Abstract
Objective
Currently, there is a lack of objective means to quantify myofascial trigger points (MTrPs) and their core features. Our research compares (1) MTrPs and surrounding myofascial tissue using two-dimensional grayscale ultrasound (2DGSUS) and vibration sonoelastography (VSE); (2) the accuracy of both modes in visualizing MTrPs; (3) ‘active’ and ‘latent’ MTrPs, using VSE; and (4) the accuracy of both modes in visualizing deep and superficially located MTrPs.
Methods
Fifty participants with more than two MTrPs in their quadratus lumborum, longissimus thoracis, piriformis, and gluteus medius muscles were assigned to an active MTrP (low back pain) group or a latent (currently pain free) MTrP group. MTrP identification was based on their essential criteria. An electronic algometer measured repeatedly the tenderness of MTrPs with reference to pressure pain threshold values. A handheld vibrator was applied over MTrPs, while VSE and 2DGSUS readings were taken using an EUB-7500 ultrasound scanner.
Results
There was a significant difference between MTrP strain and that of the immediately surrounding myofascial tissue, as measured using VSE (P = 0·001). VSE visualized all superficial and deep MTrPs with an accuracy of 100% (for both groups); the blinded results obtained using 2DGSUS achieved 33% and 35% accuracy, respectively. There was no significant difference found between the tissue strain ratios of active and latent MTrPs (P = 0·929).
Discussion
Sonoelastography can visualize superficial and deep MTrPs, and differentiate them from surrounding myofascial structure through tissue stiffness and echogenicity. VSE was more accurate than 2DGSUS in visualizing and imaging MTrPs.
Keywords: Low back pain, Myofascial trigger points, Two-dimensional grayscale ultrasound, Vibration sonoelastography
Introduction
Myofascial pain syndrome (MPS) is a major myalgic progenitor in which muscle and musculotendinous pain are the primary symptoms.1 It is one of the most significant chronic problems encountered in clinical practice, with a prevalence as high as 85%–93% in patients presenting to pain management centers.2,3 Central to this syndrome is the myofascial trigger point (MTrP),4,5 with ‘active MTrPs’ (A-MTrPs) — hyperirritable nodules within a taut band of skeletal muscle that are painful on compression and give rise to a typical referred pain pattern or motor dysfunction — responsible for patient complaints. Latent MTrPs (L-MTrPs) have similar clinical features as A-MTrPs, but are not responsible for pain symptoms.6,7
There is lack of objective means in quantifying MPS core features, such as localized taut bands of increased tone enclosing MTrPs.8 Its symptoms include pain and stiffness. Physical examination and pain pressure pain threshold (PPT) values largely depend on the sensitivity, discretion, and skill of the clinician for identification. MTrPs also produce inconsistencies in the patient’s reporting of pain. Hence, there is a degree of subjectivity in using these methods.9
Improvements in therapeutic interventions depend on our ability to objectively diagnose and quantify the effects of various manual therapy treatments. Many conditions remain obscure in origin and are dependent on the clinician’s skills for identification and treatment. This situation is particularly true for the musculoskeletal system, for which MPS remains poorly defined, controversial in nature, and is dependent on qualitative criteria for diagnosis. This is not an inconsequential issue, as a lack of objectivity and quantification can result in an inability to generate an accepted diagnosis, isolate a mechanism of action, or accurately assess the benefits of manual therapy treatment.5,7,8
Emerging techniques focusing on myofascial pain emphasize the diagnostic capability of magnetic resonance elastography (MRE),10 microanalytical methods,11 and, most recently, real-time sonoelastography, an ultrasound-based imaging technique that has shown the potential to assess, quantitate, and even visualize the characteristics of MTrP.12–15
A modality of particular interest to those in the musculoskeletal field, real-time sonoelastography, has been used to assess musculoskeletal disorders, such as traumatic lesions, myositis, neuromuscular disease, and inflammatory lesions. It works on the principle that tissue compression produces strain (displacement).16–18 Strain indicates stiffness and the relative deformation, where stiff tissue shows less strain compared to softer tissue when subjected to identical force.19–21
Vibration sonoelastography (VSE) comprises a color-gradient, Doppler flow measurement system that images vibration patterns and calculates tissue strain from the low-frequency, external shear waves that propagate through deep tissue.22,23 On color-gradient VSE images, a relaxed muscle structure with normal fascia will appear mostly soft (green, yellow, red), while contracted or degenerated muscle fiber or fascia will appear hard (blue) due to the local decrease in peak vibration amplitude at the lesion.16,18,24 Color-gradient alteration detected by VSE has been used as a quantitative method in assessing muscle and tendon stiffness in numerous musculoskeletal disorders.17–19,24
There is, however, a paucity of published research investigating the ability of VSE to image MTrPs.12–15 Such studies as do exist tend to focus on MTrPs in the upper trapezius due to their high prevalence and the muscle’s easy accessibility. Applications of sonoelastography in muscles deep within the body are limited by the depth of the penetration of ultrasound, due to its physical properties, such as acoustic velocity, attenuation, acoustic impedance, reflection, and scattering.25 Hence, there is a need to investigate its capability of imaging deep MTrPs located in low back muscles, and in quantifying the degree of tissue stiffness through tissue strain measurements. Furthermore, no studies have been conducted that compare the relative accuracies of different modes of sonoelastography.
Hence, the purpose of our study was to compare: (1) MTrPs and surrounding myofascial tissue using real-time two-dimensional grayscale ultrasound (2DGSUS) and VSE in the quadratus lumborum, longissimus thoracis, piriformis, and gluteus medius muscles; (2) the accuracies of both these modalities in visualizing MTrPs; (3) active and latent MTrPs using VSE; and (4) the accuracy of both in visualizing deep and superficially located MTrPs.
