Abstract
Objective
Many past studies have suggested atypical functional and anatomical hemispheric asymmetries in autism spectrum disorder (ASD). However, almost all of these have examined only language-related asymmetries. Here, we conduct a comprehensive investigation of microstructural asymmetries across a large number of fiber tracts in ASD.
Method
We used diffusion tensor imaging for a comprehensive investigation of anatomical white matter asymmetries across the entire white matter skeleton, using tract-based spatial statistics in 41 children and adolescents with ASD and a matched group of 44 typically developing (TD) participants.
Results
We found significant asymmetries in the TD group, being rightward for fractional anisotropy and leftward for mean diffusivity (with concordant asymmetries for radial and axial diffusivity). These asymmetries were significantly reduced in the group with ASD: in whole brain analysis for fractional anisotropy (FA), and in a region where several major association and projection tracts travel in close proximity within occipital white matter for mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). No correlations between global white matter asymmetry and age or sociocommunicative abilities were detected.
Conclusion
Our findings in TD children and adolescents can be interpreted as reflecting different processing modes (more integrative in right, more specialized in left hemisphere). These asymmetries and the “division of labor” between hemispheres implied by them appear to be diminished in autism spectrum disorder.
Keywords: autism, diffusion tensor imaging, MRI, white matter, asymmetry
INTRODUCTION
Brain development is characterized by emerging functional lateralization and specialization of the cerebral hemispheres, accompanied by changes in asymmetries at the genomic,1,2 cellular,3,4 and macrostructural levels.5 The latter include volumetric distributions of grey and white matter,6,7 especially in areas associated with language.8 Language has long been known to be predominantly controlled by the left hemisphere in most individuals.9 Moreover, the Sylvian fissure and perisylvian regions surrounding it have been found to be morphologically asymmetric, with clinical implications for deviations from typical asymmetry.10–12 However, asymmetries in white matter are not as well characterized.13,14
In the past decade, diffusion tensor imaging (DTI) has become a method of choice in the study of white matter organization in vivo. DTI detects the rate of water diffusion along multiple orientations and generates indices of white matter structure based on the diffusion tensor at each voxel. Commonly examined indices of diffusion include axial diffusivity (AD), the diffusion along the principle diffusion direction within a voxel, radial diffusivity (RD), the diffusion orthogonal to the principle diffusion direction, fractional anisotropy (FA), an index of the directional preference for diffusion in the axial relative to the radial direction, and mean diffusivity (MD), the mean diffusion regardless of direction. The tensor-based model has known limitations, particularly in its ability to resolve complex fiber crossings, and results are often non-specific with regard to the underlying cellular differences.15–17 Nonetheless, DTI is able to reliably localize group differences in white matter structure so long as results are interpreted with caution.15–18 DTI studies in healthy adults have found hemispheric differences of white matter microstructure, with rightward asymmetry of AD and FA in frontal and parietal lobes, but leftward asymmetry in temporal and occipital lobes.19,20 In both left- and right-handed adults, white matter FA in the precentral gyrus was found to be greater contralateral than ipsilateral to the dominant hand.21
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by sociocommunicative deficits. Behavioral and observational findings suggest atypical asymmetries in ASD in many respects. For example, using retrospective video analysis, Esposito and Venuti22 found that infants with ASD showed greater body asymmetry when sitting, compared to typically developing (TD) infants and those with developmental delays. Ozgen et al.23 reported that atypical facial asymmetries were highly predictive of diagnostic status (ASD vs. TD). Such observations are supported by findings from neurophysiological and imaging studies. Infants at high risk for ASD show atypical leftward processing asymmetry during face perception, compared to low-risk infants.24 Atypical hemispheric asymmetries in ASD have also been reported in a number of language-related studies, using anatomical25–28 and functional magnetic resonance imaging (MRI).29–31
In a review, Lindell and Hudry32 conclude that atypical asymmetries in ASD are tied to language impairment and primarily detected in fronto-temporal language regions. However, a recent functional connectivity MRI study using independent component analysis found that numerous functional networks were affected by atypical right-hemisphere shifts in children and adolescents with ASD,33 including many non-linguistic networks (executive, attentional, and sensorimotor). Comprehensive examinations of hemispheric asymmetries in ASD beyond the language system are therefore needed.
Few studies to date have examined asymmetry of DTI indices in ASD. Fletcher et al.34 observed that rightward asymmetry of RD in arcuate fasciculus (AF), seen in male TD adolescents, was absent in a small ASD group. While this may be consistent with one subsequent study35 reporting absence of typical leftward asymmetry of FA in arcuate fasciculus (as well as in cingulum and uncinate fasciculus) in a small sample of adolescents with ASD, it is at odds with findings from younger children (ages 4–7 years) of leftward asymmetry of RD within AF in TD children that was absent in children with ASD.28 Leftward asymmetry of AF volume, as detected in TD children, was also significantly reduced in children with ASD in this study. Finally, Lange et al.36 reported marginally reversed asymmetry of tensor skewness (a potential index of crossing fibers) in superior temporal gyrus, which was rightward in children and adults with ASD, but leftward in a matched TD group. However, all of these studies largely focused on language-related tracts. No DTI study to date has provided a comprehensive investigation of microstructural asymmetries across a large number of fiber tracts in ASD. We performed such an investigation in a cohort of children and adolescents with ASD and matched TD participants. Based on previous studies (as described above), we hypothesized an overall decrease in asymmetry in children with ASD.
