Abstract
Persistent infection by EBV is explained by the germinal center model (GCM) which provides a satisfying and currently the only explanation for EBVs disparate biology. Since the GCM touches on every aspect of the virus, this chapter will serve as an introduction to the subsequent chapters. EBV is B lymphotropic, and its biology closely follows that of normal mature B lymphocytes. The virus persists quiescently in resting memory B cells for the lifetime of the host in a non-pathogenic state that is also invisible to the immune response. To access this compartment, the virus infects naïve B cells in the lymphoepithelium of the tonsils and activates these cells using the growth transcription program. These cells migrate to the GC where they switch to a more limited transcription program, the default program, which helps rescue them into the memory compartment where the virus persists. For egress, the infected memory cells return to the lymphoepithelium where they occasionally differentiate into plasma cells activating viral replication. The released virus can either infect more naïve B cells or be amplified in the epithelium for shedding. This cycle of infection and the quiescent state in memory B cells allow for lifetime persistence at a very low level that is remarkably stable over time. Mathematically, this is a stable fixed point where the mechanisms regulating persistence drive the state back to equilibrium when perturbed. This is the GCM of EBV persistence. Other possible sites and mechanisms of persistence will also be discussed.
1 Introduction
Persistent latent infection for the lifetime of the host is a defining feature of herpesviruses. Each herpesvirus has a target tissue(s) in which it persists and each has evolved a strategy for getting there and back out again. Once at the site of persistent latent infection, the strategies coalesce in the sense that the goal is to persist latently within a very small number of cells and to minimize or eliminate viral gene expression, at least at the protein level. This in turn allows the virus to evade immune regulation and persist with minimal impact on the host where it will stay for the rest of its life. Acute infection and viral reactivation to allow spread to new hosts similarly seem to have evolved for minimal impact on the host. Acute infection should occur in childhood and is often silent. It is not a coincidence that some of the human herpesviruses are so benign and non-pathogenic that they went unnoticed until the age of AIDS where chronic immunosuppression revealed their presence.
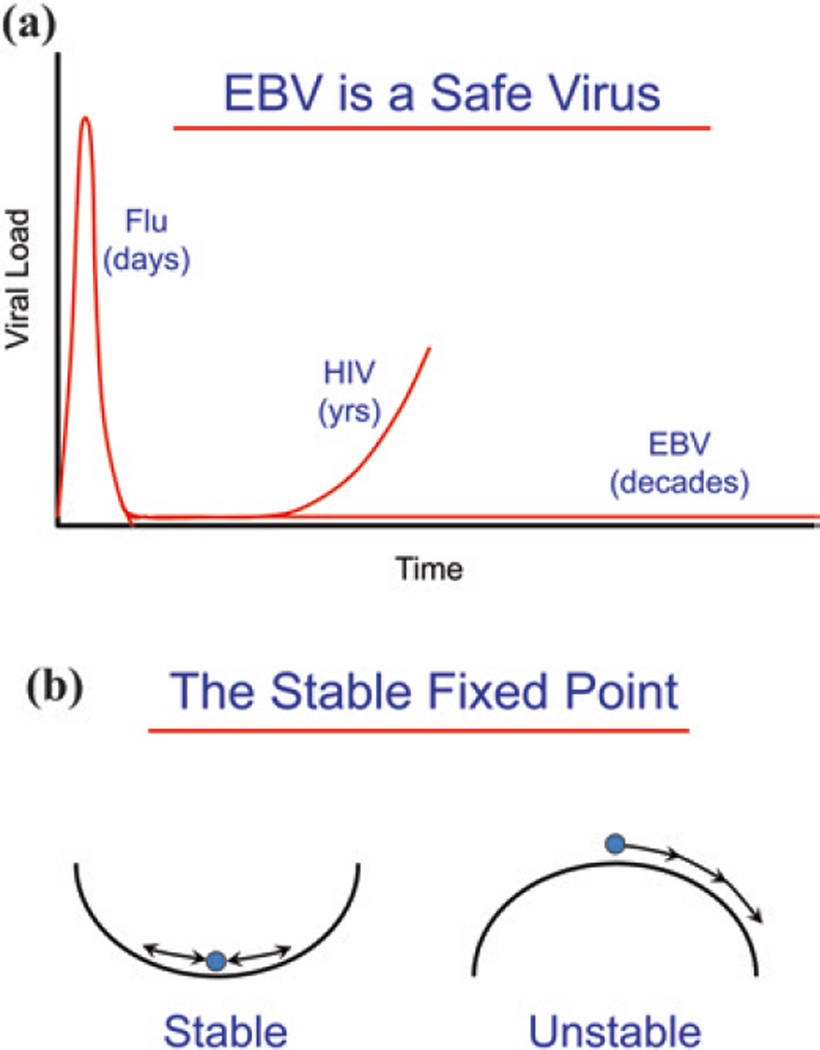
Usually, in the struggle between virus and host, one or the other wins—if it is the host, the virus is eliminated, for example influenza. Flu goes through an acute viremic stage and then is cleared within a week or two (Fig. 1a). If the virus wins, then the host dies, for example HIV. HIV also has an acute viremic stage but resolves into a low-level infection. However, this is unstable and the virus eventually returns to kill the host. EBV also has an acute viremic stage that resolves into a low-level infection, but unlike HIV the virus then simply persists stably at this very low level (something like 1 infected cell per 5 ml of blood) for the lifetime of the host (Hadinoto et al. 2009; Khan et al. 1996; Thorley-Lawson and Allday 2008). Mathematically, this is referred to as a stable fixed point. Dynamically, it is a situation that requires the mechanisms regulating the state (persistent infection) to drive it back to the fixed point whenever it is perturbed (Fig. 1b). Biologically, i.e., in the presence of perturbations, a stable fixed point is the only way to achieve stable long-term behaviors.
Fig. 1.
EBV establishes a stable, benign, low-level, lifetime persistent infection. a EBV is a safe virus. EBV establishes a persistent, benign infection in virtually every human being for their entire life. This is in comparison with a virus like flu whose infection resolves in a few days or HIV which undergoes an acute infection that resolves into a long-term low-level persistent infection that eventually returns to kill the host. EBV also undergoes acute infection but then enters into a low-level persistent infection which remains stable for the life of the host. b The stable fixed point. The type of equilibrium EBV achieves is referred to mathematically as a stable fixed point. This means that the forces regulating the system act to return it to the same place after perturbation, e.g., a marble in the bottom of a bowl, whereas in an unstable fixed point, small perturbations irrevocably destroy the fixed point, e.g., a marble on top of the bowl. In real-life biology, where there are always perturbations, the only way to achieve long-term stability is through a stable fixed point
EBV is a paradigm for studying the mechanism by which persistent infection is maintained in vivo. It is an unlikely candidate for this status. We lack an in vitro lytic system that would allow viral genetics to be studied—the production of a single viral mutant is a laborious and technically challenging task (Delecluse and Hammerschmidt 2000). Certainly, no system exists for screening large numbers of viral variants and selecting mutants of choice. For a detailed discussion on the production of EBV recombinants, see the chapter authored by Henri-Jacques Delecluse. Similarly, we lack a malleable animal model to perform these studies. The animal models available are limited to primates which are expensive, difficult to work with, and lacking in sophisticated reagents (Wang 2013) and mouse models. For a detailed discussion of primate models, see the chapter authored by Fred Wang, and for mouse models, see the chapter authored by Christian Munz. Mouse models fall into two classes: reconstitution of genetically immunocompromised mice with human cells (Chatterjee et al. 2014) and studies on the murine gammaherpesvirus MHV68 (Barton et al. 2011). Of the two systems, the latter has proved highly tractable for studying and analyzing, at the molecular, genetic, and immunological level, the basis and details of persistent infection by a gammaherpesvirus. Of the human herpesviruses, EBV is the most amenable to study in vivo because it infects readily accessible tissue, namely B lymphocytes of the lymphoid tissue (tonsils) and peripheral blood. With the advent of sophisticated and sensitive flow cytometric techniques to characterize lymphoid populations and PCR to detect very rare infected cells and their gene expression, EBV became accessible for in vivo study.
2 The Germinal Center Model (GCM) of EBV Persistence—A Historical Perspective
Epstein-Barr virus was discovered in Burkitt’s lymphoma in 1964. It is a reflection of the complex and subtle biology of the virus that 50 years later, we are only just beginning to understand the role of the virus in the development of this tumor (Speck 2002; Thorley-Lawson and Allday 2008; Vereide and Sugden 2009). By 1999, a large body of work had been accumulated pertaining to EBV’s molecular and cellular biology, immunology, virology, epidemiology, clinical manifestations, and disease associations. However, this work existed as a series of independent pieces of information that did not hang together in a consistent way to explain viral biology and persistence [for a discussion of the specific issues, see Thorley-Lawson (2005)].
For example, it has long been known that, unlike most other human herpesviruses, EBV is able to establish latent persistent infection in tissue culture (Henle et al. 1967; Pope et al. 1968). The sine qua non of EBV infection in vitro is that the virus always persists latently in proliferating B lymphoblasts whose growth is driven by viral latent proteins. This process is often referred to as “immortalization.” However, an apparent contradiction arose when it was discovered that the virus did not persist in this state in vivo but in a diametrically opposite type of cell, namely quiescent, resting memory B cells where viral protein expression has been extinguished (Babcock et al. 1998; Hochberg et al. 2004; Miyashita et al. 1997).
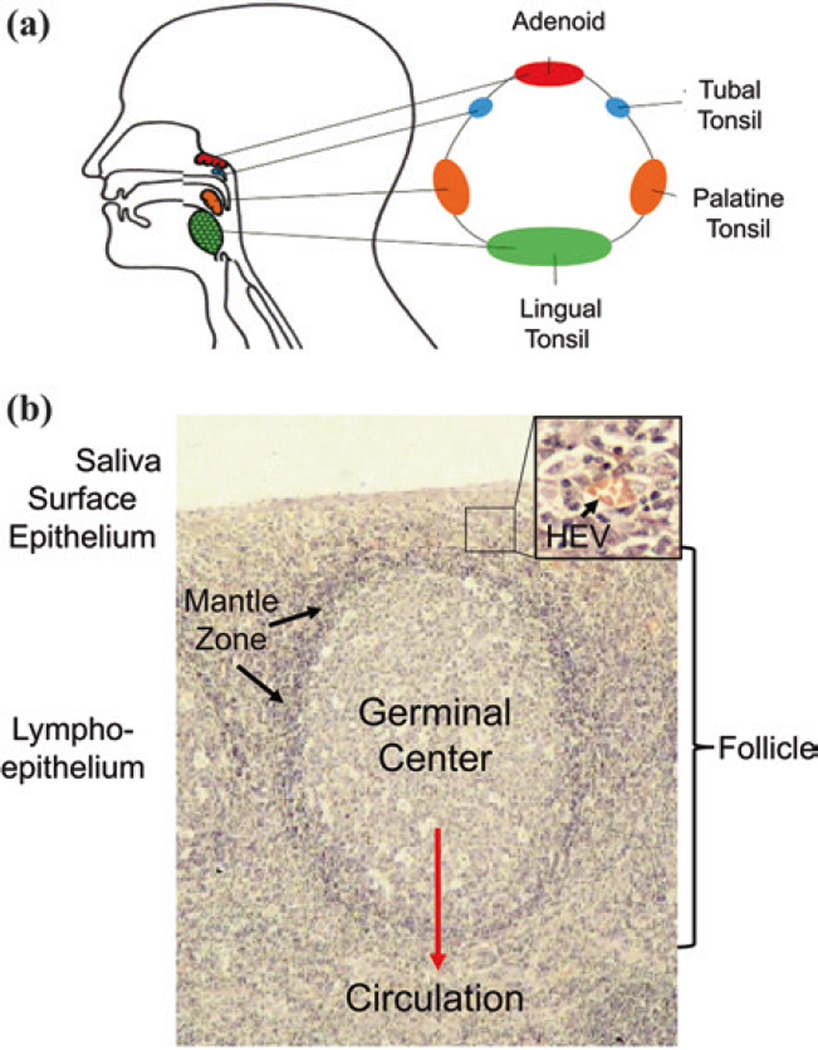
The GCM arose to resolve this contradiction (Thorley-Lawson and Babcock 1999) and in doing so provided a way to understand the complex biology of EBV. It has stood for 15 years and many tests of its reliability and predictive power (Thorley-Lawson et al. 2013). To date, it remains the only model that consistently provides a conceptual framework for understanding the complex and subtle behaviors of the virus (Thorley-Lawson and Allday 2008; Thorley-Lawson and Gross 2004). It is built on the simple idea that the virus uses the normal pathways of B cell biology in the lymphoid tissue of Waldeyer’s ring (tonsils and adenoids) (Fig. 2) to establish infection, persist, and replicate. Today, the questions that arise are not as to the validity of the general model but the extent to which the virus goes along for the ride or actively manipulates the process and whether there are additional mechanisms/sites of viral persistence.
Fig. 2.
The lymphoepithelium of the tonsil where EBV performs its biology. a Waldeyer’s ring consists of the adenoids and tonsils which form a ring of lymphoid tissue at the back of the throat. b The structure of the lymphoepithelium underlying the saliva. Inset is an expanded view of the marginal zone/epithelium. B cells exit the circulation and enter the lymphoid tissue through the HEV and migrate to the mantle zone of the follicle. Here, they reside for a period of time and then either leave or, if they see antigen, enter the follicle to undergo a GC reaction which produces memory cells that can then enter the peripheral circulation. This is the B cell system that EBV exploits. For more details, see Figs. 3 and 4 and text (Figure provided by Marta Perry)
3 EBV Infection in the Healthy Host—A Summary of the GCM
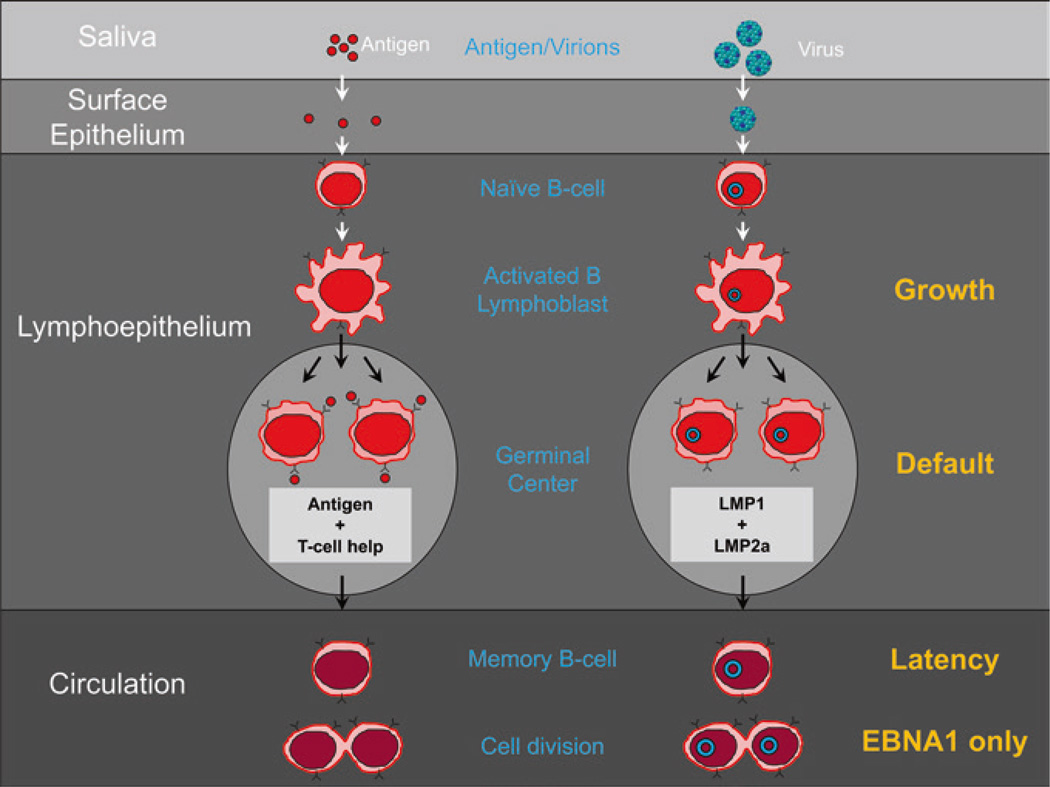
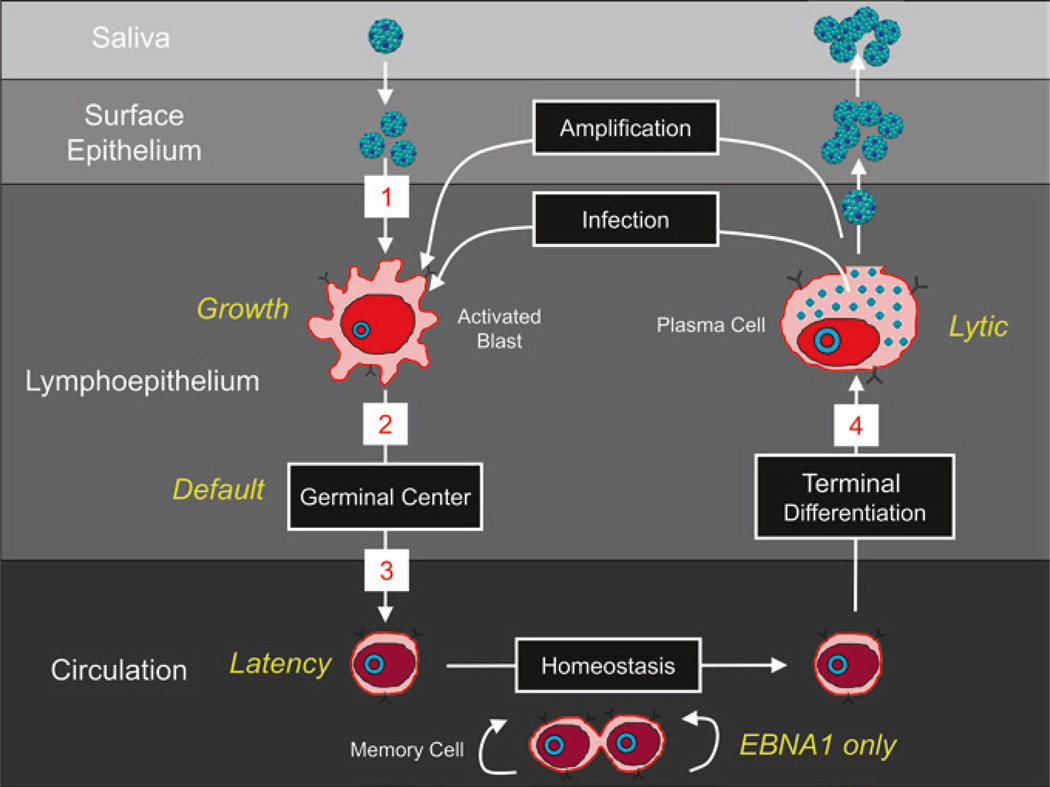
A sea change in thinking about EBV was the recognition that under normal conditions, it should not be thought of as an oncogenic virus. This despite its discovery in and association with tumors and its ability to latently infect B cells in culture and continuously drive their proliferation. The essence of its biological behavior is that it initiates, establishes, and maintains persistent infection by subtly using various aspects of normal B cell biology and has evolved to minimally perturb the normal behavior of the infected B cells. A summary of normal mature B cell biology and the parallels with EBV is given in Fig. 3, and a full description of the GCM is presented in Fig. 4. A summary of the steps from Fig. 4 is as follows:
Oral antigens enter in saliva, are sampled by the epithelium of Waldeyer’s ring, and then presented to naïve B cells in the underlying lymphoid tissue (Fig. 3). When the naïve B cells see cognate antigen, they become activated into a proliferating blast. Similarly, EBV is spread through saliva contact and crosses the epithelial barrier of Waldeyer’s ring to interact with naive B cells. Upon infection of the naïve B cell, it drives the infected cell to become a proliferating blast using the growth transcription program (a summary of the viral transcription programs is provided in Table 1).
Antigen-activated naïve blasts migrate into the follicle to initiate a GC reaction where survival of the B cell requires signals from cognate antigen and antigen-specific helper T cells. Similarly, EBV-infected naïve blasts migrate into the follicle where they switch their transcription program to the default program which provides surrogate antigen and T cell help signals.
A successful, antigen-specific, GC B cell leaves the GC to enter the memory compartment as a resting, long-lived, memory B cell which is sustained through occasional homeostatic driven division. Similarly, the latently infected GC B cells leave the follicle as resting memory B cells which are quiescent with respect to viral latent protein expression (the latency transcription program). These cells occasionally divide in the periphery. Proliferation is not driven by the virus but by the normal memory homeostatic mechanisms. At this time, the virus expresses the genome tethering protein EBNA1 which allows the viral genome to replicate with the cells (EBNA1 only program).
Antigen-specific, memory B cells in lymphoid tissue can be signaled by cognate antigen to terminally differentiate into plasma cells and produce antibody. Similarly, if an infected, resting, memory B cell latently infected with EBV returns to Waldeyer’s ring and receives signals that initiate terminal differentiation, it will trigger the release of infectious virus. The released virus can initiate a new round of naïve B cell infection or infect the epithelium. This results in transient plaques of lytic epithelial infection that greatly amplifies the amount of infectious virus that ultimately is shed into saliva for infectious spread to new hosts.