Methods
Study population and selection
This study was conducted at the Alfa Scan Radiology Center in Cairo from January 2011 until July 2012. We recruited 50 participants — male and female, with ages ranging from 25 to 45 years — from the outpatient clinic of the School of Physical Therapy at Cairo University. Individuals with more than two active or latent MTrPs in the quadratus lumborum, longissimus thoracis, piriformis, or gluteus medius muscles were assigned to one of two equally sized group s— the A-MTrPs group or the L-MTrPs group — with 25 participants in each group. At the time of entrance into our study, each participant was in the initial stage of assessment toward undergoing the standard care of treatment for his/her condition by physical therapy.
All participants provided written informed consent to participate in our study. The Board Council of Higher Education of the School of Physical Therapy, the Institutional Review Board of Higher Education and Research of Cairo University, and the Supreme Council of Universities at Egypt approved our study. The study is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12614000192684).
Inclusion and exclusion criteria
To be included in our study, participants with A-MTrPs must have experienced low back pain consistently over the 3 months before the investigation and must not have received any physical therapy for at least 3 months. The cause of the back pain was diagnosed by an orthopedic physician to be low back dysfunction; specifically, musculoskeletal strain due to postural imbalances. Its hallmark was a consistent movement loss (inability to move through full range of motion) and pain at the end of the range of movement. When the patient moved away from the end range, his/her pain decreased. An X-ray and magnetic resonance imaging scan ruled out any spinal involvement, such as herniated or prolapsed disc, and spondylolisthesis. Participants with L-MTrPs were currently ‘pain-free’ (they had experienced no low back pain over the preceding 3 months), but had been referred for treatment for other conditions. Individuals with ‘normal’ (20–24·9) and ‘overweight’ (25–29·9) body mass indexes (BMIs) were included in the study. Criteria for exclusion from our study included active rheumatoid arthritis; fractures and structural deformities of the trunk, hip, knee, and ankle joints; neurological symptoms; previous spinal surgery; pregnancy; or a BMI of class II (‘severely obese’) or III (‘very severely obese’).
MTrP identification and clinical examination
All participants underwent a physical examination carried out by a single licensed and certified manual physical therapist, who determined the presence or absence of superficial and deep A-MTrPs and L-MTrPs, according to the allocations and standard clinical criteria defined by Travell and Simons.26
Flat and pincer palpation techniques were utilized wherein each muscle was placed in a stretched-up position (lengthening and taking up the slack of muscle fibers) to open up and widen the space within which even deeply located MTrPs were found. A-MTrPs were identified by the presence of a nodule in a palpable taut band of muscle fiber that was tender upon palpation and was responsible for the patient’s present pain complaint.6,26 The L-MTrPs were identified with the same criteria of the A-MTrPs, but produced no clinical sensory complaint. In this study, any local region of myofascial tissue in which nodules were absent to palpation was defined as ‘normal’ or uninvolved.
To ensure intra-rater consistency in finding the same MTrP in each muscle, palpation was carried out three times on the identified site, locating the same MTrP every time in the identical spot each time. The examiner then marked MTrP sites and recorded them as ‘active’ or ‘latent’ and ‘superficial’ or ‘deep’. If less than two nodules were identified, palpation continued until the examiner was satisfied that only one nodule or none were present in the muscle.
For diagnostic accuracy and to establish a gold standard reference, a handheld digital electronic algometer (Force One FDI; Wagner Instruments, Greenwich, CT, USA) was used on the marked sites to measure MTrP tenderness by determining the PPT value. The examiner took three consecutive measurements of PPT levels at intervals of 20 seconds. Furthermore, the intra-rater reliability of the pressure algometer was assessed using the intraclass correlation coefficient (ICC). The value of the ICC ranged from 0 to 1, with values closer to 1 representing higher reliability. Three measurements by the pressure algometer on quadratus lumborum muscles were taken by the same examiner on 10 patients on three different occasions.
