Summary
Double hit lymphoma (DHL) and double protein-expressing (MYC and BCL2) lymphomas (DPL) fare poorly with R-CHOP; consolidative autologous stem cell transplant (ASCT) may improve outcomes. S9704, a phase III randomized study of CHOP +/−R with or without ASCT allows evaluation of intensive consolidation. Immunohistochemical analysis identified 27 of 198 patients (13.6%) with MYC IHC overexpression and 20 (74%) harboring concurrent BCL2 overexpression. Four had DHL and 16 had DPL only. With median follow-up 127 months, there is a trend favoring outcomes after consolidative ASCT in DPL and MYC protein overexpressing patients, whereas all DHL patients have died irrespective of ASCT.
Keywords: Double Hit Lymphoma, Double Protein Lymphoma, MYC, BCL2, Autologous Stem Cell transplant
Background
Diffuse large B-cell lymphoma (DLBCL), the prototype of aggressive Non-Hodgkin lymphoma (NHL), has clinical and biologic variants with diverse clinical outcomes1. Historically, dual translocations of MYC and BCL2 (or BCL6) “double hit lymphoma” (DHL) are associated with a rapid clinical course and poor survival2. DHL is fortunately uncommon, with reported frequencies of 2–8% among DLBCL patients3. The commercial availability of a reliable stain for MYC protein overexpression4 has furthered evaluation of MYC’s role in lymphomas, although the definition of overexpression varies5. In general, dual MYC and BCL protein overexpression (DPL) is also associated with a poor prognosis and is more common than DHL5.
SWOG S9704 was a phase III randomized study of aggressive NHL treated with CHOP +/−R for 5 cycles followed by either 3 additional cycles of CHOP +/−R or one additional cycle of induction chemotherapy followed by autologous stem cell transplant (ASCT) consolidation. ASCT improved progression free survival (PFS) for high-risk patients, but biologic subsets were not separately evaluated in the initial analysis6. While we have previously evaluated the impact of histopathology and MYC protein expression7 the current report includes an updated dataset with an emphasis on the impact of transplant.
Methods
This randomized intergroup trial included eligible patients 15 to 65 years with biopsy-confirmed aggressive non- Hodgkin’s lymphoma, high-intermediate or high-risk age adjusted IPI (aaIPI)8. Stratification factors and detailed treatment information have been published6.
All cases had central pathology review. 198 available patient samples were tested for MYC protein overexpression. BCL2 IHC (≥30% positive cells), COO classification (germinal center: GC versus non-germinal center: non-GC per Hans algorithm9), and FISH for MYC were performed in all MYC IHC positive cases with sufficient tissue. FISH for BCL2 rearrangements was performed in cases with a MYC rearrangement (Figure 1). A descriptive analysis of outcomes was performed using clinical annotations through SWOG statistical center, and review of S9704 database. Tissue microarrays, immunohistochemistry, FISH studies, statistical analysis were performed as previously described7 with additional details available (Supplemental Material).
Figure 1.
