Abstract
Background
Human metapneumovirus (HMPV) causes acute respiratory tract infections in infants and children. We sought to measure the clinical and economic burden of HMPV infection in hospitalized children.
Methods
We conducted a retrospective cohort study from 2007 to 2013 at Primary Children's Hospital in Salt Lake City, Utah. Children <18 years of age with laboratory-confirmed HMPV infection were included. Demographic, clinical, and financial data were abstracted from the electronic medical record.
Results
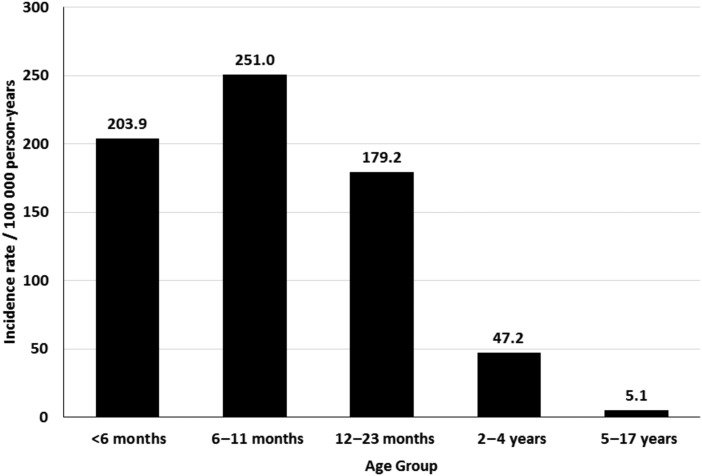
During the study period, 815 children were hospitalized with laboratory-confirmed HMPV infection: 16% <6 months, 50% 6–23 months, 23% 2–4 years, and 11% 5–17 years of age. A complex chronic condition was identified in 453 (56%) children hospitalized with HMPV infection; this proportion increased with increasing age (P < .001). There was marked variation in annual HMPV hospitalization rates, ranging from 9 of 100 000 person-years in 2012–2013 to 79 of 100 000 in 2009–2010. Hospitalization rates were highest among children <2 years (200 of 100 000 person-years) and lowest among children 5–17 years of age (5 of 100 000). Of hospitalized children, 18% were treated in the intensive care unit and 6% required mechanical ventilation. The median length of stay was 2.8 days (interquartile range [IQR], 1.8–4.6) and did not vary by age. The median total hospital cost per patient was $5513 (IQR, $3850–$9946) with significantly higher costs for patients with chronic medical conditions (P < .001).
Conclusions
Human metapneumovirus infection results in a large number of hospitalizations with substantial morbidity, resource utilization, and costs. The development of a safe and effective vaccine could reduce the clinical and economic burden of HMPV.
Keywords: complications, disease severity, HMPV, hospital cost, human metapneumovirus
In 2001, a novel respiratory virus, human metapneumovirus (HMPV), was identified from children with acute respiratory tract infections [1]. Since then, HMPV has been reported worldwide as a common cause of croup, bronchiolitis, and pneumonia in young children with the potential to cause severe disease [2–14]. Serologic surveys revealed that primary HMPV infection is common, with HMPV antibodies detected in over 90% of individuals by 5–10 years of age [15].
The clinical and economic burden associated with infections caused by other respiratory viruses, including influenza virus, parainfluenza viruses (PIVs) 1–3, respiratory syncytial virus (RSV), and adenovirus (AV) in children has been investigated. It is estimated that 58 000 and 20 000 children <5 years of age are hospitalized annually in the United States with RSV and influenza, respectively, with considerable associated morbidity and economic burden [16–20]. However, limited data describing the clinical and economic burden for children hospitalized with HMPV infection are available.
Our objective was to evaluate the incidence, outcomes, and costs of laboratory-confirmed HMPV infection among hospitalized children over a 6-year period.
PATIENTS AND METHODS
Setting and Study Population
Approval to conduct this study was granted by the institutional review boards of the University of Utah and Primary Children's Hospital (PCH). Primary Children's Hospital is a 289-bed, freestanding children's hospital that serves as both the community hospital for Salt Lake County, Utah, and as a tertiary referral center for 5 states in the intermountain west (Utah, Idaho, Wyoming, Nevada, and Montana). Primary Children's Hospital is owned and operated by Intermountain Health Care (IH), a not-for-profit, vertically integrated healthcare system.
Study Design
We performed a retrospective cohort study among Utah resident children <18 years of age who had laboratory testing for viral acute respiratory tract infections (including HMPV) at PCH over a 6-year period (July 2007 through June 2013). Respiratory specimens are routinely tested for viruses in all patients admitted to PCH with respiratory symptoms for the purposes of isolation, cohorting, and medical treatment. In addition, respiratory viral testing was performed on all febrile infants <90 days of age.
Inpatient Cohort
The inpatient cohort consisted of children with laboratory-confirmed HMPV, who were hospitalized for ≥24 hours at PCH and whose admission was principally attributable to HMPV infection. Data abstracted for all eligible patients included the following: the total hospital length of stay (LOS), the presence of complex chronic conditions (CCC) [21–23], secondary bacterial infections, intensive care unit (ICU) admission, use of mechanical ventilation, and total hospital costs. The financial transaction database contained within the IH Enterprise Data Warehouse contains detailed data about the cost of providing healthcare. This system identifies and aggregates the variable- and fixed-cost components of patient activities, hospital services, and products according to the date of service [24–26]. Hospital costs incurred in years 2007 to 2013 were standardized to 2013 US dollars by applying a yearly consumer price index adjustment for medical services [27].