METHOD
Participants
Participants were recruited through clinic referrals and advertisements. Diagnosis of ASD was performed by an expert clinical psychologist according to DSM-V criteria, using the Autism Diagnostic Interview-Revised37 and the Autism Diagnostic Observation Schedule.38 Participants with a history of medical or genetic conditions (e.g., epilepsy, tuberous sclerosis, Fragile-X, Rett syndrome) were excluded. Comorbid attention-deficit/hyperactivity disorder, obsessive-compulsive disorder, or anxiety disorder (as reported by parents on the medical history questionnaire) were not treated as exclusionary in view of the high prevalence of these diagnoses within ASD.39 In the TD group, no children with a personal or family history of autism or personal history of any other neurological or psychiatric conditions were included. Verbal and nonverbal IQ was assessed using the Wechsler Abbreviated Scale of Intelligence40 and all participants had nonverbal IQ above 50. Groups were matched for sex, handedness, verbal IQ, nonverbal IQ, and age (Table 1). Total scores on the Social Responsiveness Scale (SRS)41 were available for all ASD and all but 2 TD participants and were used in correlational analyses. The study protocol was approved by the University of California, San Diego and San Diego State University institutional review boards, and informed assent and consent were obtained from all participants and their parents prior to scanning.
Table 1.
Participant Information
| ASD (n = 41) | TD (n = 44) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Male | 32 (6 LH) | 35 (6 LH) | |||||
| Female | 9 (1 LH) | 9 (0 LH) | ||||||
| M | (SD) | Range | M | (SD) | Range | p | ||
| Age (years) | 13.68 | (2.87) | 7.4–18.0 | 13.43 | (2.75) | 8.0–17.7 | .68 | |
| Verbal IQ | 101.21 | (17.72) | 70–147 | 105.45 | (10.53) | 73–126 | .18 | |
| Nonverbal IQ | 104.79 | (18.63) | 53–140 | 104.8 | (14.0) | 62–137 | >.99 | |
| Full-scale IQ | 102.7 | (17.64) | 66–141 | 105.41 | (11.44) | 79–130 | .40 | |
| ADOS | Communication | 3.92 | (1.68) | 0–8 | ||||
| Social Interaction | 8.37 | (2.56) | 4–14 | |||||
| Repetitive Behavior | 2.32 | (1.51) | 0–5 | |||||
| ADI-R | Communication | 13.95 | (5.87) | 2–25 | ||||
| Social Interaction | 18.11 | (4.99) | 7–28 | |||||
| Repetitive Behavior | 6.41 | (2.58) | 1–12 | |||||
| SRS | Total | 82.32 | (9.17) | 62–100 | 42.50 | (4.79) | 35–52 | < .01 |
| Average translation (mm) | 0.81 | (0.16) | 0.49–1.15 | 0.87 | (0.24) | 0.49–1.71 | .17 | |
| Average rotation (rad; ×10−3) | 4.38 | (1.11) | 2.31–7 | 4.57 | (1.89) | 2.39–12.2 | .58 | |
| % Signal dropout | 0.01 | (0.02) | 0–0.06 | 0.01 | (0.02) | 0–0.09 | .91 | |
| Signal dropout severity | 1.08 | (0.18) | 1–1.66 | 1.04 | (0.10) | 1–1.41 | .19 | |
Note: Scores were unavailable for the following: Verbal IQ—2 participants with autism spectrum disorder (ASD), Nonverbal IQ—2 participants with ASD, Full Scale IQ—1 participant with ASD, Autism Diagnostic Observation Schedule (ADOS)—3 participants with ASD, Autism Diagnostic Interview-Revised (ADI-R)—4 participants with ASD, Social Responsiveness Scale (SRS)—2 typically developing (TD) participants. LH = left-handed.
We scanned 134 (72 ASD) children and adolescents for this project. Three sibling pairs (all TD) were recruited but only one from each pair was included in analyses. Additional participants were removed because of exclusionary finding on MRI (1 ASD, 2 TD), technical errors during imaging (3 ASD, 2 TD), or excessive motion (25 ASD, 11 TD) as described below. Two ASD participants recruited under DSM-IV criteria did not fully meet diagnostic criteria applied in this study (as described above) and were also excluded. The final sample consisted of 41 ASD and 44 TD participants.
