Abstract
Background
Enteric neurospheres derived from postnatal intestine represent a promising avenue for cell replacement therapy to treat Hirschsprung disease and other neurointestinal diseases. We describe a simple method to improve the neuronal yield of spontaneously-formed gut-derived neurospheres.
Materials and Methods
Enteric neurospheres were formed from the small and large intestines of mouse and human subjects. Neurosphere size, neural crest cell content, cell migration, neuronal differentiation, and neuronal proliferation in culture were analyzed. The effect of supplemental neurotrophic factors, including glial-derived neurotrophic factor (GDNF) and endothelin-3 (ET3), was also assessed.
Results
Mouse small intestine-derived neurospheres contained significantly more P75-expressing neural crest-derived cells (49.9 ± 15.3 vs. 21.6 ± 11.9%, p<0.05) and gave rise to significantly more Tuj1-expressing neurons than colon-derived neurospheres (69.9 ± 8.6 vs. 46.2 ± 15.6%, p<0.05). A similar pattern was seen in neurospheres isolated from human small and large intestine (32.6 ± 17.5 vs. 10.2 ± 8.2% neural crest cells, p<0.05; 29.7 ± 16.4 vs. 16.0 ± 13.5% enteric neurons, p<0.05). The addition of GDNF to the culture media further improved the neurogenic potential of small intestinal neurospheres (75.9 ± 4.0 vs. 67.8 ± 5.8%, p<0.05) whereas ET3 had no effect.
Conclusions
Enteric neurospheres formed from small intestine and supplemented with GDNF yield an enriched population of neural crest-derived progenitor cells and give rise to a high density of enteric neurons.
Keywords: Enteric nervous system, Enteric neural stem cells, Neurospheres, Glial cell-derived neurotrophic factor, Endothelin-3
INTRODUCTION
The enteric nervous system (ENS) is a complex network of neurons and glia which controls many essential functions of the gastrointestinal (GI) tract (1). Diseases of the ENS encompass a broad spectrum of common GI disorders including esophageal achalasia, gastroparesis, slow-transit constipation, and Hirschsprung disease (2). Current treatment for these diseases is palliative rather than curative, although cell therapy holds promise as a novel potential therapy for this group of diseases (3). Recent evidence has shown that enteric neural stem/progenitor cells (ENSCs) can be isolated from the adult gut and propagated in culture as enteric neurospheres (4), and that these neurospheres can be transplanted to aneural gut, where they give rise to new neurons that may improve bowel function (5).
However, in addition to neural and glial progenitors, enteric neurospheres also contain many other, non-neuroglial cell types (6). Many techniques have been employed to improve the neurogenic potential of enteric neurospheres. We have previously shown that co-transplantation of neurospheres with a serotonin receptor agonist can enhance neuronal differentiation and proliferation (7). Modified dissection techniques to isolate the enteric nerve plexuses from donor tissue have been described in order to generate a purer population (8). Cell sorting using neural crest cell markers have also been used to enrich the progenitor population within neurospheres (6), but this would be difficult to apply clinically and may eliminate bystander cells important to supporting neuronal growth.
Previous reports have noted that the small intestine (SI) contains more than double the number of myenteric neurons than the large intestine (LI) (9), likely owing to the longer length of the SI. The length and redundancy of the SI make it an attractive tissue source, but neurospheres derived from SI and LI have not been compared. Other factors, such as glial cell-derived neurotrophic factor (GDNF) and endothelin-3 (ET3), are both known to play an important role in ENS development, but their effect on postnatal gut-derived neurospheres has not been explored. ET3, through its receptor endothelin receptor type B (EDNRB), promotes ENSC proliferation, while GDNF, through the receptor tyrosine kinase, rearranged during transfection (RET), promotes ENSC migration, proliferation, and neuronal differentiation (10). Mutations in either the RET/GDNF or EDNRB/ET3 pathway cause Hirschsprung disease in mice and humans (11).
The goal of this study was to identify the optimal source for ENSCs and to explore methods to optimize their neurogenic potential. Our study identifies clinically useful observations for the use of neuronal cell therapy for the treatment of enteric neuropathies.
MATERIALS AND METHODS
Generation of Mouse Enteric Neurospheres
With approval from the Institutional Animal Care and Use Committee, neurospheres were generated from male and female 3-week old C57BL/6 mice (Jackson Labs, Bar Harbor, ME) according to previously published protocols (7, 12). The muscularis propria, which contains the myenteric plexus, was isolated from SI (duodenum through ileum; Fig. 1 A-B) and LI (post-cecal colon through anus, Fig. 1 D-E). Primary neurospheres were dissociated with Accutase (StemCell Technologies, Vancouver, BC) at 37 °C for 30 minutes and re-plated at 50,000 cells/mL to form secondary neurospheres (Fig. 1 C, F).