Human Metapneumovirus-Related Illness
Two authors (C. R. D. and K. A.) independently reviewed the principal International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis codes for each hospitalization. Only hospitalizations where the principal ICD-9 code was for a respiratory condition were included in the analysis. Six principal ICD-9 codes accounted for 80% of HMPV hospitalizations, which included the following: bronchiolitis, 466.19 (55%); viral pneumonia, 480.8 (10%); asthma exacerbation, 493.92 (6%); bronchiolitis due to RSV, 466.11 (3%); bacterial pneumonia, 482.9 (3%); and viral pneumonia, 480.9 (2%).
Respiratory Viral Testing
Testing for HMPV was initiated in 2007 using direct fluorescent antibody (DFA) testing (HMPV Monoclonal Antibody Analyte Specific Reagent Conjugate; Diagnostic Hybrids, Athens, OH). For DFA-negative specimens, viral culture was performed using 4 shell vials with 48-hour exit stains with specific fluorescent antibodies. Testing for rhinovirus began in January of 2008. Starting in 2009, DFA-negative samples were submitted for multiplex polymerase chain reaction testing (Luminex xTAG Respiratory Virus Panel; Luminex Diagnostics, Austin, TX). Since April 2012, all respiratory samples were tested using the FilmArray Respiratory Panel multiplexed nucleic acid amplification test (BioFire Diagnostics, Salt Lake City, UT). As described above, indications for respiratory viral testing remained constant throughout the study period.
Definition of Study Terms and Outcomes
Secondary bacterial infections were defined as the isolation of Staphylococcus aureus, Streptococcus pneumoniae, typeable Haemophilus influenzae, or Streptococcus pyogenes from a sterile site (blood, pleural fluid, or cerebrospinal fluid [CSF]), or from a respiratory tract specimen if obtained by bronchoalveolar lavage or a protected brush specimen. To evaluate CCCs that may influence disease severity among children with HMPV infection, we examined discharge codes for ICD-9 codes associated with CCCs, as previously described [22, 23]. We analyzed the burden of HMPV infection in hospitalized children by examining the following outcomes: hospitalization rates, ICU admission, LOS, complications, and hospital costs. Complications were defined as respiratory failure requiring mechanical ventilation, secondary bacterial infections, and death.
Statistical Analysis
Data were analyzed using Stata 13.1 (StataCorp LP, College Station, TX). Fisher's exact test and χ2 tests were used for categorical variables. The Wilcoxon rank-sum test was used for continuous variables. Linear and logistic regression models were constructed to compare complication rates. The results of regression analyses are presented as odds ratios (ORs) with 95% confidence intervals (95% CIs).
Population-based incidence rates were determined for Salt Lake County residents only. To estimate the incidence of HMPV-related hospitalizations, we used all children <18 years who were residents of Salt Lake County (as defined by the zip code for primary residence at the time of admission) divided by the age-specific population for the county using data obtained from the Utah Department of Health's Indicator-Based Information System for Public Health [28]. The total number of cases identified at PCH was divided by PCH's market share of Salt Lake County hospitalizations for bronchiolitis and pneumonia by age group over the study period (range, 87%–96%) to achieve representative incidence rates.
RESULTS
Seasonality and Periodicity of Laboratory-Confirmed Human Metapneumovirus Infection in Children
From 2007 to 2013, samples from 38 213 unique patients were submitted for respiratory viral testing at PCH. Fifty-two percent were positive for a respiratory virus, including the following: 1975 HMPV (5%), 7557 RSV (20%), 3101 influenza virus (8%), 1954 PIVs (5%), and 1356 AV (4%). For the period in which rhinovirus testing was performed, 6081 (17%) cases of rhinovirus were detected. The monthly proportion of respiratory specimens that were positive for HMPV varied from 0% to 26.5% across the 6 years. Overall, 815 of 1975 (41%) children who tested positive for HMPV at PCH were hospitalized.
Of the 815 children who were hospitalized and included in the study, the vast majority (97%) had only 1 visit in which HMPV was detected. However, 22 (3%) patients had 2 HMPV-positive visits and 1 (0.1%) had 3 HMPV-positive visits over the study period. The multiple visits in each patient occurred in different respiratory seasons.
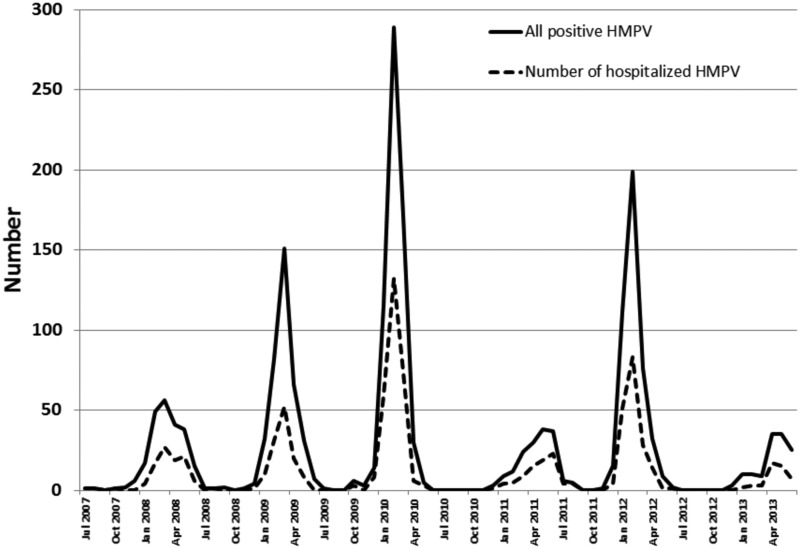
Human metapneumovirus demonstrated winter seasonality with peak activity between February and April. There was significant annual variation in HMPV positivity and the proportion of children with positive tests who were hospitalized (Figure 1).