Data Acquisition
DTI data were acquired using a GE Discovery 3.0T MR750 scanner using a single-shot diffusion-weighted echo-planar imaging pulse sequence with two degrees of diffusion weighting (b=0 and 1000 s/mm2, 61 non-collinear directions, 1.875 × 1.875 mm2 in-plane resolution with a 2-mm slice thickness) and an 8-channel head coil. Images were collected with a flip angle of 90°, repetition time of 8,500 ms, an echo time of 84.9 ms, field of view of 240 mm, 128 × 128 matrix and 68 axial slices. Field maps were collected and used to correct for geometric distortions caused by local magnetic field inhomogeneities.42
Data Analysis
Diffusion-weighted images were eddy corrected and preprocessed using the diffusion toolbox within the FMRIB Software Library, FSL version 5.0; fsl.fmrib.ox.ac.uk;43 scans were assessed for motion through both visual inspection of eddy-corrected data (for signal dropout, image noise, shifts of head placement) and quantification of artifacts. Quantitative measures included mean image translation and rotation applied during eddy correction, and severity and frequency of motion-related signal drop-outs across slices as determined by the Benner score.48–50 These quantities were combined into a total motion index (TMI) that was used as a covariate in all analyses.50 Initial visual screening excluded twenty participants (14 ASD, 8 TD); fourteen more (11ASD, 3 TD) were excluded with a TMI>6 or with more than .1% of slices affected by drop-out. The final sample included in analyses consisted of 41 participants with ASD and 44 TD participants.
Four DTI indices were calculated: FA, MD, AD, and RD. To account for anatomical differences across individuals, all DTI images were registered to the FMRIB58 FA 1mm template using FSL’s non-linear image registration tool (FNIRT). Analyses were performed using voxel-wise tract-based spatial statistics (TBSS).44 To exclude grey matter from the analysis, an FA threshold of 0.2 was set. TBSS creates an alignment-invariant “skeleton” that represents the center of each tract for an entire sample. To correct for remaining image misalignments, a vector is created between the TBSS skeleton and each participant’s local FA maxima (representative of the center of the actual tract for that individual). This vector is applied to the participant’s FA, MD, RD, and AD maps, and the DTI index value at the location of the local FA maximum is assigned to the corresponding voxel along the skeleton.
In order to test for hemispheric differences in DTI indices, a symmetrical skeleton was derived from the TBSS output using the tbss_sym tool provided in FSL. Briefly, the mean FA image was flipped, averaged with the original, skeletonized, and masked by a dilated (one voxel) original skeleton. To ensure that the skeleton was exactly symmetrical, it was masked by its flipped mirror image, creating the symmetric skeleton used by subsequent analysis. The DTI data were then projected onto this new symmetric skeleton and an asymmetry index (AI) was calculated for each voxel and for each DTI index, using the formula (2*[R−L]/[R+L])*100. A positive AI indicates that the voxel on the right hemisphere has a greater value than the corresponding voxel on the left, while a negative value indicates the opposite. To focus on cerebral hemispheric asymmetry, the thalamus, cerebellum, and brainstem were masked out prior to analyses. We localized areas of significant asymmetry and significant group differences in asymmetry by using FSL’s randomise permutation utility optimized for TBSS results. Results were controlled for multiple comparisons using threshold-free cluster enhancement.45 This correction sets a threshold based on the weighted sum of the entire cluster’s local intensity rather than setting an arbitrary hard threshold. Clusters of significant within-group effects were classified as having a right/leftward asymmetry based on the mean of that cluster. Clusters with significant group differences were described as having an increased, decreased, or reversed asymmetry in ASD when compared to TD by comparing the mean of the entire cluster.
RESULTS
Whole Brain White Matter Asymmetry
Both groups showed an overall rightward asymmetry for FA and leftward asymmetry for MD, AD, and RD when the entire TBSS skeleton was considered. The group with ASD displayed significantly reduced asymmetry for FA (p=.03; partial eta2 =.055) when compared to the TD group, but groups did not differ on whole-brain asymmetry of any other measures (Figure 1). After removal of one left-handed male participant with ASD (resulting in perfect handedness matching), this difference remained significant (p=.03; partial eta2 =.055). Asymmetry was similar in both left- and right-handed participants (see Figure S1, available online). Within-group partial correlations tested for relationships between age and average AI measures, and between Total SRS score and average AI measures. None of these survived correction for multiple comparisons in either group (all r<.35). TMI was included as a covariate in all tests.
Figure 1.
Asymmetry indices averaged across the entire white matter skeleton. Note: AD = axial diffusivity; ASD = autism spectrum disorder; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity; TD = typically developing. * p < .05.
Voxelwise Tests of Asymmetry
Regional asymmetry patterns were examined using voxelwise tests while controlling for TMI. One-sample t-tests of FA asymmetry showed a preponderance of rightward asymmetry in large portions of the skeleton in each group, accompanied by smaller clusters of leftward asymmetry (Figure 2, and see Table S1, available online). Groups did not differ significantly.