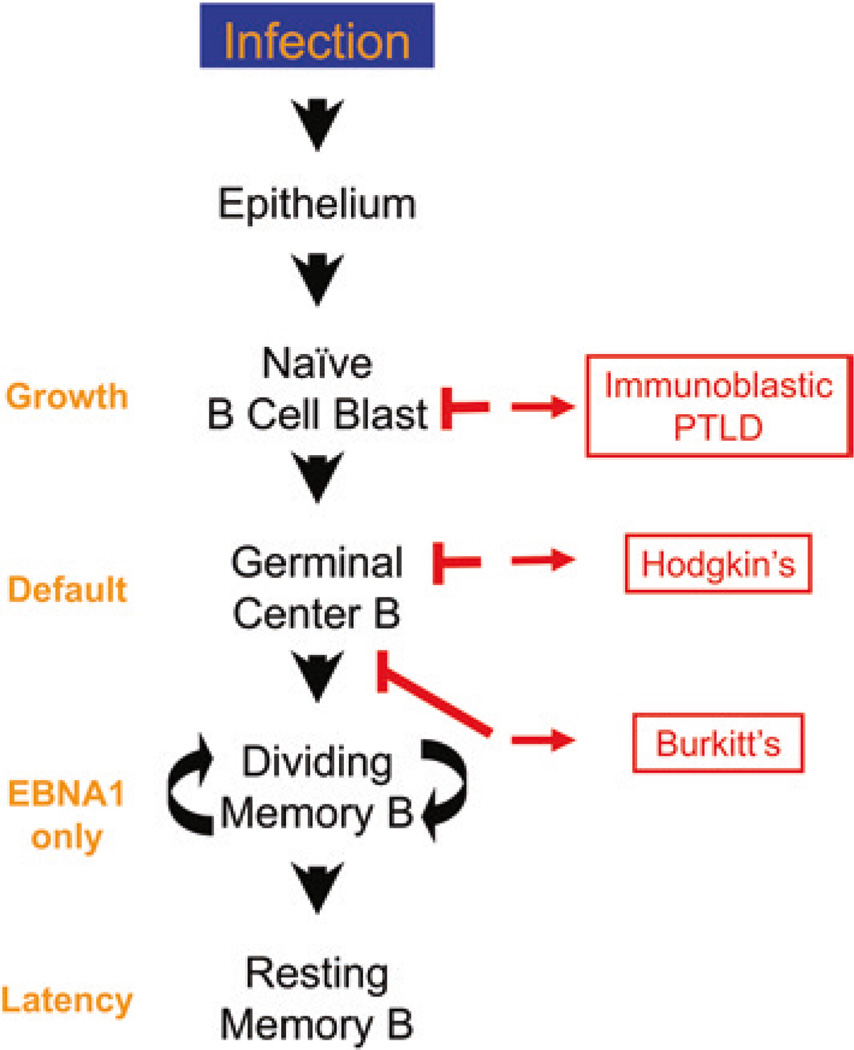
In this model, EBV gene expression is tightly regulated in a tissue-specific fashion. Dysregulation can lead to lymphomas which arise from each of the three proliferative stages of EBV infection predicted by the model. It is the context and location combined with the stage-specific viral transcription program that defines the lymphoma (Fig. 5). These are immunoblastic lymphoma (IL) from cells expressing the growth program (new infection), Hodgkin’s disease (HD) from cells expressing the default program (GC cells), and Burkitt’s lymphoma (BL) from cells expressing EBNA1 only (late GC cell).
Fig. 3.
EBV biology mirrors B cell biology. To the left is diagrammed a typical mucosal humoral immune response. Antigen in saliva crosses the epithelial barrier of the tonsil to be sampled by naïve B cells in the underlying lymphoid tissue. When naïve B cells recognize cognate antigen, they become activated blasts and migrate to the follicle to undergo a GC reaction. If they receive signals from antigen and antigen-specific Th cells, they can leave to become resting memory B cells that occasionally undergo division as part of memory B cell homeostasis. To the right is diagrammed how EBV uses the same pathways. EBV is spread through saliva, crosses the epithelial barrier, and infects naïve B cells. These become B cell blasts that enter the GC. Here, the viral latent proteins LMP1 and LMP2 have the capacity to provide surrogate antigen and Th survival signals that allow the latently infected B cells to leave the GC as resting memory cells that also divide through homeostasis. To the right are listed in orange the transcription programs used at each stage. The blue circles represent the viral DNA which is a circular episome
Fig. 4.
The germinal center model (GCM) of EBV persistence. The stages 1–4 follow those in the text from Sect. 3. “EBV Infection in the healthy host—a summary of the GCM.” For details, see the text
Table 1.
The latency transcription programs of EBV in vivo
| Program | Alternate | Site in vivo | Lymphoma | Expressed Proteinsa | ||||
|---|---|---|---|---|---|---|---|---|
| Growth | Latency 3 | Tonsil Naïve B | Immunoblastic | EBNAl (Cp) | EBNA2 | EBNA 3A–C | LMP1 | LMP2 |
| Default | Latency 2 | Tonsil GC B cell |
Hodgkin’s | EBNAl (Qp)b | LMP1 | LMP2 | ||
| EBNAl Only |
Latency 1 | Periphery dividing memory B |
Burkitt’s | EBNAl (Qp)b | ||||
| Latency | Latency 0 | Periphery resting memory B |
||||||
The non-coding RNAs which includes EBERs and the BART miRNAs are expressed in all normal and tumor cells irrespective of transcription program. The exception is a subset of BART miRNAs that are absent from the GC and memory compartments.
EBNAl is expressed from the Cp promoter in the growth program but a different promoter (Qp) in the default and EBNAl only programs.
Fig. 5.
The origin of EBV-positive lymphomas. EBV lymphomas arise from different stages of the infection process. The figure shows diagrammatically the flow of virus from infectious virions to latently infected resting memory B cell as detailed in Figs. 3 and 4 and the text. To the right are shown the 3 EBV-associated lymphomas and their proposed origin and to the left are listed the viral transcription programs expressed in the tumors and at the equivalent stage of infection. IL is proposed to arise from a latently infected blast that is unable to differentiate and so continues to proliferate. HD is derived from a GC B cell, and BL is a GC cell that has left the follicle. Note that a tumor is proposed to arise from each of the three stages of EBV biology that involve proliferation
The following sections will discuss evidence and relevant information for each of these 4 steps in more detail.
3.1 Crossing the Epithelial Barrier and the Activation/Infection of naïve B Cells
3.1.1 Crossing the Epithelial Barrier
It is generally believed that EBV is spread through salivary contact (Hoagland 1955) and that the virus enters through the epithelium that lines the nasopharynx. The lymphoid system that surrounds the nasopharyngeal region includes the adenoids and tonsils and is called Waldeyer’s ring (Fig. 2a). Together with the overlying epithelium, it forms a continuous structure referred to as the lymphoepithelium (Fig. 2b) (Perry and Whyte 1998). The epithelium is invaginated to form crypts below which resides the lymphoid tissue (Perry 1994; Tang et al. 1995). Deep in the crypts, the epithelium can be only a single epithelial cell in thickness. Environmental antigens are sampled directly through the epithelium (Perry and Whyte 1998; Brandtzaeg et al. 1999a, b). The involuted nature of the crypts allows for a massive surface area for detecting antigens as they come in with food and, when exposed to EBV-bearing saliva, provides a large target for EBV infection. It is likely that the virus, in saliva, enters the crypts and crosses the thin layer of epithelial cells to infect naïve B cells that reside below. The sponge-like nature and deep invaginations of the crypts ensure that all of the lymphocytes in the underlying lymphoid tissue are effectively close to the surface where EBV crosses the epithelium. How the virus crosses the epithelial barrier is unclear although there is evidence that the virus may cross passively via transcytosis (Tugizov et al. 2013). It has been speculated that the virus actually infects the epithelial cells, replicates, and then is released to infect B cells in the underlying areas, but there is no direct evidence for this and epithelial cells appear to be resistant to infection from the apical (i.e., mucosal) side (Tugizov et al. 2003).
3.1.2 The Activation/Infection of Naïve B Cells
As far as we know whenever EBV encounters and infects a resting B cells, it always latently infects it and uses the growth program to drive that cell to become a proliferating lymphoblast (Thorley-Lawson and Mann 1985). Phenotypically, the newly infected B cells look remarkably like antigen-activated B cell blasts (Thorley-Lawson et al. 1982, 1985; Nilsson 1979); however, in this case, the B cell is activated not through interaction with antigen and T cell help but through the activity of the latent proteins encoded by the growth program (Kempkes et al. 1995). The population that expresses the growth program in the tonsils of healthy carriers of the virus is activated, naive B cells (Joseph et al. 2000a; Babcock et al. 2000). These cells express CD19 (B cell lineage marker) CD23 and CD80 (B cell activation markers) and IgD (a marker of naïve B cells) and all of the latent proteins associated with the growth program. They lack CD10 (GC cell marker) and CD27 (memory B cell marker). Therefore, the target of the incoming virus is the resting naive B cell. This is the first example we will encounter of a latent gene transcription program used in lymphoma, being found in a normal infected B cell counterpart in vivo. In this case, the growth program, which is used in immunoblastic lymphoma (IL), is found in newly infected naïve B cell blasts (Table 1, Fig. 5).
Naïve B cells continuously recirculate throughout the body. They extravasate from the peripheral circulation into secondary lymphoid tissue such as the tonsils through specialized structures called high endothelial vesicles (HEVs) which reside in the lymphoepithelium (inset in Figs. 2b and 6). The naïve B cells migrate through the epithelium to the mantle zone (Fig. 2b) of the follicles which resides just below the epithelium. They remain there for a few days and then reenter the circulation (Brandtzaeg et al. 1999a) unless they encounter cognate antigen in which case they migrate into the follicle.
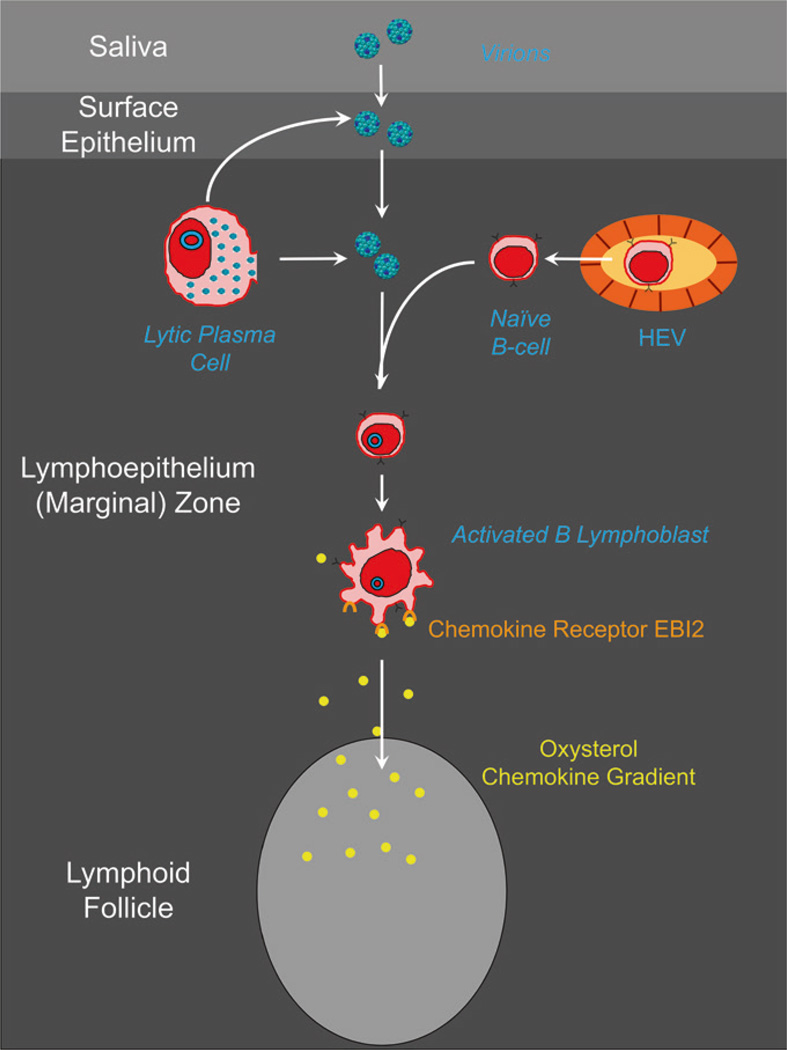
Fig. 6.
The first steps of EBV infection. Naïve B cells emerge from the HEV and migrate toward the mantle zone of the follicle. On the way, they encounter EBV that either has crossed the epithelial barrier or is derived from lyrically infected plasma cells. The newly infected lymphoblast upregulates the chemokine receptor EBI2 and follows a gradient of oxysterol chemokine into the follicle
The migration of naïve B cells from HEV in the epithelium to the mantle zone is critical for them to become exposed to the virus. This is because microdissection studies reveal that virus production and infection of new naive B cells occur in the intraepithelial layer not the mantle zone (Roughan et al. 2010). Thus, naive B cells are becoming infected, as they traverse the epithelium, by EBV that has either crossed the epithelial barrier during primary infection or been produced by the lymphoepithelium during persistent infection (Fig. 6). It follows that by the time the infected B cell arrives at the follicle, it will already be a blast so will not migrate to the mantle zone.
3.1.3 The Growth Program
Because the target for EBV infection is a resting cell, the virus must initiate latent gene transcription in a quiescent environment. It infects cells through the interaction of the viral glycoproteins gp350/220 with CD21 (Nemerow et al. 1985; Fingeroth et al. 1984) and gp42/gH/gL with MHC class II on the B cell (Li et al. 1997). For a detailed discussion of viral entry, see the chapter authored by Lindsey Hutt-Fletcher. CD21 is a receptor for C3d (a component of complement) and forms part of a multimeric signal transduction complex with CD19, CD81 (TAPA-1), and Leu-13 (Matsumoto et al. 1993). The high density of gp350/220 on the virion (Thorley-Lawson and Poodry 1982) ensures that the binding of viral particles will cause extensive cross-linking of the CD21 signaling complex which provides the signal to begin moving the resting B cells from G0 into the G1 phase of the cell cycle (Sinclair and Farrell 1995). During this time, the earliest expressed latent protein (EBNA2) is detected (Allday et al. 1989; Rooney et al. 1989). This protein is expressed from a promoter (Wp) that is present in multiple copies in the viral genome and may be designed to function in the transcriptionally sparse environment of a resting B cell (Woisetschlaeger et al. 1990). EBNA2 drives the cells through the first G1 (Sinclair et al. 1994). EBNA2 is a transcription factor that activates the promoters necessary to produce all nine of the latent proteins expressed in the growth program [reviewed in Kieff and Rickinson (2007)]. For a detailed discussion of EBNA2, see the chapter authored by Bettina Kempkes. At this point, transcription of the EBNA2 gene switches from Wp to Cp (Woisetschlaeger et al. 1990), a promoter that works optimally in B lymphoblasts and allows expression of all the EBNA proteins. The result is that infected normal B cells become activated lymphoblasts and begin to proliferate in response to the actions of viral latent proteins. Although they should not be thought of as classically transformed cells, such as are obtained with other DNA tumor viruses (e.g., SV40, papillomavirus, and adenovirus) (Allday et al. 1995), EBV-driven cells are not completely normal either as evidenced by deregulation of their cell cycle control that can result in immortal growth in culture (O’Nions and Allday 2003; Wade and Allday 2000) [reviewed in Allday (2013), O’Nions and Allday (2004)]. Thus, they rather should be thought of as undergoing a hyperplastic or preneoplastic proliferation that can develop into full-blown neoplasia if allowed to proceed unchecked and accumulate additional oncogenic mutations. However, at this point, it is necessary to mention an important caveat to these studies. Almost all have been conducted with the B95-8 strain of EBV that is often referred to as the “wild-type” “strain.” In fact, this is not a wild-type strain, but a highly defective laboratory strain that is carried in marmoset cells and was selected for its ability to transform those cells in culture and make them oncogenic in marmosets, not a natural host for the virus. This virus has multiple genomic deletions (Raab-Traub et al. 1980) among which are those that deregulate expression of the major glycoproteins (Edson and Thorley-Lawson 1981) and delete virtually all of the miRNAs (Skalsky et al. 2012). The latter, in particular, are of concern for interpreting studies on how EBV makes B cells grow and how and to what extent the virus deregulates cell cycle controls.
The nine latent proteins of the growth program include six nuclear proteins (EBNAs—Epstein-Barr virus nuclear antigens—1, 2, 3a, 3b, 3c, and LP) and three membrane proteins (LMPs—latent membrane proteins) [reviewed in Kieff and Rickinson (2007) but see this textbook for the most recent information]. Several of the latent proteins have potent growth-promoting activity and can act as oncogenes. These include EBNA2 (Kempkes et al. 1995), EBNA3a (Hickabottom et al. 2002), EBNA3c (Parker et al. 1996), and LMP1 (Wang et al. 1985).
In addition to the nine latent proteins, EBV-infected lymphoblasts express two small non-polyadenylated RNAs, termed EBER1 and EBER2 (Arrand and Rymo 1982), and ~40 microRNAs. Neither EBERs nor the miRNAs are essential for EBV infection in vitro, suggesting that their functions are most important in vivo (Kuzembayeva et al. 2014). For a detailed discussion of EBV-encoded non-translated RNAs, see the chapter authored by Bryan Cullen.
The latent genes are transcribed from the viral genome which exists as a covalently closed episomal circle (Adams and Lindahl 1975). For a detailed description of genomic structure, see the chapter authored by Paul Farrell. The linear genome from the virion forms this circle when the newly infected cell begins proliferating (Hurley and Thorley-Lawson 1988). Interestingly, only a single episome forms upon initial infection, but this then begins to amplify over time as the infected cells proliferate till a steady-state distribution of episomes is found in cells that have proliferated extensively (Hurley and Thorley-Lawson 1988; Roughan et al. 2010). The forces that produce this distribution are not well understood (Nanbo et al. 2007), but it serves as a useful marker to distinguish cells that have been recently infected from ones that have proliferated extensively. Thus, the status of the viral genome in a tissue provides a considerable amount of useful information. Linear genomes indicate viral replication, whereas episomal genomes, in the absence of linear genomes, are indicative of latently infected cells (Decker et al. 2001) and the episomal copy number is a measure of proliferation history (Roughan et al. 2010).
The viral growth program has evolved to drive the activation and proliferation of new latently infected human B cells. It achieves this, not through some rare random event, such as the integration of the viral genome and disruption of cellular genes employed by retroviruses, but by a highly intricate transcriptional program that is uniquely designed to control the growth of human B cells. This ensures that EBV will efficiently and predictably establish latency and initiate cell growth whenever it encounters a resting naive B cell in the lymphoepithelium of the nasopharynx. This program puts the host, in which the virus intends to persist, at risk for developing neoplastic disease (see Sect. 6.3.1), but it is essential, so the virus can drive the newly infected cell into a state, the proliferating blast, from where it can differentiate into a resting memory B cell. Once there, the virus can shut down, become non-pathogenic, and persist for the life of the host. How does an antigen-activated B blast and, by analogy, the EBV-infected B blast become a resting memory B cell?
3.2 Migration to the Follicle and the Germinal Center (GC) Reaction
To understand how latently infected, naive B lymphoblasts expressing the growth program can become resting memory B cells, with no viral gene expression, it is first necessary to describe how a normal naive B cell blast becomes a memory cell.
3.2.1 Entering the Follicle
Naïve B cells, activated by antigen, migrate toward the GC following a gradient of the oxysterol lipid 7a,25-dihydroxycholesterol. This lipid is produced by follicular lymphoid stromal cells and is recognized by the chemokine receptor EBI2, also known as G protein-coupled receptor 183, on the activated B cell (Gatto and Brink 2013). When EBV activates the newly infected naïve B cell with the growth program, one of the phenotypic changes it causes involves induction of EBI2 (Birkenbach et al. 1993), thus insuring that the virus-infected blasts will migrate toward the follicle (Fig. 6).
3.2.2 The Germinal Center Reaction
Once an antigen-specific B cell enters the follicle as an activated blast, it undergoes a period of rapid expansion for about 3 days, with a cell division time ~8–12 h to form the GC which consists of antigen-specific B cells (Figs. 2 and 3) [reviewed in Liu and Arpin (1997), MacLennan (1994), Victora and Nussenzweig (2012)]. These cells loose surface IgD and acquire GC-specific markers including CD10, CD77, and CD38 and they express AID and bcl-6. AID is an enzyme of the APOBEC family that is highly expressed in GCs. It is the enzyme necessary to initiate somatic hypermutation (SHM) and class switch recombination (CSR) (Muramatsu et al. 2007), functions of the GC. bcl-6 on the other hand is the master transcription factor of the GC (Basso and Dalla-Favera 2010). Its expression is restricted to GC cells (Cattoretti et al. 1995), it is required for GC production (Ye et al. 1997), and its downregulation is essential for B cells to leave the GC (Calame et al. 2003). When proliferating, the cells reside in the dark zone (DZ) of the germinal center and are referred to as centroblasts. Here, the cells undergo CSR to express a single isotype, which can be IgM, IgG, IgA, or IgE and they also undergo SHM. After several divisions, the cells rest and migrate to the light zone (LZ) of the GC. These cells are referred to as centrocytes, and they compete for help delivered by antigen-specific T helper (Th) cells (Schwickert et al. 2011). The Th cell delivers its rescue signal to the B cell through the interaction of CD40 ligand on Th cells with CD40 on B cells (Banchereau et al. 1994). Signaling through CD40 also turns off expression of bcl-6 and turns on bcl-2 which allows the cell to leave the GC and differentiate (Calame et al. 2003).
Cells in the GC go through multiple rounds of proliferation, migration, and selection so that ultimately those expressing the highest affinity B cell receptor (BCR) are selected—a process referred to as affinity maturation. Migration between the light and dark zones is controlled through the expression of specific chemokine receptors CXCR4 and CXCR5 and their cognate ligands (SDF1 and BLC, respectively) (Allen et al. 2004). The cells that survive ultimately have two fates depending on the length and type of exposure to Th cells and specific lymphokines (Banchereau et al. 1994). They can either terminally differentiate into antibody-secreting plasma cells or enter the long-lived memory compartment as resting isotype-switched memory B cells. As the name implies, these cells carry immunological memory and are responsible for a heightened secondary response upon reexposure to the specific antigen.
Unswitched, IgM+/IgD+, memory cells also exist, but they do not arise through the GC (Weill et al. 2009; Weller et al. 2004). These are generally referred to as marginal zone memory B cells because they were originally described in the marginal zone of the spleen (Spencer et al. 1985, 1998) and in the circulation (Weller et al. 2004). A phenotypically related population has also been described in the epithelium of the tonsil (Dono et al. 2003; Spencer et al. 1998); however, they appear to be functionally distinct (Weill et al. 2009).
What is clear then is that a series of events must occur if an EBV-infected naive B lymphoblast, expressing the growth program, is to become a memory cell. First, the cells should enter the GC where the latent genes that drive proliferation are turned off, and then the cells must receive the requisite survival signals and finally leave as resting memory B cells.
3.2.3 EBV-infected Cells Reside and Participate in the GC
Newly infected B cells are driven by the growth program to undergo an initial phase of rapid expansion with a division time of ~8 h for ~3 days—closely mimicking the dynamics of the early phase of GC development (Nikitin et al. 2010; Thorley-Lawson and Strominger 1978). In vitro, such cells then switch to long-term indefinite proliferation as lymphoblasts with a division time of ~24 h. However, in vivo, the cells do not continue to proliferate driven by the growth program; instead, they become GC cells and switch to a more limited form of viral gene expression—the default program.