Figure 1.
The overall number of children with laboratory-confirmed human metapneumovirus (HMPV) infection and the number hospitalized at Primary Children's Hospital from July 2007 through June 2013.
Demographics and Clinical Characteristics
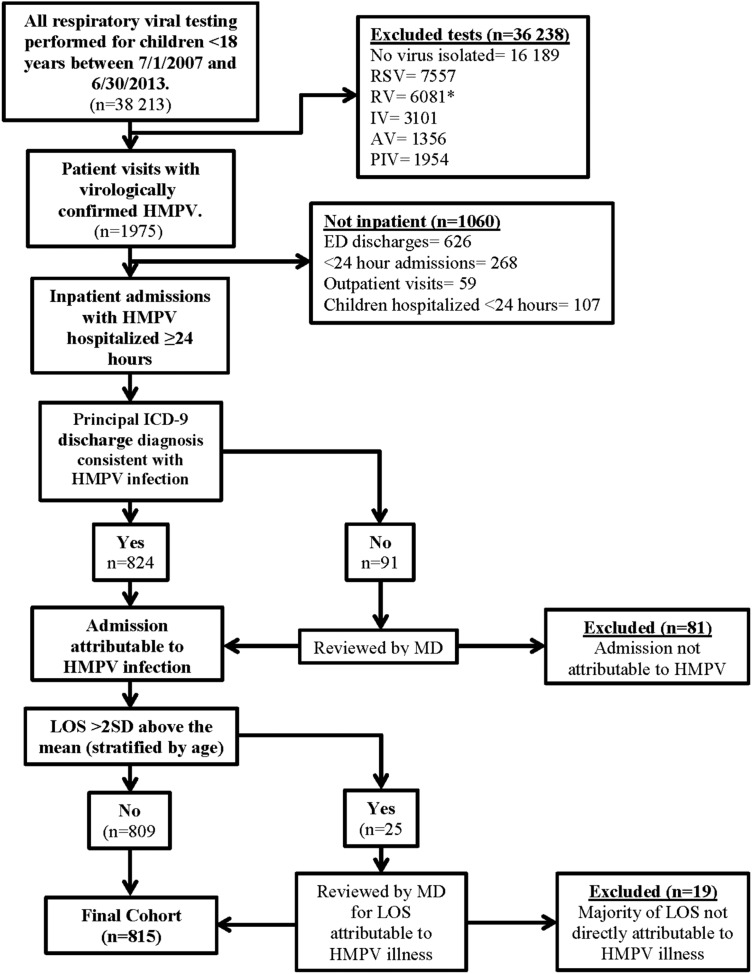
Eight hundred fifteen hospitalized patients were included in the cohort (Figure 2). Demographic characteristics of the hospitalized children are shown in Table 1. The majority of hospitalized children (65%) were <2 years, and 89% were <5 years. There was a slight male predominance (56.3%).
Figure 2.
Method for ascertaining hospitalizations attributable to human metapneumovirus (HMPV). AV, adenovirus; ED, emergency department; MD, medical doctor; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RTU, rapid treatment (and observation) unit; RV, rhinovirus. *RV testing started in 2008.
Table 1.
Characteristics and Chronic Medical Conditions of Children Hospitalized With Laboratory-Confirmed HMPV Infection (N = 815)
| Characteristics | No. of Patients |
|---|---|
| Age (yrs) | |
| Median (IQR) | 1 (0–2) |
| Age group | |
| <6 months | 129 (15.8%) |
| 6–11 months | 171 (21.0%) |
| 12–23 months | 234 (28.7%) |
| 2–4 years | 191 (23.4%) |
| 5–17 years | 90 (11.0%) |
| Sex | |
| Male | 459 (56.3%) |
| Race/ethnicity | |
| White | 424 (52.0%) |
| Hispanic | 186 (22.8%) |
| Pacific Islander | 63 (7.7%) |
| Black | 22 (2.7%) |
| American Indian | 10 (1.2%) |
| Asian | 9 (1.1%) |
| Other | 71 (8.7%) |
| Unknown | 30 (3.7%) |
| Any complex chronic condition by age group | |
| Chronic condition among children <6 months | 47 (36.4%) |
| Chronic condition among children 6–11 months | 73 (42.7%) |
| Chronic condition among children 6–23 months | 135 (57.7%) |
| Chronic condition among children 2–4 years | 123 (64.4%) |
| Chronic condition among children 5–17 years | 75 (83.3%) |
| Specific complex chronic conditions | |
| Respiratory | 379 (46.5%) |
| Asthma | 222 (58.6%) |
| Chronic pulmonary disease (other than asthma)a | 157 (41.4%) |
| Cardiac | 139 (17.0%) |
| Central nervous system | 95 (11.6%) |
| Genetic | 66 (8.1%) |
| Immunosuppression | 43 (5.3%) |
| Seizures | 40 (4.9%) |
| Metabolic | 38 (4.7%) |
| Neuromuscular | 11 (1.4%) |
| Sickle cell disease | 6 (0.7%) |
| Renal | 6 (0.7%) |
| ≥2 chronic medical conditions | 361 (44.2%) |
Abbreviations: HMPV, human metapneumovirus; IQR, interquartile range.
aOther chronic pulmonary diseases are listed in Appendix 1.