We identified multiple sites according to the criteria of Travell and Simons’ Trigger Point Manual for superficial and deep MTrPs:6,26
-
•
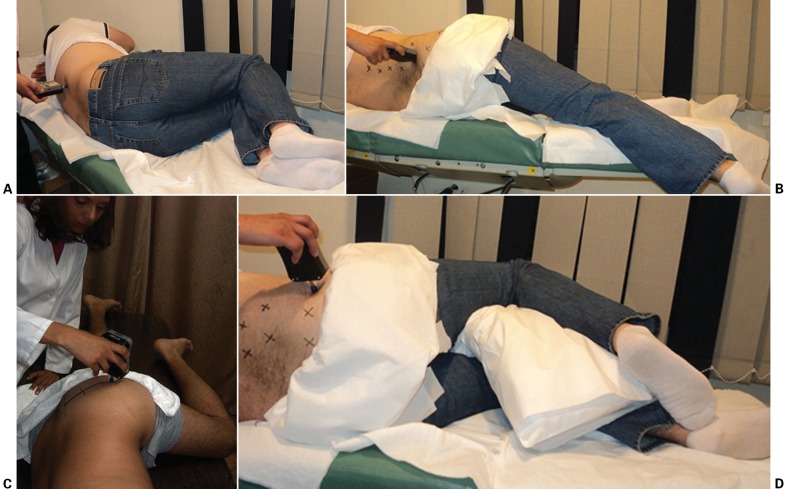
longissimus thoracis — the participants were positioned lying on his/her side with the knees taken toward the chest (Fig. 1A). MTrPs were identified in two regions: the lower thoracic region, near thoracic vertebrae T10 and T11, and the upper lumbar region, near lumbar vertebra L1, where both MTrPs were considered superficial ones;
-
•
quadratus lumborum — the participants were positioned lying on his/her side with the uppermost arm raised above the head (to elevate their rib cage) and the uppermost leg extended and adducted (to pull the side of the pelvis distally, lowering the iliac crest). MTrPs were located in two regions: deep MTrPs were found in the angle where the crest of ilium and paraspinal muscle mass meet, near the level of the L4 transverse process; superficial MTrPs were found in the angle where the paraspinal muscle mass and the twelfth rib meet (Fig. 1B);
-
•
piriformis — the participants were positioned lying on his/her side with the uppermost thigh adducted and flexed at a 90° angle. The piriformis line was identified (from the upper border of the greater trochanter through the sacroiliac cephalic end of the greater sciatic foramen at the sacrum), and then divided into equal thirds. MTrPs were located in two regions: at the medial end of the piriformis line, medial to the greater sciatic foramen, and lateral to the junction of the middle and lateral thirds of the piriformis line. All piriformis MTrPs were considered deep (Fig. 1C);
-
•
gluteus medius — the participants were positioned lying on his/her side with the uppermost thigh flexed. MTrPs were located in two regions: deep MTrPs were found just below the iliac crest, in the posterior portion of the muscle near the sacroiliac joint; superficial MTrPs were found centrally along the length of the crest until near the anterior superior iliac spine below the iliac crest (Fig. 1D).
Figure 1.
MTrP identification and PPT measurement, while muscles placed in stretched-up position: (A) longissimus thoracis; (B) quadratus lumborum; (C) piriformis; (D) gluteus medius.
Real-time sonoelastography imaging procedure
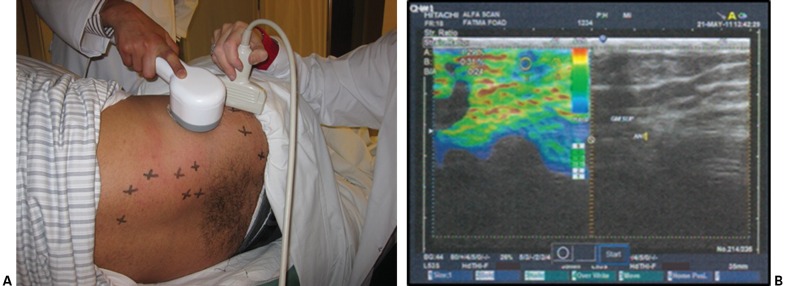
Following the MTrP clinical examination and after algometric measurements were obtained, each participant underwent a sonoelastographic examination, incorporating an EUB-7500 sonoelastography device (Hitachi Medical Systems, Tokyo, Japan) with a 10-cm linear transducer head that was applied perpendicularly above the MTrP sites. Each muscle was placed in the same stretched position used for the algometric measurements. A calibrated, handheld, mechanical massager (Beurer, Soflinger, Germany; 230 V, 11 W, ∼70 Hz) was applied within 1–2 cm of each site in order to induce shear waves, while sonoelastographic readings were taken (Fig. 2A). A certified radiologist scanned the MTrP sites, locating and scoring the MTrPs when the degree of vibration reached ‘4’ on the scale. Both modes — VSE (E-color mode) and 2DGSUS (B-mode) — were performed at the same time (Fig. 2B).
Figure 2.
(A) Positioning of the mechanical massager and the sonoelastography transducer head; (B) screenshot of sonoelastography modes.
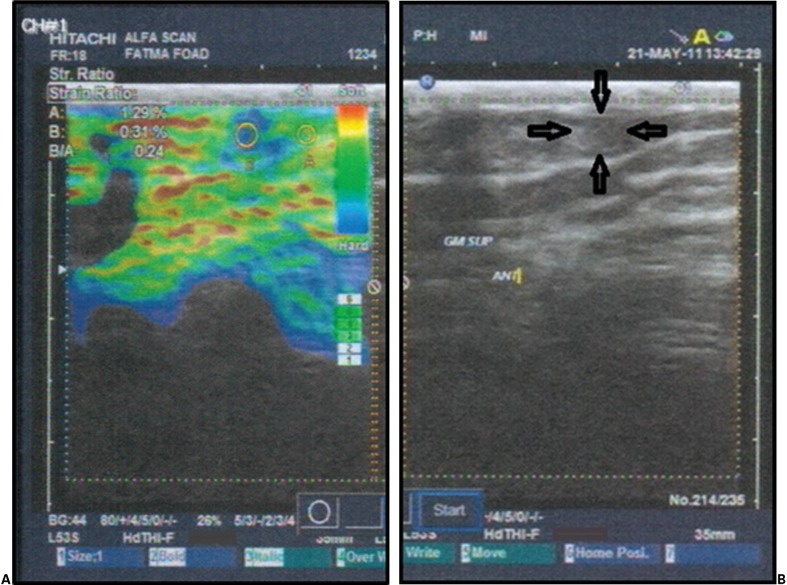
The VSE measured and calculated the tissue strain of the MTrPs and surrounding myofascial structure, as well as the strain ratio, through a built-in computer. It identified stiffness by way of a colored image, as seen in Fig. 3A, in which the blue coloring represented the stiffest tissue and the green, yellow, and red areas represented the softer tissue.24
Figure 3.