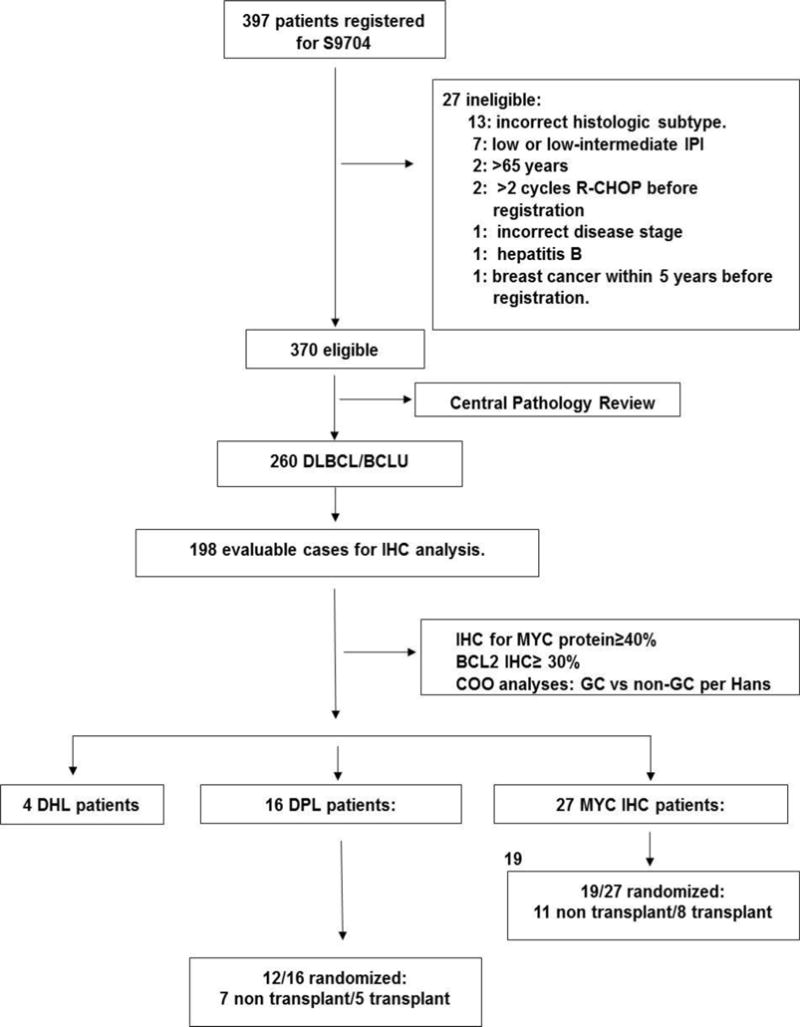
Consort Diagram showing disposition of patients. Of the 397 patients registered for S9704, 370 were eligible, 198 cases were evaluable for IHC analysis and 27 MYC IHC positive patients were identified.
Results
As previously published, there were no significant differences between randomized groups, and early ASCT improved PFS for high-intermediate-risk or high-risk disease with 2-year PFS of 69% and 55%, respectively. Among 370 eligible patients from S9704, 260 had DLBCL or B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL (BCLU) and 198 cases had available tissue for the current analysis. Twenty-seven MYC IHC positive patients were identified. Among 27 MYC IHC positive patients, 8 received CHOP. 16/27 had concurrent BCL2 overexpression by IHC and were classified as DPL. Four patients had DHL with associated dual protein expression. Seven of 27 were MYC positive only by IHC without DPL or DHL (Figure 1).
Patient Characteristics and Outcomes
Median age, aaIPI, bulky disease and elevated LDH were similar between MYC IHC positive and DPL patients. COO was performed in 17/27 MYC IHC positive patients and 11 had GC and 6 had non-GC DLBCL. In the DPL group, COO was evaluable in 10/16 patients and 4 had GC and 6 had non-GC DLBCL (Supplemental Table 1).
The median follow-up is 127 months (range, 93.8–158.2 months). In an analysis of actual treatment received, two year PFS for the transplant and non-transplant group was 63% and 16%, respectively (p*=0.02; Figure 2a). Median PFS was 6 months (95% CI: 4.5–9.0) for no transplant, and 31 months for transplant (95% CI; 6.3, not reached). Two-year OS for transplant and non-transplant group was 63% and 16%, respectively (p*=0.04; Figure 2b). Similarly, in the DPL group, 9 patients in the no transplant group and 3 patients in the transplant group have progressed or died; the median PFS was 7 months (95% CI: 2.8, 13.9) versus 31 months (95% CI: 16.3, not reached) for non-transplanted versus transplanted patients, respectively. The Kaplan Meier estimate of 2 year PFS, OS for transplant and non-transplant groups were 60% and 18%, respectively (p*=0.19 for PFS and p*=0.25 for OS; Figures 2c and 2d).
Figure 2.