One or more CCCs were documented in 453 (56%) children hospitalized with HMPV infection. Pulmonary disorders were the most common, accounting for 83% of CCCs; 222 (27%) children had a history of asthma. The proportion of children hospitalized with HMPV who had a CCC increased with age (36% among children <6 months to 83% among children 5–17 years, P < .001).
One hundred forty-three (18%) children were treated in the ICU, and 46 (6%) required intubation and mechanical ventilation (Table 2). In univariable analyses, children with CCCs were significantly more likely to require ICU admission (OR, 4.3; 95% CI, 2.7–6.7) and mechanical ventilation (OR, 7.1; 95% CI, 2.8–18.2). Older children were also more likely to require ICU admission (OR, 1.1; 95% CI, 1.0–1.1). In multivariable analyses when the presence of a CCC was added as a covariate, CCCs were associated with ICU admission (adjusted OR [aOR], 4.0; 95% CI, 2.6–6.4) and mechanical ventilation (aOR, 7.5; 95% CI, 2.9–19.3), but age was no longer significant (p > .2).
Table 2.
Resource Utilization and Outcomes of 815 Children Hospitalized with HMPV Infection at PCH, Salt Lake City, Utah, 2007–2013
| Age | All | Any Antibiotics | Bacterial Coinfection | ICU | Intubation With Mechanical Ventilation | Death |
|---|---|---|---|---|---|---|
| <6 months | 129 (15.8%) |
69 (53.5%) |
2 (1.6%) |
25 (19.2%) |
10 (7.8%) |
1 (0.8%) |
| 6–23 months | 405 (49.7%) |
251 (62.0%) |
10 (2.5%) |
61 (15.0%) |
18 (4.4%) |
1 (0.3%) |
| 2–4 years | 191 (23.4%) |
126 (66.0%) |
6 (3.1%) |
34 (17.8%) |
11 (5.8%) |
0 (0%) |
| 5–17 years | 90 (11.0%) |
64 (71.1%) |
0 (0%) |
24 (26.7%) |
7 (7.8%) |
0 (0%) |
| Total | 815 (100%) |
510 (62.6%) |
18 (2.2%) |
143 (17.6%) |
46 (5.6%) |
2 (0.2%) |
Abbreviations: HMPV, human metapneumovirus; ICU, intensive care unit; PCH, Primary Children's Hospital.
One or more additional respiratory viruses were detected in 11% of children with HMPV infection; 43% of coinfections were with rhinovirus. Sixty-two percent of children with laboratory-confirmed HMPV infection received antibiotics. Bacterial cultures of blood, CSF, or pleural fluid were obtained in 403 (49%) children. A bacterial pathogen was isolated in 18 children (4.5% of children with cultures obtained; 2.2% of all hospitalized children with HMPV), including the following: 8 S pyogenes, 5 S pneumoniae, 1 S aureus, 1 Enterococcus faecalis, and 3 others. Six children had bacteremia alone, 2 had bacteremic pneumonia, and 10 had parapneumonic empyema. No bacteria were isolated from the 48 (6%) CSF samples submitted for culture.
There were 2 deaths among children hospitalized with HMPV. Both deaths were in young patients: 1 had significant underlying cardiac disease, and the other was healthy. Both presented with cardiorespiratory failure outside the hospital. The first patient was a 49-day-old female who presented to a clinic with a history of poor feeding with significant diaphoresis and perioral cyanosis with feeds. She was admitted at PCH and discovered to have truncus arteriosus type 1 with congestive heart failure. Two days before admission, she tested positive for HMPV because of respiratory distress. She underwent emergent heart surgery after acute clinical decompensation. After surgery, she developed multisystem organ failure and a massive intracranial hemorrhage. Medical support was withdrawn after a patient care conference. The second patient was a 5-month-old previously healthy male, who presented to an outside hospital with (1) a day's history of poor feeding and (2) respiratory distress with significant diaphoresis. After cardiac arrest and resuscitation, he was transferred to PCH the same day. Upon arrival, his electroencephalogram showed low voltage consistent with a severe anoxic brain injury, and magnetic resonance imaging revealed evidence of ischemia. His subsequent cardiac catheterization did not reveal evidence of anomalous coronary arteries. After 3 days without significant change, the parents elected to withdraw support. Viral testing was performed at the time of admission and the following day, both of which were positive for HMPV. With the 2 deaths, blood cultures and tests for other viral pathogens were negative. The extent to which HMPV contributed to the deaths is unknown.
Length of Stay and Hospital Costs
Hospital LOS and costs for children hospitalized with HMPV are shown in Table 3. Overall, the median hospital LOS was 2.8 days (interquartile range [IQR], 1.8–4.6 days) and the median cost was $5513 (IQR, $3850–$9946). There was no difference in LOS by age. However, costs were significantly higher among children 5–17 years compared with other age groups ($6198 vs $5272: P = .038). Compared with healthy children, those with CCCs had significantly longer LOS (3.1 vs 2.5 days) and higher costs ($6671 vs $4738; P < .001 for both).
Table 3.
