Abstract
Importance
Constipation is a common cause of pediatric abdominal pain and emergency department (ED) presentation. Despite the high prevalence, there is a dearth of clinical information and wide practice variation in childhood constipation management in the ED.
Objective
To assess the efficacy and safety of soap suds enema (SSE) in the treatment of fecal impaction in children with abdominal pain within the pediatric emergency department (ED) setting. The primary outcome was stool output following SSE. Secondary outcomes were adverse events, admissions, and return visits within 72 hours.
Methods
This is a retrospective cross-sectional study performed in the ED at a quaternary care children’s hospital of patients seen over a 12-month period who received a SSE for fecal impaction.
Results
Five hundred twelve patients (53% female, median age 7.8 years, range: 8 months-23 years) received SSE therapy over a 1-year period. Successful therapy (bowel movement) following SSE occurred in 419 (82%). Adverse events included abdominal pain in 24 (5%) and nausea/vomiting in 18 (4%). No SSE-related serious adverse events were identified. Following SSE, 405 (79%) were subsequently discharged, of which 15 (3.7%) returned to the ED for re-evaluation within 72 hours.
Conclusions and Relevance
SSE is an efficacious and safe therapeutic option for the acute treatment of childhood fecal impaction in the ED setting.
Keywords: Pain, Constipation, Fecal Impaction, Emergencies, Enema, Laxatives
INTRODUCTION
Abdominal pain is a leading chief complaint for patients seen in the emergency department (ED) and constipation is a frequent etiology.1 Published guidelines, such as those from the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN), on the evaluation and treatment of childhood constipation are primarily focused on the outpatient, non-acute evaluation and management of constipation.2-4 The optimal therapy for pediatric fecal disimpaction in the acute setting remains unknown.
In the ED, there is often a need to rapidly evaluate for potential life-threatening etiologies of abdominal pain, including appendicitis.5-7 This may result in a diagnostic and therapeutic approach toward suspected constipation that varies from than algorithms used in the standard outpatient setting. Children undergoing disimpaction in the acute setting are more likely to experience pain relief in comparison to less aggressive therapies, and thus disimpaction per rectum is often employed.7-12 Published guidelines support oral therapies, such as polyethylene glycol 3350 (PEG), and rectal enemas as equally effective for fecal disimpaction; however, oral therapy may take several days.2,7,12 Children may tolerate rectal therapy enemas well without adverse events, and these may be employed for a more rapid onset of action.11
Soap suds enema (SSE) is a hypertonic solution that provides a large-volume and detergent-based mucosal irritation to stimulate defecation. SSE has been the standard enema therapy administered in the ED setting of our institution with good anecdotal results. However, information regarding its efficacy is based primarily on small case reports, many of which report adverse events such as discomfort, colitis and hemorrhage following SSE.13-18 To our knowledge, large studies reporting SSE usage in children are unavailable. We hypothesized that SSE is safe and effective; thus, the objective of this study was to evaluate the safety and efficacy of SSE for fecal impaction in a large population of children presenting to a pediatric ED.
METHODS
This study was conducted based on data from a quaternary children’s hospital ED with an average annual volume of approximately 85,000 visits. This retrospective cross-sectional study was approved by the Baylor College of Medicine Institutional Review Board. All patients receiving SSE in the ED during one calendar year from June 2011 to June 2012 were included. Subjects were identified from electronic medical record orders for SSE within the ED. Included subjects presented with abdominal pain and were clinically suspected of having a fecal impaction by the attending physician. Patient demographics, medical history, disposition, return visits within 72 hours and stool output were systematically recorded from review of nursing and physician notes. A significant medical history was defined as the presence of a prominent comorbidity including cystic fibrosis, cerebral palsy or muscle disorders, hypothyroidism, spina bifida or other spine anomalies, gastric anomalies, anal atresia or Hirschsprung’s disease, cardiac anomalies, or previous abdominal surgery. Data abstractors were trained by the principal investigator. To minimize bias associated with data abstraction, we used specific, restrictive key words for subjective data fields. Unavailable data were coded as missing except for medical history and laboratory testing, for which the absence of specific description was interpreted as “not present” or “not done.” When multiple documentation sources were present, that of the most senior physician was used. Historical variables were documented before reviewing the outcome, investigations, adverse events, laboratory results, and disposition status.
