Abstract
Sphingolipid involvement in infectious disease is a new and exciting branch of research. Various microbial pathogens have been shown to synthesize their own sphingolipids and some have evolved methods to “hijack” host sphingolipids for their own use. For instance, Sphingomonas species are bacterial pathogens that lack the lipopolysaccharide component typical but instead contain glycosphingolipids (Kawahara 1991, 2006). In terms of sphingolipid signaling and function, perhaps the best-studied group of microbes is the pathogenic fungi.
Pathogenic fungi still represent significant problems in human disease, despite treatments that have been used for decades. Because fungi are eukaryotic, drug targets in fungi can have many similarities to mammalian processes. This often leads to significant side effects of antifungal drugs that can be dose limiting in many patient populations. The search for fungal-specific drugs and the need for better understanding of cellular processes of pathogenic fungi has led to a large body of research on fungal signaling. One particularly interesting and rapidly growing field in this research is the involvement of fungal sphingolipid pathways in signaling and virulence. In this chapter, the research relating to sphingolipid signaling pathogenic fungi will be reviewed and summarized, in addition to highlighting pathways that show promise for future research.
Sphingolipid Synthesis
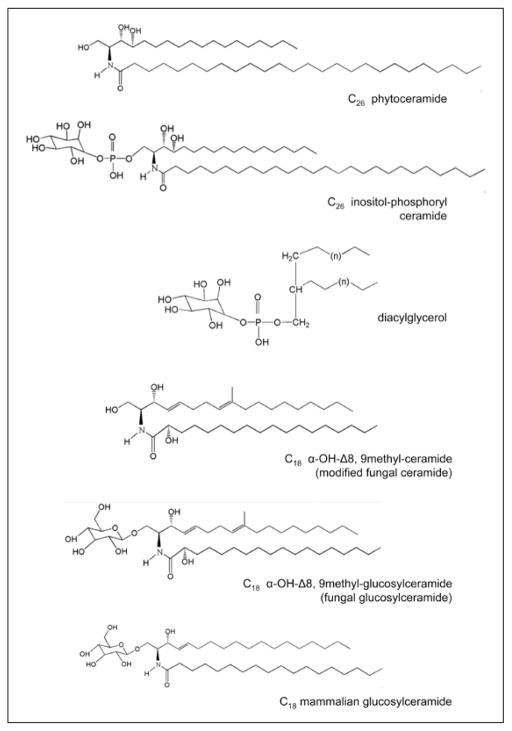
Sphingolipid synthesis in pathogenic fungi is largely conserved among species. Early steps in the process, such as the condensation of palmitoyl-CoA with serine to form 3-ketodihydrosphingosine, are the same in Saccharomyces cerevisiae. S. cerevisiae is often the gold standard for model systems, but in this context, that system is limited by the fact that S. cerevisiae has very different sphingolipid metabolism from most of the significant fungal pathogens. Both S. cerevisiae and fungal pathogens like Candida albicans make phytoceramide for instance (a modified ceramide with a carbon 4 hydroxylation on its backbone instead of the 3,4 desaturation). Phytoceramide is often conjugated with very long chain fatty acids and can be used to make more complex sphingolipids such as inositol-phosphoryl ceramide (IPC). In addition to this pathway, most pathogenic fungi also synthesize a different ceramide that contains the 3,4 desaturation and cannot be referred to as phytoceramide. This ceramide is typically conjugated to a 16–18 carbon acyl chain and undergoes additional modifications. These modifications include an additional sphingoid backbone desaturation between the 8th and 9th carbons and a methylation of that backbone on the 9th carbon. Typically, the acyl chain is hydroxylated at the α-carbon position. This modified ceramide is the substrate for an enzyme called glucosylceramide synthase (Gcs1), which glycosylates this molecule on the hydroxyl group of the 1st carbon, creating glucosylceramide (GlcCer). Though there is no direct evidence of cross talk between the glucosylceramide synthase pathway and the pathway leading to the synthesis of inositol-phosphoryl ceramide-containing lipids, this possibility cannot be ruled out. The structures of major fungal sphingolipids are found in Figure 1.
