemotherapy, though without improvement in OS [7]. In the unresectable setting, two randomized trials failed to accrue due to the technically demanding nature of re-treatment [11]. Given the significant toxicity of re-RT, national guidelines remain ambiguous on its indications in the recurrent setting [12].
In an attempt to identify patients who may benefit most from re-RT, prior studies have investigated different prognostic factors in re-RT [13–17]. Tanvetyanon et al. formulated a nomogram identifying patient characteristics such as comorbid disease, organ dysfunction, recurrence beyond the neck, tumor bulk, and time between RT (i.e., time between first course of RT and re-RT) to predict OS for patients receiving re-RT [18]. Although OS is an important endpoint, loco-regional progression is the major cause of death, and prior studies have suggested both OS and distant metastases (DM) are associated with advanced local disease [19–21]. Furthermore, local disease is associated with significant morbidity, including tumor bleeding, intractable pain, and asphyxiation, and has significant effects on quality of life, ranging from organ impairment to tumor odor and visibility [22]. Given that it is predictive of OS and morbidity and that the intent of RT is to control locoregional disease, LRC is a critical endpoint in evaluating the effectiveness of re-RT.
We thus evaluated our institutional experience and constructed a nomogram to predict the efficacy of curative-intent treatment in providing durable loco-regional control. This tool can be used by physicians in the clinic to detail the benefits and risks of re-RT with patients and facilitate a decision on curative or palliative intent to treatment. Ultimately, patients with negative prognostic factors for LRC may benefit more from palliative intent re-irradiation than intensive 2-month curative-intent radiotherapy and its attendant toxicities.
Methods
Patients
From 7/96 to 4/11, 348 patients with recurrent HNC underwent fractionated re-RT with significant overlapping area with their prior RT. Exclusion criteria included Karnofsky performance status (KPS) <60 (n = 4), melanoma or sarcoma histology (n = 28), hypo-fractionated RT, <6 months between courses of RT (n = 8), and distant metastases at time of salvage (n = 51). After exclusions, 257 patients were eligible for this analysis.
Patients were evaluated by a radiation oncologist, medical oncologist, and head and neck surgeon prior to re-RT. Pretreatment evaluations consisted of history and physical, complete blood count, chemistry, chest X-ray, dental evaluation, and imaging: computed tomography (CT), magnetic resonance imaging (MRI), and/or positron emission tomography (PET) before re-RT. For those receiving chemotherapy, urinalysis, creatinine clearance, electrocardiogram, and audiogram were obtained. The institutional review board issued a waiver of informed consent for this study.
Radiation therapy
RT was delivered as previously described [14]. For IMRT or three-dimensional conformal RT, simulation was performed by 3-mm slice CT with intravenous contrast when indicated. When possible, PET/CT simulation was performed. Beams were generally selected such that 95% of the dose encompassed the target volume, and constraints for critical tissues such as the spinal cord and brain stem were almost always respected. No efforts were made to spare the parotid glands as most patients had baseline xerostomia.
Treatment volumes and techniques have been previously described [14,23]. No attempt was made to encompass the prophylactic subclinical regions at risk in the re-RT IMRT fields. For patients with unresectable disease, gross tumor volume (GTV) was defined as visible disease on physical examination or imaging. For patients whose disease was resected, clinical tumor volume (CTV) was defined as the preoperative GTV and postoperative bed. Volumes and critical structures were defined slice by slice on axial CT. In the first few years, a margin of 1–2 cm was added to the GTV and CTV to define the planning target volume (PTV). However, more recently we have decreased this margin to 0.3 cm. The PTV expansion was reduced in regions near critical structures.
Chemotherapy, surgery, and follow-up
Chemotherapy was given at the medical oncologist’s discretion. If the tumor was resectable, the surgeon performed gross total resection. Patients were evaluated weekly during treatment, every 2–3 months in the first 2 years, and every 4–6 months thereafter by a member of the multidisciplinary team. Surveillance imaging with CT, MRI, or PET was done 2–4 months after treatment and then as indicated clinically.
Data collection
Recurrent disease was staged based on the American Joint Committee on Cancer, seventh edition [24]. The National Social Security Death Index was used to verify patient deaths. Acute toxicities and late complications were assessed retrospectively by reviewing patient records according to the Common Toxicity Criteria, version 3.0. [25]. Similarly, comorbid status and organ dysfunction were evaluated through retrospective review using definitions described by Tanvetyanon et al. [18]. Organ dysfunction included patients requiring feeding tube or tracheostomy or with soft tissue defect, fistula, or osteonecrosis. Prophylactic feeding tube placed prior to re-RT was not identified as organ dysfunction. Surgical margins were considered close if tumor was within 1 mm of the margin for larynx or tonsil subsites, or otherwise within 5 mm of the margin. Patient outcomes were calculated with intention to treat.