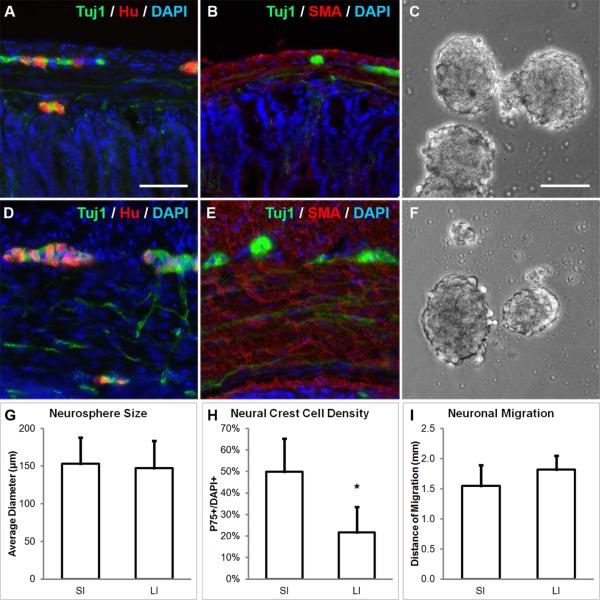
FIGURE 1. Neurospheres can be generated from the small and large intestine of mice.
Enteric ganglia containing Tuj1+ and Hu+ neurons are found in both small intestine (SI, A) and large intestine (LI, D). Tuj1+ neurons in the myenteric plexus were isolated together with the surrounding SMA+ muscularis propria from SI (B) and LI (E) and neurospheres were formed (C, F; respectively). While neurospheres from both sources were similar in size (G), SI-derived neurospheres contained significantly more P75+ neural crest-derived cells (H). Migratory distance of neurons from SI- and LI-derived neurospheres was similar (I). Scale bar in A is 100 μm and applies to A-B and D-E. Scale bar in C is 100 μm and applies to C and F.
*p<0.05; SMA, smooth muscle actin; SI, small intestine; LI, large intestine
Generation of Human Enteric Neurospheres
With approval from the Institutional Review Board, 1-3 cm2 pieces of SI or LI tissue was obtained from 5 patients (1 month-old to 21 years-old) undergoing bowel resection, including SI and LI tissue from a 17 year-old male undergoing ileocecal resection (Table 1). Neurospheres were generated based on previously published protocols (4, 13). In brief, the muscularis propria was isolated and digested for 90 minutes at 37 °C in dispase (250 μg/mL; StemCell Technologies) and collagenase XI (1 mg/mL; Sigma Aldrich, St. Louis, MO) and then filtered through a 70 μm filter. Cells were cultured in a 1:1 mix of mouse conditioned media (obtained from the supernatant of cultured mouse neurospheres) and human proliferation media, consisting of Neurocult Human Basal Medium (StemCell Technologies) supplemented with 10% Neurocult Human Proliferation Supplement (StemCell Technologies), 20 ng/mL epidermal growth factor, 20 ng/mL basic fibroblast growth factor, 0.0002% Heparin, 50 μg/mL metronidazole (Sigma Aldrich), 2 μL/mL Primocin (Invitrogen, Carlsbad, CA). After 7 days, primary neurospheres were dissociated with Accutase at 37 °C for 30 minutes and re-plated at 50,000 cells/mL in a 96-well round bottom plate (Corning, Kennebunk, ME), which was centrifuged at 480 g for 2 minutes to encourage cell aggregation. Secondary neurospheres formed after 7 days in culture.
TABLE 1.
Human tissue sources.