Cells in the GC latently infected with EBV are by all measures true GC B cells. They express the classic GC surface phenotype CD10+, CD77+, CD38+, the functional markers AID and bcl-6 (Roughan and Thorley-Lawson 2009), and the correct set of chemokine receptors being CXCR4+ CXCR5+ and CCR7−. The latter ensure that the cells will be retained in and migrate throughout the germinal center. They are positive for the proliferation marker Ki67 and undergo multiple rounds of cell division (≥20) (Roughan et al. 2010). Despite this, microdissection studies revealed that there are only on average 3–4 latently infected cells per GC (for reference, there are about 105 total B cells in a typical GC). Consequently, the vast majority of latently infected cells produced from the GC must die; otherwise, the memory compartment would be overwhelmed. This death could represent some version of affinity maturation/selection (if the emerging memory cells are truly antigen-selected) or simply destruction by CTL. However, functional CTLs do not appear to enter GCs (Quigley et al. 2007), so the cells would have to be continuously leaving and then killed.
Taken together, these data imply that latently infected B cells in the GC are truly undergoing a GC reaction, that the virus is having a minimal impact on the process and the cells may even be undergoing some form of affinity maturation and selection. Confirmation of this mechanism has come from studies with another B lymphotropic gammaherpesvirus: MHV68 in the mouse. The ability to genetically manipulate both host and virus in this system has allowed for a direct and convincing demonstration that latently infected B cells traverse the GC in order to enter memory (Barton et al. 2011; Collins and Speck 2014).
3.2.4 EBV-Infected Cells in the GC Express the Default Not the Growth Program
Microdissection and flow cytometric analysis have provided compelling and unequivocal evidence that the EBV-infected cells in the GC express the default program not the growth program (Babcock et al. 2000; Roughan and Thorley-Lawson 2009). The demonstration that infected GC cells express the default program means that this latency transcription program is consistent with the retention of GC phenotype and functionality in vivo. This is crucial because it identifies the critical intermediate between the lymphoblastoid growth program and the resting memory B cells. It is known that direct infection and the growth program ablate GC functionality and phenotype, i.e., they are not consistent with GC function (Babcock et al. 2000; Siemer et al. 2008). Thus, for a newly infected naïve blast to differentiate into memory, it must switch to the default program in the GC. This is the second example we will encounter of a latent gene transcription program used in lymphoma, being found in a normal infected B cell counterpart in vivo. In this case, the default program, which is used in Hodgkin’s disease (HD), is found in latently infected GC cells (Table 1, Fig. 5). The default program involves only three of the nine latent proteins, EBNA1, LMP1, and LMP2a (Kieff and Rickinson 2007; Thorley-Lawson 2001). Here, the Q promoter (Qp) is employed so that EBNA1 may be expressed without the other EBNAs (Tsai et al. 1995; Schaefer et al. 1995; Nonkwelo et al. 1996). EBNA1 is essential because it is required for retaining the viral genome by tethering it to cellular DNA and allowing it to be replicated (Yates et al. 1985). For a detailed discussion of EBNA1, see the chapter authored by Lori Frapier.
3.2.5 Turning Off the Growth Program
When EBNA2 is turned off in the presence of an activated c-myc, which is expressed in GC cells (Dominguez-Sola et al. 2012; Martinez-Valdez et al. 1996), the cells downregulate surface markers’ characteristic of B blasts, such as CD23, and acquire GC-specific markers, such as CD10 (Polack et al. 1996). Therefore, the infected lymphoblast appears free to acquire a GC phenotype once the differentiation block, imposed by EBNA2, is removed. One of the direct targets of EBNA2 is c-myc, a known regulator of cell growth and apoptosis (Kaiser et al. 1999). We can assume therefore that upon arrival in the follicle, the EBV lymphoblast receives a signal that turns EBNA2 and the growth program off while allowing c-myc expression to continue. How this is achieved remains unknown, but there is in vitro evidence to suggest that it may depend in part upon signals originating in the GC from cytokines such as IL-10, IL-21, and Type 1 IFN in combination with CD40 ligand (CD40L) (Kis et al. 2006, 2010; Salamon et al. 2012).
The actual mechanism by which cells switch from the growth to the default program probably depends on a negative feedback loop involving EBNA2 and the EBNA3s. These are believed to act as functional homologues of the intracellular components in the Notch signaling pathway (Kempkes et al. 1995; Speck 2002). For a detailed discussion of this hypothesis, see Thorley-Lawson and Allday (2008), and for a review of the Notch system, see Artavanis-Tsakonas et al. (1995). Upon infection of B cells, the first viral protein expressed is EBNA2 which interacts with the enhancer elements of cellular and viral latent genes to block differentiation and drive cellular proliferation. At the same time, EBNA2 activates the major EBV latent promoter Cp which leads directly to expression of all the EBNAs including EBNA3a and 3c. For a detailed discussion of the EBNA3 proteins, see the chapter authored by Martin Allday. Based on their known functions, EBNA3a and 3c could displace EBNA2 from Cp (Zimber-Strobl and Strobl 2001) and recruit repressor proteins that would lead to the stable epigenetic silencing of Cp and the suppression of EBNA2 production (Hickabottom et al. 2002; Knight et al. 2003; Radkov et al. 1999; Touitou et al. 2001). For a detailed discussion of EBV-associated chromatin and epigenetics, see the chapters authored by Paul Lieberman and Wolfgang Hammerschmidt. Cessation of EBNA2 production would cause growth arrest and allow the cells to assume a GC phenotype and express the default program. In this model, growth driven by EBV is a self-regulating feedback loop involving EBNA2 and the EBNA3s where the balance is tilted in favor of growth arrest by signaling from T cell-associated cytokines and CD40L (Kis et al. 2006, 2010; Salamon et al. 2012). It follows that the in vitro phenomenon of immortalization may be a biological artifact where the balance has been shifted in favor of EBNA2 by the absence of T cell-derived signals and the powerful selection pressure of in vitro growth.
3.2.6 EBV Can Provide the Rescue Signals—LMP1 and LMP2
Once the growth program is turned off, we have good evidence that the expression of LMP1 and LMP2 in the default program is capable of providing the two signals, T cell help and BCR, necessary to rescue the GC cell into memory.
LMP1 is a membrane protein that acts as a ligand-independent, constitutively activated receptor (Gires et al. 1997). For a detailed discussion of LMP1, see the chapter authored by Arnd Kieser. It does this by engaging signaling molecules (Izumi and Kieff 1997; Mosialos et al. 1995) which normally transmit signals from CD40 when it engages its ligand on Th cells [reviewed in Lam and Sugden (2003)]. Thus, in principle, LMP1 is able to deliver a Th signal to the infected B cell in the absence of Th cells. The parallel between CD40-mediated Th and LMP1 signaling extends to the ability of LMP1 to drive immunoglobulin class switching (He et al. 2003; Uchida et al. 1999). LMP1, like CD40, also turns off expression of bcl-6 (Panagopoulos et al. 2004) and turns on bcl-2 (Henderson et al. 1991). Through its ability to regulate bcl-2 and bcl-6, LMP1 (Carbone et al. 1998) almost certainly plays a role in driving the latently infected B cell to leave the GC and differentiate into a memory cell (Fig. 7).
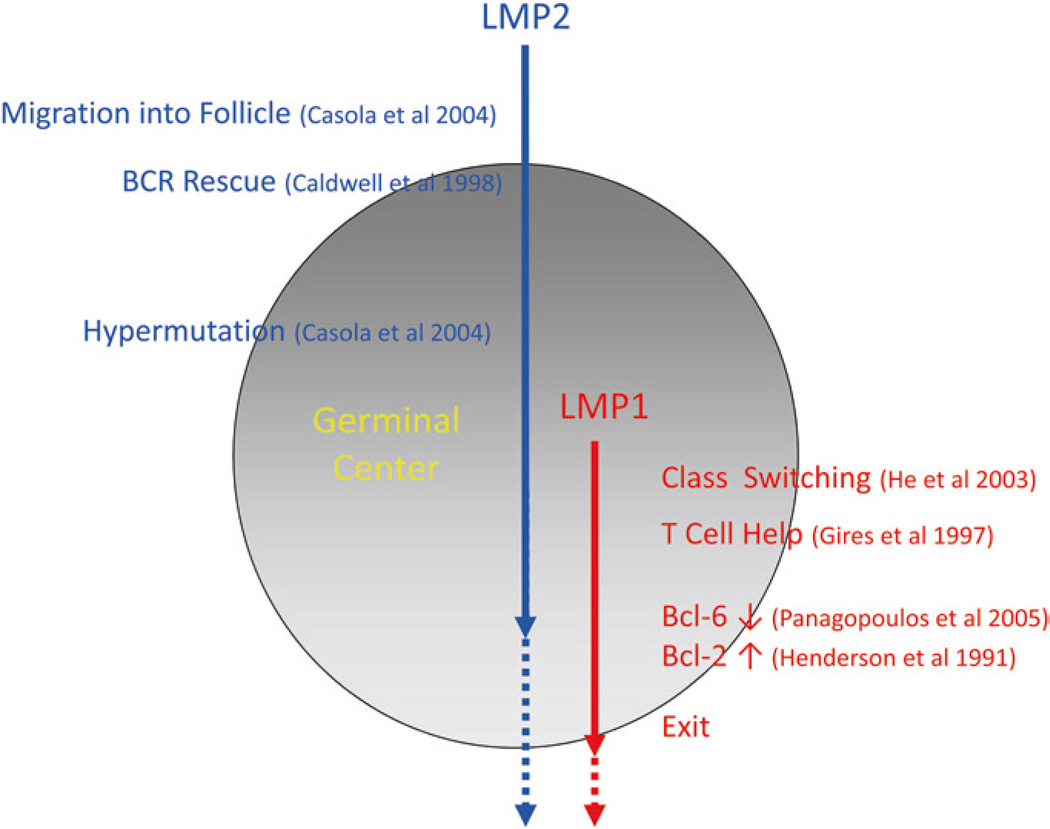
Fig. 7.
A summary of the functions of LMP1 and LMP2a demonstrated in vitro or in vivo with transgenic mice that could contribute to the GC processing of a latently infected B cell
LMP2 is also a membrane protein, but it delivers a constitutive, ligand-independent BCR signal (Caldwell et al. 1998). For a detailed discussion of LMP2, see the chapter authored by Richard Longnecker. LMP2a contains the same signaling motifs (ITAMs) (Beaufils et al. 1993), as the α- and β-chains of the BCR. These motifs allow it to engage signaling molecules employed by the BCR (Miller et al. 1995; Kurosaki 1999). The BCR produces two types of signals (MacLennan 1998): One (tonic) is required to ensure the survival of resting B cells (Lam et al. 1997; Maruyama et al. 2000), while the other (activating) leads to cellular activation, proliferation, and ultimately differentiation into immunoglobulin-secreting plasma cells (MacLennan 1994; Liu and Arpin 1997). LMP2a is able to provide the tonic but not the activating signal (Caldwell et al. 1998) and in the absence of a BCR is able to drive GC formation in mucosal tissue where the cells show evidence of having undergone mutation of their immunoglobulin genes (Casola et al. 2004b). Thus, LMP2a almost certainly plays a role in driving the latently infected B cell into and through the GC (Fig. 7).
In sum, LMP1 and LMP2a have the capacity to provide the latently infected B cell with a whole range of signals associated with GC development (Fig. 7).
3.2.7 Does EBV Do It All—The Conundrum of LMP1 and LMP2
One critical remaining question is: does EBV do it all? The signaling properties of LMP1 and LMP2 imply that together they could potentially provide all the signals necessary to rescue a latently infected B cell from the GC into memory, bypassing the normal mechanisms of antigen selection. If so, the immunoglobulin genes of latently infected memory B cells should either be unmutated or show an unselected pattern of mutations. However, the expressed immunoglobulins in latently infected memory B cells from the blood have undergone CSR, have no stop codons, and display the SHM pattern expected for antigen-selected memory cells (Souza et al. 2005, 2007). Thus, it seems that the expression of LMP1 and LMP2 has little discernible impact on the selection process as EBV-infected cells transit the GC into memory.
Experiments involving the expression of either LMP1 or LMP2 in the B cell compartment of transgenic mice indicate that alone these molecules can have devastating physiologic effects. In such studies, LMP1 could exclude B cells from the GC (Uchida et al. 1999) and even drive the development of B cell lymphomas (Kulwichit et al. 1998). LMP2 on the other hand was shown to replace the BCR allowing BCR-negative B cells to survive and enter the periphery (Caldwell et al. 1998) (a particularly relevant observation for Hodgkin’s lymphoma see below) and in some models break tolerance allowing autoreactive cells to survive in the periphery (Chang et al. 2012; Swanson-Mungerson and Longnecker 2007; Swanson-Mungerson et al. 2005). These observations suggest that deregulated expression of LMP1 or LMP2 may play an important role in the pathogenesis of lymphoma and autoimmune disease development but seemed strangely at odds with the striking lack of B cell lymphoma and autoimmunity in the vast population of EBV-infected people. However, in humans, LMP1 and LMP2 are usually expressed together and a follow-up study on double transgenic mice revealed that now the mice did not develop lymphoma or autoimmune disease and their B cells were able to comfortably transit the GC, undergo affinity maturation, and enter the memory compartment (Vrazo et al. 2012).
Thus, it seems that LMP1 and LMP2, when coexpressed in vivo, can modulate each other’s signaling. For example, in vitro, LMP1 when expressed alone can downregulate bcl-6 (Panagopoulos et al. 2004) and upregulate bcl-2, yet in the GC, LMP1 expression, in the presence of LMP2, is fully compatible with bcl-6 expression and is not associated with the upregulation of bcl-2 (Roughan and Thorley-Lawson 2009). What then is the role of these proteins in the GC? Because their functions are so tuned to the requirements of the GC and they are specifically expressed there, it seems certain that they must play some important role. What could this be? A clue comes from the analysis of the small subset of bcl-2-positive cells in the GC, those about to leave, which revealed that they only express LMP1, not LMP2, i.e., LMP1 expression in vivo, just as in vitro, is associated with upregulation of bcl-2 but only in the absence of LMP2. It seems likely therefore that the expression of LMP1 or LMP2 alone in the GC is strictly regulated to occur only at specific moments to achieve specific ends. Based on what we know so far, LMP2 expression alone in latently infected cells would ensure that the cells form GCs in mucosal epithelium; LMP1 and LMP2a together drive CSR and SHM and provide the requisite survival signals, and LMP1 alone ensures exit from the GC and terminal differentiation by switching off bcl-6 and switching on bcl-2 (Fig. 7). To test this hypothesis will require careful dissection of infected GC populations. Previous attempts at this showed no differences (Babcock et al. 2000; Roughan and Thorley-Lawson 2009), but were based on the now discredited marker CD77 (Victora et al. 2010) and were therefore artifacts. Recently, an accurate phenotype for GC subsets has been described (Victora and Nussenzweig 2012; Victora et al. 2010), making these studies now feasible.
3.3 EBV Persistence in the Peripheral Memory B Cell Compartment
How the transition from GC to resting long-lived memory B cell is achieved for any cell is not fully understood, but we may assume that once the mechanism is uncovered, we will find that the virus exploits it to gain access to the memory compartment.
3.3.1 The Resting Memory B cell
EBV, in the peripheral blood, is found only in B cells (Miyashita et al. 1995) that have the phenotype expected of a latently infected, long-lived, GC-derived, resting, memory B cell, i.e., classical memory B cells (Table 2) (Babcock et al. 1998; Decker et al. 1996; Joseph et al. 2000b; Miyashita et al. 1997). Persistence in memory B cells, first demonstrated for EBV, may be a common strategy for all B lymphotropic gammaherpesviruses (Barton et al. 2011). Restriction of EBV in the periphery to the GC-derived memory compartment is so tight that less than 1 in 10,000 latently infected cells in the blood are in the naïve compartment (Hochberg et al. 2004). They have the phenotypic hallmarks of classical GC-derived memory B cells being CD27+ (Joseph et al. 2000b; Klein et al. 1998) and having undergone CSR and SHM (Babcock et al. 1998; Joseph et al. 2000b; Souza et al. 2007). They are also CD23− and CD80− (B cell activation markers) (Miyashita et al. 1995), and >90 % are in the G0 stage of the cell cycle (Miyashita et al. 1997; Hochberg et al. 2004) all characteristics of resting B cells.
Table 2.
Phenotype of EBV Infected Cells in the Blood
| Phenotype | Implication |
|---|---|
| CD19+, CD20+, CD3− | B Cell |
| CD23−, CD80−, Ki67−, G0 stage of cell cycle | Resting Cell |
| CD27+, Ig genes hypermutated and class switched | GC Derived Memory Cell |
| IgD− | Not marginal zone B cells |
| CD5− | Not B1 cells |
| Episomal viral genomes, no linear form | Latently Infected |
The latently infected cells occupy a skewed niche within the memory compartment, being excluded from the IgD+ memory subset, but otherwise are evenly distributed among B cells carrying the different immunoglobulin isotypes. This suggests that once they enter memory, the EBV-infected cells cannot be distinguished from uninfected cells by host homeostasis mechanisms. The pattern of SHM (Souza et al. 2005) they display is that expected for antigen-selected memory cells (Souza et al. 2005). They tend to accumulate more mutations than their uninfected counterparts and actually showed a reduced proclivity to be self-reactive (Tracy et al. 2012). However, these differences were modest and may simply reflect differences between mucosal (EBV+) and splenic (peripheral) derived memory B cells. What is apparent though is that EBV does not significantly disrupt the normal processing of latently infected cells into memory. Deviations from normal B cell biology are not tolerated in these cells despite the potentially potent signaling capacities of LMP1 and LMP2.
EBV is not found in the CD5+ B1 subset (Joseph et al. 2000b), nor in circulating IgD+/IgM+/CD27+ marginal zone memory cells (Joseph et al. 2000b; Souza et al. 2007). These are both long-lived compartments of B cells (Youinou et al. 1999; Kantor 1991) that frequently have specificity for polyantigens such as bacterial cell wall components (Hardy 2008) but neither of which develop through GCs. The absence of EBV from these subsets provides further support for the conclusion that transit of the GC is required for the production of memory B cells latently infected with EBV. Studies claiming to find EBV preferentially in IgA-bearing B cells (Ehlin-Henriksson et al. 1999) or in IgD+ memory cells (Chaganti et al. 2009) were technically flawed and have not been substantiated [for a detailed discussion of the issues, see Joseph et al. (2000b) and Thorley-Lawson et al. (2013), respectively].
Memory cells latently infected with EBV in the peripheral blood do not express any of the known latent proteins (Hochberg et al. 2003a; Hochberg and Thorley-Lawson 2005). This is an important point to stress. Several studies have identified EBV latent gene expression in the peripheral blood based on RT-PCR analysis. However, these were not quantitative studies and were performed on bulk preparations of B cells (Babcock et al. 1999; Chen et al. 1995; Qu and Rowe 1992; Tierney et al. 1994). Because the assays used are so sensitive and variable in their sensitivity, it is impossible to know whether the signals are from rare infected cells expressing the transcript or are representative of the whole infected population of cells. It turns out that the former is true. By performing a limiting dilution RT-PCR analysis (Hochberg et al. 2003a; Hochberg and Thorley-Lawson 2005), it was possible to show that >99 % of the infected cells do not express transcripts for any of the known latent proteins. Indeed, the single-cell analysis afforded by this approach revealed that when latent gene transcripts were found, they were not part of any known transcription programs, indicating that they almost certainly are residual transcripts of no biological significance.
We may conclude therefore that the memory B cell is the site of long-term viral persistence. Here, it can remain for the lifetime of the host because immunological memory is for life, but the virus is no longer pathogenic to the host because the genes that drive cellular proliferation and threaten neoplastic disease are turned off. Similarly, the virus is safe from immunosurveillance because no viral proteins are expressed to act as targets of the immune system. The transcription program used in these cells, where no viral proteins are expressed, is called the latency program (Hochberg et al. 2003a and Table 1) reflecting its role at the site of latent persistence.
The frequency of infected memory B cells for an individual healthy carrier is very stable over time (Hadinoto et al. 2009; Khan et al. 1996). However, the level of infected cells in a population ranges widely from 5 to 3000 for every 107 memory B cells both in the peripheral blood (mean 110/107) and in Waldeyer’s ring (mean 175/107—the virus is evenly distributed throughout the ring) (Laichalk et al. 2002). The level of infected cells is similar between peripheral blood and Waldeyer’s ring but at least 20-fold lower in the other lymphoid tissue tested (spleen and mesenteric lymph node) (Laichalk et al. 2002), suggesting preferential homing to the lymphoepithelium of Waldeyer’s ring. Based on these measurements, the total body load calculates to 104–107 (mean 0.5 × 106) infected memory B cells per person representing a small, stable, and, most critically, “safe” pool of infected cells that guarantees long-term persistence. Only ~1 % of these cells reside in the peripheral blood.
3.3.2 Memory B Cell Homeostasis—The Maintenance of Long-Term Memory and Persistent Infection
The survival of memory B cells requires a tonic signal from the BCR (Maruyama et al. 2000), and the number of cells is controlled by homeostasis mediated by cytokines such as BAFF (Mackay and Schneider 2009; Stadanlick and Cancro 2008). The tonic BCR signal can be completely replaced by LMP2 (Caldwell et al. 1998), raising the possibility that persistently infected cells could be BCR independent. However, this is not the case, infected cells in the periphery do not express LMP2 (Hochberg et al. 2003a), and as already noted, they express a functional, possibly, antigen-selected BCR. A number of independent lines of evidence suggest that memory B cells latently infected with EBV are also maintained by homeostasis:
EBV-infected memory B cells in the periphery of adult humans are >90 % in a resting state, but at any given time, around 2–3 % of the cells are undergoing cell division (Miyashita et al. 1997; Hochberg et al. 2004). This is exactly the same rate as has been reported (2.7 %) for normal memory B cells (Hochberg et al. 2004; Macallan et al. 2005; Miyashita et al. 1997).
The half-life of both EBV-infected and EBV-uninfected memory B cells is virtually identical −7.5 ± 3.7 days (Hadinoto et al. 2008) and 11 ± 4 days (Macallan et al. 2005), respectively.
Latently infected memory cells in the periphery express no viral latent proteins. Therefore, when they divide, it must be driven by normal homeostasis signals.