Statistical methods
Primary endpoints were 2-year actuarial LRC, time for freedom from distant metastasis (FFDM), and OS. Elapsed time was calculated from the first day of re-RT. LRC and FFDM were assessed based on routine physical exam and imaging. LRC was defined as time to local (primary site) or regional (other head and neck) progression or recurrence. Actuarial estimates were calculated with the Kaplan–Meier method and a Cox proportional hazards model was used to determine predictive factors of outcome. Prognostic factors investigated were whether or not patients had surgery, concurrent chemotherapy, high-dose RT or brachytherapy boost, IMRT, radiotherapy site (isolated primary, isolated neck, or both), Charlson comorbidity score, presence of organ dysfunction, histology, oral cavity, nasopharynx or other subsite, number of recurrences prior to re-RT, time elapsed since first course of RT, whether or not disease was a new primary, recurrent stage, sex, and age.
Multivariate models were built with stepwise variable selection. The R software package (version 2.15.1) was used to construct a nomogram based on independent predictors of LRC. As it has been previously used as a definition for successful salvage therapy for recurrent HNC, we used LRC at 2 years as the predictive endpoint. A bootstrap corrected calibration plot with data split into quintiles was generated and the concordance index for the nomogram was computed (see Supplementary data for more details).
Results
The median patient age was 61 years (range, 21–89). Initial sites of disease were larynx (n = 57), oral cavity (n = 52), oropharynx (n = 43), nasopharynx (n = 38), paranasal sinus (n = 23), or other (n = 44). A minority of patients (15.6%) developed a new primary in a previously irradiated field. Sites of recurrence were primary site (n = 131), neck (n = 54), or both (n = 72). The majority of patients (73%) had a KPS of 80 or greater, and 77% had no signs of organ dysfunction at time of re-treatment. Similarly, more than half the cohort had no significant medical co-morbidities by Charlson score. Full patient characteristics are presented in Table 1.
Table 1.
Patient characteristics at time of recurrence.
| N | % | |
|---|---|---|
| Gender | ||
| Male | 177 | 68.9 |
| Female | 80 | 31.1 |
| Smoking history | ||
| >10-pack years | 130 | 50.6 |
| <10 pack-years | 103 | 40.1 |
| Unknown | 24 | 9.3 |
| Karnofsky performance status | ||
| 80–100 | 188 | 73.2 |
| 60–70 | 40 | 15.6 |
| Unknown | 29 | 11.2 |
| Organ dysfunction | ||
| Yes | 55 | 21.4 |
| No | 198 | 77 |
| Unknown | 4 | 1.6 |
| Initial site of disease | ||
| Larynx | 57 | 22.2 |
| Oral cavity | 52 | 20.2 |
| Oropharynx | 43 | 16.7 |
| Nasopharynx | 38 | 14.8 |
| Paranasal sinus | 23 | 8.9 |
| Other | 44 | 17.1 |
| New primary | ||
| Yes | 40 | 15.6 |
| No | 217 | 84.4 |
| Recurrent T stage | ||
| 0–2 | 112 | 43.6 |
| 3–4 | 136 | 52.9 |
| Unknown | 9 | 3.5 |
| Recurrent N stage | ||
| 0–1 | 164 | 63.8 |
| 2–3 | 84 | 32.7 |
| Unknown | 9 | 3.5 |
| Overall stage | ||
| I–III | 99 | 38.5 |
| IV | 152 | 59.1 |
| Unknown | 6 | 2.3 |
| Histology | ||
| SCC | 220 | 85.6 |
| Other | 37 | 14.4 |
| Number of recurrences | ||
| 1 | 159 | 61.9 |
| 2 | 71 | 27.6 |
| ≥3 | 27 | 10.5 |
| Charlson score | ||
| 0 | 172 | 66.9 |
| 1 | 43 | 16.7 |
| 2 | 15 | 5.8 |
| ≥3 | 27 | 10.5 |
SCC, squamous cell cancer.
Treatment
Two hundred one (78%) patients received IMRT. Median prior dose was 65 Gy (range 19.2–77.5 Gy) and median time between RT courses was 32.4 months (range, 6–420 months). Median re-RT dose was 59.4 Gy (range, 1.8–72 Gy). Twenty-three patients did not complete re-RT. The median cumulative radiation dose was 122.4 Gy (43.4–142.2 Gy). The cumulative dose delivered to the spinal cord and brainstem was limited to 50 Gy and 60 Gy, respectively. At the discretion of the treating physician, 13 patients received brachytherapy boost (range 15–20.4 Gy; median 20 Gy), 9 with nasopharyngeal carcinoma and 4 with oropharyngeal carcinoma.
One hundred fifteen patients (44%) had salvage surgery before re-RT. Among these patients, 19% had negative margins, 20% had close margins (<5 mm), 38% had positive margins, and margin status was unavailable in 28%. One hundred eighty-four patients received chemotherapy, with 172 receiving concurrent chemotherapy. The most common concurrent chemotherapy regimens were cisplatin (29.1%), carboplatin and paclitaxel (20.3%), and cetuximab (13.4%). Forty-two patients received induction chemotherapy, most commonly carbo/taxol (19%) and cisplatin/5-fluorouracil (19%).