| Age | Sex | Operation | Indication | Tissue |
|---|---|---|---|---|
| 1 month old | M | Colon resection | necrotizing enterocolitis with colonic stricture | Colon |
| 6 years old | F | Colostomy closure | cloaca | Colon |
| 16 years old | M | Ileostomy revision | Crohn's disease | Ileum |
| 17 years old | M | Ileocecal resection | Crohn's disease with ileocecal stricture | Ileum, Colon |
| 21 years old | M | Ileostomy closure | ulcerative colitis | Ileum |
Tissue Preparation and Immunohistochemistry
Tissue preparation and immunohistochemistry were performed as previously described (7). Cells and tissues were fixed in 4% paraformaldehyde. For cryosection, sections were cut at 12 μm thickness with a Leica CM3050 S cryostat (Leica, Buffalo Grove, IL). For immunohistochemistry, cells and tissues were permeabilized with 0.1% Triton X-100 and blocked with 10% donkey serum for 30 minutes. Primary antibodies included human anti-neuronal nuclear antibody-1 (Hu; 1:16,000; generous gift from Dr. Vanda Lennon), mouse anti-neuronal class III β-tubulin (Tuj1; 1:500; Covance, Dedham, MA), rabbit anti-p75 neurotrophin receptor (P75; 1:500; Promega, Madison, WI), rabbit anti-S100 calcium-binding protein B (S100; 1:100; NeoMarkers, Fremont, CA), and rabbit anti-α-smooth muscle actin (SMA; 1:100; Abcam, Cambridge, MA). Secondary antibodies included donkey anti-mouse Alexa Fluor 488, donkey anti-rabbit Alexa Fluor 546, and donkey anti-human Alexa Fluor 546 (Life Technologies, Carlsbad, CA). Cell nuclei were stained with DAPI (Vector Labs, Burlingame, CA). EdU incorporation was detected using the Click-iT EdU Imaging Kit (Invitrogen, Carlsbad, CA). Images were taken using a Nikon Eclipse TS100 or 80i microscope (Nikon Instruments, Melville, NY).
Characterization of Neurospheres
Cell migration was quantified by plating secondary neurospheres onto slides coated with 20 μg/mL fibronectin (Biomedical Technologies, Ward Hill, MA) and culturing for 7 days in either human or mouse differentiation media, consisting of Neurocult Human or Mouse Basal Medium supplemented with 10% Neurocult Human or Mouse Differentiation Supplement (StemCell Technologies), 10% fetal bovine serum, and 100 U/mL penicillin-streptomycin. Neurons were visualized using Tuj1 immunoreactivity and cell migration was measured as the distance from the edge of the neurosphere to the farthest neuronal nucleus using ImageJ software (National Institutes of Health, Bethesda, Maryland). The average distance to the 3 farthest neurons in each orthogonal direction was measured, accounting for 12 measurements per neurosphere with at least 3 neurospheres analyzed per condition.
To quantify the number of neural crest-derived cells in the neurosphere, 7 day-old secondary neurospheres were dissociated with Accutase incubation for 30 minutes at 37 °C and centrifuged (800 g for 2 minutes; Shandon Cytospin 3) onto a poly-L-lysine-coated slide. Neural crest-derived cells were visualized using P75 immunoreactivity. To quantify neurogenesis and cell proliferation, dissociated neurospheres were re-plated at 5,000 cells/mL in differentiation media onto fibronectin-coated slides. Cells were cultured for 7 days and 10 μM EdU was added to the culture media 24 hours prior to fixation. Neurons were visualized with Tuj1 immunoreactivity and proliferation rate determined based on the proportion of cells incorporating EdU. To test the effect of supplemental factors, ET3 (100 ng/mL) and/or GDNF (50 ng/mL) were added to secondary neurospheres at the beginning of the culture period and again following neurosphere dissociation, at the time of plating onto fibronectin-coated slides. Four to 10 non-overlapping images were taken of each cell preparation and each preparation was repeated at least twice. Numbers of cells were counted using ImageJ software.
Quantitative PCR
Total mRNA was extracted from conditioned media of secondary neurospheres using the RNeasy Mini Kit (Qiagen, Santa Clarita, CA) and cDNA synthesized with the Superscript III Reverse Transcription Kit (Invitrogen). Gdnf, Et3, Ret, and Ednrb expression levels were measured using qPCR with Gapdh as the internal standard. Relative expression was calculated by ΔCt. The primers used where: Gdnf forward, GCCGGACGGGA; Gdnf reverse, CGTCATCAAAC; Et3 forward, GTGAGAGGATT; Et3 reverse, TGTCCTTGTAA; Ret forward, TCAAGGGATGCTTACTGGGAG; Ret reverse, GGTAGACGCCATAGAGATGCT; Ednrb forward, TCGGACTACAAAGGAAAGCC; Ednrb reverse, AGTAGAAACTGAACAGCCACC.
Statistics
Data are presented as mean ± standard deviation. Neuronal migration, neurosphere size, and relative expression were compared using Student's t-test. All other results were compared using repeated measures ANOVA. Intestinal tissue was pooled from 3-5 animals for each murine experiment. The number of replicates for each neurosphere culture condition is stated in the Results section. Statistical significance was considered at p<0.05. Statistical analysis was performed using JMP version 12 (SAS Institute, Cary, NC).