SSE was ordered and delivered in the standard manner described below for the treatment of clinically suspected fecal impaction in children with abdominal pain. The decision to use SSE and the amount given were made by the treating physician and administered by the nursing team. The SSE consisted of an average of 20 mL/kg (maximum: 1 L) tap water and one packet of soap (Castile soap, Amsino International, Inc. Pomona, CA) administered by gravity using an enema bucket and a clear catheter tip inserted (up to 10 cm) into the rectum.19 One packet of soap was added regardless of volume administered thus detergent concentration varied. The patient was asked to retain the liquid as long as possible until they felt they must evacuate the liquid and stool.
The primary outcome was presence (treatment success) or absence (failure) of stool output following administration of the enema. Any stool output was considered a treatment success. If no stool output was documented, there was assumed to be no stool output, and this was coded as a failure. Secondary outcomes were adverse events, admissions, and return emergency visits. Mild adverse events were defined as nausea, vomiting, abdominal pain, or any untoward effect documented by the physician or the nurse attributed to the SSE following enema administration. Serious adverse events were defined a priori as severe colitis, hypotension, or hemorrhage. All patients with adverse events were reviewed by the first two authors to determine severity.
Statistical Analysis
Comparisons of baseline demographic data, comorbidities, laboratory, treatment effect, and adverse events were evaluated using the χ2 or Fisher exact test comparing those with vs. without successful output. Descriptive statistics including mean, median, and proportional frequencies were used to analyze the adverse events as appropriate. Continuous data including age were evaluated using the 2-sided independent Student t test with parametric data and Mann-Whitney U with non-parametric data. A p-value of <0.05 was considered statistically significant using SPSS version 21 (SPSS, Inc.; Chicago, IL).
RESULTS
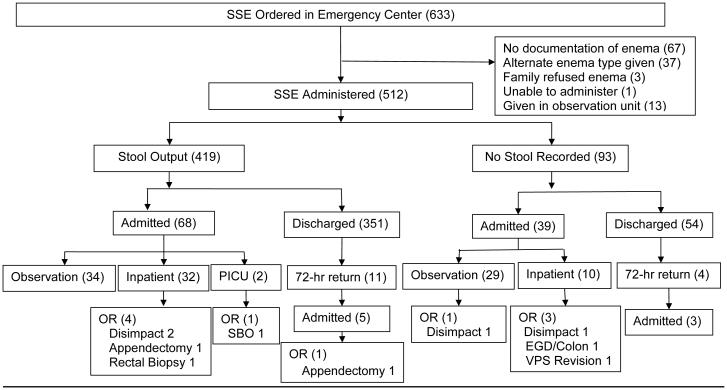
Six hundred thirty-three potential patients were reviewed, of which 512 received SSE in the ED (Figure 1). The median age was 7.8 years (range 8 months - 23 years); with a slight female predominance (Table 1). A majority of children had a significant medical history, including 193 (38%) with a history of constipation (Table 1).
Figure 1. Study Flowchart.
Disimpact=manual disimpaction; EGD/Colon=esophagogastroduodenoscopy and colonscopy; OR=operating room; PICU=pediatric intensive care unit; SBO=small bowel obstruction; SSE=soap suds enema; VPS= ventriculoperitoneal shunt
Table 1.
Demographic and Clinical Characteristics of Children Receiving Soap Suds Enema Therapy
| Number of patients | 512 |
|---|---|
| Mean age in years (range), No. (%) | 7.8 (0.7-23) |
| Girls, No. (%) | 270 (53) |
| Race/Ethnicity, No. (%) | |
| Hispanic, No. (%) | 243 (47) |
| African American, No. (%) | 102 (20) |
| Caucasian, No. (%) | 382 (75) |
| Other, No. (%) | 28 (5) |
| Constipation history, No. (%) | 193 (38) |
| Enema dose, mL/kg (range), No. (%) | 16.4 (2-35) |
| Significant medical history, No. (%)* | 314 (61) |
| Developmental delay, No. (%) | 83 (16) |
| Gastrointestinal, No. (%) | 72 (14) |
| Abdominal surgery, No. (%) | 54 (11) |
| Cardiac disease, No. (%) | 11 (2) |
| Sickle cell disease, No. (%) | 11 (2) |
| Urinary tract infections, No. (%) | 8 (2) |
Including 249 (79%) with multiple diagnoses
Efficacy and Discharges Following SSE Therapy
Productive stool was documented in 419 (82%) following SSE (Figure 1). Gender, age, and previous history of constipation were not associated with SSE therapy success or failure (data not shown). A large majority of subjects were discharged from the ED following SSE therapy (Figure 1). Of the 405 patients initially discharged from the ED following enema administration, 15 (3.7%) returned to the ED within 72 hours. This included 11 patients who had been discharged following stool output from an SSE and 4 discharged without documented output following SSE. Of those returning, 8 (53%) were admitted: 7 for observation with medical management and 1 for appendectomy. Patients with pain improvement were discharged according to provider’s clinical impression.