Figure 1.
Chemical structures of C26 phytoceramide, C26 inositol phosphoryl ceramide, diacylglycerol, C18 α-hydroxy-Δ8, 9methyl-ceramide, C18 α-hydroxy-Δ8, 9methyl-glucosylceramide (fungal glucosylceramide) and C18 glucosylceramide (mammalial glucosylceramide).
Cryptococcus Neofomans: Model of Sphingolipid Signaling in Fungi
Cryptococcus neoformans is an encapsulated fungal pathogen that primarily affects immunocompromised patients (ex. HIV/AIDS patients, transplant candidates and patients on long-term steroid treatments). This environmental yeast is inhaled to the lungs, where it can live extracellularly or intracellularly (inside the phagolysosomes of alveolar macrophages). Infection with this organism is known as cryptococcosis. In some patients, the fungus disseminates to the bloodstream, seeding many organ systems, but eventually proliferating in the central nervous system. This scenario represents a significant medical emergency, as C. neoformans is the leading cause of fungal meningoencephalitis in the world and the disease is lethal if left untreated. Cryptococcus has several recognized virulence factors, such as the production of melanin and the polysaccharide capsule.
Sphingolipid studies in C. neoformans have revealed some interesting implications for signaling pathways involving these molecules. Beyond understanding fungal biology on a cellular and biochemical level, the study of the fungal sphingolipid pathway is advantageous due to the fact that many of the enzymes and products are distinct in structure and function from their mammalian counterparts. This distinction makes them great candidates for drug targets.
The best-studied example of this paradigm is inositol-phosphoryl ceramide synthase (Ipc1). This enzyme uses phytoceramide and phosphatidylinositol (PI) as substrates, transferring the phosphorylinositol moiety to phytoceramide. In addition to the generation of inositol-phosphoryl ceramide (IPC), diacylglycerol (DAG) is also released as a product of this reaction. Early studies on Ipc1 in C. neoformans implicated the enzyme in virulence pathways. In strains where Ipc1 is downregulated, melanin production is impaired and the strain has growth deficits when inside alveolar macrophages. When tested in mouse models of cryptococcosis, the strain lacking Ipc1 was less virulent in comparison to the wildtype C. neoformans. Studies on IPC metabolism have also given clues to the role of this reaction in virulence. Inositol phosphosphingolipid-phospholipase C (Isc1) is the enzyme that catalyzes the reverse reaction of Ipc1, which is to remove the phosphotidylinositol component from IPC. A strain of C. neoformans in which this enzyme is deleted (Δisc1) shows reduced virulence in immunocompromised mouse models. However, when macrophages are depleted in this model, Δisc1 will disseminate and cause meningoencephalitis. The Ipc1/Isc1 balance seems to play a role in the interaction between C. neoformans and the alveolar macrophages.
Further studies into the mechanism underlying the connection between Ipc1 and virulence of C. neoformans have shown that the production of DAG is a common step of at least two separate determinants of virulence in this fungus. As mentioned, early studies downregulating Ipc1 showed impairments in melanin production. This interaction was found to be mediated by cryptococcal protein kinase C (Pkc1). DAG, the byproduct of Ipc1 activity, was found to bind to the C1 domain of Pkc1. This binding led to an increase in Pkc1 activity and that activation was abolished by the selective deletion of the C1 domain. It is known that Pkc1 and several pathway components are required for proper cell wall integrity, including the function of some cell wall-associated enzymes. One such enzyme is laccase, which is responsible for the synthesis of melanin. The defect in melanin synthesis observed in Ipc1-downregulated strains was caused by improper localization of laccase to the cell wall, due to reduction in DAG-dependent Pkc1 activity.