(A) E-color mode (A = strain of normal myofascial tissue, B = strain of MTrP, B/A = strain ratio); (B) B-mode (arrows mark the boundaries of the MTrP).
The 2DGSUS, with a frequency of 7–14 MHz, was applied to the clinically identified MTrPs in order to assess the echogenicity of the MTrPs and their surrounding myofascial structure (Fig. 3B). Tissue imaging scores were assigned as ‘0’, for uniform echogenicity (gray-white shadow); ‘1’, for a focal hypoechoic region with a stiff nodule (gray-black contour shadow); and ‘2’, for multiple hypoechoic regions with stiff nodules (multiple dark gray-black contour shadows).14
To compare the relative accuracies of both modes, the images were printed out and a second radiologist was asked to identify, locate, and score the MTrPs on the 2DGSUS image. This radiologist was blinded from the colored VSE image at first, and then shown the colored E-mode image and asked to locate and score MTrPs again.
Data analysis
Statistical analysis was completed using IBM SPSS Statistics 21·0 software. Descriptive statistics were used to compare the means and standard deviations of the participants’ characteristics. The independent t-test and paired-samples t-test were used for comparison within and between groups. The Wilcoxon matched pairs (signed-ranks) test was used to compare echo scores. The level of significance was accepted as P<0·05. Positive and negative predictive values (PPVs, NPVs) of the receiver-operating characteristic curve were used to measure accuracy. The ICC test was used to measure the intra-rater reliability of the pressure algometer. A statistical power analysis suggested that the sample size of 50 participants was adequate to achieve more than 80% power.
Results
Table 1 lists the general physical characteristics of the 50 participants in our study. A total of 153 A-MTrP and 159 L-MTrP sites were evaluated using the electronic algometer and imaged via real-time sonoelastography. Of these, 79 A-MTrPs and 85 L-MTrPs were located superficially and 74 A-MTrPs and 74 L-MTrPs were located deeply in the longissimus thoracis, quadratus lumborum, gluteus medius, and piriformis muscles.
Table 1.
Physical characteristics of patients in both groups (A-MTrPs, L-MTrPs)
| Characteristics | A-MTrP |
L-MTrP |
Comparison |
||||
|---|---|---|---|---|---|---|---|
| M | ±SD | M | ±SD | t value | P value | S/NS* | |
| Age (years) | 34·28 | ±6·17 | 35·8 | ±5·91 | 0·88 | 0·37 | NS |
| Weight (kg) | 66·56 | ±9·91 | 70·21 | ±10·59 | 1·25 | 0·21 | NS |
| Height (m) | 1·64 | ±0·09 | 1·66 | ±0·08 | 1·02 | 0·3 | NS |
| BMI (kg/m2) | 24·54 | ±2·75 | 25·07 | ±2·41 | 0·73 | 0·46 | NS |
Note: *S/NS, significant, non-significant; A-MTrPs, active myofascial trigger points; L-MTrPs, latent myofascial trigger points.
The ICC for intra-rater reliability of the pressure algometer on quadratus lumborum was 0·96, which indicates high reliability. Comparing between groups, active sites had significantly lower PPT than latent sites. Average PPT repeated measurements are listed in Table 2 (P<0·05).
Table 2.
Mean, standard deviation, t, and P values of average of PPT repeated measurement
| LT super. | LT super. | QL super. | QL super. | QL deep | QL deep | GMed super. | GMed super. | GMed deep | GMed deep | PF deep | PF deep | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMTrP | LMTrP | AMTrP | LMTrP | AMTrP | LMTrP | AMTrP | LMTrP | AMTrP | LMTrP | AMTrP | LMTrP | |
| M | 0·45 | 3·81 | 0·6 | 3·08 | 0·53 | 3·4 | 0·35 | 3·5 | 0·39 | 3·39 | 0·38 | 2·86 |
| ±SD | ±0·24 | ±1·11 | ±0·59 | ±1·27 | ±0·33 | ±1·43 | ±0·1 | ±1·55 | ±0·02 | ±1·6 | ±0·17 | ±1·25 |
| M diff. | 3·36 | 2·47 | 2·87 | 2·7 | 2·99 | 2·47 | ||||||
| t value | 9·3 | 6·68 | 8·87 | 5·74 | 7·39 | 7·83 | ||||||
| P value | 0·0001 | 0·0001 | 0·0001 | 0·0001 | 0·0001 | 0·0001 |
Note: PPT, pressure pain threshold; LT, longissimus thoracis; QL, quadratus lumborum; GMed, gluteus medius; PF, piriformis; super., superficial; M diff., mean difference.
Figure 4 shows within-group comparisons of the VSE results. We found a significant difference in the paired t-tests, in which A-MTrP (Table 3) and L-MTrP (Table 4) had lower strain scores than those of normal tissue, with a P value <0·0001. Comparing between groups, we found no significant difference between the tissue strains of the A-MTrPs and those of the L-MTrPs (Table 5).
Figure 4.
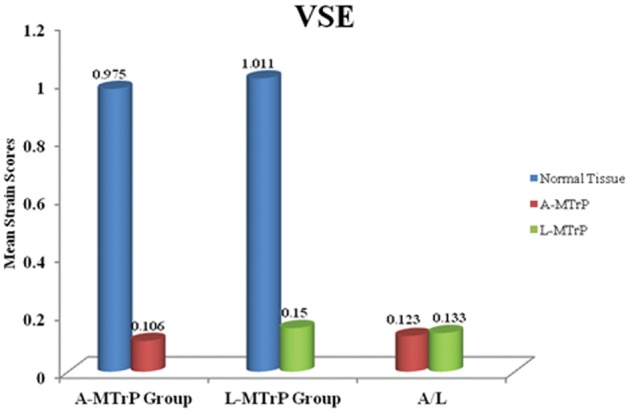
Means of the VSE strain scores. MTrPs, myofascial trigger points; PPT, pressure pain threshold; VSE, vibration sonoelastography.