LRC
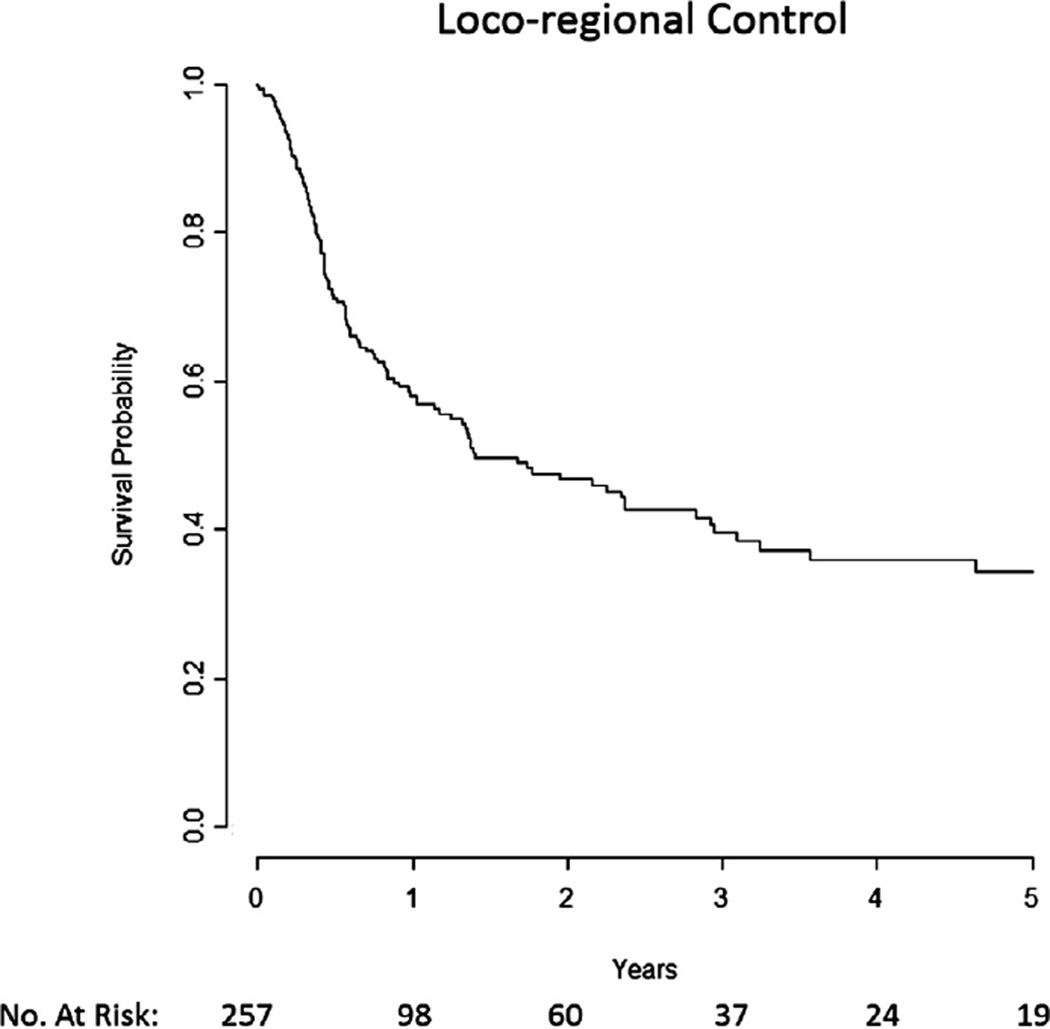
Median time to loco-regional failure was 16.8 months, and 2-year and 5-year LRC were 47% and 34%, respectively (Fig. 1). On univariate analysis, IMRT, RT dose >50 Gy or brachytherapy boost (Supp. Fig. 1a), surgical resection (Supp. Fig. 1b), KPS ≥ 80, male gender, and nasopharynx subsite were associated with statistically significant improved LRC (Table 2). Organ dysfunction (Supp. Fig. 1c), oral cavity subsite, >1 episode of recurrence, and recurrent T4 and overall stage 4 were associated with decreased LRC.
Fig. 1.
Median loco-regional control was 16.8 months.
Table 2.