RESULTS
Neurospheres derived from mouse and human SI contain more ENSCs and generate more neurons than LI-derived neurospheres
ENSCs were isolated from the muscular layers of the SI and LI of adult mice (Fig. 1A-B, D-E) and passaged to form secondary neurospheres (Fig. 1C, F). No difference was observed in the average diameter of neurospheres from SI and LI (153 ± 35 vs. 147 ± 36 μm, n=30; Fig. 1G). SI-derived neurospheres did contain significantly more P75+ neural crest-derived cells (49.9 ± 15.3%) than LI-derived neurospheres (21.6 ± 11.9%, p<0.05, n=5; Fig. 1H), representing a 2.3-fold greater density of P75+ cells from the SI. Neurons from both SI and LI migrated a similar distance in culture (1.55 ± 0.34 vs. 1.82 ± 0.23 mm, n=3; Fig. 1I).
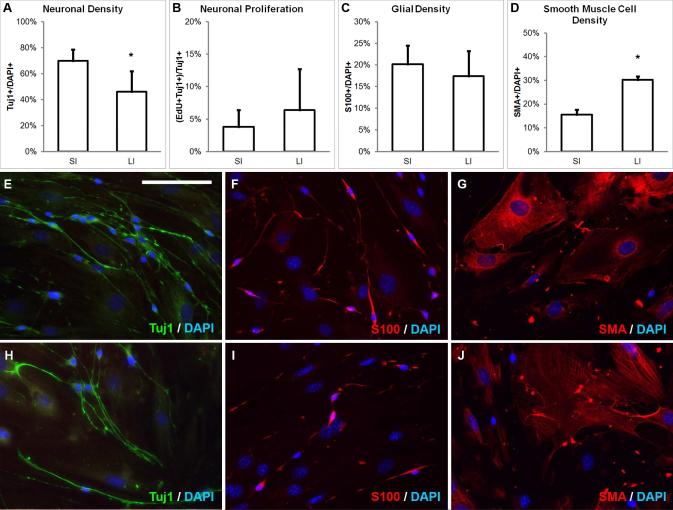
SI-derived neurospheres from mice gave rise to significantly more Tuj1+ neurons than LI (69.9 ± 8.6 vs. 46.2 ± 15.6%, p<0.05, n=7; Fig. 2A), but the rate of neuronal proliferation did not differ significantly (3.8 ± 2.6 vs. 6.4 ± 6.3%, n=7; Fig. 2B). In contrast, the percentage of glial cells did not differ between SI- and LI-derived neurospheres (20.2 ± 4.3 vs. 17.4 ± 5.8%, n=2; Fig. 2C). Interestingly, SI-derived neurospheres gave rise to significantly fewer SMA+ smooth muscle cells (15.6 ± 2.0 vs. 30.2 ± 1.5%, p<0.05, n=2; Fig. 2D). The overall gross morphology of cells did not differ between SI and LI sources (Fig. 2E-J).
FIGURE 2. Mouse SI- and LI-derived neurospheres differ in neurogenic potential.
SI-derived neurospheres give rise to a significantly greater percentage ofTuj1+ neurons than LI-derived neurospheres (A), but neuronal proliferation (B) and glial cell density (C) are not different. LI neurospheres give rise to a significantly greater percentage of smooth muscle cells (D). The morphology of Tuj1+ neurons, S100+ glia, and SMA+ smooth muscle cells from SI (E-G) are not grossly different from LI (H-J). Scale bar in E is 100 μm and applies to E-J.
*p<0.05; SMA, smooth muscle actin; SI, small intestine; LI, large intestine
The same experiments were repeated using SI- and LI-derived neurospheres generated from human intestine obtained from 5 patients, including 3 SI and 3 LI samples (Table 1). Human SI-derived neurospheres generated 28.5 ± 18.0% Tuj1+ neurons with 6.4 ± 3.6% proliferating (Table S1; median age 17, range 16 to 21 years), while neurospheres from human LI generated 15.2 ± 2.6% Tuj1+ neurons with 12.5 ± 14.2% proliferating (Table S1; median age 6, range 1 month to 17 years). SI-derived neurospheres thus generated 88% more neurons than LI, although this difference did not reach statistical significance, possibly due to the large variability in results among human patients.