Admissions Following SSE Therapy
Following the enema, 107 patients (21%) were subsequently admitted.Children who failed to have output following SSE were more likely to be admitted (39/93) than those who had a productive stool (68/419; P<0.001). Of those admitted, 83 (78%) had a significant medical history and 52 (49%) had a history of constipation. The majority of admissions were to the observation unit (N=63, 59%). In the observation unit, 47 (75%) received a cleanout using polyethylene glycol solution and were discharged home without event.
Overall in those admitted further constipation therapies (of any type) were used in 75 (70%). Of these, PEG therapy failed for 4 patients, who required a manual disimpaction by the surgical team, and an additional 5 patients went to the operating room for other reasons (Supplement). Non-abdominal pain-related admissions occurred in 6 patients (Supplement), and the remaining admissions, both observation and inpatient, were related to serial abdominal examinations for abdominal pain with subsequent discharge. Patients were discharged according to provider clinical judgement with improvement in pain.
Adverse Events
Adverse events were documented in 37 (7.2%) patients and included nausea, vomiting, and abdominal pain (Table 2). Those may all be attributed to the primary problem of increased stool burden as well. Children with adverse events were more likely to be admitted following SSE than children without an adverse event (28/37 vs. 79/475, respectively; p<0.001). No serious adverse events were noted that were attributed to SSE.
Table 2.
Patient-reported Adverse Events in Subjects Receiving Soap Suds Enema
| Adverse Events† | N (%) |
|---|---|
| Abdominal pain | 24 (5%) |
| Nausea/vomiting | 18 (3%) |
| Headache | 1 (<1%) |
| Respiratory distress | 2 (<1%) |
| Total patients with adverse events | 37 |
Some subjects experienced more than one adverse event.
Respiratory complications unrelated to SSE occurred in 2 (0.4%) patients, who were subsequently transferred to the intensive care unit. A14-year-old female with nausea and abdominal pain developed hives with respiratory distress, as well as lip and eye swelling 4 hours after enema administration subsequently attributed to ondansetron by the allergy and immunology service. A second patient with a ventriculoperitoneal shunt (VPS) developed respiratory distress following morphine administration. The respiratory distress was attributed to morphine and improved after naloxone administration. The same patient required an exploratory laparotomy for small bowel obstruction attributed by the attending surgeon to adhesions at the abdominal VPS site 3 days later. There was a 7-year-old patient that had 4 large bowel movements after SSE, tolerated 26 ounces of Gatorade and reported no abdominal pain on discharge that returned the next day with a perforated appendicitis. The appendicitis was not attributed to the SSE therapy by the attending surgeon.
SSE volume was recorded in 413 of the subjects, with the median volume of enema given 20 mL/kg (range 2-35 mL/kg; 10-20 mL/kg [25-75%]). In those with a recorded SSE volume, the volume did not differ in those with productive stool versus those without (data not shown). The volume of SSE given did not differ between those with or without adverse events (data not shown).
DISCUSSION
Identifying rapid, safe, and efficacious modalities for therapy of fecal impaction prompting ED visits for pain in the pediatric setting is important. To our knowledge, our study is the largest of its kind to evaluate SSE as a therapeutic modality in any population. We found that SSE is efficacious in a large majority of children receiving SSE for suspected fecal impaction in the ED setting. This was seen both with our primary outcome of documented stool output as well as our secondary outcome of initial discharge from the ED. In addition, we found a limited number of adverse events. None were serious in nature, suggesting SSE is safe to use in the ED setting for childhood constipation.
SSE therapy is not included in recent guidelines for functional constipation.3 In addition recent NASPGHAN pediatric gastroenterology guidelines for the treatment of constipation recommend against the use of SSE due to potential toxicity though did not grade the evidence for the recommendation.1 We suspect that SSE’s omission is based on a prior lack of large studies to date evaluating its efficacy and safety. Rather the literature on SSE is primarily based on case reports, with a few papers identifying severe colitis as an adverse event; often in adult patients with significant underlying medical conditions.13-16 However caustic colitis from SSE is believed to be rare because the strength of the enema solution is seldom strong enough to cause corrosive effects on the colon.15 Our study, using a readily available SSE solution, supports the rare nature of serious adverse events as we found none in over 500 SSE administrations. Rather, all adverse events attributable to SSE were mild and occurred at a rate comparable to the 10-30% reported in other enema types in children in the ED setting.8, 11 Given the relatively low number of adverse events related to SSE in our study, we suggest SSE may be as safe as other commonly used disimpaction therapies in the ED setting.