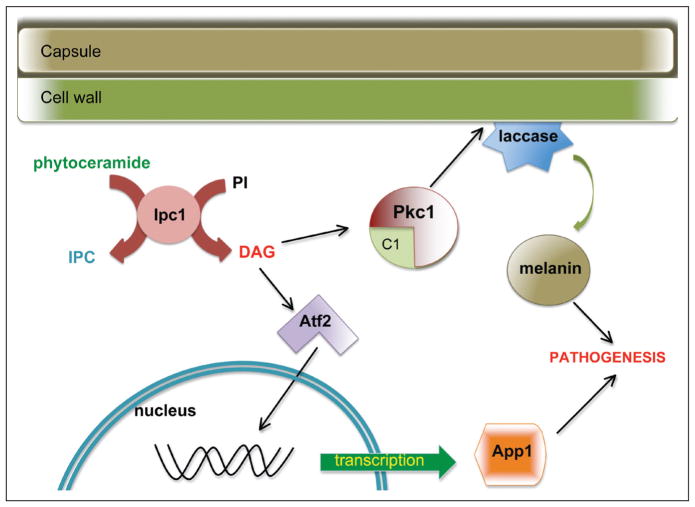
Another way in which DAG production has been linked to virulence involves the fungal-macrophage interaction. C. neoformans has methods to avoid phagocytosis by alveolar macrophages when necessary, including the polysaccharide capsule. One such method is the production of anti-phagocytic protein 1 (App1). When App1 is deleted, the resulting strain (Δapp1) shows reduced virulence in immunocompromised mice. The production of App1 is driven, transcriptionally, by the presence of DAG. DAG binds and activates the transcription factor Atf2. Atf2 activation promotes the transcription of App1 and thus evasion of phagocytosis leading to increased virulence. App1, in addition to regulating phagocytosis, has been shown to bind host complement receptors CR2 and CR3, suggesting even more complex fungal-host interactions affected by production of DAG in C. neoformans. The downstream effects of Ipc1 activity are summarized in Figure 2.
Figure 2.
Sphingolipid signaling in Cryptococcus neoformans. Inositol phosphoryl ceramide synthase 1 (Ipc1) in C. neoformans produces diacylglycerol (DAG) in addition to inositol phosphoryl ceramide IPC). DAG binds to the C1 domain of protein kinase C1 (Pkc1), which is important for cell wall integrity. This integrity is crucial for localization of laccase, the enzyme responsible for melanin synthesis. In addition, DAG also activates the transcription factor Atf2, which leads to transcription of the antiphagocytic protein 1 (App1). Both App1 and melanin regulate pathogenicity of C. neoformans.
Sphingolipid Signaling in Other Pathogenic Fungi
While many groups have discovered and characterized sphingolipid components of other pathogenic fungi, few have delved into the role of these lipids in signaling or cellular processes. One such fungus is Candida albicans. C. albicans is a dimorphic fungus that normally lives as a commensal organism in the human gut and urogenital tract. In immunocompromised patients, C. albicans can cause significant disease, including systemic dissemination. Recent evidence in Candida albicans has shown possible sphingolipid involvement in endocytosis and plasma membrane functions. Sur7 is a membrane bound enzyme known to be involved in sphingolipid membrane makeup in S. cerevisiae. When the homolog of Sur7 was deleted in C. albicans, the resulting strain showed defects in hyphal morphogenesis, endocytosis and cell wall formation. Confirming the role of sphingolipids in this process, blocking sphingolipid synthesis resulted in disruption of Sur7 patches in the plasma membrane. Though this has yet to show the definitive role of sphingolipids in this process, their involvement is clear. Also, the function and localization of some multidrug resistance proteins in C. albicans have been shown to be dependent on membrane sphingolipid composition.