Loco-regional control univariate and multivariate analysis.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Variable | ||||
| Hazard ratio | P value | Hazard ratio | P value | |
| T stage (4 vs. 1–3) | 1.71 (1.19–2.45) | 0.003 | ||
| N stage (2–3 vs. 0–1) | 1.15 (0.78–1.69) | 0.49 | ||
| Overall stage (4 vs. 1–3) | 1.80 (1.23–2.66) | 0.003 | 1.77 (1.19–2.63) | 0.005 |
| Initial site | ||||
| Other | (reference) | (reference) | ||
| Nasopharynx | 0.54 (0.31–0.95) | 0.03 | 0.59 (0.31–1.13) | 0.11 |
| Oral cavity | 1.61 (1.05–2.48) | 0.03 | 2.17 (1.38–3.42) | <0.001 |
| Histology (SCC vs. other) | 1.18 (0.75–1.86) | 0.47 | ||
| Number of recurrences (1 vs. ≥2) | 0.64 (0.45–0.92) | 0.02 | ||
| Time since first radiation therapy | 0.90 (0.80–1.02) | 0.09 | ||
| Gender (male vs. female) | 0.69 (0.48–0.99) | 0.05 | ||
| Age (>70 vs. <70) | 0.86 (0.55–1.34) | 0.51 | ||
| KPS (≥80 vs. <80) | 0.36 (0.23–0.57) | <0.001 | ||
| Organ dysfunction | 2.04 (1.36–3.07) | <0.001 | 2.14 (1.39–3.29) | <0.001 |
| Charlson score (>2 vs. ≤1) | 0.73 (0.43–1.24) | 0.25 | ||
| Surgery | 0.64 (0.45–0.92) | 0.02 | 0.47 (0.32–0.69) | <0.001 |
| Concurrent chemotherapy | 1.31 (0.89–1.93) | 0.17 | ||
| Radiation type (IMRT vs. other) | 0.48 (0.33–0.70) | <0.001 | ||
| Radiation dose (>50 Gy vs. ≤50 Gy) | 0.53 (0.36–0.77) | <0.001 | 0.57 (0.38–0.85) | 0.006 |
IMRT, image-guided radiation therapy; KPS, Karnofsky performance status; SCC, squamous cell cancer.
On multivariate analysis, RT dose >50 Gy or brachytherapy boost, and surgical salvage resection were independently associated with statistically significant improved LRC. Oral cavity subsite, recurrent overall stage 4, and organ dysfunction were independently associated with decreased LRC (Table 2).
FFDM
Median time for FFDM was not reached. The 2-year and 5-year FFDM were 70% and 62.8%, respectively. Univariate analysis found that KPS ≥ 80, N stage 0–1, overall stage 1–3, and longer time since first course of radiotherapy were significantly associated with better FFDM. Multivariate analysis showed KPS, overall stage, and time since first course of radiotherapy to be independently prognostic.
OS
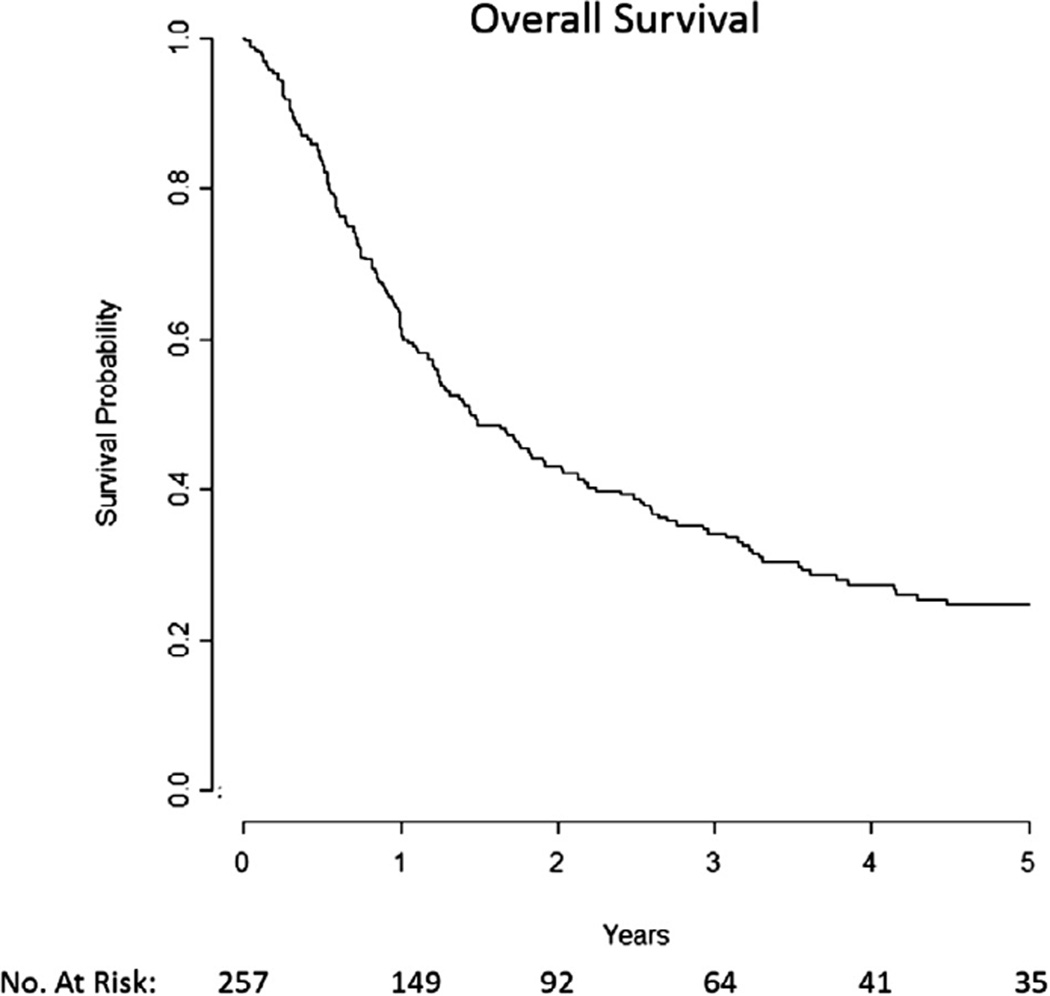
The median OS was 17.3 months with a 2-year and 5-year OS of 43% and 25%, respectively (Fig. 2). Median follow-up was 32.6 months. On univariate analysis, RT dose (Supp. Fig. 2a), salvage surgery (Supp. Fig. 2b), KPS, organ dysfunction (Supp. Fig. 2c), N stage, initial site of disease, number of recurrences, and time since first radiotherapy were significant prognostic factors associated with OS (Table 3). On multivariate analysis each of these factors remained independently significant except for number of recurrences and time since first RT (Table 3).