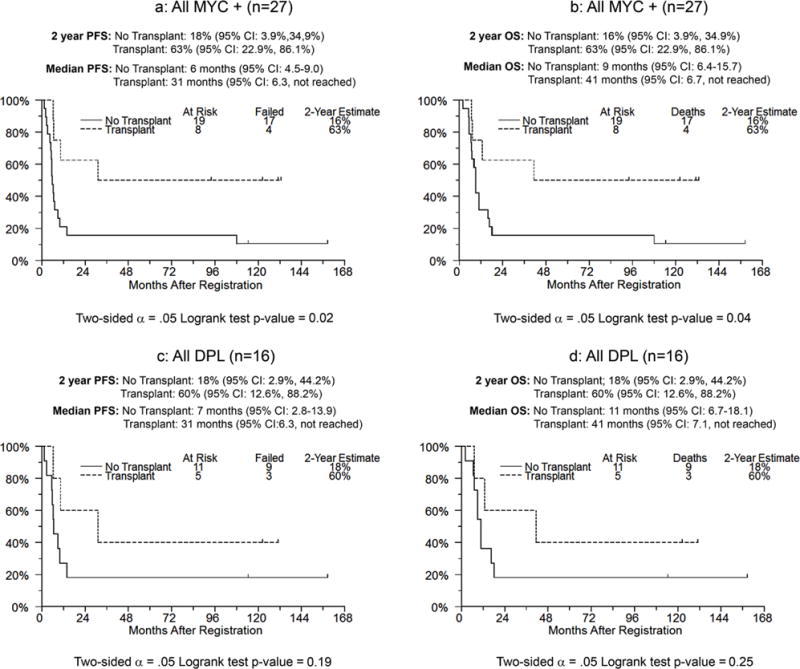
Progression free survival and overall survival of all patients with and without transplant for MYC IHC positive patients (Figures 2a and 2b), and DPL patients (Figures 2c and 2d) respectively.
In MYC positive patients, 19/27 patients could be randomized, with disease progression precluding randomization in others. 11/19 patients did not receive transplant, and their 2 year PFS was 27% compared to 8/19 patients who received transplant with a 2 year PFS of 63% (p*=0.11; Figure S1a); similarly, the 2-year OS for the non-transplant group was 27% and the transplant group was 63%, respectively (p*=0.17; Figure S1b). Among patients with DPL, 12/16 patients were randomized; the 2-year PFS and OS was 29% for the non-transplant group and 60% for the transplant group, respectively (p*=0.43 for PFS and p*=0.53 for OS); (Figure S1c and S1d). p* (two-sided Logrank).
Three of four DHL patients survived to randomization, and one was randomized to transplant. All progressed and died with a median overall survival of 5.9 months (95% CI: 5.3, 6.7 months).
Discussion
DHL and DPL are associated with inferior outcomes following standard chemoimmunotherapy10. When correlating DHL with COO, DHL appears associated with the GC type of DLBCL whereas several groups have observed that non-GC DLBCL is enriched for DPL, and provocatively suggest that the major underlying reason for poor outcomes in non-GC DLBCL is due to MYC and BCL2 protein overexpression11. Our analysis sought to determine the frequency of DHL and DPL among a MYC IHC positive transplant-eligible population, and to evaluate the impact of consolidative ASCT in a prospective dataset. The key observations are the rarity of both DHL and DPL in younger patients, the universally dismal outcome of DHL, and the suggestion that consolidative ASCT may be useful in DPL patients.
The overall incidence of MYC IHC positivity was low in our population, identifying only 27/198 (13.6%) cases with available tissue. This is in contrast to other retrospective series in which the incidence of MYC IHC positivity occurs in 30–50% of cases5. Similarly, we had 4 cases of DHL and 16 cases (8% of the available samples) of DPL. This discrepancy may be due to differences in the median age across series and additional cases that were MYC IHC negative may not have been captured. The median age of S9704 patients was 51 years (range, 18–66 years), reflecting a transplant-eligible population. In contrast, most retrospective DHL and DPL series demonstrate that over half of the patients with DPL are older than 60 years, and some report a median age of 71 years12. Thus, despite enrolling high IPI and advanced stage patients in S9704, the low frequency of DPL in our series suggests a strong correlation with advanced age that may impact future trials in this population.
Despite general consensus that dual MYC and BCL2 protein expression confer a negative prognosis in DLBCL, the optimal IHC cut-point defining overexpression is not uniform5, 13. The definition of BCL2 expression was 30% in the Hans Classifier9; however, the largest series to study DPL used a cut-point of 70%12. We used a cutoff of 30%; despite this conservative cut-point, we still identified a relatively small sample size.