Conclusion
The signaling pathway involving Ipc1 and the production of DAG is clearly related to virulence in more ways than one. What other sphingolipid metabolic pathways could be involved in virulence of C. neoformans and other pathogenic fungi? Recall that in addition to IPC-based sphingolipids, most pathogenic fungi synthesize glucosylceramide that is not based on a phytoceramide backbone. The synthesis of these GlcCers requires enzymes that introduce the desaturation of the sphingoid backbone between carbons 8 and 9 as well as the methylation of the ninth carbon. Examinations into the function of these enzymes as well as glucosylceramide synthase have suggested major roles in biology and virulence. In Candida, for instance, the sphingolipid Δ8-desaturase, responsible for the backbone desaturation at that position, is required for proper hyphal growth. The function of the methyltransferase responsible for the C9 methylation seen in most fungi was studied in the plant pathogen Fusarium graminerum. Disruption of the enzyme encoding this enzyme resulted in a strain that showed defects in virulence, growth and differentiation. When the gene for glucosylceramide synthase is deleted in C. neoformans, the resulting strain shows condition-dependent growth defects as well as a lack of virulence in inhalation mouse models. Taken together, these studies are beginning to uncover the roles of the “glucosylceramide branch” of sphingolipids in pathogenic fungi. Like enzymes involved in IPC production, many of these enzymes are unique to fungi and thus represent attractive possibilities as therapeutic targets. Though the signaling mechanisms involved are unclear, these observations may represent the beginning of new understandings into the role of sphingolipid signaling in pathogenic fungi.
References
- Hanada K. Sphingolipids in infectious diseases. Jpn J Infect Dis. 2005;58(3):131–148. [PubMed] [Google Scholar]
- Heung LJ, Luberto C, Del Poeta M. Role of sphingolipids in microbial pathogenesis. Infect Immun. 2006;74(1):28–39. doi: 10.1128/IAI.74.1.28-39.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K, Sato N, Tsuge K, Seto Y. Confirmation of the anomeric structure of galacturonic acid in the galacturonosyl-ceramide of Sphingomonas yanoikuyae. Microbiol Immunol. 2006;50(1):67–71. doi: 10.1111/j.1348-0421.2006.tb03763.x. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Moll H, Knirel YA, et al. Structural analysis of two glycosphingoliids from the lipopolysaccharide-lacking bacterium Sphingomonas capsulata. Eur J Biochem. 2000;267(6):1837–1846. doi: 10.1046/j.1432-1327.2000.01189.x. [DOI] [PubMed] [Google Scholar]
- Heitman J, GFS, Edwards JEJ, Mitchell AP. Molecular Principles of Fungal Pathogenesis. Washington: American Society of Microbiology; 2006. [Google Scholar]
- Marr KA. New approaches to invasive fungal infections. Curr Opin Hematol. 2003;10(6):445–450. doi: 10.1097/00062752-200311000-00009. [DOI] [PubMed] [Google Scholar]
- Rapp RP. Changing strategies for the management of invasive fungal infections. Pharmacotherapy. 2004;24(2 Pt 2):4S–28S. quiz 29S–32S. [PubMed] [Google Scholar]
- McQuiston TJ, Haller C, Del Poeta M. Sphingolipids as targets for microbial infections. Mini Rev Med Chem. 2006;6(6):671–680. doi: 10.2174/138955706777435634. [DOI] [PubMed] [Google Scholar]
- Rhome R, McQuiston T, Kechichian T, et al. Biosynthesis and immunogenicity of glucosylceramide in Cryptococcus neoformans and other human pathogens. Eukaryot Cell. 2007;6(10):1715–1726. doi: 10.1128/EC.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matmati N, Hannun YA. Thematic review series: sphingolipids. ISC1 (inositol phosphosphingolipid-phospholipase C), the yeast homologue of neutral sphingomyelinases. J Lipid Res. 2008;49(5):922–928. doi: 10.1194/jlr.R800004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid LM, Okamoto Y, Mao C. Yeast sphingolipids: metabolism and biology. Biochim Biophys Acta. 2002;1585(2–3):163–171. doi: 10.1016/s1388-1981(02)00337-2. [DOI] [PubMed] [Google Scholar]