Total Hospital Length of Stay and Hospital Costs by Age Group Among Children Hospitalized With Laboratory-Confirmed HMPV Infection
| Number (N) | LOS, days | Hospital Cost, ($)1 | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| All ages | 815 | 2.8 (1.8–4.6) | 5513 (3850–9946) |
| Healthya | 362 | 2.5 (1.7–3.6) | 4738 (3428–6673) |
| Any chronic conditiona | 453 | 3.1 (2.1–5.7) | 6671 (4186–13 682) |
| 0–6 months (all)b | 129 | 2.8 (1.8–4.5) | 5272 (3770–9020) |
| Healthyc | 82 | 2.4 (1.6–3.8) | 4852 (2451–6621) |
| Any chronic conditionc | 47 | 3.7 (2.3–7.6) | 7160 (4695–16 981) |
| 6–23 monthsb | 405 | 2.9 (1.9–4.9) | 5726 (3948–10 252) |
| Healthyd | 197 | 2.6 (1.8–3.9) | 5058 (3580–7123) |
| Any chronic conditiond | 208 | 3.6 (2.1–5.9) | 6922 (4382–14 302) |
| 2–4 yearsb | 191 | 2.6 (1.8–3.7) | 5013 (3379–8526) |
| Healthye | 68 | 2.0 (1.5–2.7) | 3983 (3063–5127) |
| Any chronic conditione | 123 | 2.9 (2.1–5.3) | 6329 (3960–11 678) |
| 5–17 yearsb | 90 | 2.8 (1.8–4.5) | 6198 (4166–11 402) |
| Healthyf | 15 | 2.8 (1.8–4.2) | 5309 (4021–7003) |
| Any chronic conditionf | 75 | 2.8 (1.8–4.5) | 6449 (4166–11 998) |
Abbreviations: HMPV, human metapneumovirus; IQR, interquartile range; LOS, length of stay.
1Hospital cost, $ (adjusted for inflation to 2013 U.S. dollars).
aMann-Whitney test for pairwise comparison, P < .001 (LOS) and P < .001 (Cost).
bLinear regression, P = .7 (LOS) and P = .4 (Cost).
cMann-Whitney test for pairwise comparison, P < .001 (LOS) and P < .001 (Cost).
dMann-Whitney test for pairwise comparison, P < .001 (LOS) and P < .001 (Cost).
eMann-Whitney test for pairwise comparison, P < .001 (LOS) and P < .001 (Cost).
fMann-Whitney test for pairwise comparison, P = .9 (LOS) and P = .3 (Cost).
Among children with a CCC, those with chronic pulmonary conditions other than asthma, neuromuscular, neurologic, genetic, and metabolic conditions had longer LOS and higher costs compared with those with asthma and cardiac conditions (Supplemental Table). Children with 2 or more CCCs had significantly longer hospital LOS and higher hospital costs compared with children with only 1 CCC (P < .001 for both).
Hospitalization Rates
A total of 613 (75%) children hospitalized with HMPV at PCH were Salt Lake County residents. The overall hospitalization rate was 36 of 100 000 person-years among children <18 years, 108 of 100 000 person-years among children <5 years of age, and 200 of 100 000 person-years for children <2 years of age. Hospitalization rates were highest in children <2 years, and these rates dropped sharply with increasing age (Figure 3).
Figure 3.
Population-based incidence of hospitalization for human metapneumovirus infection among children, Salt Lake County, Utah, 2007–2013.
DISCUSSION
In this 6-year study of children hospitalized with laboratory-confirmed HMPV, we found evidence of substantial clinical and economic burden of HMPV infection as demonstrated by hospitalization rates, severity, and total hospital costs. The average incidence of hospitalization for HMPV among Salt Lake County residents was 36.4 of 100 000 children <18 years and 200 of 100 000 children <2 years, similar to influenza incidence rates [18, 29]. More than half of children hospitalized with HMPV had a CCC, the majority with chronic pulmonary conditions. If the observed hospitalization rates and costs are extrapolated to the US population using mean hospitalization rates and median hospital costs, we estimate that HMPV may result in ∼27 000 hospitalizations annually in children <18 years with a mean estimated medical cost of $277 million (95% CI, $246–$308 million).
Human metapneumovirus was detected in 5% of children with a respiratory sample submitted for viral testing during the study period, representing the fourth most common respiratory virus detected after RSV (20%), rhinovirus (17%), and influenza (8%), and similar to that of PIV (5%). In previous studies of HMPV in children ≤5 years, HMPV was detected in 2.5%–11.3% of respiratory samples and was the second or third most commonly detected respiratory virus [14, 30–33]. Our findings reinforce the important role of HMPV in acute respiratory infections in children.
Previous studies reported observing alternating year epidemics of HMPV [34, 35]. In this study, years with high HMPV activity in the winter were occasionally followed by years with low HMPV activity that peaked during the spring. The cause of this biannual periodicity remains unknown. However, annual changes and replacement of the predominant circulating HMPV subgroups (A1, A2a, A2b, B1, and B2) and short-term herd immunity may play a role [36].
In our study, 18% of children hospitalized with HMPV required ICU admission and 7% required mechanical ventilation, comparable with previously published reports and similar to influenza [18, 22, 37–40]. Patients with CCCs were significantly more likely to require ICU admission and mechanical ventilation. In this study, there were 2 deaths (0.2%), which is consistent with previous reports describing the low case-fatality rate associated with pediatric HMPV infections, despite the large proportion of children with CCCs [14, 22, 38].