Fig. 2.
Median overall survival was 17.3 months.
Table 3.
Overall survival univariate and multivariate analysis.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | |
| T stage (4 vs. 1–3) | 1.17 (0.87–1.58) | 0.31 | ||
| N stage (2–3 vs. 0–1) | 1.39 (1.02–1.90) | 0.04 | 1.72 (1.24–2.39) | 0.001 |
| Overall stage (4 vs. 1–3) | 1.32 (0.98–1.78) | 0.07 | ||
| Initial site | ||||
| Other | (reference) | (reference) | ||
| Nasopharynx | 0.50 (0.32–0.79) | 0.003 | 0.50 (0.30–0.81) | 0.005 |
| Oral cavity | 1.54 (1.07–2.20) | 0.02 | 1.81 (1.22–2.68) | 0.003 |
| Histology (SCC vs. other) | 1.48 (0.99–2.18) | 0.05 | ||
| Number of recurrences (1 vs. ≥2) | 0.71 (0.53–0.95) | 0.02 | ||
| Time since first radiation therapy | 0.90 (0.81–0.99) | 0.04 | ||
| Gender (male vs. female) | 0.93 (0.69–1.27) | 0.65 | ||
| Age (>70 vs. <70) | 1.17 (0.83–1.63) | 0.37 | ||
| KPS (≥80 vs. <80) | 0.38 (0.26–0.56) | <0.001 | 0.41 (0.27–0.63) | <0.001 |
| Organ dysfunction | 2.19 (1.57–3.06) | <0.001 | 1.89 (1.31–2.75) | <0.001 |
| Charlson score (>2 vs. ≤1) | 1.00 (0.67–1.48) | 0.98 | ||
| Surgery | 0.57 (0.43–0.77) | <0.001 | 0.37 (0.27–0.52) | <0.001 |
| Concurrent chemotherapy | 1.20 (0.88–1.62) | 0.26 | ||
| Radiation type (IMRT vs. other) | 0.72 (0.52–1.00) | 0.05 | ||
| Radiation dose (>50 Gy vs. ≤50 Gy) | 0.72 (0.52–0.98) | 0.04 | 0.65 (0.47–0.92) | 0.01 |
IMRT, image-guided radiation therapy; KPS, Karnofsky performance status; SCC, squamous cell cancer.
Late toxicity
Of the 209 patients who survived 6 months after re-RT, 166 were assessable for long-term toxicity. The overall rate of any grade 3 or greater toxicity was 31.3% (Supp. Table 1). The most common grade 3 toxicity was dysphagia requiring a percutaneous endoscopic gastrostomy (PEG) tube for nutrition in 20.4% of patients. Moderate xerostomia and neck fibrosis were common. Trismus was also common; however, only 3% of patients developed trismus significant enough to require feeding tube placement.
Seventeen percent of patients developed grade 1 or greater radiation necrosis (osteoradionecrosis, temporal lobe necrosis, or at other sites); however, most cases were asymptomatic (radiographically detected) or managed conservatively. Seven percent of all patients required surgical management for necrosis. Four percent of patients developed grade 3 or greater hearing loss and 1 patient developed unilateral blindness. Two patients developed esophageal fistulas that required reconstructive surgery. There were three grade 5 toxicities, two due to carotid injury and one secondary to osteoradionecrosis of the clivus.