The ED setting differs from a typical outpatient setting in many ways. This may include more frequent vital sign monitoring, nursing assessments, and potential therapies such as intravenous fluids. These and other potential differences may help ensure safety of SSE therapy in the acute care setting. Future studies in other outpatient settings are needed before SSE therapy is adopted outside the ED.
Recent NASPGHAN guidelines on the treatment of functional constipation state that enema therapy is as effective as PEG-based oral therapies for disimpaction.2, 6 While one productive stool following enema was the measure of success in this study, a complete rectal disimpaction was unlikely completed in most children. This outcome measure was chosen in the ED setting, as the resulting clinical improvement following a bowel movement may be reassuring to both clinicians and families with respect to the correct diagnosis and therapy needed. Therefore, we felt this outcome best exemplified whether SSE therapy was successful as an end point lending to a safe disposition for the child as others have done when evaluating ED-based therapies.8 The large number of children able to be discharged home with only a few returning to the ED reinforces the efficacy of this therapy in the ED setting. Further oral therapy was required in the majority of discharged children, a finding consistent with previously reported laxatives on discharge.8
As expected SSE was not completely successful in all cases. We hypothesize that some children were too impacted to respond sufficiently to one SSE enema alone. Other possibilities include an incorrect diagnosis of fecal impaction as a contributing factor for the abdominal pain, insufficient enema volume used, or other technical issues such as lack of retention of the enema following administration. Future studies investigating diagnostic algorithms or higher volumes or concurrent therapies with SSE may be warranted in this population.
There are several strengths to the study. First, this is the largest cohort of patients in which SSE therapy has been reported to date. Second, the data was obtained from the medical record; as such the results are likely to be generalizable and applicable in the clinical setting as the SSE therapy was given as part of routine clinical practice. SSE provides a less expensive and readily available enema alternative. Additionally, in our setting, this enema solution does not come from pharmacy and thus is more rapidly administered than other enema types. Finally, outcomes related to short-term efficacy as well as longer-term efficacy with relation to return visits allowed for an increased opportunity to capture a wide evaluation of efficacy and safety of the SSE therapy.
The primary limitation to this retrospective study is the lack of prospective standardization (e.g., SSE volume administered, concentration, diagnostic criteria, and discharge criteria.) There may have been bias in how some variables (e.g., adverse events, quantification for amount of stool output) were documented, as it is based on provider and nurse determinations. However, we thoroughly reviewed all ED provider and nursing notes as well as hospital notes from admitted patients in an attempt to capture all documented events. In addition, there is the possibility that the discharged patients sought care elsewhere and were not captured on follow-up. Future prospective trials, perhaps comparing SSE to other therapeutic modalities will be needed to capture outcome evaluations more thoroughly.
In conclusion, SSE is an effective means of treating fecal impaction in the ED setting. The adverse events of SSE are comparable to those seen with other laxative therapies, and no serious attributable adverse events were found in over 500 SSE administrations. Given this, we suggest consideration of SSE as a potential therapy for constipation in the ED setting.
Supplementary Material
Summary.
Abdominal pain is a leading complaint in the emergency department, and constipation a frequent etiology.
Optimal therapy for pediatric fecal disimpaction in the acute setting remains unknown.
The current literature on soap suds enemas is primarily based on small case reports in adults describing adverse events.
Impact on Clinical Practice.
This study is the largest study of soap suds enema use in children in the acute setting.
Soap suds enema was effective in 82% of children with fecal impaction.
No serious attributable adverse events occurred.
Soap suds enemas offers an effective and safe therapy for fecal impaction in the acute setting.
Footnotes
Conflicts of Interest/Disclosures: None of the authors have conflicts of interest, including relevant financial interests. BPC is a NIH K23 recipient unrelated to this study.
AUTHOR CONTRIBUTIONS
CEC and BPC conceived the study. CEC, EBH, and BPC supervised the conduct of the study and data collection. KLV and EBH performed data abstraction and managed the data, while EBH and CEC provided quality control. BPC provided statistical advice on study design and analyzed the data. CEC drafted the manuscript, and all authors contributed substantially to its revision. CEC takes responsibility for the paper as a whole. This study was presented in part at the Pediatric Academic Society Annual Meeting in Washington, DC, in May 2013 and at Digestive Disease Week in May 2013 in Orlando, FL.