A secondary bacterial infection was diagnosed in 4.5% of children who had specimens obtained for bacterial culture (2.2% of the entire study population). When compared with other respiratory viruses, rates of secondary bacterial infection with HPMV were slightly higher than those reported for influenza (<2%) [18, 41, 42], RSV (<1%) [43], or AV (1.4%) [44]. Interactions between respiratory viruses and subsequent bacterial infections have been well documented; however, there are very few data describing the interactions between bacteria and HMPV [45, 46]. Like infections with influenza, we previously reported a correlation between seasonal epidemics of HMPV and increases in invasive pneumococcal disease in children [47]. The relatively frequent identification of S pyogenes among children with primary HMPV infection in this study is intriguing. Similar increases in S pyogenes infection have been reported after influenza and varicella infection [47–49]. Further studies are needed to evaluate the interaction between HMPV and S pyogenes.
Complex chronic conditions have been previously reported to be associated with severe HMPV infection. The proportion of children requiring hospitalization for HMPV who also had CCCs in our study is comparable to those previously observed [22, 23, 50]. Similar to Hahn et al [22], medical conditions such as asthma, chronic pulmonary disorders, cardiac disorders, neurologic/neuromuscular disorders, and genetic/metabolic disorders were most commonly identified.
The proportion of children with CCCs increased with age, suggesting these conditions are important risk factors for hospitalization for HMPV among older children. Complex chronic conditions were associated with longer hospital LOS and higher costs, except for children 5–17 years of age.
The high rates of HMPV-associated hospitalizations we observed were similar to those observed in the Centers for Disease Control and Prevention's New Vaccine Surveillance Network (NVSN) of 300, 200, 100, and 100 per 100 000 children <6 months, 6–11 months, 12–23 months, and <5 years, respectively [50]. Compared with other respiratory viruses, the hospitalization rates we observed are higher than influenza hospitalization rates reported by the NVSN from 2004–2007 [51] as well as in Salt Lake County from 2001–2004 [18]. Even so, our study findings are lower than hospitalization rates reported in a 1-year study from the United Kingdom of 126 of 100 000 in children <6 years [30] and 260 of 100 000 children <36 months of age in Spain over a 3-year period [52]. Altogether, our estimates are consistent with others when one factors in the different seasons studied, the duration of our study, seasonal variations in the incidence of HMPV, and various viral detection methods.
The total cost for children hospitalized with HPMV varied annually, ranging from a low of $651 000 in 2010–2011 to a high of $3 million in 2009–2010. The total direct costs associated with HMPV hospitalizations were $7.9 million over the study period. Children <2 years, 2–4 years, and 5–17 years accounted for 63.1%, 24.4%, and 12.5%, respectively, of the total hospital cost. Extrapolating our data to the US population, we estimate that each year approximately 27 000 children <18 years in the United States are hospitalized with HMPV at a cost of $277 million (95% CI, $246–$308 million). For children <5 years, we estimate that 22 000 children are hospitalized each year with HMPV, which is similar to that reported by Edwards et al [50]. Our estimates for HMPV are also similar to those reported for influenza, which is estimated to result in 8000–30 000 hospitalized children each year; however, national estimates for the costs of influenza hospitalizations were lower ($44–$163 million) [18, 53]. Our estimates of the burden of HMPV are comparable with those reported for PIV (63 000–70 000 hospitalizations annually at a cost of $190–$215 million) [52, 53], and the estimates are somewhat lower than those reported for RSV (86 000 children <5 years hospitalizations annually at a cost of $390 million) [54].
With the increasing appreciation of the clinical and economic burden associated with HMPV infection, there is ongoing research into the development of preventive vaccines and other monoclonal antibody therapies [55–57]. Our data have the potential to inform the selection of priority groups for potential vaccination. Vaccination of all children <2 years has the potential to provide coverage for 79% of children hospitalized with HMPV, and expanding vaccines to older children with CCCs would increase coverage to 94% (Supplemental Figure).
This study has several limitations. Although we excluded patients whose primary reason for admission was not directly attributable to HMPV infection, not all outcomes, LOS, or hospital costs are necessarily due entirely to HMPV. However, outliers for hospital cost and LOS were reviewed. We did not systematically collect data on CCCs for children with HMPV who were not admitted so we were unable to examine risk factors for hospitalization. Our estimates of the economic burden of HMPV hospitalizations do not account for indirect medical costs such as parental work absences. It is possible that we may have missed important outcomes such as positive bacterial cultures performed at an outside facility before transfer to our hospital. Lastly, viral testing methods changed during the study period, which may have resulted in an underestimation of the incidence of HMPV during the first 2 years of the study due to the use of less sensitive DFA testing methods.
CONCLUSIONS
Human metapneumovirus is a common respiratory pathogen among young children. Hospitalization rates were lower than those for RSV, similar to those for influenza, and higher than those for PIV. Unlike RSV and PIV and similar to influenza, HMPV infection affects a broader age group, including school-aged children. Human metapneumovirus infection was associated with substantial morbidity, including bacterial superinfection, resource utilization and costs, particularly in children with chronic medical conditions. The development of a safe and effective vaccine has the potential to reduce the significant clinical and economic burden of HMPV.