- Sims KJ, Spassieva SD, Voit EO, Obeid LM. Yeast sphingolipid metabolism: clues and connections. Biochem Cell Biol. 2004;82(1):45–61. doi: 10.1139/o03-086. [DOI] [PubMed] [Google Scholar]
- Garcia J, Shea J, Alvarez-Vasquez F, et al. Mathematical modeling of pathogenicity of Cryptococcus neoformans. Mol Sys Biol. 2008;4:183–195. doi: 10.1038/msb.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittershaus PC, Kechichian TB, Allegood J, et al. Glucosylceramide is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest. 2006;116(6):1651–1659. doi: 10.1172/JCI27890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Perfect JR. Cryptococcus neoformans. Washington, DC: ASM Press; 1998. pp. 381–405. [Google Scholar]
- Luberto C, Toffaletti DL, Wills EA, et al. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev. 2001;15(2):201–212. doi: 10.1101/gad.856001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea J, Kechichian TB, Luberto C, Del Poeta M. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C (Isc1) confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect Immun. 2006;74(10):5977–5988. doi: 10.1128/IAI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heung LJ, Luberto C, Plowden A, et al. The sphingolipid pathway regulates protein kinase C 1 (Pkc1) through the formation of diacylglycerol (DAG) in Cryptococcus neoformans. J Biol Chem. 2004;279(20):21144–21153. doi: 10.1074/jbc.M312995200. [DOI] [PubMed] [Google Scholar]
- Heung LJ, Kaiser AE, Luberto C, Del Poeta M. The role and mechanism of diacylglycerol-protein kinase C1 signaling in melanogenesis by Cryptococcus neoformans. J Biol Chem. 2005;280(31):28547–28555. doi: 10.1074/jbc.M503404200. [DOI] [PubMed] [Google Scholar]
- Gerik KJ, Donlin MJ, Soto CE, et al. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol Microbiol. 2005;58(2):393–408. doi: 10.1111/j.1365-2958.2005.04843.x. [DOI] [PubMed] [Google Scholar]
- Luberto C, Martinez-Marino B, Taraskiewicz D, et al. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J Clin Invest. 2003;112(7):1080–1094. doi: 10.1172/JCI18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mare L, Iatta R, Montagna MT, et al. APP1 transcription is regulated by IPC1-DAG pathway and is controlled by ATF2 transcription factor in Cryptococcus neoformans. J Biol Chem. 2005;280(43):36055–36064. doi: 10.1074/jbc.M507285200. [DOI] [PubMed] [Google Scholar]
- Tommasino N, Villani M, Qureshi A, et al. Atf2 transcription factor binds to the APP1 promoter in Cryptococcus neoformans: stimulatory effect of diacylglycerol. Eukaryot Cell. 2008;7(2):294–301. doi: 10.1128/EC.00315-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stano P, Williams V, Villani M, et al. App1: an antiphagocytic protein that binds to complement receptors 3 and 2. J Immunol. 2009;182(1):84–91. doi: 10.4049/jimmunol.182.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Douglas LM, Rosebrock A, et al. The Sur7 protein regulates plasma membrane organization and prevents intracellular cell wall growth in Candida albicans. Mol Biol Cell. 2008;19(12):5214–5225. doi: 10.1091/mbc.E08-05-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasrija R, Panwar SL, Prasad R. Multidrug transporters CaCdr1p and CaMdr1p of Candida albicans display different lipid specificities: both ergosterol and sphingolipids are essential for targeting of CaCdr1p to membrane rafts. Antimicrob Agents Chemother. 2008;52(2):694–704. doi: 10.1128/AAC.00861-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oura T, Kajiwara S. Disruption of the sphingolipid Delta8-desaturase gene causes a delay in morphological changes in Candida albicans. Microbiology. 2008;154(Pt 12):3795–3803. doi: 10.1099/mic.0.2008/018788-0. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy V, Cahoon EB, Thokala M, et al. Sphingolipid C-9 methyltransferases are important for growth and virulence but not for sensitivity to antifungal plant defensins in Fusarium graminearum. Eukaryot Cell. 2009;8(2):217–229. doi: 10.1128/EC.00255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75(10):4792–4798. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]