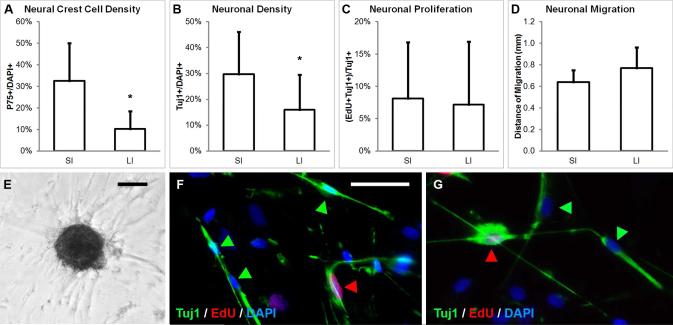
From a single patient who underwent ileocecal resection, a direct comparison of SI and LI was performed. Ileum-derived neurospheres contained significantly more P75+ neural crest cells than cecum-derived neurospheres (32.6 ± 17.5 vs. 10.2 ± 8.2%, p<0.05; Fig. 3A) and gave rise to significantly more Tuj1+ neurons in culture (29.7 ± 16.4 vs. 16.0 ± 13.5%, p<0.05; Fig. 3B). As with mouse enteric neurospheres, neuronal proliferation from ileum and cecum-derived neurospheres did not differ significantly (8.1 ± 8.7% vs. 7.2 ± 9.7%; Fig. 3C), nor did neuronal migration (0.64 ± 0.11 vs. 0.77 ± 0.19 mm; Fig. 3D).
FIGURE 3. Human SI and LI-derived neurospheres differ in their neurogenic potential.
Neurospheres generated from human ileum contain significantly more P75+ neural crest-derived cells (A, E) and give rise to significantly more Tuj1+ neurons (B) than neurospheres from the cecum of the same patient. However, neuronal proliferation (C) and migration (D) are not statistically different. Proliferating neurons from SI (F) and LI (G) are immunoreactive to Tuj1 (green arrows) and incorporate EdU (red arrows). Scale bar in E is 100 μm. Scale bar in F is 50 μm and applies to F and G.
*p<0.05; SI, small intestine; LI, large intestine
Neurogenic potential is enhanced by GDNF
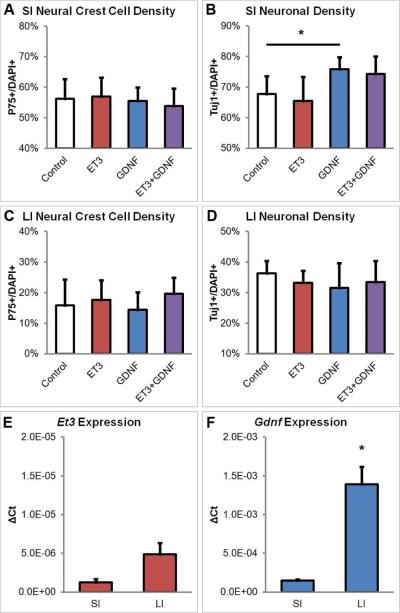
The effect of factors known to promote ENSC proliferation during embryonic ENS development was tested by adding GDNF, ET3, or both to cultured mouse neurospheres. The proportion of P75+ neural crest-derived cells did not differ significantly between control, ET3, GDNF, or ET3+GDNF treated cultures for SI-derived neurospheres (56.3 ± 6.4 vs. 56.9 ± 6.3 vs. 55.5 ± 4.4 vs. 53.9 ± 5.7%, respectively, n=5; Fig. 4A) or LI-derived neurospheres (15.9 ± 8.4 vs. 17.6 ± 6.4 vs. 14.4 ± 5.7 vs. 19.7 ± 5.2%, respectively, n=5; Fig. 4C). However, SI-derived neurospheres cultured with GDNF gave rise to significantly more Tuj1+ neurons (75.9 ± 4.0%) compared to control neurospheres cultured with no additives (67.8 ± 5.8%, p<0.05, n=5; Fig. 4B), representing an increase of 12% in neuronal density. No significant difference in neuronal density was observed in the presence of ET3 (65.4 ± 7.9%) or ET3+GDNF (74.3 ± 5.7%). In contrast, LI-derived neurospheres cultured with either no additives, ET3, GDNF, or ET3+GDNF all gave rise to equivalent numbers of Tuj1+ neurons (36.4 ± 4.0 vs. 33.2 ± 4.0 vs. 31.6 ± 8.0 vs. 33.5 ± 6.9%, respectively, n=4; Fig. 4D). The rate of neuronal proliferation was not significantly different among the various treatment groups for SI-derived (4.1 ± 3.1 vs. 3.7 ± 2.5 vs. 3.6 ± 2.0 vs. 6.8 ± 4.5%, respectively, n=5; data not shown) or LI-derived neurospheres (8.8 ± 7.6 vs. 7.2 ± 7.2 vs. 4.8 ± 4.1 vs. 8.3 ± 7.4%, respectively, n=4; data not shown).