We may conclude therefore that the pool of latently infected memory B cells is indistinguishable to the host from normal memory B cells.
When EBV-infected cells divide, they must express EBNA1 because the viral genome cannot replicate in its absence (Yates et al. 1985). Predictably, therefore, latently infected memory cells in the periphery express EBNA1 when they undergo cell division (Hochberg et al. 2003a). This is the third example we will encounter of a latent gene transcription program used in lymphoma, being found in a normal infected B cell counterpart in vivo. In this case, the EBNA1 only program, which is used in Burkitt’s lymphoma (BL), is found in dividing, latently infected memory B cells in the blood (Table 1, Fig. 5). EBNA1 expression during cell division is the only potential point of attack for the immune system against the pool of latently infected memory cells. It is perhaps not surprising therefore that EBNA1 has evolved so as not to be processed and presented efficiently to the immune system [Levitskaya et al. (1995, 1997) reviewed recently in Daskalogianni et al. (2014)], thus minimizing the risk of attack.
3.4 Viral Replication—Plasma Cell Differentiation, Stress, and the Role of Epithelial Cells
3.4.1 Terminal Differentiation—Maintenance of Stable Antibody Production and Viral Shedding
The last component of persistent infection to be discussed is that infectious virus is continuously shed into the saliva (Golden et al. 1973; Hadinoto et al. 2009). Unlike the level of latently infected memory cells, which is strikingly stable over long time periods, virus shedding fluctuates dramatically. The level of shedding is relatively stable over short periods (hours–days) but varies through 3.5–5.5 orders of magnitude over longer periods (Hadinoto et al. 2009). This variation means, contrary to what is generally believed, that the definition of high and low shedder is not so much a function of variation between individuals but within individuals over time. Also an important but simple insight, that had gone unrealized in the field, was that EBV shedding into saliva must be continuous and rapid. This is because the virus must be replaced ~2 min which is how frequently, on average, a normal individual swallows. Thus, the mouth is not, as often cited, a reservoir of virus but a conduit through which a continuous flow stream of virus passes in saliva (Fig. 8). Consequently, virus is being shed at a much higher rate than is generally appreciated.
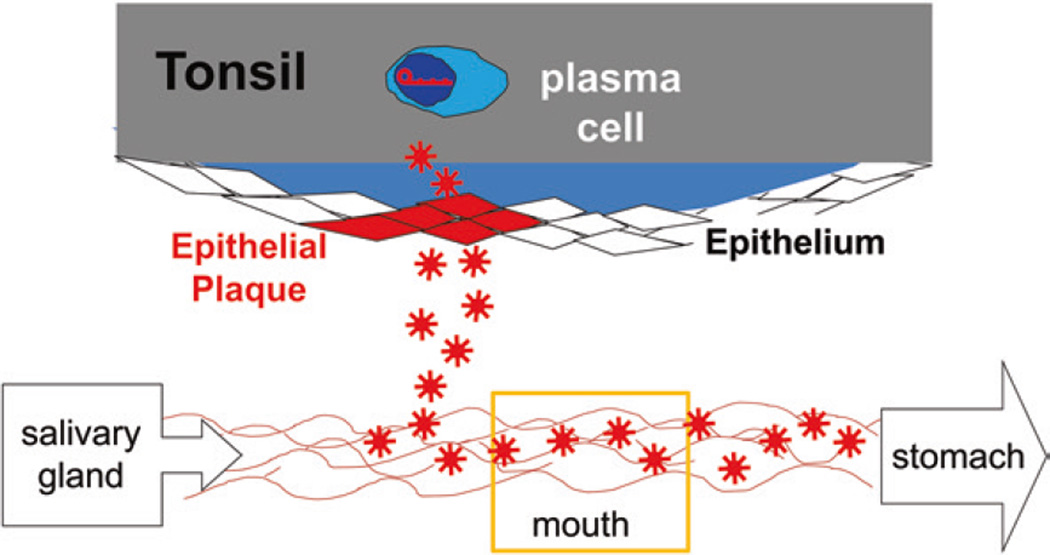
Fig. 8.
A model of EBV reactivation and shedding. The known data fit a model where a single latently infected memory B cell in the tonsil occasionally differentiates into a plasma cell and releases virus that infects epithelial cells. The infection spreads exponentially through the epithelium, resulting in the shedding of virus. The plaque is eventually eliminated by the immune response. Meanwhile, another plaque initiates elsewhere in the Waldeyer’s ring. The data are consistent with their being no more than three such plaques in Waldeyer’s ring at any one time. Virus is continuously shed into the mouth where it mingles with saliva for about 2 min before being swallowed. Thus, the mouth is a flow stream of EBV not a static reservoir
Memory B cells, transiting the nasopharyngeal lymphoid tissue, presumably must occasionally initiate virus replication and release the virus. From cell surface phenotyping of fractionated tonsil cells, it is clear that the B cells replicating the virus in the lymphoepithelium of the tonsils are plasma cells (CD38hi, CD10−, CD19−, CD20lo, slg−, and clg+) (Laichalk and Thorley-Lawson 2005), a conclusion consistent with histological observations (Niedobitek et al. 2000; Anagnostopoulos et al. 1995). Quantitative estimates suggest that somewhere in the region of −250 cells are undergoing replication in Waldeyer’s ring at any one time (Hawkins et al. 2013; Laichalk and Thorley-Lawson 2005). However, sequentially fewer cells express the immediate early, early, and then late antigens of the lytic cycle such that only −10 % of the cells complete the replicative cycle. Thus, only a handful of B cells are actually releasing virus in Waldeyer’s ring at any given time. This sequential diminution in the numbers of cells replicating the virus as they proceed through the cycle may indicate that replication is frequently abortive or may be the result of aggressive immunosurveillance by CTL (Callan et al. 1998b). This despite mechanisms that the virus employs during its lytic cycle to reduce CTL surveillance (Ressing et al. 2008). For a detailed discussion of immune evasion by EBV, see the chapter authored by Emmanuel Wietz.
It has been shown that differentiation into plasma cells, and not the signals that induce differentiation, initiates viral replication (Laichalk and Thorley-Lawson 2005). Again, the biology of the virus is intimately tailored and responsive to normal B cell biology. This was confirmed by in vitro studies in cells showing that the promoter for BZLF1, the gene that begins viral replication, becomes active only after memory cells differentiate into plasma cells, that it is active in plasma cell lines and is activated by the plasma cell-associated transcription factors XBP-1 and Blimp1. The molecular mechanism behind this activation process has been comprehensively reviewed recently (Kenney and Mertz 2014). For a detailed discussion, see the chapter authored by Ayman El-Guindy.
The signal that causes latently infected memory B cells to undergo terminal differentiation is unclear. It has been suggested that immunological B cell memory may be sustained through bystander T cell help (Bernasconi et al. 2002) such that a memory B cell transiting through a lymph node will, when it encounters bystander T cell help, undergo a cell division that will generate one memory cell and one plasma cell. This ensures the stability of the memory pool, while a continuous supply of plasma cells is produced that will guarantee stable production of antibody. Applied to EBV, this could explain how the population of latently infected memory cells could be maintained for years, while, through the generation of plasma cells, virus can also be continuously produced.
An alternate hypothesis is that the generation of plasma cells replicating EBV is stimulated by cognate antigen and T cell help. This hypothesis has the attractive feature that latency would need to be established in antigen-specific memory cells in the tonsil. These cells would then enter the peripheral circulation where they would maintain persistent infection. As these cells reenter secondary lymphoid tissue, the site where they would most likely reencounter cognate antigen would be the tonsil. This would provide a mechanism for preferential homing and reactivation of the latently infected memory cells in the tonsil compared to other lymph nodes. Although it is difficult to conceive of a mechanism by which the virus could access antigen-specific naive B cells with a high enough probability and frequency to be feasible, this model is very consistent with the observation that the latently infected memory cells appear to bear antigen-selected BCRs.
3.4.2 Stress—An Alternate Pathway to Viral Replication
Indications of a second pathway to viral replication come from in vitro studies that a number of stress-inducing agents including TGFb and chemotherapy agents, BCR cross-linking and hypoxia can also initiate viral replication in cell lines (Kenney and Mertz 2014). In these systems, however, there is acute activation of the BZLF1 promoter within minutes of receiving the stimulus, and not surprisingly, the cells do not undergo plasma cell differentiation prior to viral replication. In a similar fashion, explanted infected peripheral memory cells will acutely undergo spontaneous reactivation (Rickinson et al. 1977), presumably in response to the stress induced upon being placed in culture. What these systems have in common is the induction of apoptosis in the B cells in response to the stress signal (Inman et al. 2001). However, EBV encodes a homologue of the antiapoptotic gene bcl-2 that is expressed during viral replication in vitro (Henderson et al. 1993) and this protects these cells from stress-induced death and apoptosis, while the virus replicates (Inman et al. 2001). It is known that B cells are particularly prone to apoptosis. It seems therefore that in addition to replication in plasma cells located in the epithelium of the tonsil for infectious spread, the virus has developed an escape hatch that allows it to exit any infected B cell that may begin to die by apoptosis.
3.4.3 Replication in Epithelial Cells
Although we lack a direct demonstration that EBV replicates in epithelial cells in vivo, the indirect evidence is compelling:
The strongest evidence comes from numerical arguments. Put simply, there are not enough B cells replicating the virus in Waldeyer’s ring to account for either the amount or extreme variability of EBV shedding in saliva. For a detailed discussion of the numbers, see Hadinoto et al. (2009). The dynamics of virus shedding is most simply explained by single B cells sporadically releasing virus that infects neighboring epithelial cells (Fig. 8). (This mechanism is analogous to the neurotropic herpesviruses (HSV and VZV) that persist silently in ganglia but when reactivated travel down the neurons to replicate in fibroblasts.) Epithelial infection by EBV spreads at an exponential rate and is terminated randomly, resulting in infected plaques of epithelial cells ranging in size from 1 to 105 cells, more than sufficient to account for the observed rate of shedding. At any one time, there would be a very small number (≤3) of such infected epithelial plaques in the entire Waldeyer’s ring that would be transient and usually small, explaining why they have previously gone undetected.
Cell cultures of primary epithelial cells from tonsils carry already infected cells that are both latently infected and replicating the virus (Pegtel et al. 2004).
EBV is found in oral hairy leukoplakia which represents a lesion where EBV is actively replicating in the epithelium of the tongue (Greenspan et al. 1985). This indicates that EBV can replicate in epithelial cells in vivo.
The glycoprotein patterns on the virus differ depending on whether the virus emerges from a B cell or an epithelial cell (Borza and Hutt-Fletcher 2002). This happens in such a way that the virus bears an epithelial tropic pattern of viral glycoproteins when it emerges from B cells and a B lymphotropic pattern when it emerges from epithelial cells. These results imply that the virus has evolved to efficiently shuttle back and forth between epithelial and B cells.
A unique receptor, α5β1 integrin, for EBV is expressed on epithelial cells that allows infection only on the basolateral surface (Tugizov et al. 2003). As with the glycoprotein patterns, this implies that epithelial cell infection by EBV is only used in one direction, in this case specifically restricted to exit by the virus.
Taken together, this evidence presents a strong circumstantial argument that tonsillar epithelium is actively infected with replicating EBV as an ongoing part of normal viral persistence and provides an explanation for the presence of the virus in the associated diseases of epithelial cells.
4 The Cyclic Pathogen Refinement of GCM
If a biological model is correct, then it should be logically rigorous and able to be expressed mathematically. Mathematical modeling is not really that different from how biology has always been done, it is just a more rigorous way to organize data and a more logical way to make testable predictions based on hypotheses. However, most biological systems are not well enough characterized quantitatively to be amenable to this type of analysis. This is under appreciated by biologists who tend to see the failing of modeling (despite its obvious utility in other more quantitative sciences such as physics and engineering) as a consequence of the limitations of modeling itself rather than the lack of rigor in understanding the biological system being studied. Persistent EBV infection is an exception.
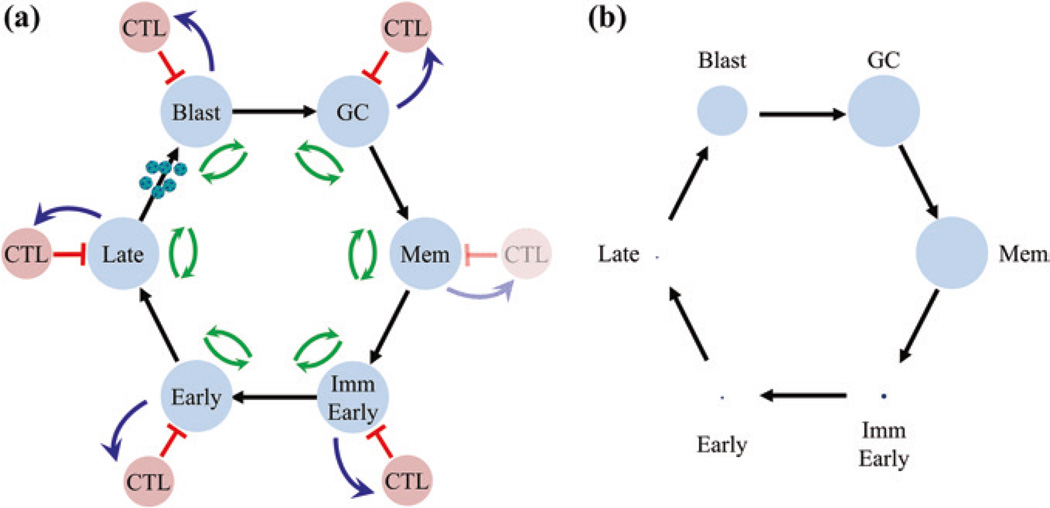
The GCM, as generalized in Fig. 9, can be described by a system of differential equations—the cyclic pathogen model (CPM) (Delgado-Eckert and Shapiro 2011) for which there is one and only one solution that is stable and biologically credible. We have sufficient quantitative information to be able to know, derive precisley, or estimate approximately values for all the parameters (rate constants) governing these equations. When solved with this parameter set, the model very precisely replicates the actual dynamics of the infection (Hawkins et al. 2013). This includes predicting which and to what extent each infected stage is recognized by CTL and even precisely predicting the expected sizes of the infected memory and GC populations and the extent to which they vary between infected individuals. Furthermore, when marginally non-biological values are assigned to parameters, the model fails to replicate infection. This is an important result that seems to have gone unappreciated in the biological community. The chances that one could randomly pluck a complex model such as the one shown in Fig. 9 and have it predict correctly when and only when biological values are applied are vanishingly small. The fact that this model works so well is a convincing argument for the biological accuracy of the GCM in explaining EBV persistence.
Fig. 9.
The cyclic pathogen model (CPM). a CPM is a mathematical description of the GCM. It consists of a cycle of 6 infected stages (blue circles based on the biological GCM illustrated in Fig. 4). These are blast, GC, memory and immediate early, early and late lyrically infected B cells, each of which is potentially controlled by the immune response (red circles). The single lytic stage in the GCM is broken down into three discrete stages which are known to be recognized independently by the immune response. Biologically, there is never a CTL response against the memory stage; however, the model allows analysis of theoretical conditions such as the memory compartment being regulated by CTL. This model can be described by a system of differential equations employing rate constants for the stimulation of CTL (blue arrows), killing of CTL targets (red arrows), and the proliferation and death of each stage (green arrows). For this system, there is one and only one mathematical solution that is stable and biologically credible. This solution accurately describes biologically persistent infection. b Shows the infected populations as circles whose area is proportional to their frequency within all tonsils (1:5:1.5.102:104:104:0.5.104, Late:Early:ImmEarly:Memory:GC:Blast). This highlights the very large range in the sizes of these populations
The mathematical description of the cyclic pathogen model and its subsequent analysis also provided important new insights including:
There are two possible mechanisms for EBV persistence in B cell memory. In one, the virus persists through homeostasis independently of new infection and addition to that compartment. In the second (predicted by the CPM), it is the cycle of infected states that accounts for persistence. Aggressive intervention with antivirals should distinguish these since they should have no impact on the memory compartment if the former is true but will reduce the overall level of viral infection if the latter is true. Indeed, long-term treatment with antivirals, which dramatically reduce viral shedding, produced a parallel decline in the level of infected memory B cells (Hoshino et al. 2009). This confirms the prediction from the CPM. For a detailed discussion of antiviral interventions, see the chapter authored by Richard Ambinder.
Based on the same arguments, CPM predicts that an effective vaccine against primary EBV infection will also be effective over time in reducing and eventually eliminating persistent infection because it will interdict the cycle of infection required for long-term persistence. For a detailed discussion of vaccine strategies, see the chapter authored by Rajiv Khanna.
To a biological eye, it is apparent that EBV persists because it can attain latent infection of resting memory B cells that are invisible to the immune system. However, the CPM provides a different interpretation, fully compatible with all the biological data, namely that it is the cycle of infection that allows persistence. Persistence is possible even if the memory compartment were highly immunogenic; however, the overall structure and dynamics of the persistent infection would look nothing like what is actually observed (in passing, it is worth noting the utility of modeling in allowing such biologically impossible experiments to be performed mathematically). This is a further validation of the accuracy of the mathematical model. Thus, access to the immunologically protected memory compartment defines the overall pattern, features, and dynamics of persistent infection but alone does not account for it.
It explains how infection can be stable at a very low level. This is crucial for both the host and the virus because it imposes the minimum burden on the host within which EBV wants to persist for life. For an average person, there is ~1 infected cell per 5 ml of blood. Such a low level of infection leaves the virus vulnerable to extinction through stochastic variation, yet the value only varies by a factor of perhaps ±25 % over many years (Hadinoto et al. 2009). This is because the cycle of infection ensures that an obliterated population can be rapidly repopulated returning the system to the same equilibrium as before.
The absolute levels of infection are defined by the level of the immune response against viral proteins. This includes cytotoxic responses to infected cells expressing latent and lytic proteins and neutralizing antibody against infectious virus. The prediction that the immune system only moderates the overall viral load, not the form of persistence, is confirmed in studies of immunosuppressed individuals. Here, in the presence of a minimally effective immune response, the levels of virus-infected memory B cells increase on average 50-fold (Babcock et al. 1999). However, the regulation of viral persistence is intact and the virus in the blood remains restricted to resting memory B cells. This means that the immune response per se plays no role in regulating the mechanisms of viral persistence, but it only regulates absolute levels of the infection.
That the system is a simple circle is amply demonstrated by studies on acutely infected individuals. Here, the system is allowed to run unchecked until the immune response is activated. In this case, as many as a staggering 50 % of all memory cells may become latently infected with EBV until the immune system begins to reduce the overall load of infection (Hadinoto et al. 2008; Hochberg et al. 2004). Impressively, the regulation still holds and the virus remains restricted to resting memory cells in the blood again highlighting that the immune system only functions to regulate the level not the form of the infection.
5 The Model of Persistence—A Summary
In summary, persistent infection by EBV can be seen as a self-perpetuating circle of infection, differentiation, persistent infection, reactivation, and reinfection (Figs. 4 and 9) that exploits virtually every aspect of mature B cell biology. The expansion of the virus is counterbalanced by the immune response. It is this cycle of infection together with the quiescent infection of peripheral memory B cells that allows the virus to be maintained at the extremely low and stable levels characteristic of persistent infection. In doing so, EBV does not disrupt the normal processing of latently infected cells into memory, and in so far as the presence of the virus may cause deviations from normal B cell biology, they are not detectable by the time the cells enter the memory compartment.
6 Disease Pathogenesis—Insights from the GCM
The GCM explains that EBV needs to transit the GC to access the resting memory compartment. EBV-infected and GC B cells are tightly regulated because they both proliferate rapidly—a risk factor for cancer. GC cells also actively undergo DNA breakage and mutagenesis during CSR and SHM, additional risk factors for tumor development and the production of autoreactive B cells. Furthermore, in the GC, EBV expresses LMP1, a growth-promoting potential oncogene, and LMP2, a pro-survival molecule able to rescue autoreactive B cells. Thus, the presence of EBV in GC B cells presents a nexus for disease risk, especially cancer (EBV-positive Hodgkin’s disease and Burkitt’s lymphoma both arise from EBV-infected GC cells) and autoimmunity. It is not surprising, therefore, that EBV has been linked with a number of such diseases.
6.1 Infectious Mononucleosis—Acute Infection (AIM)
Delayed infection by EBV can cause infectious mononucleosis (AIM). Why adolescence and adults get AIM is not clear. It is likely immunopathologic in nature, meaning the disease symptoms are caused by the inflammatory response of the immune system rather than the virus itself. For a detailed discussion of AIM, see the chapter authored by Kristin Hogquist. The intensity of the disease varies but can last for weeks or months before finally resolving (Hoagland 1967). IM is characterized by a lymphocytosis (Wood and Frenkel 1967) due to the appearance of large numbers of “atypical” lymphocytes which are predominantly CD8+ T cells, representing a vigorous CTL response to the virus (Strang and Rickinson 1987; Callan et al. 1998b).
6.1.1 AIM and the GCM
Virologically and immunologically, we know nothing about what is happening in the newly infected host until they arrive at the clinic with symptoms some 5 weeks into the infection (Hoagland 1964). We may assume though that when the virus initially infects, there is nothing to control the cycle of infection, latency, reactivation, and reinfection, shown in Fig. 4. Consequently, the memory compartment begins to fill up with latently infected B cells. A staggering level of infection is achieved that can reach ≥50 % of all memory B cells (Hochberg et al. 2004). Despite this overwhelming invasion of the B cell compartment by EBV no cells expressing the lymphoblastoid form of latency/infection are detected in the periphery, the virus remains restricted to resting memory B cells. This is fully consistent with the GCM which predicts that the lymphoblastoid form of latency is restricted to the lymphoid tissue and tightly regulated such that the cells rapidly transit into the GC to become memory cells before entering the circulation.
By the time patients experience symptoms and arrive at the clinic, the infection is always resolving (Hadinoto et al. 2008). Viral shedding and the levels of newly infected B cells are all falling. All that is left is the massive level of infection in the memory compartment. Since these cells are not seen by the immune response, their levels decrease simply by attrition as they initiate viral replication and are immediately killed by CTLs that recognize the immediate early lytic antigens. Consequently, at this time, as many as half of all the CTLs in the body are directed against EBV-infected cells expressing these targets (Callan et al. 1998b). It is most likely that this destruction of large numbers of infected B cells is responsible for the inflammatory response leading to the fever and malaise characteristic of IM.