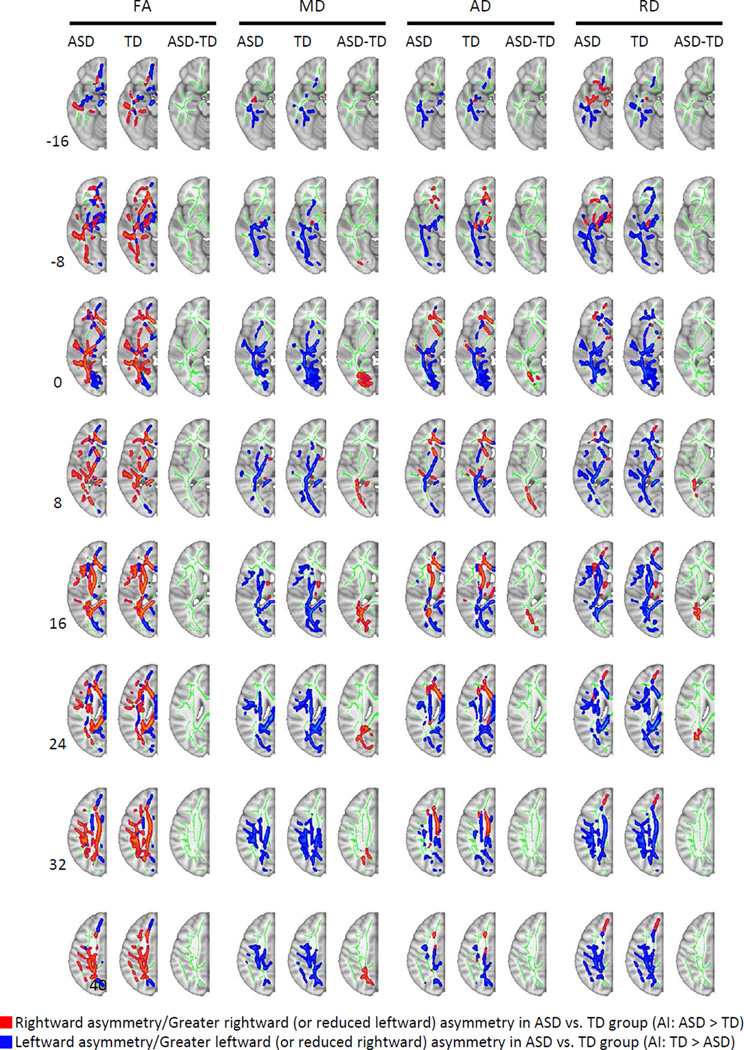
Figure 2.
Within- and between-group significance maps of white matter asymmetry. Note: Effects are presented on a single hemisphere of the symmetric tract-based spatial statistics (TBSS) skeleton (depicted in green). Within-group maps show significant rightward asymmetry (right [R]>left [L]) in red and leftward (L>R) in blue. Between-group maps indicate higher asymmetry indices (autism spectrum disorder [ASD]>typically developing [TD]) in red and lower indices (ASD<TD) in blue. Slice levels are indicated on the left. All effects p < .05 (corr.). AD = axial diffusivity; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity.
For MD, one-sample t-tests revealed leftward asymmetry in both groups throughout most of the skeleton (except for forceps minor and frontal white matter). The between-group t-tests revealed a large continuous cluster (3167 mm3) of reduced or reversed asymmetry in ASD (TD AI: M = −6.0; ASD AI: M = 0.1) extending through much of occipital and parietal lobes, including the posterior thalamic radiation, and corona radiata (Figure 2, and see Table S2, available online). Several major association tracts lie in close proximity in this region,46,47 and posterior portions of the inferior longitudinal and inferior fronto-occipital fasciculi may have also contributed to this cluster.
Asymmetry patterns of AD and RD were largely consistent (leftward) with those for MD for both groups, although rightward asymmetry was present in anterior regions (frontal radiate white matter, anterior corona radiata and internal capsule). Between-group differences were less extensive for AD compared to MD but spatially overlapping, falling mostly in occipital white matter (total volume of significant clusters: 668 mm3). Group differences were centered in occipital lobe for RD as well, additionally affecting the posterior portion of the superior longitudinal fasciculus (SLF) and the tapetum (cluster volume: 513 mm3).
Voxelwise between-group t-tests were also performed on the DTI measures themselves (FA, MD, AD, RD), rather than AI, to determine whether one hemisphere was a primary driver of AI effects. However, no significant clusters were identified in either hemisphere for any of the dependent measures.
DISCUSSION
The current study examined the hemispheric asymmetry of cerebral white matter microstructure in children and adolescents with ASD. Across much of the TBSS skeleton, we detected an overall rightward asymmetry for FA in TD and children and adolescents with ASD. This asymmetry was significantly reduced in ASD when averaged across the entire brain. In addition, voxel-wise analyses revealed robust leftward asymmetry for MD in both groups, driven by concordant asymmetry for RD and (more moderately) AD. These asymmetries were significantly reduced in the ASD group in a region of occipital white matter where several major association and projection tracts travel in close proximity. Parietal white matter showed a similar reduction in asymmetry for the MD measure (Figure 1). ASD and TD groups were well matched for head motion, which can otherwise bias diffusion results.48,49
The overall finding for FA in the TD group reflected a preponderance of rightward asymmetry across many segments of the white matter skeleton, likely affecting multiple major tracts (Figure 2). This was, however, accompanied by inverse effects (leftward asymmetry) in superior frontal white matter and posterior portions of the occipital lobe. These findings are partially consistent with previous studies in healthy adults. Takao et al.20 observed rightward asymmetry of FA in corona radiata, internal capsule, posterior thalamic radiation, and parts of the SLF. Thiebaut de Schotten et al.50 further reported rightward asymmetry of FA in the anterior segment of the AF, while Iwabuchi19 found rightward FA asymmetry in parietal and leftward asymmetry in occipital tracts. However, inverse effects of leftward asymmetry for FA have also been seen, as for example in anterior thalamic radiation,20 uncinate,51 cingulum,52 and inferior longitudinal fasciculus (ILF).50 Apart from inconsistencies among these adult DTI studies themselves, the overall more robust rightward FA asymmetry in our study and differences in local findings could be in part attributed to maturational changes from childhood to adulthood. Studies in TD children have, however, also reported mixed DTI asymmetry findings, with leftward asymmetry for FA in the superior corona radiata, centrum semiovale, and cingulum, but rightward asymmetry in frontal white matter when examined with single-slice ROIs53 and tractography studies finding rightward asymmetry in the AF,54 and the fronto-parietal segment of the AF, but leftward asymmetry in corticospinal tract (CST), uncinate, and temporal part of the AF.55 It is therefore likely that additional demographic or methodological differences (e.g., inclusion of small group-matched subsamples of left-handed participants) may have resulted in the more extensive rightward asymmetries detected in our TD group. One possibility relates to the creation of a white matter skeleton in TBSS, which puts focus on a limited number of voxels with peak FA most likely to fall onto white matter tracts, contrary to more inclusive region of interest and tractography procedures practiced in the previous studies cited above.