FIGURE 4. GDNF enhances neurogenic potential of SI-derived neurospheres.
Mouse SI- and LI-derived neurospheres were cultured with endothelin-3 (ET3, 100 ng/mL), glial cell-derived neurotrophic factor (GDNF, 50 ng/mL), both factors, or no additives (Control). Neither ET3 nor GDNF has an effect on the density of P75+ neural crest cells in SI-derived neurospheres (A). Addition of GDNF alone significantly increases the proportion of neurons generated from SI-derived neurospheres compared to control and ET3 (B). In LI-derived neurospheres, neither ET3 nor GDNF affect the density of P75+ neural crest cells or Tuj1+ neurons (C-D). The level of Et3 expression does not differ significantly between SI- and LI-derived neurospheres (E). However, the level of Gdnf expression in SI-derived neurospheres is significantly diminished relative to LI-derived neurospheres (F).
*p<0.05; SI, small intestine; LI, large intestine; ET3, endothelin-3; GDNF, glial cell-derived neurotrophic factor
In order to understand the differential effect of GDNF on SI and LI, we examined Gdnf and Et3 transcript levels in SI- and LI-derived neurospheres. The relative expression of Et3 transcript did not differ significantly between SI and LI (1.25 × 10−6 ± 4.15 × 10−7 vs. 4.88 × 10−6 ±1.46 × 10−6; Fig. 4E). Interestingly, however, the relative expression of Gdnf was significantly lower in SI neurospheres than in LI neurospheres (1.48 × 10−4 ± 1.41 × 10−5 vs. × 1.39 × 10−3 ± 2.26 × 10−4, p<0.05; Fig. 4F), representing a 10-fold difference in expression. The ratio of the relative expression of Gdnf compared to Et3 was also significantly lower in SI neurospheres than LI neurospheres (119 ± 11 vs. 285 ± 46; p<0.05). The relative expression of Ednrb did not differ significantly between SI and LI (7.98 × 10−4 ± 3.52 × 10−4 vs. 2.40 × 10−3 ± 2.65 × 10−5, data not shown), nor did the expression of Ret (2.88 × 10−5 ± 4.29 × 10−6 vs. 4.70 × 10−5 ± 3.52 × 10−6, data not shown).
DISCUSSION
Enteric neurospheres represent a potential treatment option for neurointestinal diseases by serving as an enriched source of ENSCs to replace missing or abnormal enteric neurons in patients with enteric neuropathies. Identifying the optimal source of these cells and developing efficient methodologies for their isolation and cultivation are important goals in order to achieve clinical application of this promising technology. Our results demonstrate that SI-derived neurospheres from mice and humans contain more than double the density of neural crest-derived progenitor cells as LI-derived neurospheres and subsequently give rise to significantly more neurons in culture. Furthermore, the neurogenic potential of those SI-derived neurospheres is substantially enhanced by the addition of the neurotrophic factor, GDNF. These findings will help to refine current strategies for generating ENSCs for use as cell therapy.
SI represents a readily available source for ENSC harvest in the clinical setting. The SI is easily accessible for biopsy endoscopically or laparoscopically. When more tissue is needed, a large segment of SI can be surgically resected without adverse effects (14). Furthermore, SI is usually normally ganglionated in Hirschsprung disease and other enteric neuropathies, allowing harvest of autologous cells. Our findings suggest that SI may also possess other advantages over LI. The greater progenitor purity and neurogenic potential of SI-derived neurospheres compared to LI-derived neurospheres is an important observation. This may be due to the thinner muscular layer in the SI, as SI neurospheres produced significantly more neurons and less smooth muscle cells than their LI counterparts. The differences between SI and LI-derived neurospheres do not appear to be due to any innate differences in their neural crest-derived cells, as neuronal proliferation did not differ between SI and LI in our study. Similarly, the rate of neuronal proliferation has been shown to remain constant along different regions of the gut in embryonic mice (15). However, SI may inherently contain a greater density of ENSCs than LI, although this remains unclear. Though it accounts for a relatively small portion of the distal colon, the sacral neural crest contributes to the myenteric plexus of LI (16) and is limited in its ability to form enteric ganglia compared to the vagal neural crest (17). This difference in SI and LI innervation may explain some of the differences in enteric neurospheres formed from the two regions. Further study is needed to determine if the distal gut truly contains fewer ENSCs and whether this underlies the differences in SI- and LI-derived neurospheres.