Table 3.
Strain scores (VSE) of A-MTrPs versus normal tissue
| LT sup. | LT sup. | QL sup. | QL sup. | QL deep | QL deep | GMed sup. | GMed sup. | GMed deep | GMed deep | PF deep | PF deep | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A-MTrP | Normal tissue | A-MTrP | Normal tissue | A-MTrP | Normal tissue | A-MTrP | Normal tissue | A-MTrP | Normal tissue | A-MTrP | Normal tissue | |
| M | 0·08 | 0·85 | 0·1 | 0·83 | 0·09 | 1·07 | 0·15 | 0·9 | 0·1 | 0·96 | 0·12 | 1·24 |
| ±SD | ±0·07 | ±0·43 | ±0·09 | ±0·47 | ±0·11 | ±0·58 | ±0·09 | ±0·29 | ±0·11 | ±0·45 | ±0·12 | ±0·44 |
| M diff. | 0·77 | 0·73 | 0·97 | 0·74 | 0·85 | 1·11 | ||||||
| t value | 5·69 | 6·58 | 8·19 | 9·29 | 8·57 | 10·8 | ||||||
| P value | 0·0001 | 0·0001 | 0·0001 | 0·0001 | 0·0001 | 0·0001 |
Note: LT, longissimus thoracis; QL, quadratus lumborum; GMed, gluteus medius; PF, piriformis; super., superficial; M diff., mean difference.
Table 4.
Strain scores (VSE) of L-MTrPs versus normal tissue
| LT super. | LT super. | QL super. | QL super. | QL deep | QL deep | GMed super. | GMed super. | GMed deep | GMed deep | PF deep | PF deep | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-MTrP | Normal tissue | L-MTrP | Normal tissue | L-MTrP | Normal tissue | L-MTrP | Normal tissue | L-MTrP | Normal tissue | L-MTrP | Normal tissue | |
| M | 0·19 | 1·16 | 0·13 | 0·91 | 0·13 | 0·94 | 0·14 | 0·97 | 0·15 | 0·97 | 0·16 | 1·12 |
| ±SD | ±0·16 | ±0·37 | ±0·05 | ±0·38 | ±0·13 | ±0·4 | ±0·12 | ±0·45 | ±0·14 | ±0·58 | ±0·13 | ±0·47 |
| M diff. | 0·97 | 0·78 | 0·8 | 0·83 | 0·82 | 0·95 | ||||||
| t value | 13·14 | 7·34 | 10·57 | 9·58 | 8·13 | 10·91 | ||||||
| P value | 0·0001 | 0·0001 | 0·0001 | 0·0001 | 0·0001 | 0·0001 |
Note: LT, longissimus thoracis; QL, quadratus lumborum; GMed, gluteus medius; PF, piriformis; super., superficial; M diff., mean difference.
Table 5.
Strain scores (VSE) of A-MTrPs and L-MTrPs
| LT super. | LT super. | QL super. | QL super. | QL deep | QL deep | GMed super. | GMed super. | GMed deep | GMed deep | PF deep | PF deep | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A-MTrP | L-MTrP | A-MTrP | L-MTrP | A-MTrP | L-MTrP | A-MTrP | L-MTrP | A-MTrP | L-MTrP | A-MTrP | L-MTrP | |
| M | 0·13 | 0·13 | 0·13 | 0·14 | 0·1 | 0·15 | 0·18 | 0·13 | 0·1 | 0·12 | 0·1 | 0·13 |
| ±SD | ±0·11 | ±0·1 | ±0·11 | ±0·07 | ±0·11 | ±0·12 | ±0·11 | ±0·09 | ±0·11 | ±0·07 | ±0·09 | ±0·09 |
| M diff. | 0·006 | 0·01 | 0·05 | 0·05 | 0·01 | 0·03 | ||||||
| t value | 0·14 | 0·38 | 1·23 | 1·28 | 0·48 | 1·19 | ||||||
| P value | 0·88 | 0·7 | 0·22 | 0·2 | 0·62 | 0·24 |
Note: LT, longissimus thoracis; QL, quadratus lumborum; GMed, gluteus medius; PF, piriformis; super., superficial; M diff., mean difference.
With the 2DGSUS (B-mode), non-blinded method, we found that MTrPs (active or latent) appeared as one focal hypoechoic region (0·0), whereas the normal, immediately surrounding myofascial tissue showed uniform echogenicity (1·0), with a P value <0·001. The accuracy of locating, visualizing, and imaging the superficial and deep, active and latent MTrPs by VSE (Table 6) was 100% sensitive. However, the accuracy of the 2DGSUS in locating MTrPs using the blinded method was 33% for active and 35% latent sites; it was 100% sensitive with the non-blinded method (Table 7).
Table 6.