In our ASD group, many skeletal segments also showed rightward FA asymmetry, but these effects were less robust resulting in a significant decrease in asymmetry when the entire skeleton was considered as a whole. Voxel-wise analyses did not reveal significant clusters, however, suggesting that reduced asymmetry in ASD may be diffuse but locally variable.
A further finding was significant leftward asymmetry of MD in both groups. Although widespread across the white matter skeleton, this effect was especially pervasive in posterior regions. This MD asymmetry was significantly reduced in the ASD group compared to TD in white matter of the occipital and, to some extent, parietal lobes. Comparison to electronic atlases shows the significant cluster falling in a region where multiple association, projection, and commissural tracts lie in close proximity, with fibers running largely in parallel to form a sheet of anterior to posterior running axons.56 The ILF, inferior fronto-occipital fasciculus (IFOF), sagittal stratum, posterior thalamic radiation, and forceps major all pass through this white matter band and cannot be fully disentangled with in vivo imaging. This proximity has led to substantial confusion in the anatomical literature with argument as to the mere existence of some tracts or their distinction from adjacent fibers.
The leftward asymmetry of MD seen in the TD group reflected concordant asymmetry for both radial and axial diffusion, although these latter measures also showed a contrasting rightward asymmetry in anterior white matter. Our findings in children and adolescents are consistent with the general pattern of leftward asymmetry observed in 5–19-year-old TD participants by Bonekamp et al.,53 but their effects were found primarily in anterior regions. On the other hand, Joseph et al.,54 in an examination limited to the AF, found no significant MD asymmetry in TD or ASD groups, possibly related to smaller sample size or younger age of participants. Furthermore, our findings did not coincide with those in Peterson et al.,57 who reported rightward asymmetry of MD in TD participants (not tested statistically) and increased MD in the left hemisphere in 8–12-year-old children with ASD compared to TD children. However, a direct test of the asymmetry index produced no significant group difference. This study did not perform voxelwise tests of asymmetry and used broad regions of interest, again differing from the TBSS skeleton tested in our study, which puts selective focus on white matter regions most likely to fall within major tracts.
The asymmetry index reflects a ratio between the two hemispheres. Group differences in AI could arise from marked alteration in a single hemisphere or subtler, but opposing, differences in both hemispheres. Within-hemisphere comparisons did not reveal any group differences in our sample, suggesting that white matter is affected bilaterally, but differentially, in ASD. This could arise for differences during early neurodevelopment or as an outcome of altered functional specialization of the hemispheres.
Our findings for TD children and adolescents could be misinterpreted as indicating greater white matter integrity (higher FA, reduced MD and RD) in the right compared to the left hemisphere. More accurately, the pattern of findings may reflect a greater degree of inter-regional integration in the right hemisphere, compared to greater functional specialization in the left. This interpretation is consistent with findings by Iturria-Medina et al.58 who used diffusion-weighted MRI and graph theory and detected greater efficiency and interconnectivity within the right (compared to the left) hemisphere in healthy right-handed adults. Although FA is often considered an index of tract integrity, it cannot be uniquely attributed to any single neural parameter. Lower FA in one hemisphere (which would affect the AI) may reflect reduced axonal density or reduced myelination, but it can also result from greater tract complexity because multiple fiber orientations within a voxel will reduce FA.59 The findings by Iturria-Medina et al.,58 as well as our findings in TD children and adolescents, may reflect a dichotomy between more specialized and segregated network organization in the left, but more integrative organization in the right hemisphere. This is also consistent with more local functional specialization of the left and more integrative and global function of the right hemisphere.60,61 Our findings in children and adolescents with ASD, as well as those from an earlier functional MRI study,33 suggest that this typical hemispheric specialization is impaired in autism. Since hemispheric asymmetries are the outcome of prolonged maturational changes, it is however unlikely that immediate translational conclusions can be drawn from our findings (e.g., intervention with transcranial magnetic or direct current stimulation).