There then ensues a parallel decrease in the number of latently infected memory B cells (dying as they enter viral replication and are destroyed by CTL) and the number of CTLs that they stimulate (Catalina et al. 2001; Hadinoto et al. 2008). For the next few weeks, there is an exponential decrease in the levels of latently infected memory B cells (half-life ~7.5 days) and CTL against the IE proteins (half-life ~73 days). Eventually, the level of infected memory B cells drops to a point where the rate of attrition is matched by the steady-state low-level production of newly infected memory B cells. At this time, the level of infected memory cells and CTL against IE proteins begins to stabilize. Thus, the acute infection is eventually limited by the immune response but at an excruciatingly slow rate. The ensuing events are strikingly different from infection with most other viruses. Shedding of EBV does not stop, but continues for life (Hadinoto et al. 2009; Yao et al. 1985). B cells expressing the growth and default programs persist in Waldeyer’s ring (Babcock et al. 2000), and latently infected memory cells, expressing the latency program, remain in the blood for life (Babcock et al. 1998; Khan et al. 1996). At the same time, levels of neutralizing antibodies (Henle and Henle 1979) and CTL (Callan et al. 1998a; Steven et al. 1996) also continue at significant and stable levels for the lifetime of the host. For a detailed discussion of the immune responses that regulate EBV, see the chapters authored by David Nadal, Martin Rowe, Jaap Middeldorp, and Andrew Hislop et al. It is apparent therefore that the immune response ameliorates and counterbalances the infection but never clears it. An equilibrium is established between the immune response and the various states of viral latency allowing the virus to persist at stable levels without causing significant impairment to the host. The CPM states that these countervailing forces are responsible for maintaining the stable fixed point that mathematically describes EBV persistence.
6.1.2 Why Do Adolescents Get AIM?
The age dependence of symptoms has led to the suggestion that IM is a disease of a mature immune response. What this means mechanistically is less clear. It has also been suggested that the tonsil is an immune-privileged site during AIM, since the majority of circulating CTLs at this time lack the requisite mucosal homing receptors to enter the tonsil (Hislop et al. 2005). However, a much simpler and more likely explanation for this observation is that the majority of EBV-infected cells reside in the spleen during acute infection as evidenced by the well-documented symptom of splenomegaly. Thus, most of the CTL should be homing to this non-mucosal site until the infection resolves.
A more compelling explanation for AIM is the theory of heterologous immunity (Clute et al. 2010; Selin et al. 1998). In this theory, the CTL response to new infections early in life is by naive T cells which produce high-affinity CTLs that efficiently and rapidly clear the virus infection and then become memory CTL. As the organism ages, memory CTLs accumulate to a variety of pathogens. Exposure to a novel infection later in life is more likely to trigger cross-reacting memory CTL than naive CTL. These memory CTLs are of lower affinity and may produce an ineffectual response allowing extensive viral replication and spread that triggers a massive inflammatory response that lasts until the virus is finally brought under control.
6.2 Autoimmune Disease
EBV has variously been linked with a number of autoimmune diseases including SLE (James et al. 1997), rheumatoid arthritis (Lotz and Roudier 1989), Sjorgen’s syndrome (Fox et al. 1987), and most recently and aggressively with multiple sclerosis [reviewed in Ascherio and Munger (2010)]. For a detailed discussion of EBV and autoimmune disease, see the chapter authored by Alberto Ascherio. The driving concept behind these associations is the knowledge that EBV can cause the activation and proliferation of infected B cells in an antigen-independent fashion and that such cells are immortal in tissue culture. This raises the possibility that, by infecting them, EBV infection could allow the survival of autoreactive clones of B cells. However, EBV does not persist by immortalizing B cells in vivo, but by differentiating the infected cells into a resting memory state. Consequently, the fundamental rationale for the association must be modified to suggest that the EBV latent genes may rescue a forbidden clone from a GC into the memory compartment. Such a latently infected cell itself could not produce antibodies because upon plasma cell differentiation it would produce infectious virus and die. However, theoretically, such a cell could present autoimmune antigens and break tolerance. There is evidence to support this idea in that LMP2, which is expressed in the GC, is capable of breaking tolerance in a mouse model of autoreactivity (Swanson-Mungerson and Longnecker 2007; Swanson-Mungerson et al. 2005), and has even been shown to exacerbate disease in a mouse model of MS (Chang et al. 2012). Such behavior must be anomalous, however, because the expressed immunoglobulins of latently infected memory cells in healthy humans are, if anything, skewed away from self-reactivity (Tracy et al. 2012).
Demonstrating experimentally a causative role for EBV in autoimmune disease is difficult because infection usually occurs early in life and by adulthood >90 % of the population is infected. Analysis is further complicated by the fact that EBV is carried in the peripheral circulation by infected memory B cells, so sensitive tests will detect EBV in any inflamed tissue, regardless of the virus’s role in causing the inflammation. The GCM/CPM adds another layer of complication which is that EBV uses virtually every aspect of mature B cell biology to establish and maintain persistent infection and virus shedding which is counterbalanced by the immune response to produce a defined stable level of infection. The corollary is that EBV is exquisitely sensitive to changes in the immune system. Any disease that affects the immune system will have an impact on the regulation of EBV persistence. This could result in an increase in the numbers of infected cells in the blood (peripheral blood burden) and/or an increase in virus shedding. Thus, changes in virological or immunological parameters of EBV infection associated with an autoimmune disease are most likely an indirect effect of a compromised immune system caused by the disease as is the case with SLE (Gross et al. 2005) rather than a cause of the disease.
6.3 Cancer
The motivating force behind associating EBV with cancer is obvious. It is well established that EBV has latent proteins that can drive cellular proliferation, at least in B lymphocytes, and it is highly likely that inappropriate or deregulated expression of these genes could play a causative role in tumor development. EBV-associated cancers fall into three discrete groups.
Tumors for which there are claims that remain to be substantiated. These would include, but not be limited to, breast (Bonnet et al. 1999) and hepatocellular carcinoma (Sugawara et al. 1999). In these cases, the doubt usually exists through the inability of investigators to reproducibly detect the virus in the tumor cells. Since the assays used are usually based on either PCR or immunohistochemistry, they are subject to the vagaries of those techniques which include a high level of false positives and dependence on technical skill to perform well-controlled studies. Thus, it becomes difficult to resolve whether conflicting results are caused by false-positive artifacts or technical inconsistencies.
Tumors for which there is strong supportive evidence, but the tumors arise in cell types for which no latently infected biological equivalent has been established. Such tumors may arise through accidental infection leading to inappropriate viral latent gene expression. This includes such tumors as nasopharyngeal (Raab-Traub 2002), gastric (Shibata et al. 1991; Shibata and Weiss 1992), and salivary gland (Raab-Traub et al. 1991) carcinomas. For a detailed discussion of EBV-positive carcinoma, see the chapter authored by Nancy Raab-Traub. Also included are leiomyosarcoma (van Gelder et al. 1995; Timmons et al. 1995) and T and NK lymphomas (Chiang et al. 1996; Tao et al. 1995). In these cases, there is a high degree of correlation between the disease and EBV [e.g., 100 % of undifferentiated NPC contains EBV (Andersson-Anvret et al. 1977)] and reproducible detection of the virus in the tumor cells. Since the viral episome is lost from cells absent some selective pressure for its retention (Kirchmaier and Sugden 1995), the consistent presence of viral DNA in any tumor is prime facie evidence that the virus is playing a role in the growth/survival of the tumor cells (Vereide and Sugden 2009). These tumors also frequently express the latent gene LMP1 which is known to be highly oncogenic when constitutively expressed (Baichwal and Sugden 1988; Moorthy and Thorley-Lawson 1992; Nicholson et al. 1997; Uchida et al. 1999; Wang et al. 1985). This type of tumor is relevant to the GCM which posits that EBV latent gene expression patterns have evolved to be regulated in concert with normal B cell activation and differentiation, with the ultimate goal of establishing persistent infection in a memory B cell where the latent genes are no longer expressed. It follows that if EBV fortuitously gains access to a cell type which is not a natural target of infection, i.e., a non-B cell, this could lead to aberrant latent gene expression that would not be regulated appropriately. This could result in constitutive expression of LMP1 for example.
Tumors for which there is good evidence linking EBV. These are the lymphomas IL, HD, and BL. There is convincing epidemiological, serological, and molecular biological evidence associating EBV with these tumors. The GCM provided the first and, to date, only explanation for the origin of these lymphomas and the reason they express restricted patterns of latent proteins (Thorley-Lawson and Gross 2004). Indeed, it is supportive of the GCM and cannot be a coincidence that tumors arise from each of the three proliferative stages of EBV infection predicted by the model (Fig. 5). These are IL from cells expressing the growth program (new infection), HD from cells expressing the default program (GC cells), and BL from cells expressing EBNA1 only (late GC cell).
6.3.1 Lymphoma in the Immunosuppressed—IL
Patients who are immunosuppressed are at risk for diseases such as post-transplant lymphoproliferative disease (PTLD) in organ transplant patients and immunoblastic lymphoma in AIDS patients. These are a heterogeneous collection of B cell disorders [reviewed in Hopwood and Crawford (2000)] that usually carry the virus and express the growth program (Thomas et al. 1990). The obvious explanation for PTLD is that suppression of the immune response allows uninhibited growth of EBV-infected cells; however, it is not that simple. If EBV was able to freely drive cell growth in the absence of an immune response, there would be several consequences. First, all immunosuppressed patients who are EBV-infected should develop the disease. Second, it should be a polyclonal and disseminated disease since EBV would drive the growth of many infected cells throughout the body. Finally, the lymphomas would be expected to arise most frequently in the places where infected cells are known to express the growth program—Waldeyer’s ring (Joseph et al. 2000a). In reality, the disease has none of these features. Only a small fraction of patients (0.1–10 % depending on the setting) develop the disease, it is usually oligoclonal arising from one or a few infected cells, and it often occurs in extranodal sites such as the brain and gut (Penn 1998; Hopwood and Crawford 2000). This indicates that the disease is not simply EBV-driven growth but involves a rare event where EBV infection has gone wrong.
The GCM states that the growth program of EBV is used specifically to activate newly infected naïve B cells in Waldeyer’s ring, so they can then differentiate into resting memory B cells. It follows that for a lymphoblastoid cell, proliferating due to the growth program, to survive and evolve into a lymphoma, the cell must be unable to exit the cell cycle. This could occur if infection of the wrong B cell type or in the wrong location occurs (inappropriate infection).
IL in the immunosuppressed is therefore a consequence of two events. The rare specific event is the expression of the growth program in a B cell that cannot exit the cell cycle. The global event is immunosuppression that prevents the elimination of these rare cells. At this stage of the disease, the tumor cells are still susceptible to immunosurveillance and regression can be achieved by reducing immunosuppression (Starzl et al. 1984) or by treatment with autologous CTL (Rooney et al. 1995). For a detailed discussion of the application of adoptive transfer for treatment of EBV tumors, see the chapter authored by Stephen Gottschalk and Cliona Rooney. However, in the absence of T cell immunity, the proliferating cells acquire additional genetic damage and more malignant clones arise (Knowles et al. 1995). These cells ultimately become unresponsive to reduced immunosuppression or immunotherapy and are usually fatal.
6.3.2 Hodgkin’s Disease (HD)
For a detailed discussion of HD, see the chapter authored by Paul Murray and Andy Bell. HD is a tumor of germinal center cells (Kuppers 2012; Kuppers and Rajewsky 1998) and is characterized by the unusual Hodgkin’s Reed–Sternberg (HRS) tumor cells. AIM and elevated antibody titers to EBV are both risk factors for HD (Ambinder 2007; Henle and Henle 1979; Hjalgrim et al. 2007), and up to 40 % of the tumors contain EBV (Glaser et al. 1997). The virus in the tumors is clonal and expresses the default transcription program (Oudejans et al. 1996; Deacon et al. 1993; Herbst et al. 1991; Niedobitek et al. 1997), the same transcription program used by latently infected GC B cells (Babcock et al. 2000). Thus, the cell origin and the viral gene expression data agree that HD arises from an EBV-infected GC B cell expressing the default program (Fig. 5). In effect, the viral gene expression pattern in HD is not created within and selected by the tumor, but is a natural consequence of the cellular origin of the tumor. The presence of EBV in ~40 % of the tumors would seem to rule out a chance association of the virus with the tumor. But this does not take into account that the levels of EBV-infected B cells reach extremely high levels during AIM, frequently 10–50 % (Hochberg et al. 2003b), so there is a very high probability (as high as 50 %) that the premalignant GC cell will have EBV in it by chance. Therefore, it remains a possibility that it is the immunological disruption of AIM which is the risk factor and EBV is simply a passenger that plays no role in tumor development.
However, retention of the virus in HD strongly argues that it must be contributing something to tumor cell survival/growth (Vereide and Sugden 2009). One specific contribution has been identified for the subset of HD tumors that express immunoglobulin genes crippled by mutation. These cases are almost universally EBV positive (Bechtel et al. 2005) and express LMP2 which has been shown independently to replace the missing BCR-derived tonic signal necessary for the survival of B cells with crippled BCRs (Mancao and Hammerschmidt 2007).
6.3.3 Burkitt’s Lymphoma (BL)
For a detailed discussion of BL and diffuse large B cell lymphoma, see the chapters authored by Ann Moorman and Rosemary Rochford and Sandeep Dave. BL has the pedigree of being the tumor in which EBV was originally discovered, but its contribution to BL still remains enigmatic. The defining genetic lesion in BL is deregulated activation of the c-myc oncogene due to reciprocal translocation with one of the immunoglobulin genes (Klein 1983; Leder 1985; Manolov and Manolova 1972). BL can occur without EBV, and expression in transgenic mice of c-myc, in the context of the immunoglobulin translocation, has been shown to be sufficient to produce Burkitt’s lymphoma-like tumors (Kovalchuk et al. 2000), suggesting that deregulated c-myc is sufficient to produce the tumors. This raises the question as to what role EBV may play. The most compelling evidence of EBV’s involvement in BL is the retention of the genome by the tumors (Vereide and Sugden 2009) and the high frequency of tumors carrying the virus (de-Thé 1985) in the endemic (eBL) regions of Africa (>95 % contain EBV DNA). The frequency is lower (15–85 %) in the sporadic form of the tumor (sBL). The presence of clonal EBV in the tumors (Gulley et al. 1992) has also been interpreted as evidence of EBV’s role, but in actuality, this only means that EBV was present prior to the last event that produced the tumor. Curiously, none of the viral growth-promoting latent genes are expressed in the tumor cells, the only latent protein present being EBNA1 (Gregory et al. 1990), along with the non-coding small RNAs EBERs and the microRNAs. Transcriptionally, this looks like a situation where the virus is just along for the ride since EBNA1 has to be expressed to allow duplication of the viral genome. However, there is evidence for all the EBV genes expressed in BL that they may contribute to pathogenesis, usually by limiting sensitivity to apoptosis (Iwakiri 2014; Kennedy et al. 2003; Vereide et al. 2014; Wilson et al. 1996).
What is required for understanding how EBV may predispose to BL is an explanation for why only EBNA1 is expressed. In the case of HD, we have shown that the default program is expressed because the tumor derives from an EBV-infected GC cell and the default program is what the virus naturally expresses in a GC cell. Applying this thinking to BL, there is currently only one way known to produce the “EBNA1-only” phenotype of BL in a non-tumor cell. This is when a latently infected GC cell that becomes a memory cell expressing the latency program, i.e., no latent proteins, divides, as part of normal B cell homeostasis (Figs. 3 and 4). At this time, the virus turns on expression of “EBNA1 only” to ensure replication of the viral DNA with the cell. BL has the phenotypic (Gregory et al. 1987) and gene expression profile of a light zone (LZ) GC cell (Victora et al. 2012). The LZ is where GC cells express c-myc (Dominguez-Sola et al. 2012) before they begin to proliferate again and also where GC cells reside prior to exit. Thus, this would be the location where viral gene expression would be expected to shut down prior to exiting the GC. Lastly, although BL has the characteristics of a GC cell, the tumor actually grows in extrafollicular locations (Klein et al. 1995). Therefore, a consistent scenario is that BL is derived from a LZ GC cell that has left the follicle to become a resting memory cell but cannot achieve this because it continues to proliferate due to an activated c-myc and therefore constitutively expresses EBNA1 only. This scenario also accounts for the presence of clonal EBV in the tumors because the virus would already be present when the major transformation event, c-myc translocation, occurs.
There are two major infectious players in the predisposition to eBL: malaria and EBV. Recently, experimental evidence has been presented to account for this based on the GCM of EBV and the notion that BL arises from a latently infected GC B cell (Thorley-Lawson and Allday 2008; Torgbor et al. 2014). It is known that expression of the growth program that occurs prior to entry into the GC includes the epigenetic silencing of proapoptotic functions, including bim, an important regulator of myc-induced apoptosis (Allday 2009, 2013). This, together with the antiapoptotic activities associated with the EBNA1, EBERs and micro-RNAs expressed in latently infected GC cells leave these cells more resistant to apoptosis induced by a translocated/deregulated myc gene. The myc translocation itself is believed to be mediated by the enzyme AID which is uniquely expressed in the GC (Ramiro et al. 2004; Robbiani et al. 2008). Infection by P. falciparum malaria has two consequences. First, it increases the viral burden of EBV, resulting in higher numbers of latently infected cells transiting the GC and able to resist apoptosis. Second, it drives deregulated expression of AID in B cells, potentially increasing the frequency of translocation events (Torgbor et al. 2014). Taken together, the increased levels of virus-infected cells and rate of myc translocations in the GC induced by malaria can account for the close association of eBL with malaria and EBV.
6.3.4 X-linked Lymphoproliferative Disease—XLP
For a detailed discussion of EBV infection in primary immunodeficiencies, see the chapter authored by Jeffrey Cohen. XLP is a rare X-linked immunodeficiency (Purtilo et al. 1975; Seemayer et al. 1995) which frequently results in lymphoma or fulminating AIM [reviewed in Bassiri et al. (2008), Purtilo et al. (1975), Seemayer et al. (1995)]. Responses to other virus infections are typically normal, but ~75 % of the boys typically succumb within one month of primary EBV infection. Death is due to the accumulation of EBV-infected B cells expressing the growth program in tissues such as the liver or subsequently by the widespread tissue damage associated with a pronounced virus-associated hemophagocytic syndrome. Surviving boys typically have severely disrupted immune systems, resulting in varying degrees of hypogammaglobulinemia. The XLP gene itself, SH2D1A or SAP (Sayos et al. 1998; Coffey et al. 1998), encodes for a small signaling molecule of 128 amino acids that consists essentially of a single SH2 domain with a small C-terminal extension that is an important regulator of T and NK cell interactions and activation. It has been suggested that the inefficient recognition of SAP-deficient B cells, the target cell for EBV-driven growth, accounts for the disease (Dupre et al. 2005). However, studies in SAP-deficient mice and humans have demonstrated defects in long-term B cell memory (Crotty et al. 2003; Ma et al. 2006) due to their inability to develop functional GCs. This suggests an alternative scenario based on the GCM, namely that these patients may be unable to process latently infected blasts into memory because of their defective GCs. This would result in the infected cells being permanently stuck in the proliferative phase driven by the growth program which, together with the defective T cell response, could lead to uncontrolled proliferation and death.
7 Other Sites of EBV Persistence
7.1 The Epithelium
The role of the epithelium in persistence as a site of viral replication is now broadly accepted. Whether it itself is an independent site of persistent infection is less clear. Two early studies suggested that it is not. Patients undergoing complete bone marrow ablation as part of a bone marrow transplant lost their EBV (Gratama et al. 1988), and patients with XLA, an X-linked genetic disorder where the patients lack B cells, showed no signs of being infected (Faulkner et al. 1999). However, the technical aspects of these papers leave much to be desired and there is a need to reproduce them using modern sensitive, quantitative techniques. This is especially true in light of recent work from the laboratory of Katherine Luzuriaga (Renzette et al. 2014). They have presented intriguing evidence from deep sequencing of EBV within infected individuals that variants are primarily generated over time in saliva not in the blood. This is completely consistent with the GCM concept that the site of latency is a resting memory B cell in the blood which divides only rarely and therefore would accumulate variants extremely slowly, whereas the virus replicates in the epithelium, providing a site for the more rapid accumulation of mutations. However, since (1) the variants would arise as single virions and need to be amplified by reinfection to be detected and (2) the variants are retained over time, this is striking evidence suggesting that the epithelium may be a site where the virus can persist through continuous reinfection and replication in new epithelial plaques.
7.2 The Tonsil Intraepithelial (Marginal Zone) B Cell—A Second Route to Persistence?
The study of persistent infection by EBV has been driven from the start by the property that EBV is able to establish latent persistent infection in vitro by driving newly infected B cells to become latently infected proliferating lymphoblasts expressing the growth program. As a consequence, it was initially assumed that proliferating latently infected lymphoblasts represented the mechanism by which the virus persisted in vivo. We now know from the GCM that this is incorrect. Rather EBV uses lymphoblastoid activation of newly infected naïve B cells transiently in vivo to gain access to the resting memory compartment—the actual site of viral persistence. However, lymphoblastoid activation by EBV infection in vitro is not transient; it results in indefinite proliferation. The question remains therefore: Does extended lymphoblastoid proliferation driven by EBV have a biologically relevant role in vivo? Is this an in vitro counterpart of an in vivo infected, proliferating cell where the virus might persist or is it an artifact of selection for growth in culture?
For a proliferating blast to persist for a long period of time in vivo, it would need to evade CTL, but it turns out that is not so hard to do. One can envision a scenario where infection occurs in vivo, driving the expansion of latently infected lymphoblasts that subsequently stimulate a robust CTL response. The CTLs begin to kill the blasts reducing their numbers, and therefore the antigenic load, leading in turn to attrition of the CTLs. Thus, both populations will be reduced till they reach a point where the time it takes for a CTL to find its target, the lymphoblastoid cell expressing the growth program, exactly equals the time it takes for that cell to die and an equilibrium will have been established (Hawkins et al. 2013). Besides avoiding CTL, long-term proliferation of lymphoblastoid cells also requires that latent proteins have functions specifically evolved to override the cellular mechanisms that normally limit proliferation, i.e., the virus would have to fundamentally change the nature of the B cell (Allday 2013; Price and Luftig 2014). It is now clear that in vitro at least this is indeed the case since EBNA3A and 3C specifically act to override cell cycle checkpoints (Allday 2013). Moreover, this activity is not required for the initial phase of rapid proliferation only coming into play as late as 7 days post-infection. Clearly, this activity must exist to sustain long-term proliferation. Confirmation that these arguments are correct requires that such cells must be demonstrated to exist in vivo. What type of cell might this be?