Accuracy of VSE (E-mode)
| A-MTrP | L-MTrP | Superficial MTrP | Deep MTrP | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | Present | No. | Absent | No. | Present | No. | Absent | No. | Present | No. | Absent | No. | Present | No. | Absent | No. |
| Positive | True +ve | 153 | False +ve | 0 | True +ve | 159 | False +ve | 0 | True +ve | 166 | False +ve | 0 | True +ve | 146 | False +ve | 0 |
| Negative | False −ve | 0 | True −ve | 115 | False −ve | 0 | True −ve | 91 | False −ve | 0 | True −ve | 133 | False −ve | 0 | True −ve | 51 |
| Total | 153 | 115 | 159 | 91 | 166 | 133 | 146 | 51 | ||||||||
| Sensitivity | 100% | 100% | 100% | 100% | ||||||||||||
| Specificity | 100% | 100% | 100% | 100% | ||||||||||||
| PPV | 100% | 100% | 100% | 100% | ||||||||||||
| NPV | 100% | 100% | 100% | 100% |
Table 7.
Accuracy of two-dimensional grayscale ultrasound (B-mode)
| Mode | Blinded radiologist | Non-blinded radiologist | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MTrP | A-MTrP | L-MTrP | A-MTrP | L-MTrP | ||||||||||||
| Test | Present | No. | Absent | No. | Present | No. | Absent | No. | Present | No. | Absent | No. | Present | No. | Absent | No. |
| Positive | True +ve | 51 | False +ve | 0 | True +ve | 55 | False +ve | 0 | True +ve | 153 | False +ve | 0 | True +ve | 159 | False +ve | 0 |
| Negative | False −ve | 102 | True −ve | 115 | False −ve | 104 | True −ve | 91 | False −ve | 0 | True −ve | 115 | False −ve | 0 | True −ve | 91 |
| Total | 153 | 115 | 159 | 91 | 153 | 115 | 159 | 91 | ||||||||
| Sensitivity | 33% | 35% | 100% | 100% | ||||||||||||
| Specificity | 100% | 100% | 100% | 100% | ||||||||||||
| PPV | 100% | 100% | 100% | 100% | ||||||||||||
| NPV | 53% | 47% | 100% | 100% |
Discussion
Our study investigated the ability of a real-time sonoelastographic technique to visualize and image superficially and deeply located active or latent MTrPs in low back muscles, and to discriminate them from normal myofascial tissue in the surrounding area. We found that VSE was an effective method for imaging the relative distribution of vibration amplitude in the normal myofascial tissue and for detecting localized regions of stiffness (where MTrPs were represented by blue colored areas, discriminating them from normal myofascial tissue, which was represented by green, red, and yellow colored areas) with 100% sensitivity.
This quantitative method also measured and distinguished site type through calculating and comparing strain ratios. Comparing tissue strains, we found that the MTrPs (active and latent) had lower tissue strains than those of the normal, contiguous myofascial tissue, indicating that MTrPs are much stiffer than normal myofascial tissue in terms of tissue strain.
Results from the sonoelastography imaging of both modes confirmed that significant tissue abnormalities and morphological changes are associated with MTrPs. Differences in the tissue stiffness and echogenicity of the MTrPs, compared to those of the surrounding myofascial tissue, suggest a disruption of normal muscle fiber structure and a change in local tissue characteristics. The lower strain ratios and hypoechoic regions of the nodules may be indicative of contraction knots resulting from increased muscle fiber contraction and recruitment, local injury, and/or localized regions of ischemia.5,6
However, we were unable to image or visualize the taut bands associated with the MTrPs, either through the colored images or by using strain measurements. A reason for this might be that the existence of a taut band was demonstrated by MRE, and it was found that the stiffness of the taut bands may be 50% greater than that of the surrounding muscle tissue10 — hence, the taut band itself is not sufficiently stiff, as is the MTrP, to be differentiated from the normal, immediately surrounding myofascial tissue.
We found no significant difference between the A-MTrP and L-MTrP strain ratios, indicating that A-MTrPs and L-MTrPs have the same degree of tissue stiffness, regardless of whether it is painful or not. In contrast, we found that A-MTrPs were more tender (with lower PPT values) than L-MTrPs. It would seem logical to suppose that, as the stiffness of tissue increases beyond the norm, there would be an associated increase in pain sensitivity. However, in our study, we found no correlation between the stiffness of MTrPs and PPT scores for either latent or active sites.
This finding implies that, although A-MTrPs had lower PPT scores (more tender) than L-MTrPs, stiffness and pain are not directly linked. The data suggest that other mechanisms could be contributing independently to MTrP stiffness and pain sensitivity. For example, the PPT may depend on the presence of a sensitizing biochemical, such as one of the catecholamines or neuropeptides.11
The integrated trigger point hypothesis proposes that MTrPs form as a result of muscle overuse.6 That is, patients usually complain of pain that is linked to MTrPs following acute, repetitive, extended, or chronic muscle overload. For example, a decrease in PPT in L-MTrPs was found after 20 minutes of continuous piano playing.27 A separate study found that continuously typing on a computer for 30 minutes also resulted in MTrP development.28
Even the lowest level of normal muscle contractions associated with various movement patterns results in an increase in Ca2+ release, increased metabolic demands, energy exhaustion, the release of multiple cytokines, and capillary constriction.5 With continuous low-level contractions, intramuscular pressure increases significantly, particularly near muscle insertions or attachments, resulting in an impaired local circulation. This ultimately leads to hypoxia and ischemia, which results in muscle fiber degeneration and the development of MTrPs.4,5,29,30 Ischemia and hypoxia at the site of an MTrP sensitizes peripheral and central nociceptors causing increased pain and tenderness.31
The biochemical environment of A-MTrPs has been found to be more acidic, featuring elevated levels of inflammatory mediators, catecholamines, neuropeptides, and proinflammatory cytokines.11,32 These biochemicals are known to be associated with persistent pain states, where myofascial tenderness, intercellular signaling, and inflammation may help to explain the sensory abnormalities associated with the increased sensitivity A-MTrPs when compared to L-MTrPs, in our study.