We did not find any significant correlations between DTI asymmetry indices and age or sociocommunicative abilities (SRS) for the entire white matter skeleton. Such links with white matter asymmetry may exist, but occur only at a tract-specific or regional level. Effects may go in different directions (e.g., asymmetry becoming more leftward with age in one region, but more rightward in another). Longitudinal data not available here may be necessary to investigate such regionally specific and relatively subtle effects.
A particular strength of the current study was the careful matching of groups for in-scanner head motion. Recent studies have made it clear that systematic group differences in motion can have confounding effects on diffusion findings, even when motion is subtle. Clinical groups, such as those with ASD, often move more than TD populations during the extended acquisition times required for diffusion imaging, and this can lead to false findings.48,49 Our study quantified motion in each participant, ensured that groups were matched on these measures, and included a summary measure of motion, TMI, as a covariate throughout analyses. This ensures that the group differences identified here were not artifacts of motion, and may help to explain differences between our findings and some findings from studies that did not implement such motion matching (reviewed in Travers et al., 201262). In particular, no such steps were taken in previous studies of diffusion asymmetry in ASD, suggesting greater validity of our findings due to reduced motion bias.
A few limitations of the current study design should be mentioned. First, two demographic variables that were not considered in our study were pubertal status and socioeconomic status, both of which can influence rates and patterns of neurodevelopment.63,64 Future studies with larger samples should include and control for these additional variables to identify independent or modulatory contributions. The increasing availability of large imaging databases on autism (e.g. National Database for Autism Research, https://ndar.nih.gov; Autism Brain Imaging Data Exchange65) will no doubt improve sensitivity to subtle demographic factors and relatively small subpopulations, which may show distinct developmental trajectories (e.g. females66). Second, the segment of participants with ASD included in our study was limited by the need for minimal head motion during scanning, and our findings may therefore not apply to the lower-functioning end of the autism spectrum. We use pre-scan training in a mock scanner to reduce the frequency of motion-related image artifacts. Implementation of a similar or more extensive behavioral training regimen as well as imaging sequences designed to recover from subject motion67 may help to extend the functional range of participants who can be included in future studies.68,69 Third, for inter-subject alignment of scans, our TBSS analyses utilized the FMRIB58_FA template that was based on a sample of 20–50-year-olds. A better approach given our sampled age range (7–17-year-olds) would be a study-specific registration template. However, since subsequent processing steps derived the white matter skeleton directly from our sample, and diffusion data were mapped onto this skeleton rather than the FMRIB58_FA template, any misalignment issues due to use of the FMRIB58_FA template would be expected to be relatively minor. Finally, using TBSS, our analyses focused only on white matter voxels with relatively high FA, from which the tract skeleton was created. With this approach, issues related to complex fiber orientations (which affect FA in ambiguous ways) could be somewhat mitigated, as regions with multiple fiber orientation will tend to have low FA and will thus be excluded from the skeleton.
In conclusion, our findings indicate reductions in anatomical white matter asymmetries in ASD, particularly in posterior white matter, suggesting that a neurotypical “division of labor” – between more integrative processing in the right, and more specialized processing in the left hemisphere – may be reduced or lacking in ASD.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants R01-MH081023 and K01-MH097972.
The authors especially thank the children and families who participated in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Disclosure: Drs. Carper and Müller, Mr. Treiber, and Ms. DeJesus report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Dr. Ruth A. Carper, Brain Development Imaging Laboratory at San Diego State University, San Diego, CA.
Mr. Jeffrey M. Treiber, School of Medicine, University of California, San Diego.
Ms. Shannon Yandall DeJesus, Brain Development Imaging Laboratory at San Diego State University, San Diego, CA.
Dr. Ralph-Axel Müller, Brain Development Imaging Laboratory at San Diego State University, San Diego, CA.