A critical, as yet unanswered, question in DHL and DPL is the optimal initial therapy and whether or not consolidative ASCT improves outcomes. Most data are retrospective series or registry databases, and focused primarily on DHL rather than DPL. These series collectively show that R-CHOP is insufficient therapy with median overall survival of 10 months or less; Compared to augmented or intensified regimens, the use of R-CHOP appears inferior14. All four patients with DHL in our series have died of disease with a median survival of six months despite consolidative ASCT in one, emphasizing the role of an effective induction.
The role of high dose therapy in DPL and DHL is undefined. Some have proposed that achievement of CR determines outcome rather than consolidative ASCT15. However, these studies evaluated DHL and not DPL; our small analysis thus offers one of the first reports to address consolidative transplant in DPL. Of note, the median age of the 16 DPL patients was 56.3 years, placing these patients among the oldest of the entire initial series that had a median age of 51 years. Furthermore, only 12 could be randomized, mainly due to progressive disease during induction. While numbers are small and confidence intervals wide, DPL patients randomized to ASCT had superior outcomes (median PFS 29 months versus 3 months).
In summary, this is a subset analysis of the S9704 trial, one of the largest available data sets to prospectively address the question of upfront ASCT in patients with MYC positive lymphomas in the rituximab era. While our data are limited by small numbers, we observed a trend that MYC IHC positive and DPL patients consolidated with ASCT may have improved outcomes; however, nearly one-third of MYC IHC positive patients were unable to be randomized due to early progression or death. True FISH-defined DHL were infrequent even in this high-risk cohort and had a dismal prognosis. Clearly, MYC positive aggressive B-cell lymphomas, DHL and DPL represent unmet needs and constitute the basis of an intergroup study under development.
Supplementary Material
Supplemental Figure 1: Progression free survival and overall survival of patients randomized to transplant vs no transplant for MYC IHC positive patients (Figures S1a and S1b), and DPL patients (Figures S1c and S1d) respectively.
Acknowledgments
This investigation was supported in part by National Institutes of Health (NIH)/National Cancer Institute (NCI)/National Clinical Trials Network (NCTN) grants CA180888, CA180819, CA180846, CA180835, CA180863, CA180821, CA180820; NIH/NCI Community Oncology Research Program (NCORP) grants CA189953, CA189808, CA189954, CA189804, CA189952, CA189957, CA189854, CA189860, CA189822, CA189872, CA 180801; and in part by Bristol-Myers Squibb
Footnotes
Author contributions:
Soham D. Puvvada: designed research, performed research, analyzed/interpreted data, and wrote the manuscript. Patrick J. Stiff: designed research, performed research, analyzed/interpreted data, and wrote the manuscript. Michael Leblanc: analyzed and interpreted data, performed statistical analysis, wrote the manuscript. James R. Cook: designed research, performed research, analyzed/interpreted data. Stephen Couban: performed research, analyzed and interpreted the data. John P. Leonard: performed research, analyzed and interpreted the data. Brad Kahl: performed research, analyzed and interpreted the data. Deborah Marcellus: performed research, analyzed and interpreted the data. Thomas C. Shea: performed research, analyzed and interpreted the data. Jane N. Winter: performed research, analyzed and interpreted the data. Li Hongli: analyzed and interpreted data, performed statistical analysis, wrote the manuscript. Lisa M. Rimsza: designed research, performed research, analyzed/interpreted data. Jonathan W. Friedberg: designed research, performed research. Sonali M. Smith: designed research, performed research, analyzed/interpreted data, and wrote the manuscript.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. 4th. Lyon, France: IARC Press; 2008. [Google Scholar]