Regarding the accuracy of the visualization and imaging of MTrPs by both modes, we found the VSE had advanced diagnostic accuracy in locating, visualizing, and imaging all superficial and deep A-MTrPs and L-MTrPs (with 100% sensitivity), compared to that of the 2DGSUS. We found that, when the radiologist was blinded from the VSE colored image, the accuracy of locating MTrPs using 2DGSUS was 33% and 35% sensitivity for A-MTrPs and L-MTrPs, respectively — and, when the same radiologist was shown the colored VSE image, accuracy was 100%.
These results indicate that the typical presentation of MTrPs using 2DGSUS alone cannot be produced easily through imaging the defined areas of all participants. One reason for this might be that assigning numbers to heterogeneous echo texture relies on the ability to observe differences in the echogenicity and to interpret the grayscale image. Comparatively, VSE offered more diagnostic accuracy through its resultant colored image, which was easily interpretable through color differentiation. Furthermore, it objectively quantified MTrPs based on actual deformation when put under force by calculating their strain and comparing it to that of the normal, contiguous myofascial tissue.
There are several clinical conclusions that might be drawn from our results. The first is that real-time VSE can be used as a powerful diagnostic tool providing a detailed, accurate, and sensitive approach to detect even deeply located MTrPs and differentiate them from the surrounding, normal tissue. This should help to establish a gold standard diagnostic method likely to be more reliable, sensitive, and specific than physical examination alone. Second, confirmation of the existence of MTrPs by means of their visualization through color gradient alteration allows for the subsequent development of objective outcome measures, following therapeutic interventions and, thereby assessing the value of various methods. Finally, VSE enables cost-effective imaging, compared to MRE or microanalytic techniques.
Limitations and generalizability
Because of the clinical nature of the study, we did encounter some difficulties. The clinical identification of MTrPs was performed by a single, certified therapist: inter-rater reliability was not established. Also, clinical identification was based on palpation and with reference to Travell and Simons,6,26 essential and confirmatory criteria of A-MTrPs and L-MTrPs. Although these are the reference standards for detecting and classifying MTrPs, they are still considered to be subjective measures. To quantitatively assess MTrPs, we used PPT scores. However, this utilizes the perception of what each patient or study participant determines the pain threshold to be. Hence, universal generalization to MPS and MTrPs is still premature.
Future work needs to focus on inter-rater reliability of sonoelastography, and on developing objective and repeatable diagnostic tests for evaluating and tracking the changes in MTrPs before and after treatment. Such measures can be used to accurately diagnose and provide a better understanding of the physiological environment in the muscle tissue and to overcome the subjectivity and limitations of digital palpation. In addition, linking the physical properties of MTrPs’ stiffness and tenderness with the biochemical changes in A-MTrP and L-MTrP sites may provide important clues to the pathogenesis or pathophysiological mechanisms of MTrPs.
Disclaimer Statements
Contributors Dr Jean-Michel Brismée, PT, ScD, Fellow of the American Academy of Orthopaedic Manual Physical Therapists and Associate Professor at Texas Tech University, has contributed to this work, where he revised the paper before submission and provided valuable insights in editing the manuscript.
Funding None.
Conflicts of interest I am pleased to submit an original research article entitled ‘A comparison between different modes of real-time sonoelastography in visualizing myofascial trigger points in low back muscles’ by Mary Takla for consideration for publication in the Journal of Manual & Manipulative Therapy. This study was my PhD thesis and it was personally funded, and all authors denoted have helped in reviewing this paper, after I gathered the content and wrote the paper. This study was presented in the Thirteenth American Association of Orthopedic Manual Physical Therapy Conference held on 20 October 2013, in Cincinnati, OH, USA. I personally held the platform presentation. Only the abstract was published on their online book of abstracts. This manuscript has not been published and is not under consideration for publication in any journal elsewhere.
Ethics approval In order of approve my PhD thesis after my proposal, all of the Board council of Higher education of the School of Physical Therapy, the Institutional Review Board of Higher Education and Research of Cairo University, and the Supreme Council of Universities at Cairo, Egypt, approved this study.