REFERENCES
- 1.Sun T, Collura RV, Ruvolo M, Walsh CA. Genomic and evolutionary analyses of asymmetrically expressed genes in human fetal left and right cerebral cortex. Cerebral cortex. 2006 Jul;16(Suppl 1):i18–i25. doi: 10.1093/cercor/bhk026. [DOI] [PubMed] [Google Scholar]
- 2.Sun T, Patoine C, Abu-Khalil A, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005 Jun 17;308(5729):1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes TL, Lewis DA. Hemispheric differences in layer III pyramidal neurons of the anterior language area. Archives of neurology. 1993 May;50(5):501–505. doi: 10.1001/archneur.1993.00540050053015. [DOI] [PubMed] [Google Scholar]
- 4.Hayes TL, Lewis DA. Anatomical specialization of the anterior motor speech area: hemispheric differences in magnopyramidal neurons. Brain and language. 1995 Jun;49(3):289–308. doi: 10.1006/brln.1995.1035. [DOI] [PubMed] [Google Scholar]
- 5.Hugdahl K, Westerhausen R. The two halves of the brain: information processing in the cerebral hemispheres. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- 6.Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 7.Pujol J, Lopez-Sala A, Deus J, et al. The lateral asymmetry of the human brain studied by volumetric magnetic resonance imaging. NeuroImage. 2002;17(2):670–679. [PubMed] [Google Scholar]
- 8.Toga AW, Thompson PM. Mapping brain asymmetry. Nature reviews. Neuroscience. 2003;4(1):37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 9.Finger S, Roe D. Gustave Dax and the early history of cerebral dominance. Arch Neurol-Chicago. 1996;53:806–813. doi: 10.1001/archneur.1996.00550080132021. [DOI] [PubMed] [Google Scholar]
- 10.Hellige JB, Taylor KB, Lesmes L, Peterson S. Relationships between brain morphology and behavioral measures of hemispheric asymmetry and interhemispheric interaction. Brain Cogn. 1998;36:158–192. doi: 10.1006/brcg.1997.0951. [DOI] [PubMed] [Google Scholar]
- 11.Badcock NA, Bishop DV, Hardiman MJ, Barry JG, Watkins KE. Co-localisation of abnormal brain structure and function in specific language impairment. Brain and language. 2012;120(3):310–320. doi: 10.1016/j.bandl.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witelson SF, Kigar DL. Sylvian Fissure Morphology and Asymmetry in Men and Women: Bilateral Differences in Relation to Handedness in Men. J Comp Neurol. 1992;323(3):326–340. doi: 10.1002/cne.903230303. [DOI] [PubMed] [Google Scholar]
- 13.Bonilha L, Nesland T, Rorden C, Fridriksson J. Asymmetry of the structural brain connectome in healthy older adults. Frontiers in psychiatry. 2014;4:186. doi: 10.3389/fpsyt.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song JW, Mitchell PD, Kolasinski J, Ellen Grant P, Galaburda AM, Takahashi E. Asymmetry of White Matter Pathways in Developing Human Brains. Cerebral cortex. 2014;25(9):2883–2893. doi: 10.1093/cercor/bhu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in biomedicine. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 16.Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR in biomedicine. 2010;23(7):803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- 17.Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 18.Tournier J-D, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65:1532–1556. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwabuchi SJ, Häberling IS, Badzakova-Trajkov G, et al. Regional differences in cerebral asymmetries of human cortical white matter. Neuropsychologia. 2011;49(13):3599–3604. doi: 10.1016/j.neuropsychologia.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Takao H, Abe O, Yamasue H, et al. Gray and white matter asymmetries in healthy individuals aged 21–29 years: a voxel-based morphometry and diffusion tensor imaging study. Hum Brain Mapp. 2011;32:1762–1773. doi: 10.1002/hbm.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchel C. White Matter Asymmetry in the Human Brain: A Diffusion Tensor MRI Study. Cereb Cortex. 2004;14:945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- 22.Esposito G, Venuti P, Maestro S, Muratori F. An exploration of symmetry in early autism spectrum disorders: analysis of lying. Brain and Development. 2009 Feb;31(2):131–138. doi: 10.1016/j.braindev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Ozgen H, Hellemann GS, de Jonge MV, Beemer FA, van Engeland H. Predictive value of morphological features in patients with autism versus normal controls. J Autism Dev Disord. 2013 Jan;43(1):147–155. doi: 10.1007/s10803-012-1554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keehn B, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Atypical hemispheric specialization for faces in infants at risk for autism spectrum disorder. Autism Res. 2015 Apr;8(2):187–198. doi: 10.1002/aur.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Fosse L, Hodge SM, Makris N, et al. Language-association cortex asymmetry in autism and specific language impairment. Annals of neurology. 2004 Dec;56(6):757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- 26.Herbert MR, Ziegler DA, Deutsch CK, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain : a journal of neurology. 2005 Jan;128(Pt 1):213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- 27.Wan CY, Marchina S, Norton A, Schlaug G. Atypical hemispheric asymmetry in the arcuate fasciculus of completely nonverbal children with autism. Annals of the New York Academy of Sciences. 2012;1252:332–337. doi: 10.1111/j.1749-6632.2012.06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph RM, Fricker Z, Fenoglio A, Lindgren KA, Knaus TA, Tager-Flusberg H. Structural asymmetries of language-related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging Behav. 2013;8(1):60–72. doi: 10.1007/s11682-013-9245-0. [DOI] [PubMed] [Google Scholar]
- 29.Kleinhans NM, Müller R-A, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knaus TA, Silver AM, Kennedy M, et al. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Lang. 2010;112(2):113–120. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen JA, Zielinski BA, Fletcher PT, et al. Abnormal lateralization of functional connectivity between language and default mode regions in autism. Mol Autism. 2014;5(1):8. doi: 10.1186/2040-2392-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindell AK, Hudry K. Atypicalities in cortical structure, handedness, and functional lateralization for language in autism spectrum disorders. Neuropsychol Rev. 2013;23(3):257–270. doi: 10.1007/s11065-013-9234-5. [DOI] [PubMed] [Google Scholar]