When naïve B cells are infected in culture, they become activated proliferating blasts that express AID and the memory cell marker CD27 and undergo SHM (Siemer et al. 2008). However, they are unable to undergo CSR (Heath et al. 2012), remain IgD+, and do not express bcl-6 (Siemer et al. 2008), and hence, they would be unable to enter a GC (Kitano et al. 2011). Thus, the phenotype of a lymphoblastoid cell derived from an infected naïve B cell in vitro is IgD+, CD27+, AID+, and bcl-6−, with Ig genes that are somatically mutated but not class-switched [for a complete gene expression profile, see White et al. (2010) and http://www.epstein-barrvirus.org/]. This is reminiscent of resident, tonsil intraepithelial (marginal zone) B cells (Dono et al. 2003; Spencer et al. 1985; Weill et al. 2009; Xu et al. 2007) and completely distinct from GC cells, which are bcl-6+ and undergoing Ig class switching, and GC-derived memory cells, which are IgD− and AID−, and have class-switched Ig genes (Table 3). Can we find such cells in tonsils? The answer is tentatively yes. We have found a population of IgD+ CD27+ B cells in the tonsil that express the growth program and have proliferated extensively (Torgbor and Thorley-Lawson unpublished observations). If it can be confirmed that they are also AID+ and bcl-6− this will be compelling evidence that EBV is able to enter into and persist for some period of time in the resident tonsil intraepithelial (marginal zone) memory B cell compartment.
Table 3.
Lymphoblasts transformed in vitro by EBV most closely resemble activated marginal zone memory B cells
| Marker | Lymphoblasts | Marginal Zone B Cells | Memory B Cells | GC B Cells |
|---|---|---|---|---|
| AID | + | + | − | + |
| bcl-6 | − | − | − | + |
| CD27 | + | + | + | +/− |
| SHM | + | + | + | + |
| CSR | − | − | + | + |
It is important to reiterate that what we are discussing here is the resident, marginal zone-like, intraepithelial, memory compartment of the tonsils. These are thought to be distinct from circulating marginal zone memory cells (Weill et al. 2009; Weller et al. 2004) which have been shown in repeated studies to lack EBV (Joseph et al. 2000b; Souza et al. 2007). The potential presence of EBV in the tonsil subset and absence from the circulating subset support a separate origin for these two types of cells.
There remains much work to be done to investigate this hypothesis not the least of which is whether lymphoblastoid proliferation is a form of long- or short-term persistence, do the cells somehow transition to a resting state, why do they not usually enter the periphery, and how does the virus get back out again from these cells. But the central question remains: What is the biological significance, if any, of long-term proliferation driven by EBV in vivo?
7.3 GC-Independent Maturation of Infected Naïve Blasts
As noted above, direct infection of naïve B cells leads them to become blasts that have many characteristics of memory cells including somatically mutated Ig genes and expression of CD27. This has led to the suggestion that EBV could drive the differentiation of infected naïve B cells all the way to a memory phenotype without the need to access the GC (Heath et al. 2012). This model does not provide a mechanism for the cells to leave the cell cycle and does not account for the different viral latency programs nor the origin of the different lymphomas. More critically, the cells produced in vitro did not undergo CSR and presumably do not express antigen-selected patterns of SHM, two well-known characteristics of the latently infected memory B cells seen in vivo. Rather than contradicting the GCM, these studies actually provide an elegant refinement because the authors reported that the cells would undergo CSR if provided exogenous T cell help which can only be found in the GC (Victora and Nussenzweig 2012). Thus, these studies suggest that infection of naïve B cells in vivo can initiate the GC process, but the cells need to migrate into and through a GC to emerge as class-switched memory B cells with antigen-selected patterns of SHM.
7.4 Two Pathways to Persistence?
The results discussed in the two previous sections raise the intriguing possibility that a newly infected naïve B cell in vivo in Waldeyer’s ring may have two routes to persistence (Fig. 10). The key may lie in the observation that upon initial infection in vitro, cells undergo a brief period (~3 days) of rapid proliferation before transitioning to a stable slower proliferative state that goes on indefinitely—the lymphoblastoid cell line [Nikitin et al. (2010), Thorley-Lawson and Strominger (1978) and see Sect. 3.2.3]. If this occurs in vivo, then the initial phase of rapid proliferation may indicate the initiation of the GC reaction; hence, the cells express AID and begin SHM in the absence of bcl-6 (see preceding sections). Because these cells lack bcl-6, they likely will be unable to physically enter the GC (Kitano et al. 2011). Instead, they will arrest at the T cell/B cell boundary of the GC. The speculation is that if they access T cell help and/or other signals at this time, they would become bcl-6+, proceed into the GC, switch to the default program, and undergo CSR and some version of affinity maturation. It is interesting to note here that one of the latent genes expressed in the growth program, EBNA3B, specifically activates the expression of cytokines that would attract Th cells (White et al. 2012). Eventually, they exit into the periphery as a latently infected GC-derived memory cell as described by the GCM. If, however, after 2–3 days of hyperproliferation, the cells cannot access the necessary signals at the T/B cell boundary, they would transition to the phase of slower, long-term proliferation, remain bcl-6 negative, fail to enter the GC, and instead remain in the inter-follicular lymphoepithelium as latently infected marginal zone B cells.
Fig. 10.
Are there 2 pathways to persistence? The current data suggest the following possible hypothetical model. Infected naive blasts will migrate to the follicle because they express the chemokine receptor EBI2. They express AID and undergo SHM but will not enter the GC because they are bcl-6 negative. If they receive the necessary signals (cytokines/T cell help), they will enter the follicle switch on bcl-6, undergo CSR, and eventually leave as resting memory B cells as described by the GCM (Route 1). If, however, the cells do not receive the necessary signal to turn on bcl-6, they will continue to proliferate as marginal zone memory B cells (Route 2). The ultimate fate of such cells is unclear. For example, to be biologically relevant, they would need to release infectious virus at some point. What is clear is that they appear capable of extensive proliferation despite the presence of CTL
7.5 Direct Infection of Memory Cells
Direct infection of memory B cells was first raised as a possibility over 15 years ago (Babcock et al. 1998) and was subsequently proposed by Rajewsky and coworkers (Kurth et al. 2000, 2003). However, no further evidence for or explanation of a mechanism behind this idea has been produced. Problems with the model include the following:
In repeated experiments, we have never detected evidence for the presence of directly infected memory B cells in the tonsil.
It fails to provide an explanation for the different latency transcription programs and especially why EBV would have a program (the default program) specifically designed to allow the survival of GC B cells.
It has failed to provide evidence or a mechanism for how the directly infected memory B cells transit to a resting state.
It does not explain why EBV in the periphery is restricted only to GC-derived memory B cells.
Predictions made by the model were incorrect when tested experimentally, instead supporting the GCM. Thus, infected GC B cells express the viral default transcription program in vivo (Babcock et al. 2000; Roughan and Thorley-Lawson 2009) (as predicted by the GCM), not the growth program [as predicted by the direct infection model (Siemer et al. 2008)], and in a transgenic mouse model, one of the EBV latent proteins expressed in the GC (LMP2a) was shown to drive B cells to form GCs in the absence of antigen as required by the GCM and contrary to the idea that EBV directly infects memory cells (Casola et al. 2004a).
8 Conclusions
The GC model of EBV infection demonstrates that persistent infection by EBV is a self-renewing circle of infection, differentiation, persistent infection, reactivation, and reinfection (Fig. 4) that elegantly exploits virtually every aspect of mature B cell biology to:
Establish persistent infection (B cell activation with the growth program and GC differentiation with the default program);
Maintain persistent infection (latency in the long-lived memory pool, maintained and regulated through the processes of homeostasis);
Replicate for reinfection and infectious spread (reactivation of viral replication in response to terminal differentiation into plasma cells).
It is this cycle of infection together with the quiescent infection in memory that allows the virus to be maintained at the extremely low and stable level of infection observed. In doing so, EBV does not detectably disrupt the normal processing of latently infected cells into memory.
This remains the only model, consistent with experimental observation that provides a framework for uniting and understanding the disparate behaviors of EBV, for example:
Why does EBV drive the activation and proliferation of B cells which put the host at risk for neoplastic disease? Because the latently infected resting naïve B cell has to become activated so that it can subsequently differentiate through the GC to become a resting memory B cell where it can persist in a state that is no longer a pathogenic risk to the host.
EBV uses a different transcription program in different forms of lymphoma (IL, HD, and BL). Why? The pattern of genes expressed by the different lymphomas is indicative of the infected cell of origin. The existence of lymphomas expressing all three of the transcription programs associated with the proliferation of infected B cells—growth program (IL), default program (HD), and EBNA1 only (BL)—suggests that each of these stages in the EBV life cycle is vulnerable to deregulation leading to lymphoma.
EBV infects and persists in >90 % of the adult human population almost always benignly despite its ability to make cells grow. The proliferating cells are short-lived and not normally a pathogenic threat because the virus is programmed to ensure that they rapidly differentiate into resting memory cells.
LMP1 and LMP2 have signaling properties analogous to T cell help and the BCR, respectively. Why would two EBV latent proteins mimic B cell survival and differentiation signals? LMP1 and LMP2 have these properties because they are replicating the signals that are normally used to rescue and differentiate normal GC B cells into memory. Hence, LMP1 and LMP2 are the only viral regulatory proteins expressed in infected GC cells.
The epitopes on the latent proteins recognized by cytotoxic T cells are conserved (Khanna et al. 1997). Why would the virus do this? Once the virus has colonized the memory compartment, any infected cell that continues to express the growth program is a threat to the host. The virus ensures that any cell population that continues to expand due to the growth program will be eliminated by conserving the targets of EBV-specific CTL.
The GCM has also provided insights into the behavior of memory B cells. Notably, it has overturned the belief that memory cells do not recirculate (Gray et al. 1982) because latently infected memory B cells clearly do recirculate (Laichalk et al. 2002). In addition, the restriction of EBV to the isotype-switched GC-derived memory pool and absence from the marginal zone memory pool in the periphery support the view that these marginal zone memory B cells arise independently of the GC (Weill et al. 2009; Weller et al. 2004). Lastly, the presence of EBV in tonsil intraepithelial (marginal) zone B cells supports the idea that this subset is functionally distinct from the circulating/splenic marginal zone B cell.
9 To Be Continued
Although the details of the GCM are likely to change and much is still to be learned, it seems certain that an ultimate understanding of EBV infection will involve a model, whereby EBV uses the normal biology of mature B lymphocytes to establish and maintain persistent infection. The most interesting unanswered questions that remain about EBV persistence are as follows:
Is/are there GC-independent mechanisms/sites of persistent infection?
What, if any, is the biological significance in vivo of the in vitro phenomenon of long-term lymphoblastoid proliferation?
Why does the virus encode for a BCR surrogate if it is persisting in B cells with an apparently normal BCR?
What is the relative contribution of viral latent proteins (especially LMP1 and LMP2a) and physiologic signals (Th and BCR) to the production of latently infected memory cells? Could the requirement for both be providing us new insights into the complexities involved in producing and maintaining immunological memory?
10 Final Thought—EBV Is Not As Safe As You Might Think!
EBV seems like a pretty safe virus. It infects virtually every human being for life, and the infection is almost always benign. However, XLP arises during acute EBV infection and almost always results in death. It is caused by mutations in the SH2D1A gene (Sayos et al. 1998; Coffey et al. 1998). So all that stands between EBV switching from a benign lifetime persistent infection to a life-threatening acute disease is a single point mutation in the XLP gene. Put another way, the reader of this chapter would likely have expired and not be around to read this if it was not for that single mutation.
Acknowledgments
The work described here is in large part the consequence of research carried out by a number of graduate students in my own laboratory too numerous to mention individually but hopefully appropriately referenced in the text. I would also like to express my thanks to Michael Lawson for a very careful and thorough editing of the text. To the extent that this chapter is comprehendible, it is due to him. Finally, I would like to acknowledge NIH, who have supported my laboratory continuously through Public Health Service grants R01 CA65883 and R01 AI18757.
Abbreviations
- AID
Activation-induced cytidine deaminase
- AIM
Acute infectious mononucleosis
- APOBEC
Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like
- BAFF
B cell activating factor
- BCR
B cell receptor
- BL
Burkitt’s lymphoma
- BLC
B lymphocyte chemoattractant CXCL13
- CD40L
CD40 ligand
- cIg
Cytoplasmically expressed immunoglobulin
- CPM
Cyclic pathogen model
- CtBP
C-terminal-binding protein
- CTL
Cytotoxic T cell
- DZ
Dark zone
- eBL
Endemic Burkitt’s lymphoma
- EBV
Epstein-Barr virus
- EBNA
Epstein-Barr virus nuclear antigen
- GC
Germinal center
- GCM
Germinal center model
- HD
Hodgkin’s disease
- HEV
High endothelial venules
- HIV
Human immunodeficiency virus
- IE
Immediate early
- Ig
Immunoglobulin
- IL
Immunoblastic lymphoma
- LMP
Latent membrane protein
- LZ
Light zone
- RBPJk
Recombining binding protein
- RTPCR
Real-time polymerase chain reaction
- SDF1
Stromal cell-derived factor 1 CXCL12
- sIg
Surface-expressed immunoglobulin
- sBL
Sporadic Burkitt’s lymphoma
- Th
CD4+ T helper cell
References
- Adams A, Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci USA. 1975;72:1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allday MJ. How does Epstein-Barr virus (EBV) complement the activation of Myc in the pathogenesis of Burkitt’s lymphoma? Semin Cancer Biol. 2009;19:366–376. doi: 10.1016/j.semcancer.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allday MJ. EBV finds a polycomb-mediated, epigenetic solution to the problem of oncogenic stress responses triggered by infection. Front Genet. 2013;4:212. doi: 10.3389/fgene.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allday MJ, Crawford DH, Griffin BE. Epstein-Barr virus latent gene expression during the initiation of B cell immortalization. J Gen Virol. 1989;70:1755–1764. doi: 10.1099/0022-1317-70-7-1755. [DOI] [PubMed] [Google Scholar]
- Allday MJ, Sinclair A, Parker G, Crawford DH, Farrell PJ. Epstein-Barr virus efficiently immortalizes human B cells without neutralizing the function of p53. EMBO J. 1995;14:1382–1391. doi: 10.1002/j.1460-2075.1995.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- Ambinder RF. Epstein-barr virus and hodgkin lymphoma. Hematol Am Soc Hematol Educ Program. 2007;2007:204–209. doi: 10.1182/asheducation-2007.1.204. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85:744–750. [PubMed] [Google Scholar]
- Andersson-Anvret M, Forsby N, Klein G, Henle W. Relationship between the Epstein-Barr virus and undifferentiated nasopharyngeal carcinoma: correlated nucleic acid hybridization and histopathological examination. Int J Cancer. 1977;20:486–494. doi: 10.1002/ijc.2910200403. [DOI] [PubMed] [Google Scholar]
- Arrand JJ, Rymo L. Characterisation of the major Epstein-Barr virus specific RNA in Burkitt lymphoma derived cells. J Virol. 1982;41:376–389. doi: 10.1128/jvi.41.2.376-389.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Epstein-Barr virus and multiple sclerosis: epidemiological evidence. Clin Exp Immunol. 2010;160:120–124. doi: 10.1111/j.1365-2249.2010.04121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- Babcock GJ, Decker LL, Freeman RB, Thorley-Lawson DA. Epstein-barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med. 1999;190:567–576. doi: 10.1084/jem.190.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- Baichwal VR, Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988;2:461–467. [PubMed] [Google Scholar]
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, Van KC, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Barton E, Mandal P, Speck SH. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol. 2011;29:351–397. doi: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- Bassiri H, Janice YEOWC, Rothman J, Koretzky GA, Nichols KE. X-linked lymphoproliferative disease (XLP): a model of impaired anti-viral, anti-tumor and humoral immune responses. Immunol Res. 2008;42:145–159. doi: 10.1007/s12026-008-8048-7. [DOI] [PubMed] [Google Scholar]
- Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol. 2010;105:193–210. doi: 10.1016/S0065-2776(10)05007-8. [DOI] [PubMed] [Google Scholar]
- Beaufils P, Choquet D, Mamoun RZ, Malissen B. The (YXXL/I)2 signalling motif found in the cytoplasmic segments of the bovine leukaemia virus envelope protein and Epstein-Barr virus latent membrane protein 2A can elicit early and late lymphocyte activation events. EMBO J. 1993;12:5105–5112. doi: 10.1002/j.1460-2075.1993.tb06205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel D, Kurth J, Unkel C, Kuppers R. Transformation of BCR-deficient germinal-center B cells by EBV supports a major role of the virus in the pathogenesis of Hodgkin and posttransplantation lymphomas. Blood. 2005;106:4345–4350. doi: 10.1182/blood-2005-06-2342. [DOI] [PubMed] [Google Scholar]
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol. 1993;67:2209–2220. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet M, Guinebretiere JM, Kremmer E, Grunewald V, Benhamou E, Contesso G, Joab I. Detection of Epstein-Barr virus in invasive breast cancers. J Natl Cancer Inst. 1999;91:1376–1381. doi: 10.1093/jnci/91.16.1376. [DOI] [PubMed] [Google Scholar]
- Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med. 2002;8:594–599. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Baekkevold ES, Farstad IN, Jahnsen FL, Johansen FE, Nilsen EM, Yamanaka T. Regional specialization in the mucosal immune system: what happens in the micro-compartments? Immunol Today. 1999a;20:141–151. doi: 10.1016/s0167-5699(98)01413-3. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Farstad IN, Haraldsen G. Regional specialization in the mucosal immune system: primed cells do not always home along the same track. Immunol Today. 1999b;20:267–277. doi: 10.1016/s0167-5699(99)01468-1. [DOI] [PubMed] [Google Scholar]
- Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. Epub 2001 Dec 19. [DOI] [PubMed] [Google Scholar]
- Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- Callan MF, Annels N, Steven N, Tan L, Wilson J, McMichael AJ, Rickinson AB. T cell selection during the evolution of CD8+ T cell memory in vivo. Eur J Immunol. 1998a;28:4382–4390. doi: 10.1002/(SICI)1521-4141(199812)28:12<4382::AID-IMMU4382>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Callan MF, Tan L, Annels N, Ogg GS, Wilson JD, O’Callaghan CA, Steven N, McMichael AJ, Rickinson AB. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus In vivo. J Exp Med. 1998b;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone A, Gaidano G, Gloghini A, Larocca LM, Capello D, Canzonieri V, Antinori A, Tirelli U, Falini B, Dalla-Favera R. Differential expression of BCL-6, CD138/syndecan-1, and Epstein-Barr virus-encoded latent membrane protein-1 identifies distinct histogenetic subsets of acquired immunodeficiency syndrome-related non-Hodgkin’s lymphomas. Blood. 1998;91:747–755. [PubMed] [Google Scholar]
- Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004a;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004b;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- Catalina MD, Sullivan JL, Bak KR, Luzuriaga K. Differential evolution and stability of epitope-specific CD8(+) T cell responses in EBV infection. J Immunol. 2001;167:4450–4457. doi: 10.4049/jimmunol.167.8.4450. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Chang CC, Cechova K, Zhang J, Ye BH, Falini B, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. BCL-6 protein is expressed in germinal-center B cells. Blood. 1995;86:45–53. [PubMed] [Google Scholar]
- Chaganti S, Heath EM, Bergler W, Kuo M, Buettner M, Niedobitek G, Rickinson AB, Bell AI. Epstein-Barr virus colonization of tonsillar and peripheral blood B-cell subsets in primary infection and persistence. Blood. 2009;113:6372–6381. doi: 10.1182/blood-2008-08-175828. [DOI] [PubMed] [Google Scholar]