Nomogram
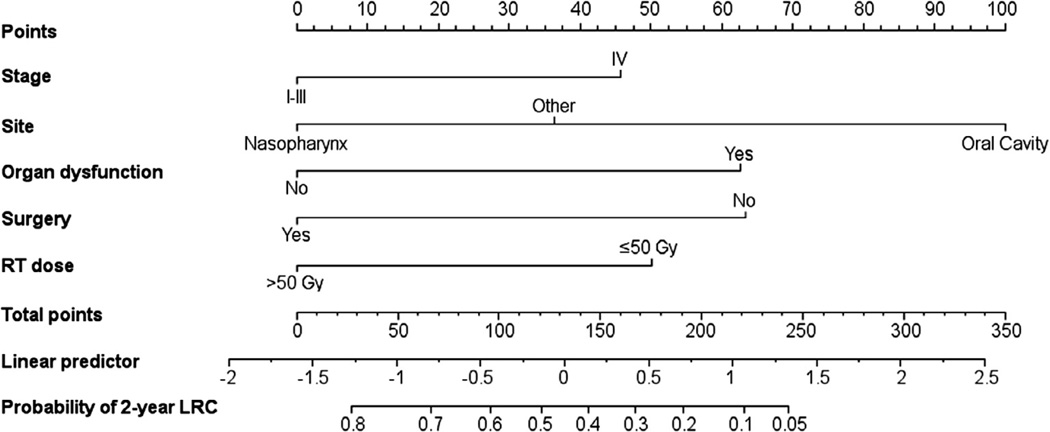
Prognostic factors significant for LRC on multivariate analysis were ranked according to predictive accuracy and the most predictive variables used to create a nomogram to predict the 2-year probability of LRC (Fig. 3). The model with greatest predictive accuracy comprised the following variables: recurrent stage, site of recurrence, presence of organ dysfunction, salvage surgery, and radiotherapy dose. The concordance index of the nomogram was 0.68 and a calibration plot demonstrated good agreement between predicted and actual probability of recurrence. In addition to using Fig. 3 to compute the probability of 2-year LRC, an excel spreadsheet is available in the Supplementary data online that can compute the probability.
Fig. 3.
The Nomogram predicts 2-year probability of loco-regional control (LRC) after re-irradiation. Stage is at time of recurrence. Organ dysfunction is defined as patients requiring feeding tube or tracheostomy, or those with soft-tissue defect, fistula, or osteonecrosis. Surgery indicates those patients who underwent surgical salvage. Radiation therapy (RT) dose indicates those who received greater than 50 Gy and/or can receive a brachytherapy boost.
Discussion
This single-institution study represents the largest HNC re-RT cohort published to date and the first study to include a majority of patients treated with modern radiation techniques. We found a 2-year LRC and OS of 47% and 43%, respectively, and a 5-year LRC and OS of 34% and 25%, respectively. Other modern re-RT series have reported 2-year OS from 15% to 58%, with the wide range secondary to differences in case mix and in frequency of surgically salvaged patients [7,9,10,17,18]. Overall, these results compare favorably to the 2-year OS results for chemotherapy alone [6]. More importantly, we now have 35 patients alive beyond 5 years, confirming that long-term survival is possible in this setting.
However, these encouraging outcomes were not achieved without considerable morbidity. Grade 3 or higher toxicity was reported in 31% of patients. The majority of late grade 3 toxicity was feeding-tube related, although 7% of patients required surgical intervention for radiation necrosis and two suffered carotid blowouts. It is difficult to directly compare toxicity rates between series due to differences in scales and reporting, but generally late grade 3 or greater toxicity appears to be decreasing with improved radiotherapy technique [26–28]. Also, as it has become clear that most failures are in-field, margin reduction with better setup techniques (i.e., image guided radiotherapy) should lead to smaller fields and further reduction in late complications [29,30].
Given the significant toxicity from treatment, identifying ideal candidates for this aggressive modality is imperative. To help guide decision-making, we sought to identify prognostic factors and develop a nomogram to identify optimal candidates for loco-regional control using re-RT. Our study showed that many disease and patient characteristics are predictive for improved LRC, and are therefore useful in selecting patients who are most likely to benefit from aggressive re-RT. These included performance status, gender, disease location, number of recurrences, organ dysfunction, and recurrent stage of disease. On multivariate analysis, organ dysfunction, location of disease, and recurrent stage all remained independently significant.
Many prognostic factors incorporated into our nomogram have been previously found to be associated with improved oncologic outcomes. Baseline organ dysfunction due to prior treatment and disease such as soft tissue defects, feeding tube dependence, and tracheostomy has been previously found to be critical [18]. Unsurprisingly, the volume of disease at time of recurrence has been previously shown to be prognostic, whether recorded by stage, axial imaging measurements, or recurrent tumor volumes [16,18,30–32]. The original location of disease is also important as others have shown the curability of recurrent nasopharyngeal cancer [14,31,32].We also found recurrent oral cavity cancer to be predictive of significantly worse outcome. Generally, beyond the patient’s functional status, interval since prior RT is one of the most common factors considered, and has been a key criterion in clinical trials [7,9,10,18]. We did not find this to be significant, most likely due to exclusion of patients with early recurrences.