Supplementary Data
Acknowledgments
Financial support. This work was supported by grants from the National Institute of Allergy and Infectious Diseases (Grant U01A1082482 [to K. A. and C. L. B.], Grant U01 AI074419 [to C. L. B. and A. T. P.], Grant U01AI082184 [to A. J. B. and A. T. P.], Grant K23-AI079401 [to A. J. B.]) and the Centers for Disease Control Prevention (Grant U18-IP000303 [to C. S., K. A., C. L. B., A. J. B., and A. T. P.]). This project was further supported by the University of Utah, Department of Pediatrics, through the Children's Health Research Center and the Pediatric Clinical and Translational Research Scholars Program, and the Primary Children's Medical Center Foundation. C. L. B. is supported by the H.A. and Edna Benning Presidential Endowment and C. S. is supported by the American Foundation for Pharmaceutical Education's Clinical Pharmaceutical Sciences Fellowship.
Potential conflicts of interest. K. A., C. L. B., A. L. H., A. J. B., and A. T. P. collaborate with BioFire Diagnostics, Inc. (formerly Idaho Technology, Inc.) on several projects funded by the National Institutes of Health and the Centers for Disease Control and Prevention (see Funding). C. L. B. and A. J. B. have intellectual property in and receive royalties from BioFire Diagnostics, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.van den Hoogen BG, de Jong JC, Groen J et al. . A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 2001; 7:719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esper F, Martinello RA, Boucher D et al. . A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis 2004; 189:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAdam AJ, Hasenbein ME, Feldman HA et al. . Human metapneumovirus in children tested at a tertiary-care hospital. J Infect Dis 2004; 190:20–6. [DOI] [PubMed] [Google Scholar]
- 4.Gray GC, Capuano AW, Setterquist SF et al. . Multi-year study of human metapneumovirus infection at a large US Midwestern Medical Referral Center. J Clin Virol 2006; 37:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowe JE., Jr Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr Infect Dis J 2004; 23(11 Suppl):S215–21. [DOI] [PubMed] [Google Scholar]
- 6.Williams JV, Harris PA, Tollefson SJ et al. . Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. New Engl J Med 2004; 350:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boivin G, De Serres G, Cote S et al. . Human metapneumovirus infections in hospitalized children. Emerg Infect Dis 2003; 9:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nissen MD, Siebert DJ, Mackay IM et al. . Evidence of human metapneumovirus in Australian children. Med J Aust 2002; 176:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockton J, Stephenson I, Fleming D, Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis 2002; 8:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung JY, Han TH, Kim BE et al. . Human metapneumovirus infection in hospitalized children with acute respiratory disease in Korea. J Korean Med Sci 2006; 21:838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ordas J, Boga JA, Alvarez-Arguelles M et al. . Role of metapneumovirus in viral respiratory infections in young children. J Clin Microbiol 2006; 44:2739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peiris JS, Tang WH, Chan KH et al. . Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis 2003; 9:628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf DG, Greenberg D, Kalkstein D et al. . Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J 2006; 25:320–4. [DOI] [PubMed] [Google Scholar]
- 14.Williams JV, Edwards KM, Weinberg GA et al. . Population-based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis 2010; 201:1890–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung J, Esper F, Weibel C, Kahn JS. Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. J Clin Microbiol 2005; 43:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glezen WP, Greenberg SB, Atmar RL et al. . Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 2000; 283:499–505. [DOI] [PubMed] [Google Scholar]
- 17.Henrickson KJ, Kuhn SM, Savatski LL. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin Infect Dis 1994; 18:770–9. [DOI] [PubMed] [Google Scholar]
- 18.Ampofo K, Gesteland PH, Bender J et al. . Epidemiology, complications, and cost of hospitalization in children with laboratory-confirmed influenza infection. Pediatrics 2006; 118:2409–17. [DOI] [PubMed] [Google Scholar]
- 19.Neuzil K, Mellen B, Wright P et al. . The effect of influenza on hospitalizations, outpatients visits, and courses of antibiotics in children. New Engl J Med 2000; 342:225–31. [DOI] [PubMed] [Google Scholar]
- 20.Hall CB. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Tar 2012; 12:92–7. [DOI] [PubMed] [Google Scholar]
- 21.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics 2000; 106(1 Pt 2):205–9. [PubMed] [Google Scholar]
- 22.Hahn A, Wang W, Jaggi P et al. . Human metapneumovirus infections are associated with severe morbidity in hospitalized children of all ages. Epidemiol Infect 2013; 141:2213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Principi N, Esposito S. Paediatric human metapneumovirus infection: epidemiology, prevention and therapy. J Clin Virol 2014; 59:141–7. [DOI] [PubMed] [Google Scholar]
- 24.Classen D, Pestotnik S, Evans R et al. . Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277:301–6. [PubMed] [Google Scholar]
- 25.Harbarth S, Burke J, Lloyd J et al. . Clinical and economic outcomes of conventional amphotericin B-associated nephrotoxicity. Clin Infect Dis 2002; 35:e120–7. [DOI] [PubMed] [Google Scholar]
- 26.Oderda G, Evans R, Lloyd J et al. . Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage 2003; 25:276–83. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Bureau of Labor Statistics. CPI Inflation Calculator. Available at: http://www.bls.gov/cpi/home.htm. Accessed 2 September 2014. [Google Scholar]