- Chang RA, Miller SD, Longnecker R. Epstein-Barr virus latent membrane protein 2A exacerbates experimental autoimmune encephalomyelitis and enhances antigen presentation function. Sci Rep. 2012;2:353. doi: 10.1038/srep00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee B, Leung CS, Munz C. Animal models of Epstein Barr virus infection. J Immunol Methods. 2014;40:80–87. doi: 10.1016/j.jim.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Chen F, Zou JZ, Di RL, Winberg G, Hu LF, Klein E, Klein G, Ernberg I. A subpopulation of normal B cells latently infected with Epstein-Barr virus resembles Burkitt lymphoma cells in expressing EBNA-1 but not EBNA-2 or LMP1. J Virol. 1995;69:3752–3758. doi: 10.1128/jvi.69.6.3752-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang AK, Tao Q, Srivastava G, Ho FC. Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin’s disease. Int J Cancer. 1996;68:285–290. doi: 10.1002/(SICI)1097-0215(19961104)68:3<285::AID-IJC3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Clute SC, Naumov YN, Watkin LB, Aslan N, Sullivan JL, Thorley-Lawson DA, Luzuriaga K, Welsh RM, Puzone R, Celada F, Selin LK. Broad cross-reactive TCR repertoires recognizing dissimilar Epstein-Barr and influenza A virus epitopes. J Immunol. 2010;185:6753–6764. doi: 10.4049/jimmunol.1000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E, Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini P, Sylla B, Zollo M, Franco B, Bentley DR, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- Collins CM, Speck SH. Expansion of murine gammaherpesvirus latently infected B cells requires T follicular help. PLoS Pathog. 2014;10:e1004106. doi: 10.1371/journal.ppat.1004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- Daskalogianni C, Pyndiah S, Apcher S, Mazars A, Manoury B, Ammari N, Nylander K, Voisset C, Blondel M, Fahraeus R. Epstein-Barr virus-encoded EBNA1 and ZEBRA: targets for therapeutic strategies against EBV-carrying cancers. J Pathol. 2014;235:334–341. doi: 10.1002/path.4431. [DOI] [PubMed] [Google Scholar]
- Deacon EM, Pallesen G, Niedobitek G, Crocker J, Brooks L, Rickinson AB, Young LS. Epstein-Barr virus and Hodgkin’s disease: transcriptional analysis of virus latency in the malignant cells. J Exp Med. 1993;177:339–349. doi: 10.1084/jem.177.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker LL, Klaman LD, Thorley-Lawson DA. Detection of the latent form of Epstein-Barr virus DNA in the peripheral blood of healthy individuals. J Virol. 1996;70:3286–3289. doi: 10.1128/jvi.70.5.3286-3289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker LL, Babcock GJ, Thorley-Lawson DA. Detection and discrimination of latent and replicative herpesvirus infection at the single cell level in vivo. Methods Mol Biol. 2001;174:111–116. doi: 10.1385/1-59259-227-9:111. [DOI] [PubMed] [Google Scholar]
- Delecluse HJ, Hammerschmidt W. The genetic approach to the Epstein-Barr virus: from basic virology to gene therapy. Mol Pathol. 2000;53:270–279. doi: 10.1136/mp.53.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Eckert E, Shapiro M. A model of host response to a multi-stage pathogen. J Math Biol. 2011;63:201–227. doi: 10.1007/s00285-010-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de-Thé G. Epstein-Barr virus and Burkitt’s lymphoma worldwide: the causal relationship revisited. In: Olweny CLM, Lenoir GM, O’conor GT, editors. Burkitt’s Lymphoma a human cancer model. New York: Oxford University Press; 1985. [PubMed] [Google Scholar]
- Dominguez-Sola D, Victora GD, Ying CY, Phan RT, Saito M, Nussenzweig MC, Dalla-Favera R. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol. 2012;13:1083–1091. doi: 10.1038/ni.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dono M, Zupo S, Colombo M, Massara R, Gaidano G, Taborelli G, Ceppa P, Burgio VL, Chiorazzi N, Ferrarini M. The human marginal zone B cell. Ann N Y Acad Sci. 2003;987:117–124. doi: 10.1111/j.1749-6632.2003.tb06039.x. [DOI] [PubMed] [Google Scholar]
- Dupre L, Andolfi G, Tangye SG, Clementi R, Locatelli F, Arico M, Aiuti A, Roncarolo MG. SAP controls the cytolytic activity of CD8+ T cells against EBV-infected cells. Blood. 2005;105:4383–4389. doi: 10.1182/blood-2004-08-3269. [DOI] [PubMed] [Google Scholar]
- Edson CM, Thorley-Lawson DA. Epstein-Barr virus membrane antigens: characterization, distribution, and strain differences. J Virol. 1981;39:172–184. doi: 10.1128/jvi.39.1.172-184.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlin-Henriksson B, Zou JZ, Klein G, Ernberg I. Epstein-Barr virus genomes are found predominantly in IgA-positive B cells in the blood of healthy carriers. Int J Cancer. 1999;83:50–54. doi: 10.1002/(sici)1097-0215(19990924)83:1<50::aid-ijc10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Faulkner GC, Burrows SR, Khanna R, Moss DJ, Bird AG, Crawford DH. X-Linked agammaglobulinemia patients are not infected with Epstein-Barr virus: implications for the biology of the virus. J Virol. 1999;73:1555–1564. doi: 10.1128/jvi.73.2.1555-1564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Biro PA, Fearon DT. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci USA. 1984;81:4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RI, Chilton T, Scott S, Benton L, Howell FV, Vaughan JH. Potential role of Epstein-Barr virus in Sjogren’s syndrome. Rheum Dis Clin North Am. 1987;13:275–292. [PubMed] [Google Scholar]
- Gatto D, Brink R. B cell localization: regulation by EBI2 and its oxysterol ligand. Trends Immunol. 2013;34:336–341. doi: 10.1016/j.it.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Gires O, Zimber-Strobl U, Gonnella R, Ueffing M, Marschall G, Zeidler R, Pich D, Hammerschmidt W. Latent membrane protein 1 of Epstein-Barr virus mimics a con-stitutively active receptor molecule. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, Pallesen G, Gulley ML, Khan G, O’Grady J, Hummel M, Preciado MV, Knecht H, Chan JK, Claviez A. Epstein-Barr virus-associated Hodgkin’s disease: epidemiologic characteristics in international data. Int J Cancer. 1997;70:375–382. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Golden HD, Chang RS, Prescott W, Simpson E, Cooper TY. Leukocyte-transforming agent: prolonged excretion by patients with mononucleosis and excretion by normal individuals. J Infect Dis. 1973;127:471–473. doi: 10.1093/infdis/127.4.471. [DOI] [PubMed] [Google Scholar]
- Gratama JW, Oosterveer MA, Zwaan FE, Lepoutre J, Klein G, Ernberg I. Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency. Proc Natl Acad Sci USA. 1988;85:8693–8696. doi: 10.1073/pnas.85.22.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D, Maclennan IC, Bazin H, Khan M. Migrant mu+ delta+ and static mu+ delta-B lymphocyte subsets. Eur J Immunol. 1982;12:564–569. doi: 10.1002/eji.1830120707. [DOI] [PubMed] [Google Scholar]
- Greenspan JS, Greenspan D, Lennette ET, Abrams DI, Conant MA, Petersen V, Freese UK. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985;313:1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- Gregory CD, Tursz T, Edwards CF, Tetaud C, Talbot M, Caillou B, Rickinson AB, Lipinski M. Identification of a subset of normal B cells with a Burkitt’s lymphoma (BL)-like phenotype. J Immunol. 1987;139:313–318. [PubMed] [Google Scholar]
- Gregory CD, Rowe M, Rickinson AB. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt’s lymphoma cell line. J Gen Virol. 1990;71:1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- Gross AJ, Hochberg D, Rand WM, Thorley-Lawson DA. EBV and systemic lupus erythematosus: a new perspective. J Immunol. 2005;174:6599–6607. doi: 10.4049/jimmunol.174.11.6599. [DOI] [PubMed] [Google Scholar]
- Gulley ML, Raphael M, Lutz CT, Ross DW, Raab-Traub N. Epstein-Barr virus integration in human lymphomas and lymphoid cell lines. Cancer. 1992;70:185–191. doi: 10.1002/1097-0142(19920701)70:1<185::aid-cncr2820700129>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hadinoto V, Shapiro M, Greenough TC, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. On the dynamics of acute EBV infection and the pathogenesis of infectious mononucleosis. Blood. 2008;111:1420–1427. doi: 10.1182/blood-2007-06-093278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadinoto V, Shapiro M, Sun CC, Thorley-Lawson DA. The dynamics of EBV shedding implicate a central role for epithelial cells in amplifying viral output. PLoS Pathog. 2009;5:el000496. doi: 10.1371/journal.ppat.1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. Fundamental Immunology. 6th. Philadelphia: Lippincott Williams and Wilkins; 2008. Chapter 7: B Lymphocyte Development and Biology. [Google Scholar]
- Hawkins JB, Delgado-Eckert E, Thorley-Lawson DA, Shapiro M. The cycle of EBV infection explains persistence, the sizes of the infected cell populations and which come under CTL regulation. PLoS Pathog. 2013;9:e1003685. doi: 10.1371/journal.ppat.1003685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Raab-Traub N, Casali P, Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol. 2003;171:5215–5224. doi: 10.4049/jimmunol.171.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath E, Begue-Pastor N, Chaganti S, Croom-Carter D, Shannon-Lowe C, Kube D, Feederle R, Delecluse HJ, Rickinson AB, Bell AI. Epstein-Barr virus infection of naive B cells in vitro frequently selects clones with mutated immunoglobulin genotypes: implications for virus biology. PLoS Pathog. 2012;8:e1002697. doi: 10.1371/journal.ppat.1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W, Henle G. Seroepidemiology of the virus. In: Epstein MA, Achong BG, editors. The Epstein-Barr virus. Berlin: Springer; 1979. [Google Scholar]
- Henle W, Diehl V, Kohn G, Zur Hausen H, Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967;157:1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Herbst H, Dallenbach F, Hummel M, Niedobitek G, Pileri S, Muller LN, Stein H. Epstein-Barr virus latent membrane protein expression in Hodgkin and Reed-Sternberg cells. Proc Natl Acad Sci USA. 1991;88:4766–1770. doi: 10.1073/pnas.88.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickabottom M, Parker GA, Freemont P, Crook T, Allday MJ. Two nonconsensus sites in the Epstein-Barr virus oncoprotein EBNA3A cooperate to bind the co-repressor carboxyl-terminal-binding protein (CtBP) J Biol Chem. 2002;277:47197–47204. doi: 10.1074/jbc.M208116200. (Epub 2002 Oct 7) [DOI] [PubMed] [Google Scholar]
- Hislop AD, Kuo M, Drake-Lee AB, Akbar AN, Bergler W, Hammerschmitt N, Khan N, Palendira U, Leese AM, Timms JM, Bell AI, Buckley CD, Rickinson AB. Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. J Clin Invest. 2005;115:2546–2555. doi: 10.1172/JCI24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalgrim H, Smedby KE, Rostgaard K, Molin D, Hamilton-Dutoit S, Chang ET, Ralfkiaer E, Sundstrom C, Adami HO, Glimelius B, Melbye M. Infectious mononucleosis, childhood social environment, and risk of Hodgkin lymphoma. Cancer Res. 2007;67:2382–2388. doi: 10.1158/0008-5472.CAN-06-3566. [DOI] [PubMed] [Google Scholar]
- Hoagland RJ. The transmission of infectious mononucleosis. Am J Med Sci. 1955;229:262–272. doi: 10.1097/00000441-195503000-00003. [DOI] [PubMed] [Google Scholar]
- Hoagland RJ. The incubation period of infectious mononucleosis. Am J Public Health Nations Health. 1964;54:1699–1705. doi: 10.2105/ajph.54.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland RJ. Infectious mononucleosis. New York/London: Grune and Stratton Inc; 1967. Infectious mononucleosis. [Google Scholar]
- Hochberg DR, Thorley-Lawson DA. Quantitative detection of viral gene expression in populations of Epstein-Barr virus-infected cells in vivo. Methods Mol Biol. 2005;292:39–56. doi: 10.1385/1-59259-848-x:039. [DOI] [PubMed] [Google Scholar]
- Hochberg D, Middeldorp JM, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Demonstration of the Burkitt’s Lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci USA. 2003a;101:239–244. doi: 10.1073/pnas.2237267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg D, Vorobyova T, Catalina M, Sullivan JS, Luzuriaga K, Thorley-Lawson DA. Acute infection with Epstein-Bar virus targets and overwhelms the memory B cell compartment with latently infected cells. 2003b doi: 10.1128/JVI.78.10.5194-5204.2004. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg D, Souza T, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Acute infection with Epstein-Bar virus targets and overwhelms the peripheral memory B cell compartment with resting, latently infected cells. J Virol. 2004 doi: 10.1128/JVI.78.10.5194-5204.2004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood P, Crawford DH. The role of EBV in post-transplant malignancies: a review. J Clin Pathol. 2000;53:248–254. doi: 10.1136/jcp.53.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y, Katano H, Zou P, Hohman P, Marques A, Tyring SK, Follmann D, Cohen JI. Long-term administration of valacyclovir reduces the number of Epstein-Barr virus (EBV)-infected B cells but not the number of EBV DNA copies per B cell in healthy volunteers. J Virol. 2009;83:11857–11861. doi: 10.1128/JVI.01005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley EA, Thorley-Lawson DA. B cell activation and the establishment of Epstein-Barr virus latency. J Exp Med. 1988;168:2059–2075. doi: 10.1084/jem.168.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman GJ, Binne UK, Parker GA, Farrell PJ, Allday MJ. Activators of the Epstein-Barr virus lytic program concomitantly induce apoptosis, but lytic gene expression protects from cell death. J Virol. 2001;75:2400–2410. doi: 10.1128/JVI.75.5.2400-2410.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakiri D. Epstein-Barr virus-encoded RNAs: key molecules in viral pathogenesis. Cancers (Basel) 2014;6:1615–1630. doi: 10.3390/cancers6031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi KM, Kieff ED. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF- kappaB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997;100:3019–3026. doi: 10.1172/JCI119856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Babcock GJ, Thorley-Lawson DA. Cells expressing the Epstein-Barr virus growth program are present in and restricted to the naive B-cell subset of healthy tonsils. J Virol. 2000a;74:9964–9971. doi: 10.1128/jvi.74.21.9964-9971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Babcock GJ, Thorley-Lawson DA. EBV persistence involves strict selection of latently infected B cells. J Immunol. 2000b;165:2975–2981. doi: 10.4049/jimmunol.165.6.2975. [DOI] [PubMed] [Google Scholar]
- Kaiser C, Laux G, Eick D, Jochner N, Bornkamm GW, Kempkes B. The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J Virol. 1999;73:4481–4484. doi: 10.1128/jvi.73.5.4481-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor AB. The development and repertoire of B-1 cells (CD5 B cells) Immunol Today. 1991;12:389–391. doi: 10.1016/0167-5699(91)90136-H. [DOI] [PubMed] [Google Scholar]
- Kempkes B, Spitkovsky D, Jansen-Durr P, Ellwart JW, Kremmer E, Delecluse HJ, Rottenberger C, Bornkamm GW, Hammerschmidt W. B-cell proliferation and induction of early G1-regulating proteins by Epstein-Barr virus mutants conditional for EBNA2. EMBO J. 1995;14:88–96. doi: 10.1002/j.1460-2075.1995.tb06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy G, Komano J, Sugden B. Epstein-Barr virus provides a survival factor to Burkitt’s lymphomas. Proc Natl Acad Sci USA. 2003;100:14269–14274. doi: 10.1073/pnas.2336099100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney SC, Mertz JE. Regulation of the latent-lytic switch in Epstein-Barr virus. Semin Cancer Biol. 2014;26:60–68. doi: 10.1016/j.semcancer.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan G, Miyashita EM, Yang B, Babcock GJ, Thorley-Lawson DA. Is EBV persistence in vivo a model for B cell homeostasis? Immunity. 1996;5:173–179. doi: 10.1016/s1074-7613(00)80493-8. [DOI] [PubMed] [Google Scholar]
- Khanna R, Burrows SR, Neisig A, Neefjes J, Moss DJ, Silins SL. Hierarchy of Epstein-Barr virus-specific cytotoxic T-cell responses in individuals carrying different subtypes of an HLA allele: implications for epitope-based antiviral vaccines. J Virol. 1997;71:7429–7435. doi: 10.1128/jvi.71.10.7429-7435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E, Rickinson AB. Epstein-Barr virus and its replication. In: Knipe DM, Howley PM, editors. Fields virology. 5th. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- Kirchmaier AL, Sugden B. Plasmid maintenance of derivatives of oriP of Epstein-Barr virus. J Virol. 1995;69:1280–1283. doi: 10.1128/jvi.69.2.1280-1283.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kis LL, Takahara M, Nagy N, Klein G, Klein E. Cytokine mediated induction of the major Epstein-Barr virus (EBV)-encoded transforming protein, LMP-1. Immunol Lett. 2006;104:83–88. doi: 10.1016/j.imlet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Kis LL, Salamon D, Persson EK, Nagy N, Scheeren FA, Spits H, Klein G, Klein E. IL-21 imposes a type II EBV gene expression on type III and type I B cells by the repression of C-and activation of LMP-1-promoter. Proc Natl Acad Sci USA. 2010;107:872–877. doi: 10.1073/pnas.0912920107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Klein G. Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell. 1983;32:311–315. doi: 10.1016/0092-8674(83)90449-x. [DOI] [PubMed] [Google Scholar]
- Klein U, Klein G, Ehlin-Henriksson B, Rajewsky K, Kuppers R. Burkitt’s lymphoma is a malignancy of mature B cells expressing somatically mutated V region genes. Mol Med. 1995;1:495–505. [PMC free article] [PubMed] [Google Scholar]
- Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JS, Lan K, Subramanian C, Robertson ES. Epstein-Barr virus nuclear antigen 3C recruits histone deacetylase activity and associates with the corepressors mSin3A and NCoR in human B-cell lines. J Virol. 2003;77:4261–4272. doi: 10.1128/JVI.77.7.4261-4272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles DM, Cesarman E, Chadburn A, Frizzera G, Chen J, Rose EA, Michler RE. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995;85:552–565. [PubMed] [Google Scholar]
- Kovalchuk AL, Qi CF, Torrey TA, Taddesse-Heath L, Feigenbaum L, Park SS, Gerbitz A, Klobeck G, Hoertnagel K, Polack A, Bornkamm GW, Janz S, III, Morse HC. Burkitt lymphoma in the mouse. J Exp Med. 2000;192:1183–1190. doi: 10.1084/jem.192.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulwichit W, Edwards RH, Davenport EM, Baskar JF, Godfrey V, Raab-Traub N. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci USA. 1998;95:11963–11968. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers R. New insights in the biology of Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2012;2012:328–334. doi: 10.1182/asheducation-2012.1.328. [DOI] [PubMed] [Google Scholar]
- Kuppers R, Rajewsky K. The origin of Hodgkin and Reed/Sternberg cells in Hodgkin’s disease. Annu Rev Immunol. 1998;16:471–493. doi: 10.1146/annurev.immunol.16.1.471. [DOI] [PubMed] [Google Scholar]
- Kurosaki T. Genetic analysis of B cell antigen receptor signaling. Annu Rev Immunol. 1999;17:555–592. doi: 10.1146/annurev.immunol.17.1.555. [DOI] [PubMed] [Google Scholar]
- Kurth J, Spieker T, Wustrow J, Strickler GJ, Hansmann LM, Rajewsky K, Kuppers R. EBV-infected B cells in infectious mononucleosis: viral strategies for spreading in the B cell compartment and establishing latency. Immunity. 2000;13:485–495. doi: 10.1016/s1074-7613(00)00048-0. [DOI] [PubMed] [Google Scholar]
- Kurth J, Hansmann ML, Rajewsky K, Kuppers R. Epstein-Barr virus-infected B cells expanding in germinal centers of infectious mononucleosis patients do not participate in the germinal center reaction. Proc Natl Acad Sci USA. 2003;100:4730–4735. doi: 10.1073/pnas.2627966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzembayeva M, Hayes M, Sugden B. Multiple functions are mediated by the miRNAs of Epstein-Barr virus. Curr Opin Virol. 2014;7C:61–65. doi: 10.1016/j.coviro.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol. 2005;79:1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laichalk LL, Hochberg D, Babcock GJ, Freeman RB, Thorley-Lawson DA. The dispersal of mucosal memory B cells: evidence from persistent EBV infection. Immunity. 2002;16:745–754. doi: 10.1016/s1074-7613(02)00318-7. [DOI] [PubMed] [Google Scholar]
- Lam N, Sugden B. CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal. 2003;15:9–16. doi: 10.1016/s0898-6568(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Leder P. Translocations among antibody genes in human cancer. In: Lenoir GM, O’conor GT, Olweny CLM, editors. Burkitt’s Lymphomaa human cancer model. New York: Oxford University Press; 1985. [Google Scholar]
- Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald MP, Klein G, Kurilla MG, Masucci MG. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci MG. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Natl Acad Sci USA. 1997;94:12616–12621. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Spriggs MK, Kovats S, Turk SM, Comeau MR, Nepom B, Hutt-Fletcher LM. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71:4657–4662. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–126. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- Lotz M, Roudier J. Epstein-Barr virus and rheumatoid arthritis: cellular and molecular aspects. Rheumatol Int. 1989;9:147–152. doi: 10.1007/BF00271872. [DOI] [PubMed] [Google Scholar]
- Ma CS, Pittaluga S, Avery DT, Hare NJ, Maric I, Klion AD, Nichols KE, Tangye SG. Selective generation of functional somatically mutated IgM+ CD27+, but not Ig iso-type-switched, memory B cells in X-linked lymphoproliferative disease. J Clin Invest. 2006;116:322–333. doi: 10.1172/JCI25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macallan DC, Wallace DL, Zhang Y, Ghattas H, Asquith B, de Lara C, Worth A, Panayiotakopoulos G, Griffin GE, Tough DF, Beverley PC. B-cell kinetics in humans: rapid turnover of peripheral blood memory cells. Blood. 2005;105:3633–3640. doi: 10.1182/blood-2004-09-3740. [DOI] [PubMed] [Google Scholar]
- Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- Maclennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Maclennan IC. B-cell receptor regulation of peripheral B cells. Curr Opin Immunol. 1998;10:220–225. doi: 10.1016/s0952-7915(98)80252-5. [DOI] [PubMed] [Google Scholar]
- Mancao C, Hammerschmidt W. Epstein-Barr virus latent membrane protein 2A is a B-cell receptor mimic and essential for B-cell survival. Blood. 2007;110:3715–3721. doi: 10.1182/blood-2007-05-090142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolov G, Manolova Y. Marker band in one chromosome 14 from Burkitt lymphomas. Nature. 1972;237:33–34. doi: 10.1038/237033a0. [DOI] [PubMed] [Google Scholar]
- Martinez-Valdez H, Guret C, de Bouteiller O, Fugier I, Banchereau J, Liu YJ. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J Exp Med. 1996;183:971–977. doi: 10.1084/jem.183.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M, Lam KP, Rajewsky K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 2000;407:636–642. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]
- Matsumoto AK, Martin DR, Carter RH, Klickstein LB, Ahearn JM, Fearon DT. Functional dissection of the CD21/CD19/TAPA-1/Leu-13 complex of B lymphocytes. J Exp Med. 1993;178:1407–1417. doi: 10.1084/jem.178.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Burkhardt AL, Lee JH, Stealey B, Longnecker R, Bolen JB, Kieff E. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- Miyashita EM, Yang B, Lam KM, Crawford DH, Thorley-Lawson DA. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell. 1995;80:593–601. doi: 10.1016/0092-8674(95)90513-8. [DOI] [PubMed] [Google Scholar]