REFERENCES
- 1.Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;386:1–32. [PubMed] [Google Scholar]
- 2.Baker SS, Liptak GS, Colletti RB, et al. Evaluation and treatment of constipation in infants and children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 1999;29(5):612–26. doi: 10.1097/00005176-199911000-00029. [DOI] [PubMed] [Google Scholar]
- 3.Tabbers MM, Di Lorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58(2):258–74. doi: 10.1097/MPG.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 4.Nurko S. Advances in the management of pediatric constipation. Curr Gastroenterol Rep. 2000;2(3):234–40. doi: 10.1007/s11894-000-0066-0. [DOI] [PubMed] [Google Scholar]
- 5.Wai S, Ma L, Kim E, Adekunle-Ojo A. The utility of the emergency department observation unit for children with abdominal pain. Pediatr Emerg Care. 2013;29(5):574–8. doi: 10.1097/PEC.0b013e31828e572d. [DOI] [PubMed] [Google Scholar]
- 6.Chang YJ, Chao HC, Kong MS, et al. Misdiagnosed acute appendicitis in children in the emergency department. Chang Gung Med J. 2010;33(5):551–7. [PubMed] [Google Scholar]
- 7.Miller MK, Dowd MD, Friesen CA, et al. A randomized trial of enema versus polyethylene glycol 3350 for fecal disimpaction in children presenting to an emergency department. Pediatr Emerg Care. 2012 Feb;28(2):115–9. doi: 10.1097/PEC.0b013e3182442c0a. [DOI] [PubMed] [Google Scholar]
- 8.Miller MK, Dowd MD, Fraker M. Emergency department management and short-term outcome of children with constipation. Pediatr Emerg Care. 2007;23(1):1–4. doi: 10.1097/01.pec.0000248690.19305.a5. [DOI] [PubMed] [Google Scholar]
- 9.Hansen SE, Whitehill JL, Goto CS, et al. Safety and efficacy of milk and molasses enemas compared with sodium phosphate enemas for the treatment of constipation in a pediatric emergency department. Pediatr Emerg Care. 2011;27(12):1118–20. doi: 10.1097/PEC.0b013e31823b0088. [DOI] [PubMed] [Google Scholar]
- 10.Youssef NN, Peters JM, Henderson W, et al. Dose response of PEG 3350 for the treatment of childhood fecal impaction. J Pediatr. 2002;141(3):410–4. doi: 10.1067/mpd.2002.126603. [DOI] [PubMed] [Google Scholar]
- 11.Bekkali NL, van den Berg MM, Dijkgraaf MG, et al. Rectal fecal impaction treatment in childhood constipation: enemas versus high doses oral PEG. Pediatrics. 2009;124(6):e1108–15. doi: 10.1542/peds.2009-0022. [DOI] [PubMed] [Google Scholar]
- 12.Nurko S, Youssef NN, Sabri M, et al. PEG3350 in the treatment of childhood constipation: a multicenter, double-blinded, placebo-controlled trial. J Pediatr. 2008;153(2):254–61. doi: 10.1016/j.jpeds.2008.01.039. 261.e1. [DOI] [PubMed] [Google Scholar]
- 13.Harish K, Tony J, Sunilkumar R, et al. Severe colitis induced by soap enemas. Indian J Gastroenterol. 2006;25(2):99–100. [PubMed] [Google Scholar]
- 14.Sheibani S, Gerson LB. Chemical colitis. J Clin Gastroenterol. 2008;42(2):115–21. doi: 10.1097/MCG.0b013e318151470e. [DOI] [PubMed] [Google Scholar]
- 15.Rousseau P. No soapsuds enemas! Postgrad Med. 1988;83(4):352–3. doi: 10.1080/00325481.1988.11700208. [DOI] [PubMed] [Google Scholar]
- 16.Orchard JL, Lawson R. Severe colitis induced by soap enemas. South Med J. 1986;79(11):1459–60. doi: 10.1097/00007611-198611000-00038. [DOI] [PubMed] [Google Scholar]
- 17.Kim SK, Cho C, Levinsohn EM. Caustic colitis due to detergent enema. AJR Am J Roentgenol. 1980;134(2):397–8. doi: 10.2214/ajr.134.2.397. [DOI] [PubMed] [Google Scholar]
- 18.Schmelzer M, Case P, Chappell SM, et al. Colonic cleansing, fluid absorption, and discomfort following tap water and soapsuds enemas. Appl Nurs Res. 2000;13(2):83–91. [PubMed] [Google Scholar]
- 19.Hockenberry MJ, Wilson D. Wong’s nursing care of infants and children. 8th Mosby.; St Louis: 2012. p. 1135. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.