Treatment-related factors also impacted outcome, with patients who were able to receive more aggressive treatment having better outcomes. Patients with resectable disease and who are able to tolerate surgery are more likely to have superior disease control and survival. Prior studies have indicated that surgical resection is an independent predictor of increased LRC and OS [14,15]. Similarly, patients who received more modern or higher-dose radiotherapy had better outcomes. Dose-comparison in this study was split at 50 Gy as done previously. Prior studies have investigated higher cut-offs, as Salama et al. described improved OS and LRC [15] and Watkins et al. found improved OS with doses >58 Gy [33].
With these patient-, disease-, and treatment-related factors, we sought to develop a tool to identify patients who would be ideal candidates for re-RT. As radiation is a local modality and we have previously demonstrated the importance of LRC to survival [14], we developed a nomogram on the probability of LRC at 2 years. Each clinical parameter in our nomogram is routinely collected clinical data easily available at time of consultation. Furthermore, as discussed above, most of the features in our nomogram have been identified in multiple previous studies as prognostic for outcome.
Our findings do have disparities with the nomogram previously proposed by Tanvetyanon et al. Isolated neck recurrence and comorbidity status were not strong predictors in our data. We did not find a significant association between LRC or OS and comorbidity (Charlson index ≥1), despite having a comparable proportion of patients with comorbid disease. This may be due to the retrospective nature of the study or the greater recurrent disease burden in our cohort at presentation [18].
One limitation of our nomogram, or any nomogram based solely on disease outcomes, is that it does not account for the risk of toxicity from re-treatment. An individual’s risk from re-RT will depend on specific radiation received previously, the location of recurrence, and the doses received by the organs at risk in the vicinity. Better estimates of the risk of toxicity to a critical structure would also more clearly help weigh the pros and cons of re-RT for an individual patient. Additionally, toxicities were evaluated based on retrospective chart review, which is likely to have resulted in underreporting of complications [34]. Despite these limitations and given the repeated difficulty in performing randomized studies in this setting, decision-making will largely have to be based on retrospective data. Lastly, although the individual predictors we considered have been used by others, we are still in the process of validating our nomogram on another cohort of patients to ensure its generalizability and at the moment it should still be considered hypothesis generating.
In conclusion, we report that re-irradiation of patients with recurrent HNC or second primaries in a previously irradiated field can be curative in a substantial number of patients, particularly if well selected. We have created a clinically useful nomogram that predicts the probability of loco-regional control, which can be used to select patients most likely to benefit from re-irradiation.
Supplementary Material
Acknowledgments
This report is not supported by specific funding;
Footnotes
Presented in part at the American Society for Radiation Oncology Annual Meeting, Boston, MA, October 28–November 31, 2012.
Conflict of interest
There are no financial disclosures from any authors.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2014.06.003.
References
- 1.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91–11. Arch Otolaryngol Head Neck Surg. 2003;129:44–49. doi: 10.1001/archotol.129.1.44. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84:1198–1205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82:291–298. doi: 10.1016/j.ijrobp.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Gomez D, Cahlon O, Mechalakos J, et al. An investigation of intensity-modulated radiation therapy versus conventional two-dimensional and 3D-conformal radiation therapy for early stage larynx cancer. Radiat Oncol. 2010;5:74. doi: 10.1186/1748-717X-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong SJ, Machtay M, Li Y. Locally recurrent, previously irradiated head and neck cancer: concurrent re-irradiation and chemotherapy, or chemotherapy alone? J Clin Oncol. 2006;24:2653–2658. doi: 10.1200/JCO.2005.05.3850. [DOI] [PubMed] [Google Scholar]
- 7.Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008;26:5518–5523. doi: 10.1200/JCO.2007.15.0102. [DOI] [PubMed] [Google Scholar]
- 8.Haraf DJ, Weichselbaum RR, Vokes EE. Re-irradiation with concomitant chemotherapy of unresectable recurrent head and neck cancer: a potentially curable disease. Ann Oncol. 1996;7:913–918. doi: 10.1093/oxfordjournals.annonc.a010793. [DOI] [PubMed] [Google Scholar]
- 9.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol. 2007;25:4800–4805. doi: 10.1200/JCO.2006.07.9194. [DOI] [PubMed] [Google Scholar]
- 10.Spencer SA, Harris J, Wheeler RH, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck. 2008;30:281–288. doi: 10.1002/hed.20697. [DOI] [PubMed] [Google Scholar]
- 11.Radiation Therapy Oncology Group: RTOG Clinical Trials Study Number 0421. n.d. [Google Scholar]