References
- 1.Lavelle E, Lavelle W, Smith H. Myofascial trigger points. Med Clin North Am. 2007;91:229–39. [DOI] [PubMed] [Google Scholar]
- 2.Kao M, Han T, Kuan T, Hsieh Y, Su B, Hong C. Myofascial trigger points in early life. Arch Phys Med Rehabil. 2007;88(2):251–4. [DOI] [PubMed] [Google Scholar]
- 3.Kaergaard A, Andersen J. Musculoskeletal disorders of the neck and shoulders in female sewing machine operators: prevalence, incidence, and prognosis. Occup Environ Med. 2000;57:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerwin RD, Dommerholt J, Shah JP. An expansion of Simons’ integrated hypothesis of trigger point formation. Curr Pain Headache Rep. 2004;8:468–75. [DOI] [PubMed] [Google Scholar]
- 5.Simons D. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14:95–107. [DOI] [PubMed] [Google Scholar]
- 6.Travell J, Simons D. Travell & Simons’ myofascial pain and dysfunction: the trigger point manual. Vol. 1 Upper half of the body. 2nd ed. Baltimore, MD: Williams & Wilkins; 1999. [Google Scholar]
- 7.Gerwin RD. Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5:412–20. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez D, Rockwell P. Trigger points: diagnosis and management. Am Fam Physician. 2002;65:653–60. [PubMed] [Google Scholar]
- 9.Chesterton L, Barlas P, Foster N, Baxter G, Wright C. Gender differences in pressure pain threshold in healthy humans. Pain. 2003;101:259–66. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Bensamoun S, Basford J, Thompson J, An K. Identification and quantification of myofascial taut bands with magnetic resonance elastography. Arch Phys Med Rehabil. 2007;88:1658–61. [DOI] [PubMed] [Google Scholar]
- 11.Shah J, Gilliams E. Uncovering the biochemical milieu of myofascial trigger points using in-vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther. 2008;12(4):371–84. [DOI] [PubMed] [Google Scholar]
- 12.Shah J, Gebreab T, Gerber L, Gilliams E, Sikdar S. Poster 105: a new application of 2-dimensional gray scale ultrasound and vibration sonoelastography to image myofascial trigger points in the upper trapezius muscle. Arch Phys Med Rehabil. 2008;89(11):55. [Google Scholar]
- 13.Sikdar S, Shah J, Gilliams E, Gebreab T, Gerber L. Assessment of myofascial trigger points (MTrPs): a new application of ultrasound imaging and vibration sonoelastography. Arch Phys Med Rehabil. 2008;1:5585–9. [DOI] [PubMed] [Google Scholar]
- 14.Sikdar S, Shah J, Gebreab T, Yen R, Gilliams E, Danoff J, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90(11):1829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballyns J, Shah J, Hammond J, Gebreab T, Gerber L, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J Ultrasound Med. 2011;30:1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh A, Ueno E, Tohno E. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50. [DOI] [PubMed] [Google Scholar]
- 17.de Zordo T, Lill S, Fink C, Feuchtner G, Werner J, Bellmann-Weiler R, et al. Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. AJR Am J Roentgenol. 2009;193(1):180–5. [DOI] [PubMed] [Google Scholar]
- 18.Botar-Jid C, Damian L, Dudea S, Vasilescu D, Rednic S. The contribution of ultrasonography and sonoelastography in assessment of myositis. Med Ultrason. 2010;12:120–6. [PubMed] [Google Scholar]
- 19.Peng Q, Zhang L. Ultrasound evaluation of mechanical properties of individual muscles-tendons during active contraction. In: Proceedings of the 27th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 31 Aug–3 September 2005; Shanghai, China: IEEE Operations Center: Piscataway, NJ, USA, 2005, p. 7436–9. [DOI] [PubMed] [Google Scholar]
- 20.Bae U, Kim Y. Angular strain estimation method for elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54(12):2653–61. [DOI] [PubMed] [Google Scholar]
- 21.Souchon R. Ultrasonic elastography. In: Lemoigne Y, Caner A, Rahal R, editors. Physics for medical imaging applications. Proceedings of the NATO Advanced Study Institute on Optimising Detectors, Imaging and Computing Technologies from Nuclear Physics in General and Security Applications Dordrecht: Springer; 2007. p. 197–209. [Google Scholar]
- 22.Parker K, Gao L, Alam S, Rubens D, Lerner R. Sonoelasticity imaging: theory and applications. In: Proceedings of the IEEE Ultrasonic Symposium; 3–6 November 1996; San Antonio, TX, USA: IEEE: Piscataway, NJ, USA, 1996, p. 623–8. [Google Scholar]
- 23.Taylor L, Porter B, Rubens D, Parker K. Three-dimensional sonoelastography: principles and practices. Phys Med Biol. 2000;45:1477–94. [DOI] [PubMed] [Google Scholar]
- 24.Razek N. Real time sono-elastography in assessment of rotator cuff tendon tears: comparison of findings between elastography and MRI in healthy volunteers and patients with shoulder pain. In: Proceeding of the Radiological Society of North America 94th Scientific Assembly and Annual Meeting; 30 November–5 December 2008; Chicago, IL, USA: The Society: Oak Brook, IL, USA, 2008, SSE14-04, p. 396. [Google Scholar]
- 25.Parker K, Taylor L, Gracewski S, Rubens D. A unified view of imaging the elastic properties of tissue. J Acoust Soc Am. 2005;117(5):2705–12. [DOI] [PubMed] [Google Scholar]
- 26.Travell J, Simons D. Myofascial pain and dysfunction: the trigger point manual. Vol. 2. Baltimore, MD: Williams & Wilkins; 1992. p. 237–58. [Google Scholar]
- 27.Chen Q, Basford J, An K. Ability of magnetic resonance elastography to assess taut bands. Clin Biomech. 2008;23:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treaster D, Marras W, Burr D, Sheedy J, Hart D. Myofascial trigger point development from visual and postural stressors during computer work. J Electromyogr Kinesiol. 2006;16:115–24. [DOI] [PubMed] [Google Scholar]
- 29.Gissel H. Ca2+ accumulation and cell damage in skeletal muscle during low-frequency stimulation. Eur J Appl Physiol. 2000;83:175–80. [DOI] [PubMed] [Google Scholar]
- 30.Febbraio M, Pedersen B. Contraction-induced myokine production and release: Is skeletal muscle an endocrine organ. Exerc Sport Sci Rev. 2005;33:114–9. [DOI] [PubMed] [Google Scholar]
- 31.Prushansky T, Handelzalts S, Pevzner E. Reproducibility of pressure pain threshold and visual analog scale findings in chronic whiplash patients. Clin J Pain. 2007;23:339–45. [DOI] [PubMed] [Google Scholar]
- 32.Shah J, Phillips T, Danoff J, Gerber L. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99(5):1977–84. [DOI] [PubMed] [Google Scholar]