- 28.Population Estimates Query Module for Utah Counties and Local Health Districts. Available at: http://www.pandemicflu.utah.gov/query/selection/pop/PopSelection.html. Accessed 30 June 2014. [Google Scholar]
- 29.Poehling KA, Edwards KM, Weinberg GA et al. . The underrecognized burden of influenza in young children. New Engl J Med 2006; 355:31–40. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson KG, McNally T, Silverman M et al. . Rates of hospitalisation for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine 2006; 24:102–8. [DOI] [PubMed] [Google Scholar]
- 31.Foulongne V, Guyon G, Rodiere M, Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J 2006; 25:354–9. [DOI] [PubMed] [Google Scholar]
- 32.Sloots TP, Mackay IM, Bialasiewicz S et al. . Human metapneumovirus, Australia, 2001–2004. Emerg Infect Dis 2006; 12:1263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heikkinen T, Osterback R, Peltola V et al. . Human metapneumovirus infections in children. Emerg Infect Dis 2008; 14:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aberle SW, Aberle JH, Sandhofer MJ et al. . Biennial spring activity of human metapneumovirus in Austria. Pediatr Infect Dis J 2008; 27:1065–8. [DOI] [PubMed] [Google Scholar]
- 35.Heininger U, Kruker AT, Bonhoeffer J, Schaad UB. Human metapneumovirus infections--biannual epidemics and clinical findings in children in the region of Basel, Switzerland. Eur J Pediatr 2009; 168:1455–60. [DOI] [PubMed] [Google Scholar]
- 36.Aberle JH, Aberle SW, Redlberger-Fritz M et al. . Human metapneumovirus subgroup changes and seasonality during epidemics. Pediatr Infect Dis J 2010; 29:1016–8. [DOI] [PubMed] [Google Scholar]
- 37.Boivin G, Abed Y, Pelletier G et al. . Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis 2002; 186:1330–4. [DOI] [PubMed] [Google Scholar]
- 38.Eggleston HA, Gunville CF, Miller JI et al. . A comparison of characteristics and outcomes in severe human metapneumovirus and respiratory syncytial virus infections in children treated in an intensive care unit. Pediatric Infect Dis J 2013; 32:1330–4. [DOI] [PubMed] [Google Scholar]
- 39.Mullins JA, Erdman DD, Weinberg GA et al. . Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis 2004; 10:700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akhras N, Weinberg JB, Newton D. Human metapneumovirus and respiratory syncytial virus: subtle differences but comparable severity. Infect Dis Rep 2010; 2:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dawood FS, Chaves SS, Perez A et al. . Complications and associated bacterial coinfections among children hospitalized with seasonal or pandemic influenza, United States, 2003–2010. J Infect Dis 2014; 209:686–94. [DOI] [PubMed] [Google Scholar]
- 42.Coffin SE, Zaoutis TE, Rosenquist AB et al. . Incidence, complications, and risk factors for prolonged stay in children hospitalized with community-acquired influenza. Pediatrics 2007; 119:740–8. [DOI] [PubMed] [Google Scholar]
- 43.Bloomfield P, Dalton D, Karleka A, Kesson A, Duncan G, Isaacs D. Bacteraemia and antibiotic use in respiratory syncytial virus infections. Arch Dis Child. 2004; 89:363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rocholl C, Gerber K, Daly J, Pavia A, Byington C. Adenoviral infection in children: The impact of rapid diagnosis. Pediatrics 2004; 113:e51–e6. [DOI] [PubMed] [Google Scholar]
- 45.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003; 187:1000–9. [DOI] [PubMed] [Google Scholar]
- 46.Iverson AR, Boyd KL, McAuley JL, Plano LR, Hart ME, McCullers JA. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis. 2011; 203:880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ampofo K, Bender J, Sheng X et al. . Seasonal invasive pneumococcal disease: Role of preceding respiratory viral infection. Pediatrics 2008; 122. [DOI] [PubMed] [Google Scholar]
- 48.Peterson CL, Vugia DJ, Meyers HB et al. . Risk factors for invasive group A streptococcal infections in children with varicella: a case-control study. Pediatr Infect Dis J. 1996; 15:151–6. [DOI] [PubMed] [Google Scholar]
- 49.Poehling KA, Talbot TR, Griffin MR et al. . Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006; 295:1668–74. [DOI] [PubMed] [Google Scholar]
- 50.Edwards KM, Zhu Y, Griffin MR et al. . Burden of human metapneumovirus infection in young children. New Engl J Med 2013; 368:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poehling KA, Edwards KM, Griffin MR et al. . The burden of influenza in young children, 2004–2009. Pediatrics 2013; 131:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cilla G, Onate E, Perez-Yarza EG et al. . Hospitalization rates for human metapneumovirus infection among 0- to 3-year-olds in Gipuzkoa (Basque Country), Spain. Epidemiol Infect 2009; 137:66–72. [DOI] [PubMed] [Google Scholar]
- 53.Fairbrother G, Cassedy A, Ortega-Sanchez IR et al. . High costs of influenza: Direct medical costs of influenza disease in young children. Vaccine 2010; 28:4913–9. [DOI] [PubMed] [Google Scholar]
- 54.Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. PharmacoEconomics 2004; 22:275–84. [DOI] [PubMed] [Google Scholar]
- 55.Herfst S, Schrauwen EJ, de Graaf M et al. . Immunogenicity and efficacy of two candidate human metapneumovirus vaccines in cynomolgus macaques. Vaccine 2008; 26:4224–30. [DOI] [PubMed] [Google Scholar]
- 56.Cseke G, Wright DW, Tollefson SJ et al. . Human metapneumovirus fusion protein vaccines that are immunogenic and protective in cotton rats. J Virol 2007; 81:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levy C, Aerts L, Hamelin ME et al. . Virus-like particle vaccine induces cross-protection against human metapneumovirus infections in mice. Vaccine 2013; 31:2778–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.