The balance of ET3 and GDNF plays a pivotal role in ENS development (10) and may continue to affect postnatal ENSCs in culture. The addition of GDNF to culture media had a modest but significant effect on the density of neurons generated from SI neurospheres. In contrast, GDNF had no observable neurogenic effect on LI neurospheres. Interestingly, both the relative expression of Gdnf and the ratio of Gdnf to Et3 expression were significantly lower in SI than LI neurospheres, which may account for the response to supplemental GDNF in the former. The relative expression of Et3 was not significantly different between the two, and supplemental ET3 had no observable neurogenic effect on SI- or LI-derived neurospheres. Since both GDNF and ET3 are expressed by the gut mesenchyme during embryonic development, an increased proportion of ENSCs and decreased proportion of bystander cells, such as mesenchymal (e.g. smooth muscle) cells, may diminish the relative expression of GDNF and ET3 in the neurosphere. As the population of neural progenitors is enriched in enteric neurospheres, our results suggest that it may be necessary to supplement factors normally produced by non-neural cells in order to maximize neurogenesis.
While GDNF and ET3 have been shown to exert a synergistic effect on the growth of embryonic ENSCs (18), neither GDNF nor ET3 had an effect on postnatal ENSC density or neuronal proliferation. The lack of effect may be due to a number of factors. As discussed above, the effect of supplemental factors may not be seen until constitutive expression drops below a threshold level. In addition, changes in culture media and cell density are known to alter the effects of ET3 and GDNF (19), and the specific conditions used in this study may obscure observable changes. Finally, the effects of ET3 and GDNF may be time-sensitive. In normal development, ET3 stimulates ENSC proliferation only in early embryonic stages (20, 21), and GDNF stimulates ENSC proliferation early in ENS development and promotes neuronal differentiation at later embryonic stages (22). The effect of ET3 and GDNF on postnatal cells may be diminished as the pathways that promote postnatal enteric neurogenesis may differ from those involved in the embryonic mechanisms (23, 24). It is likely that many of the factors which promote postnatal ENSC growth remain undiscovered. More insight into the proliferation and differentiation of postnatal neurons will help develop techniques to maximize their growth in vitro.
In summary, enteric neurospheres contain ENSCs with the potential to repopulate the aganglionic gut of patients with Hirschsprung disease. Compared to LI-derived neurospheres, SI-derived neurospheres contain a greater proportion of ENSCs and subsequently give rise to more neurons. The neurogenic potential of SI-derived neurospheres can be further augmented by the addition of GDNF. Optimization of enteric neurosphere culture improves the neurogenic, and subsequently, therapeutic potential of enteric neurospheres.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Vanda Lennon (Mayo Clinic, Rochester, MN) for the kind gift of Hu antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR DISCLOSURE STATEMENTS
The authors have no conflicting interests.
AUTHOR CONTRIBUTIONS L.S.C., R.H., and A.M.G. designed the experiments. L.S.C. performed the experiments and wrote the manuscript. H.K.G. provided reagents and maintained mouse colonies. W.P. performed quantitative PCR. A.M.G. provided human tissue. All authors critically reviewed and approved of the final manuscript.
DISCLOSURE
L.S.C. is supported by the Society of University Surgeons Ethicon Surgical Research Fellowship Award. R.H. is supported by grants from the Tosteson Fund for Medical Discovery at Massachusetts General Hospital, the REACHirschsprung Foundation, and the American Neurogastroenterology and Motility Society. A.M.G. is supported by the National Institutes of Health (R01DK103785).