- Miyashita EM, Yang B, Babcock GJ, Thorley-Lawson DA. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B cell. J Virol. 1997;71:4882–4891. doi: 10.1128/jvi.71.7.4882-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy R, Thorley-Lawson DA. Mutational analysis of the transforming function of the EBV encoded LMP-1. Curr Top Microbiol Immunol. 1992;182:359–365. doi: 10.1007/978-3-642-77633-5_46. [DOI] [PubMed] [Google Scholar]
- Mosialos G, Birkenbach M, Yalamanchili R, Vanarsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Nagaoka H, Shinkura R, Begum NA, Honjo T. Discovery of activation-induced cytidine deaminase, the engraver of antibody memory. Adv Immunol. 2007;94:1–36. doi: 10.1016/S0065-2776(06)94001-2. [DOI] [PubMed] [Google Scholar]
- Nanbo A, Sugden A, Sugden B. The coupling of synthesis and partitioning of EBV’s plasmid replicon is revealed in live cells. EMBO J. 2007;26:4252–4262. doi: 10.1038/sj.emboj.7601853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow GR, Wolfert R, McNaughton ME, Cooper NR. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2) J Virol. 1985;55:347–351. doi: 10.1128/jvi.55.2.347-351.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson LJ, Hopwood P, Johannessen I, Salisbury JR, Codd J, Thorley-Lawson D, Crawford DH. Epstein-Barr virus latent membrane protein does not inhibit differentiation and induces tumorigenicity of human epithelial cells. Oncogene. 1997;15:275–283. doi: 10.1038/sj.onc.1201187. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Kremmer E, Herbst H, Whitehead L, Dawson CW, Niedobitek E, von Ostau C, Rooney N, Grasser FA, Young LS. Immunohistochemical detection of the Epstein-Barr virus-encoded latent membrane protein 2A in Hodgkin’s disease and infectious mononucleosis. Blood. 1997;90:1664–1672. [PubMed] [Google Scholar]
- Niedobitek G, Agathanggelou A, Steven N, Young LS. Epstein-Barr virus (EBV) in infectious mononucleosis: detection of the virus in tonsillar B lymphocytes but not in desquamated oropharyngeal epithelial cells. Mol Pathol. 2000;53:37–42. doi: 10.1136/mp.53.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin PA, Yan CM, Forte E, Bocedi A, Tourigny JP, White RE, Allday MJ, Patel A, Dave SS, Kim W, Hu K, Guo J, Tainter D, Rusyn E, Luftig MA. An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe. 2010;8:510–522. doi: 10.1016/j.chom.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K. The nature of lymphoid cell lines and their relationship to the virus. In: Epstein MA, Achong BG, editors. The Epstein-Barr virus. Berlin: Springer; 1979. [Google Scholar]
- Nonkwelo C, Skinner J, Bell A, Rickinson A, Sample J. Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early-passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. J Virol. 1996;70:623–627. doi: 10.1128/jvi.70.1.623-627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Nions J, Allday MJ. Epstein-Barr virus can inhibit genotoxin-induced G1 arrest downstream of p53 by preventing the inactivation of CDK2. Oncogene. 2003;22:7181–7191. doi: 10.1038/sj.onc.1206838. [DOI] [PubMed] [Google Scholar]
- O’nions J, Allday MJ. Deregulation of the Cell Cycle by the Epstein-Barr Virus. In: Vande Woude GF, Klein G, editors. Advances in Cancer Research. New York: Elsevier; 2004. [DOI] [PubMed] [Google Scholar]
- Oudejans JJ, Dukers DF, Jiwa NM, van den Brule AJ, Grasser FA, de Bruin PC, Horstman A, Vos W, van Gorp J, Middeldorp JM, Meijer CJ. Expression of epstein-barr virus encoded nuclear antigen 1 in benign and malignant tissues harbouring EBV. J Clin Pathol. 1996;49:897–902. doi: 10.1136/jcp.49.11.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos D, Victoratos P, Alexiou M, Kollias G, Mosialos G. Comparative analysis of signal transduction by CD40 and the Epstein-Barr virus oncoprotein LMP1 in vivo. J Virol. 2004;78:13253–13261. doi: 10.1128/JVI.78.23.13253-13261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GA, Crook T, Bain M, Sara EA, Farrell PJ, Allday MJ. Epstein-Barr virus nuclear antigen (EBNA)3C is an immortalizing oncoprotein with similar properties to adenovirus E1A and papillomavirus E7. Oncogene. 1996;13:2541–2549. [PubMed] [Google Scholar]
- Pegtel DM, Middeldorp J, Thorley-Lawson DA. Epstein-Barr virus infection in ex vivo tonsil epithelial cell cultures of asymptomatic carriers. J Virol. 2004;78:12613–12624. doi: 10.1128/JVI.78.22.12613-12624.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn I. The role of immunosuppression in lymphoma formation. Springer Semin Immunopathol. 1998;20:343–355. doi: 10.1007/BF00838048. [DOI] [PubMed] [Google Scholar]
- Perry ME. The specialised structure of crypt epithelium in the human palatine tonsil and its functional significance. J Anat. 1994;185(Pt 1):111–127. [PMC free article] [PubMed] [Google Scholar]
- Perry M, Whyte A. Immunology of the tonsils. Immunol Today. 1998;19:414–421. doi: 10.1016/s0167-5699(98)01307-3. [DOI] [PubMed] [Google Scholar]
- Polack A, Hortnagel K, Pajic A, Christoph B, Baier B, Falk M, Mautner J, Geltinger C, Bornkamm GW, Kempkes B. c-myc activation renders proliferation of Epstein-Barr virus (EBV)- transformed cells independent of EBV nuclear antigen 2 and latent membrane protein 1. Proc Natl Acad Sci U S A. 1996;93:10411–10416. doi: 10.1073/pnas.93.19.10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope JH, Home MK, Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968;3:857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Price AM, Luftig MA. Dynamic Epstein-Barr virus gene expression on the path to B-cell transformation. Adv Virus Res. 2014;88:279–313. doi: 10.1016/B978-0-12-800098-4.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtilo DT, Cassel CK, Yang JP, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan’s disease) Lancet. 1975;1:935–940. doi: 10.1016/s0140-6736(75)92004-8. [DOI] [PubMed] [Google Scholar]
- Qu L, Rowe DT. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992;66:3715–3724. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley MF, Gonzalez VD, Granath A, Andersson J, Sandberg JK. CXCR5+ CCR7− CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur J Immunol. 2007;37:3352–3362. doi: 10.1002/eji.200636746. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431–441. doi: 10.1016/s1044579x0200086x. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N, Dambaugh T, Kieff E. DNA of Epstein-Barr virus VIII: B95-8, the previous prototype, is an unusual deletion derivative. Cell. 1980;22:257–267. doi: 10.1016/0092-8674(80)90173-7. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N, Rajadurai P, Flynn K, Lanier AP. Epstein-Barr virus infection in carcinoma of the salivary gland. J Virol. 1991;65:7032–7036. doi: 10.1128/jvi.65.12.7032-7036.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radkov SA, Touitou R, Brehm A, Rowe M, West M, Kouzarides T, Allday MJ. Epstein-Barr virus nuclear antigen 3C interacts with histone deacetylase to repress transcription. J Virol. 1999;73:5688–5697. doi: 10.1128/jvi.73.7.5688-5697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro AR, Jankovic M, Eisenreich T, Difilippantonio S, Chen-Kiang S, Muramatsu M, Honjo T, Nussenzweig A, Nussenzweig MC. AID is required for c-myc/IgH chromosome translocations in vivo. Cell. 2004;118:431–438. doi: 10.1016/j.cell.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Renzette N, Somasundaran M, Brewster F, Coderre J, Weiss ER, McManus M, Greenough T, Tabak B, Garber M, Kowalik TF, Luzuriaga K. Epstein-Barr virus latent membrane protein 1 genetic variability in peripheral blood B cells and oropharyngeal fluids. J Virol. 2014;88:3744–3755. doi: 10.1128/JVI.03378-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressing ME, Horst D, Griffin BD, Tellam J, Zuo J, Khanna R, Rowe M, Wiertz EJ. Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products. Semin Cancer Biol. 2008;18:397–408. doi: 10.1016/j.semcancer.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Rickinson AB, Finerty S, Epstein MA. Mechanism of the establishment of Epstein-Barr virus genome-containing lymphoid cell lines from infectious mononucleosis patients: studies with phosphonoacetate. Int J Cancer. 1977;20:861–868. doi: 10.1002/ijc.2910200607. [DOI] [PubMed] [Google Scholar]
- Robbiani DF, Bothmer A, Callen E, Reina-San-martin B, Dorsett Y, Difilippantonio S, Borland DJ, Chen HT, Corcoran AE, Nussenzweig A, Nussenzweig MC. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell. 2008;135:1028–1038. doi: 10.1016/j.cell.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C, Howe JG, Speck SH, Miller G. Influence of Burkitt’s lymphoma and primary B cells on latent gene expression by the nonimmortalizing P3J-HR-1 strain of Epstein-Barr virus. J Virol. 1989;63:1531–1539. doi: 10.1128/jvi.63.4.1531-1539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, Brenner MK, Heslop HE. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- Roughan JE, Thorley-Lawson DA. The intersection of Epstein-Barr virus with the germinal center. J Virol. 2009;83:3968–3976. doi: 10.1128/JVI.02609-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan JE, Torgbor C, Thorley-Lawson DA. Germinal center B cells latently infected with Epstein-Barr virus proliferate extensively but do not increase in number. J Virol. 2010;84:1158–1168. doi: 10.1128/JVI.01780-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon D, Adori M, Ujvari D, Wu L, Kis LL, Madapura HS, Nagy N, Klein G, Klein E. Latency type-dependent modulation of Epstein-Barr virus-encoded latent membrane protein 1 expression by type I interferons in B cells. J Virol. 2012;86:4701–4707. doi: 10.1128/JVI.06829-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, Oettgen H, de Vries JE, Aversa G, Terhorst C. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- Schaefer BC, Strominger JL, Speck SH. Redefining the Epstein-Barr virus-encoded nuclear antigen EBNA-1 gene promoter and transcription initiation site in group I Burkitt lymphoma cell lines. Proc Natl Acad Sci USA. 1995;92:10565–10569. doi: 10.1073/pnas.92.23.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert TA, Victora GD, Fooksman DR, Kamphorst AO, Mugnier MR, Gitlin AD, Dustin ML, Nussenzweig MC. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med. 2011;208:1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemayer TA, Gross TG, Egeler RM, Pirruccello SJ, Davis JR, Kelly CM, Okano M, Lanyi A, Sumegi J. X-linked lymphoproliferative disease: twenty-five years after the discovery. Pediatr Res. 1995;38:471–478. doi: 10.1203/00006450-199510000-00001. [DOI] [PubMed] [Google Scholar]
- Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992;140:769–774. [PMC free article] [PubMed] [Google Scholar]
- Shibata D, Tokunaga M, Uemura Y, Sato E, Tanaka S, Weiss LM. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol. 1991;139:469–474. [PMC free article] [PubMed] [Google Scholar]
- Siemer D, Kurth J, Lang S, Lehnerdt G, Stanelle J, Kuppers R. EBV transformation overrides gene expression patterns of B cell differentiation stages. Mol Immunol. 2008;45:3133–3141. doi: 10.1016/j.molimm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Farrell PJ. Host cell requirements for efficient infection of quiescent primary B lymphocytes by Epstein-Barr virus. J Virol. 1995;69:5461–5468. doi: 10.1128/jvi.69.9.5461-5468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AJ, Palmero I, Peters G, Farrell PJ. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Corcoran DL, Gottwein E, Frank CL, Kang D, Hafner M, Nusbaum JD, Feederle R, Delecluse HJ, Luftig MA, Tuschl T, Ohler U, Cullen BR. The viral and cellular microRNA targetome in lymphoblastoid cell lines. PLoS Pathog. 2012;8:e1002484. doi: 10.1371/journal.ppat.1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza TA, Stollar BD, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Peripheral B cells latently infected with Epstein-Barr virus display molecular hallmarks of classical antigen-selected memory B cells. Proc Natl Acad Sci USA. 2005;102:18093–18098. doi: 10.1073/pnas.0509311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza TA, Stollar BD, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Influence of EBV on the peripheral blood memory B cell compartment. J Immunol. 2007;179:3153–3160. doi: 10.4049/jimmunol.179.5.3153. [DOI] [PubMed] [Google Scholar]
- Speck SH. EBV framed in Burkitt lymphoma. Nat Med. 2002;8:1086–1087. doi: 10.1038/nm1002-1086. [DOI] [PubMed] [Google Scholar]
- Spencer J, Finn T, Pulford KA, Mason DY, Isaacson PG. The human gut contains a novel population of B lymphocytes which resemble marginal zone cells. Clin Exp Immunol. 1985;62:607–612. [PMC free article] [PubMed] [Google Scholar]
- Spencer J, Perry ME, Dunn-Walters DK. Human marginal-zone B cells. Immunol Today. 1998;19:421–426. doi: 10.1016/s0167-5699(98)01308-5. [DOI] [PubMed] [Google Scholar]
- Stadanlick JE, Cancro MP. BAFF and the plasticity of peripheral B cell tolerance. Curr Opin Immunol. 2008;20:158–161. doi: 10.1016/j.coi.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP, Rosenthal JT, Hakala TR, Jr, Shaw BW, Hardesty RL, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;1:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven NM, Leese AM, Annels NE, Lee SP, Rickinson AB. Epitope focusing in the primary cytotoxic T cell response to Epstein-Barr virus and its relationship to T cell memory. J Exp Med. 1996;184:1801–1813. doi: 10.1084/jem.184.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang G, Rickinson AB. Multiple HLA class I-dependent cytotoxicities constitute the “non-HLA-restricted” response in infectious mononucleosis. Eur J Immunol. 1987;17:1007–1013. doi: 10.1002/eji.1830170717. [DOI] [PubMed] [Google Scholar]
- Sugawara Y, Mizugaki Y, Uchida T, Torii T, Imai S, Makuuchi M, Takada K. Detection of Epstein-Barr virus (EBV) in hepatocellular carcinoma tissue: a novel EBV latency characterized by the absence of EBV-encoded small RNA expression. Virology. 1999;256:196–202. doi: 10.1006/viro.1999.9619. [DOI] [PubMed] [Google Scholar]
- Swanson-Mungerson M, Longnecker R. Epstein-Barr virus latent membrane protein 2A and autoimmunity. Trends Immunol. 2007;28:213–218. doi: 10.1016/j.it.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Swanson-Mungerson MA, Caldwell RG, Bultema R, Longnecker R. Epstein-Barr virus LMP2A alters in vivo and in vitro models of B-cell anergy, but not deletion, in response to autoantigen. J Virol. 2005;79:7355–7362. doi: 10.1128/JVI.79.12.7355-7362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Hori S, Osamura RY, Tsutsumi Y. Reticular crypt epithelium and intra-epithelial lymphoid cells in the hyperplastic human palatine tonsil: an immunohistochemical analysis. Pathol Int. 1995;45:34–44. doi: 10.1111/j.1440-1827.1995.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Tao Q, Ho FC, Loke SL, Srivastava G. Epstein-Barr virus is localized in the tumour cells of nasal lymphomas of NK, T or B cell type. Int J Cancer. 1995;60:315–320. doi: 10.1002/ijc.2910600306. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Hotchin NA, Allday MJ, Amlot P, Rose M, Yacoub M, Crawford DH. Immunohistology of Epstein-Barr virus-associated antigens in B cell disorders from immunocompromised individuals. Transplantation. 1990;49:944–953. doi: 10.1097/00007890-199005000-00022. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA. EBV persistence and latent infection in vivo. In: Robertson ES, editor. Epstein-Barr Virus. Norfolk: Caister Academic Press; 2005. [Google Scholar]
- Thorley-Lawson DA, Allday MJ. The curious case of the tumour virus: 50 years of Burkitt’s lymphoma. Nat Rev Microbiol. 2008;6:913–924. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Babcock GJ. A model for persistent infection with Epstein-Barr virus: the stealth virus of human B cells. Life Sci. 1999;65:1433–1453. doi: 10.1016/s0024-3205(99)00214-3. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Mann KP. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985;162:45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Poodry CA. Identification and isolation of the main component (gp350–gp220) of Epstein-Barr virus responsible for generating neutralizing antibodies in vivo. J Virol. 1982;43:730–736. doi: 10.1128/jvi.43.2.730-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Strominger JL. Reversible inhibition by phosphonoacetic acid of human B lymphocyte transformation by Epstein-Barr virus. Virology. 1978;86:423–431. doi: 10.1016/0042-6822(78)90082-x. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Schooley RT, Bhan AK, Nadler LM. Epstein-Barr virus superinduces a new human B cell differentiation antigen (B-LAST 1) expressed on transformed lymphoblasts. Cell. 1982;30:415–425. doi: 10.1016/0092-8674(82)90239-2. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Nadler LM, Bhan AK, Schooley RT. BLAST-2 [EBVCS], an early cell surface marker of human B cell activation, is superinduced by Epstein Barr virus. Journal of Immunology. 1985;134:3007–3012. [PubMed] [Google Scholar]
- Thorley-Lawson DA, Hawkins JB, Tracy SI, Shapiro M. The pathogenesis of Epstein-Barr virus persistent infection. Curr Opin Virol. 2013;3:227–232. doi: 10.1016/j.coviro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney RJ, Steven N, Young LS, Rickinson AB. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons CF, Dawson DB, Richards CS, Andrews WS, Katz JA. Epstein-Barr virus-associated leiomyosarcomas in liver transplantation recipients. Origin from either donor or recipient tissue. Cancer. 1995;76:1481–1489. doi: 10.1002/1097-0142(19951015)76:8<1481::aid-cncr2820760828>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Torgbor C, Awuah P, Deitsch K, Kalantari P, Duca KA, Thorley-Lawson DA. A multifactorial role for P. falciparum malaria in endemic Burkitt’s lymphoma pathogenesis. PLoS Pathog. 2014;10:e1004170. doi: 10.1371/journal.ppat.1004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touitou R, Hickabottom M, Parker G, Crook T, Allday MJ. Physical and functional interactions between the corepressor CtBP and the Epstein-Barr virus nuclear antigen EBNA3C. J Virol. 2001;75:7749–7755. doi: 10.1128/JVI.75.16.7749-7755.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy SI, Kakalacheva K, Lunemann JD, Luzuriaga K, Middeldorp J, Thorley-Lawson DA. Persistence of Epstein-Barr virus in self-reactive memory B cells. J Virol. 2012;86:12330–12340. doi: 10.1128/JVI.01699-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CN, Liu ST, Chang YS. Identification of a novel promoter located within the Bam HI Q region of the Epstein-Barr virus genome for the EBNA 1 gene. DNA Cell Biol. 1995;14:767–776. doi: 10.1089/dna.1995.14.767. [DOI] [PubMed] [Google Scholar]
- Tugizov SM, Berline JW, Palefsky JM. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med. 2003;9:307–314. doi: 10.1038/nm830. [DOI] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, Palefsky JM. Epstein-Barr virus transcytosis through polarized oral epithelial cells. J Virol. 2013;87:8179–8194. doi: 10.1128/JVI.00443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida J, Yasui T, Takaoka-Shichijo Y, Muraoka M, Kulwichit W, Raab-Traub N, Kikutani H. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science. 1999;286:300–303. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- van Gelder T, Vuzevski VD, Weimar W. Epstein-Barr virus in smooth-muscle tumors. N Engl J Med. 1995;332:1719. doi: 10.1056/nejm199506223322516. [DOI] [PubMed] [Google Scholar]
- Vereide D, Sugden B. Proof for EBV’s sustaining role in Burkitt’s lymphomas. Semin Cancer Biol. 2009;19:389–393. doi: 10.1016/j.semcancer.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereide DT, Seto E, Chiu YF, Hayes M, Tagawa T, Grundhoff A, Hammerschmidt W, Sugden B. Epstein-Barr virus maintains lymphomas via its miRNAs. Oncogene. 2014;33:1258–1264. doi: 10.1038/onc.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Dominguez-Sola D, Holmes AB, Deroubaix S, Dalla-Favera R, Nussenzweig MC. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. Blood. 2012;120:2240–2248. doi: 10.1182/blood-2012-03-415380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrazo AC, Chauchard M, Raab-Traub N, Longnecker R. Epstein-Barr virus LMP2A reduces hyperactivation induced by LMP1 to restore normal B cell phenotype in transgenic mice. PLoS Pathog. 2012;8:e1002662. doi: 10.1371/journal.ppat.1002662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M, Allday MJ. Epstein-Barr virus suppresses a G(2)/M checkpoint activated by genotoxins. Mol Cell Biol. 2000;20:1344–1360. doi: 10.1128/mcb.20.4.1344-1360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. Nonhuman primate models for Epstein-Barr virus infection. Curr Opin Virol. 2013;3:233–237. doi: 10.1016/j.coviro.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova JL, Reynaud CA, Weill JC. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, Groves IJ, Turro E, Yee J, Kremmer E, Allday MJ. Extensive co-operation between the Epstein-Barr virus EBNA3 proteins in the manipulation of host gene expression and epigenetic chromatin modification. PLoS ONE. 2010;5:e13979. doi: 10.1371/journal.pone.0013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, Ramer PC, Naresh KN, Meixlsperger S, Pinaud L, Rooney C, Savoldo B, Coutinho R, Bodor C, Gribben J, Ibrahim HA, Bower M, Nourse JP, Gandhi MK, Middeldorp J, Cader FZ, Murray P, Munz C, Allday MJ. EBNA3B-deficient EBV promotes B cell lymphomagenesis in humanized mice and is found in human tumors. J Clin Invest. 2012;122:1487–1502. doi: 10.1172/JCI58092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JB, Bell JL, Levine AJ. Expression of Epstein-Barr virus nuclear antigen-1 induces B cell neoplasia in transgenic mice. EMBO J. 1996;15:3117–3126. [PMC free article] [PubMed] [Google Scholar]
- Woisetschlaeger M, Yandava CN, Furmanski LA, Strominger JL, Speck SH. Promoter switching in Epstein-Barr virus during the initial stages of infection of B lymphocytes. Proc Natl Acad Sci USA. 1990;87:1725–1729. doi: 10.1073/pnas.87.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TA, Frenkel EP. The atypical lymphocyte. Am J Med. 1967;42:923–936. doi: 10.1016/0002-9343(67)90073-3. [DOI] [PubMed] [Google Scholar]
- Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, Knowles DM, Santini PA, Cerutti A. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- Yao QY, Rickinson AB, Epstein MA. A re-examination of the Epstein-Barr virus carrier state in healthy seropositive individuals. Int J Cancer. 1985;35:35–42. doi: 10.1002/ijc.2910350107. [DOI] [PubMed] [Google Scholar]
- Yates JL, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, Leung C, Nouri-Shirazi M, Orazi A, Chaganti RS, Rothman P, Stall AM, Pandolfi PP, Dalla-Favera R. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type infammation. Nat Genet. 1997;16:161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- Youinou P, Jamin C, Lydyard PM. CD5 expression in human B-cell populations. Immunol Today. 1999;20:312–316. doi: 10.1016/s0167-5699(99)01476-0. [DOI] [PubMed] [Google Scholar]
- Zimber-Strobl U, Strobl LJ. EBNA2 and Notch signalling in Epstein-Barr virus mediated immortalization of B lymphocytes. Semin Cancer Biol. 2001;11:423–434. doi: 10.1006/scbi.2001.0409. [DOI] [PubMed] [Google Scholar]