- 12.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancer. Fort Washington, PA: n.d. [Google Scholar]
- 13.Hwang JM, Fu KK, Phillips TL. Results and prognostic factors in the retreatment of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1998;41:1099–1111. doi: 10.1016/s0360-3016(98)00164-3. [DOI] [PubMed] [Google Scholar]
- 14.Lee N, Chan K, Bekelman JE, et al. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:731–740. doi: 10.1016/j.ijrobp.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 15.Salama JK, Vokes EE, Chmura SJ, et al. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2006;64:382–391. doi: 10.1016/j.ijrobp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 16.De Crevoisier R, Bourhis J, Domenge C, et al. Full-dose reirradiation for unresectable head and neck carcinoma: experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol. 1998;16:3556–3562. doi: 10.1200/JCO.1998.16.11.3556. [DOI] [PubMed] [Google Scholar]
- 17.Sulman EP, Schwartz DL, Le TT, et al. IMRT reirradiation of head and neck cancer-disease control and morbidity outcomes. Int J Radiat Oncol Biol Phys. 2009;73:399–409. doi: 10.1016/j.ijrobp.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Tanvetyanon T, Padhya T, McCaffrey J, et al. Prognostic factors for survival after salvage reirradiation of head and neck cancer. J Clin Oncol. 2009;27:1983–1991. doi: 10.1200/JCO.2008.20.0691. [DOI] [PubMed] [Google Scholar]
- 19.Hong WK, Bromer RH, Amato DA, et al. Patterns of relapse in locally advanced head and neck cancer patients who achieved complete remission after combined modality therapy. Cancer. 1985;56:1242–1245. doi: 10.1002/1097-0142(19850915)56:6<1242::aid-cncr2820560603>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Pignon JP, le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Strojan P, Corry J, Eisbruch A, et al. Recurrent and second primary squamous cell carcinoma of the head and neck: when and how to reirradiate. Head Neck. 2013 doi: 10.1002/hed.23542. Available at: http://onlinelibrary.wiley.com/doi/10.1002/hed.23542/abstract. [DOI] [PubMed] [Google Scholar]
- 22.Sciubba JJ. End of life considerations in the head and neck cancer patient. Oral Oncol. 2009;45:431–434. doi: 10.1016/j.oraloncology.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Wong LY, Wei WI, Lam LK, et al. Salvage of recurrent head and neck squamous cell carcinoma after primary curative surgery. Head Neck. 2003;25:953–959. doi: 10.1002/hed.10310. [DOI] [PubMed] [Google Scholar]
- 24.Edge S, Byrd D, Compton C, et al. AJCC cancer staging manual. 7th. New York: Springer; 2010. [Google Scholar]
- 25.National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0. 2006 ctep.cancer.gov.
- 26.Wong SJ, Bourhis J, Langer CJ. Retreatment of recurrent head and neck cancer in a previously irradiated field. Semin Radiat Oncol. 2012;22:214–219. doi: 10.1016/j.semradonc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Kharofa J, Choong N, Wang D, et al. Continuous-course reirradiation with concurrent carboplatin and paclitaxel for locally recurrent, nonmetastatic squamous cell carcinoma of the head-and-neck. Int J Radiat Oncol Biol Phys. 2012;83:690–695. doi: 10.1016/j.ijrobp.2011.06.2010. [DOI] [PubMed] [Google Scholar]
- 28.Ozyigit G, Cengiz M, Yazici G, et al. A retrospective comparison of robotic stereotactic body radiotherapy and three-dimensional conformal radiotherapy for the reirradiation of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e263–e268. doi: 10.1016/j.ijrobp.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 29.Popovtzer A, Gluck I, Chepeha DB, et al. The pattern of failure after reirradiation of recurrent squamous cell head and neck cancer: implications for defining the targets. Int J Radiat Oncol Biol Phys. 2009;74:1342–1347. doi: 10.1016/j.ijrobp.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen AM, Farwell DG, Luu Q, et al. Prospective trial of high-dose reirradiation using daily image guidance with intensity-modulated radiotherapy for recurrent and second primary head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:669–676. doi: 10.1016/j.ijrobp.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Yu KH, Leung SF, Tung SY, et al. Survival outcome of patients with nasopharyngeal carcinoma with first local failure: a study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Head Neck. 2005;27:397–405. doi: 10.1002/hed.20161. [DOI] [PubMed] [Google Scholar]
- 32.Koutcher L, Lee N, Zelefsky M, et al. Reirradiation of locally recurrent nasopharynx cancer with external beam radiotherapy with or without brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76:130–137. doi: 10.1016/j.ijrobp.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 33.Watkins JM, Shirai KS, Wahlquist AE, et al. Toxicity and survival outcomes of hyperfractionated split-course reirradiation and daily concurrent chemotherapy in locoregionally recurrent, previously irradiated head and neck cancers. Head Neck. 2009;31:493–502. doi: 10.1002/hed.20986. [DOI] [PubMed] [Google Scholar]
- 34.Papanikolaou PN, Christidi GD, Ioannidis JP. Comparison of evidence on harms of medical interventions in randomized and nonrandomized studies. CMAJ. 2006;174:635–641. doi: 10.1503/cmaj.050873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.