REFERENCES
- 1.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 2.Burns AJ, Thapar N. Neural stem cell therapies for enteric nervous system disorders. Nat Rev Gastroenterol Hepatol. 11:317–328. doi: 10.1038/nrgastro.2013.226. [DOI] [PubMed] [Google Scholar]
- 3.Burns AJ, Goldstein AM, Newgreen DF, Stamp L, Schafer KH, Metzger M, Hotta R, Young HM, Andrews PW, Thapar N, Belkind-Gerson J, Bondurand N, Bornstein JC, Chan WY, Cheah K, Gershon MD, Heuckeroth RO, Hofstra RM, Just L, Kapur RP, King SK, McCann CJ, Nagy N, Ngan E, Obermayr F, Pachnis V, Pasricha PJ, Sham MH, Tam P, Berghe PV. White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies. Dev Biol. doi: 10.1016/j.ydbio.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzger M, Bareiss PM, Danker T, Wagner S, Hennenlotter J, Guenther E, Obermayr F, Stenzl A, Koenigsrainer A, Skutella T, Just L. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology. 2009;137:2063–2073. e2064. doi: 10.1053/j.gastro.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 5.Hetz S, Acikgoez A, Voss U, Nieber K, Holland H, Hegewald C, Till H, Metzger R, Metzger M. In vivo transplantation of neurosphere-like bodies derived from the human postnatal and adult enteric nervous system: a pilot study. PLoS One. 9:e93605. doi: 10.1371/journal.pone.0093605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder E, Natarajan D, Cooper J, Kronfli R, Cananzi M, Delalande JM, McCann C, Burns AJ, Thapar N. Enteric neurospheres are not specific to neural crest cultures: implications for neural stem cell therapies. PLoS One. 10:e0119467. doi: 10.1371/journal.pone.0119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotta R, Cheng LS, Graham HK, Nagy N, Belkind-Gerson J, Mattheolabakis G, Amiji MM, Goldstein AM. Delivery of enteric neural progenitors with 5-HT4 agonist-loaded nanoparticles and thermosensitive hydrogel enhances cell proliferation and differentiation following transplantation in vivo. Biomaterials. 88:1–11. doi: 10.1016/j.biomaterials.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundmann D, Klotz M, Rabe H, Glanemann M, Schafer KH. Isolation of high-purity myenteric plexus from adult human and mouse gastrointestinal tract. Sci Rep. 5:9226. doi: 10.1038/srep09226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development. 2003;130:2187–2198. doi: 10.1242/dev.00433. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein AM, Hofstra RM, Burns AJ. Building a brain in the gut: development of the enteric nervous system. Clin Genet. 83:307–316. doi: 10.1111/cge.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson K, Mason I, Hall S. Hirschsprung's disease: genetic mutations in mice and men. Gut. 1997;41:436–441. doi: 10.1136/gut.41.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotta R, Cheng LS, Graham HK, Pan W, Nagy N, Belkind-Gerson J, Goldstein AM. Isogenic enteric neural progenitor cells can replace missing neurons and glia in mice with Hirschsprung disease. Neurogastroenterol Motil. doi: 10.1111/nmo.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56:489–496. doi: 10.1136/gut.2006.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji G, Chu D, Wang W, Dong G. The safety of donor in living donor small bowel transplantation--an analysis of four cases. Clin Transplant. 2009;23:761–764. doi: 10.1111/j.1399-0012.2009.01072.x. [DOI] [PubMed] [Google Scholar]
- 15.Young HM, Turner KN, Bergner AJ. The location and phenotype of proliferating neural-crest-derived cells in the developing mouse gut. Cell Tissue Res. 2005;320:1–9. doi: 10.1007/s00441-004-1057-5. [DOI] [PubMed] [Google Scholar]
- 16.Burns AJ, Douarin NM. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- 17.Burns AJ, Champeval D, Le Douarin NM. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Dev Biol. 2000;219:30–43. doi: 10.1006/dbio.1999.9592. [DOI] [PubMed] [Google Scholar]
- 18.Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003;40:905–916. doi: 10.1016/s0896-6273(03)00730-x. [DOI] [PubMed] [Google Scholar]
- 19.Hearn CJ, Murphy M, Newgreen D. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev Biol. 1998;197:93–105. doi: 10.1006/dbio.1998.8876. [DOI] [PubMed] [Google Scholar]
- 20.Woodward MN, Kenny SE, Vaillant C, Lloyd DA, Edgar DH. Time-dependent effects of endothelin-3 on enteric nervous system development in an organ culture model of Hirschsprung's disease. J Pediatr Surg. 2000;35:25–29. doi: 10.1016/s0022-3468(00)80007-x. [DOI] [PubMed] [Google Scholar]
- 21.Shin MK, Levorse JM, Ingram RS, Tilghman SM. The temporal requirement for endothelin receptor-B signalling during neural crest development. Nature. 1999;402:496–501. doi: 10.1038/990040. [DOI] [PubMed] [Google Scholar]
- 22.Chalazonitis A, Rothman TP, Chen J, Gershon MD. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: expression of GFRalpha-1 in vitro and in vivo. Dev Biol. 1998;204:385–406. doi: 10.1006/dbio.1998.9090. [DOI] [PubMed] [Google Scholar]
- 23.Becker L, Peterson J, Kulkarni S, Pasricha PJ. Ex vivo neurogenesis within enteric ganglia occurs in a PTEN dependent manner. PLoS One. 8:e59452. doi: 10.1371/journal.pone.0059452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uesaka T, Nagashimada M, Enomoto H. Neuronal Differentiation in Schwann Cell Lineage Underlies Postnatal Neurogenesis in the Enteric Nervous System. J Neurosci. 35:9879–9888. doi: 10.1523/JNEUROSCI.1239-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.