- 2.Johnson NA, Savage KJ, Ludkovski O, Ben-Neriah S, Woods R, Steidl C, Dyer M, Siebert R, Kuruvilla J, Klasa R, Connors JM, Gascoyne R, Horsman DE. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114:2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HM, Boerma E, Kluin PM. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Lin P, Fayad LE, Lennon PA, Miranda RN, Yin CC, Lin E, Medeiros LJ. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Modern Pathology. 2012;25:145–156. doi: 10.1038/modpathol.2011.147. [DOI] [PubMed] [Google Scholar]
- 5.Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, Scott DW, Tan KL, Steidl C, Sehn LH, Chan WC, Iqbal J, Meyer PN, Lenz G, Wright G, Rimsza LM, Valentino C, Brunhoeber P, Grogan TM, Braziel R, Cook JR, Tubbs RR, Weisenburger D, Campo E, Rosenwald A, Ott G, Delabie J, Holcroft C, Jaffe ES, Staudt LM, Gascoyne R. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of Clinical Oncology. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, Shea TC, Porcu P, Winter JN, Kahl BS, Miller TP, Tubbs RR, Marcellus D, Friedberg JW, Barton KP, Mills GM, LeBlanc M, Rimsza LM, Forman SJ, Fisher RI. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. New England Journal of Medicine. 2013;369:1681–1690. doi: 10.1056/NEJMoa1301077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook JR, Goldman B, Tubbs RR, Rimsza L, Leblanc M, Stiff P, Fisher R. Clinical significance of MYC expression and/or “high-grade” morphology in non-Burkitt, diffuse aggressive B-cell lymphomas: a SWOG S9704 correlative study. Am J Surg Pathol. 2014;38:494–501. doi: 10.1097/PAS.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A predictive model for aggressive non-Hodgkin’s lymphoma. The international Non-Hodgkin’s Lymphoma Prognostic Factors Project. New England Journal of Medicine. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 9.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 10.Horn H, Ziepert M, Becher C, Barth TF, Bernd HW, Feller AC, Klapper W, Hummel M, Stein H, Hansmann ML, Schmelter C, Moller P, Cogliatti S, Pfreundschuh M, Schmitz N, Trumper L, Siebert R, Loeffler M, Rosenwald A, Ott G. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253–2263. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 11.Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, Liu WM, Visco C, Li Y, Miranda RN, Montes-Moreno S, Dybkaer K, Ciuh A, Orazi A, Zu Y, Bhagat G, Richards KL, Hsi ED, Choi WW, Zhao K, van Krieken JH, Huang Q, Huh J, Ai W, Ponzoni M, Ferreri AJ, Zhou F, Slack GW, Gasoyne RD, Tu M, Variakojis D, Chen W, Go RS, Piris MA, Moller MB, Medeiros LJ, Young KH. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021–4031. doi: 10.1182/blood-2012-10-460063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, Nielsen O, Gadeberg OV, Mourits-Andersen T, Fredericksen M, Pedersen LM, Moller MB. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of Clinical Oncology. 2012;30:3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 13.Perry AM, Alvarado-Bernal Y, Laurini JA, Smith LM, Slack GW, Tan KL, Sehn LH, Fu K, Aoun P, Greiner TC, Chan WC, Bierman PJ, Bociek RG, Armitage JO, Vose JM, Gascoyne RD, Weisenburger DD. MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. British Journal of Haematology. 2014;165:382–391. doi: 10.1111/bjh.12763. [DOI] [PubMed] [Google Scholar]
- 14.Petrich AM, Gandhi M, Jovanovic B, Castillo JJ, Rajguru S, Yang DT, Shah KA, Whyman JD, Lansigan F, Hernandez-Ilizaliturri FJ, Lee LX, Barta SK, Melinamani S, Karmali R, Adeimy C, Smith S, Dalal N, Nabhan C, Peace D, Vose J, Evens AM, Shah N, Fenske TS, Zelenetz AD, Landsburg DJ, Howlett C, Mato A, Jaglal M, Chavez JC, Tsai NP, Reddy N, Li S, Handler C, Flowers CR, Cohen JB, Blum KA, Song K, Sun HL, Press O, Cassaday R, Jaso J, Medieros LJ, Sohani AR, Abramson JS. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124:2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JB, Geyer SM, Lozanski G, Zhao W, Heerema NA, Jones JA, Porcu P, Christian BA, Baiocchi RA, Maddocks KJ, Flynn JM, Devine SM, Blum KA. Complete response to induction therapy in patients with Myc-positive and double-hit non-Hodgkin lymphoma is associated with prolonged progression-free survival. Cancer. 2014;120:1677–1685. doi: 10.1002/cncr.28642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Progression free survival and overall survival of patients randomized to transplant vs no transplant for MYC IHC positive patients (Figures S1a and S1b), and DPL patients (Figures S1c and S1d) respectively.