- 33.Cardinale RC, Shih P, Fishman I, Ford LM, Müller R-A. Pervasive rightward asymmetry shifts of functional networks in autism spectrum disorder: An fMRI study using independent component analysis. JAMA Psychiatry. 2013 Jul 31;70(9):975–982. doi: 10.1001/jamapsychiatry.2013.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fletcher PT, Whitaker RT, Tao R, et al. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010;51(3):1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo Y-C, Soong W-T, Gau SS-F, et al. The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: A study using diffusion spectrum imaging tractography. Psychiatry Research: Neuroimaging. 2011;192(1):60–66. doi: 10.1016/j.pscychresns.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Lange N, Dubray MB, Lee JE, et al. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 2010 Dec;3(6):350–358. doi: 10.1002/aur.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview - R. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 38.Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services; 2001. [Google Scholar]
- 39.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008 Aug;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 40.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) - Second edition. San Antonio, Texas: Psychological Corporation; 2011. [Google Scholar]
- 41.Constantino JN, Gruber CP. Social Responsiveness Scale. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- 42.Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995 Jul;34(1):65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- 43.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004 Jan 1;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 44.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multisubject diffusion data. NeuroImage. 2006 Jul 15;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 45.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 46.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mori S. Laboratory of Brain Anatomical MRI. JHU white-matter tractography atlas (e-atlas) 2012 Jan; [Retrieved from: http://fsl.fmrib.ox.ac.uk.fsl/fslwiki/%5D. 2008. [Google Scholar]
- 48.Koldewyn K, Yendiki A, Weigelt S, et al. Differences in the right inferior longitudinal fasciculus but no general disruption of white matter tracts in children with autism spectrum disorder. Proc Natl Acad Sci USA. 2014;111(5):1981–1986. doi: 10.1073/pnas.1324037111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage. 2013;88C:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thiebaut de Schotten M, Ffytche DH, Bizzi A, et al. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage. 2011;54(1):49–59. doi: 10.1016/j.neuroimage.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 51.Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Lüders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49:1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- 52.Gong G, Jiang T, Zhu C, et al. Asymmetry analysis of cingulum based on scale-invariant parameterization by diffusion tensor imaging. Human brain mapping. 2005;24(2):92–98. doi: 10.1002/hbm.20072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonekamp D, Nagae LM, Degaonkar M, et al. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. NeuroImage. 2007 Jan 15;34(2):733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joseph RM, Fricker Z, Fenoglio A, Lindgren KA, Knaus TA, Tager-Flusberg H. Structural asymmetries of language-related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging Behav. 2014 Mar;8(1):60–72. doi: 10.1007/s11682-013-9245-0. [DOI] [PubMed] [Google Scholar]
- 55.Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007 Dec;17(12):2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- 57.Peterson D, Mahajan R, Crocetti D, Mejia A, Mostofsky S. Left-hemispheric microstructural abnormalities in children with high-functioning autism spectrum disorder. Autism Res. 2015 Feb;8(1):61–72. doi: 10.1002/aur.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iturria-Medina Y, Perez Fernandez A, Morris DM, et al. Brain hemispheric structural efficiency and interconnectivity rightward asymmetry in human and nonhuman primates. Cereb Cortex. 2011;21(1):56–67. doi: 10.1093/cercor/bhq058. [DOI] [PubMed] [Google Scholar]
- 59.Johnson RT, Yeatman JD, Wandell BA, Buonocore MH, Amaral DG, Nordahl CW. Diffusion properties of major white matter tracts in young, typically developing children. Neuroimage. 2013;88C:143–154. doi: 10.1016/j.neuroimage.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain. 1997;120:1779–1791. doi: 10.1093/brain/120.10.1779. [DOI] [PubMed] [Google Scholar]
- 61.Gotts SJ, Jo HJ, Wallace GL, Saad ZS, Cox RW, Martin A. Two distinct forms of functional lateralization in the human brain. Proc Natl Acad Sci U S A. 2013;110(36):E3435–E3444. doi: 10.1073/pnas.1302581110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Travers BG, Adluru N, Ennis C, et al. Diffusion Tensor Imaging in Autism Spectrum Disorder: A Review. Autism Res. 2012 Jul 11;5(5):289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juraska JM, Willing J. Pubertal onset as a critical transition for neural development and cognition. Brain Res. doi: 10.1016/j.brainres.2016.04.012. [published online ahead of print Apr 6 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Martino A, Yan CG, Li Q, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2014 Jun;19(6):659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seunarine KK, Clayden JD, Jentschke S, et al. Sexual Dimorphism in White Matter Developmental Trajectories Using Tract-Based Spatial Statistics. Brain Connect. 2016 Feb;6(1):37–47. doi: 10.1089/brain.2015.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown TT, Kuperman JM, Erhart M, et al. Prospective motion correction of high-resolution magnetic resonance imaging data in children. NeuroImage. 2010 Oct 15;53(1):139–145. doi: 10.1016/j.neuroimage.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nordahl CW, Mello M, Shen AM, et al. Methods for acquiring MRI data in children with autism spectrum disorder and intellectual impairment without the use of sedation. J Neurodev Disord. 2016;8:20. doi: 10.1186/s11689-016-9154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnea-Goraly N, Weinzimer SA, Ruedy KJ, et al. High success rates of sedation-free brain MRI scanning in young children using simple subject preparation protocols with and without a commercial mock scanner- -the Diabetes Research in Children Network (DirecNet) experience. Pediatr Radiol. 2014;44(2):181–186. doi: 10.1007/s00247-013-2798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.