Abstract
In this study, the effects of a potentially lethal radiation exposure on the brain for long-term cognitive sequelae were investigated using Rhesus macaques (Macaca mulatta) adopted from other facilities after analysis of acute radiation response via the Centers for Medical Countermeasures against Radiation (CMCR) network. Fifty-nine animals were given the opportunity to participate in cognitive cage-side testing. The animals that received single-dose gamma irradiation were significantly less likely to engage in cognitive testing than the controls, suggesting that irradiated animals may have differences in cognitive ability. Five irradiated (6.75–8.05 Gy) and three naïve control animals self-selected, were extensively trained and administered a simple visual discrimination with reversal (SVD+R) task 2–3 times per week for 11–18 months. Each session consisted of 30 trials in which the animals were required to choose the correct visual stimulus for a food reward. After the initial presentation, the stimulus that signaled the presence of food was twice reversed once the animal reached criterion (90% accuracy across four consecutive sessions). While the limited sample size precluded definitive statistical analysis, irradiated animals took longer to reach the criterion subsequent to reversal than did control animals, suggesting a relative deficiency in cognitive flexibility. These results provide preliminary data supporting the potential use of a nonhuman primate model to study radiation-induced, late-delayed cognitive deficits.
INTRODUCTION
In addition to the recent Fukushima Daiichi nuclear disaster, increases in terroristic threats and attacks highlight the growing need to understand the late effects of acute radiation exposure on the body (1). Radiation studies to date examining cognitive function have demonstrated profound functional and structural effects on the brain, ranging from progressive cognitive impairment (2, 3) and dementia (4) to white matter lesions and degradation (5) in patients treated for cancer. However, these clinical studies have largely utilized fractionated radiation schemes involving repeated small-fraction doses, and are often confounded by concurrent chemotherapy treatment (6) and/or the presence of neoplasms (7). Data on cognitive function from single doses of radiation are limited, perhaps due to the lower radiosensitivity of the brain relative to other major organ systems that dominate acute radiation-response research (8).
Because controlled human studies using single, sublethal radiation doses are not possible, nonhuman primate (NHP) models are essential in deciphering the effects of radiation on the brain (9). Rhesus monkeys are physiologically and genetically similar to humans, as is the pattern of gene expression in the brain observed through the course of development (10), and they perform comparably to apes on many cognitive tasks (11). Moreover, findings from NHP studies are more translatable to humans than those using rodent models (12). In addition to cognitive changes due to normal aging (13), NHPs are excellent models of Alzheimer’s disease (14), Parkinson’s disease (15), depression (16), Huntington’s disease (17), schizophrenia (18, 19), as well as brain dysfunction related to ischemia (20), drug addiction (21), anxiety and emotional regulation (22). NHPs are also models for the study of radiation-induced brain pathology (9, 23, 24). Cognitive studies on the effects of single, sublethal radiation doses in NHPs have largely focused on early effects at very high (>11 Gy) doses (25, 26). With recent therapeutic advances in mitigating radiation injury (27), there is a growing need to understand the long-term cognitive sequelae of acute radiation doses in those patients who survive through pharmacologic intervention and supportive care.
A number of influencing factors that mediate the radiation response in cognitive models using fractionation schedules must be considered to accurately discern radiation effects on cognitive function. For example, time of exposure mediates the disruption in performance of working memory as adults. Monkeys exposed to radiation in utero, but not as juveniles, experience the working memory disruption (28). Fractionated whole-brain irradiation (fWBI) schemes introduce other considerations such as dose rate, fraction number and size, host factors and radiation quality (29). Moreover, tasks with higher cognitive load (i.e., the amount of cognitive resources utilized in completing the task) are needed to detect impairment (30). Adult rhesus monkeys exposed to a fWBI scheme, similar to that which cancer patients experience, show significant deficits in cognitive function seven months postirradiation for low cognitive load tasks and as early as one month postirradiation for high cognitive load tasks (30). Both age at irradiation and time since irradiation must be considered, as normal aging may make it difficult to differentiate radiation-induced cognitive effects [as reported in other animal models, e.g., rats (31)].
The current study was part of a programmatic assessment of the response of multiple bodily systems (kidneys, lungs, cardiovascular, hematopoietic, brain and gastrointestinal) in an NHP model (rhesus monkey) to significant, single-dose gamma irradiation resembling that which might occur as a result of nuclear disaster or attack. In this study, we compared the acquisition performance of total-body irradiated NHPs and age-matched, nonirradiated controls on two cognitive tasks. A simple object retrieval task (ORT) and a visual discrimination (SVD) task with visual discrimination reversal (VDR; collectively SVD+R) were employed. Rhesus monkeys have previously demonstrated the ability to perform these tasks (32, 33), which, together, are used to assess learning, reference memory, attention, cognitive flexibility and response inhibition (33, 34). As radiation is known to adversely affect these parameters (35), we hypothesized that irradiated animals would take longer to acquire the SVD+R than control animals. We further characterized the two groups in terms of their willingness to complete cognitive tasks and how readily they became familiar with assessment apparatus and tasks. We predicted that the control animals would more easily become familiar with the SVD+R and would be more likely to perform cognitive tasks in general than irradiated animals.
METHODS
Subjects
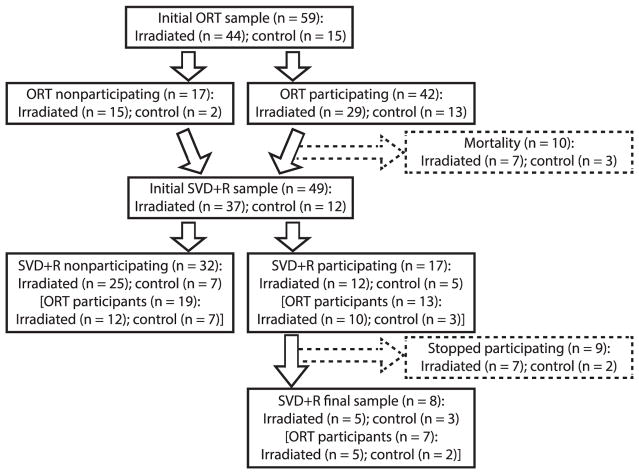
The subjects in this study were selected opportunistically from a group of 59 gamma-irradiated and nonirradiated adult male and female rhesus macaques (Macaca mulatta) from a large observational study housed at the Wake Forest School of Medicine (WFSM; see Fig. 1), similar to how participants would be studied in the clinic. Prior to arriving at the WFSM, the monkeys in the experimental group were housed and irradiated either at the University of Maryland (UMD), or the University of Illinois at Chicago (UIC) and had no prior experience in cognitive testing. Table 1 provides a list of the identification number, age at the time of this study, radiation dose (if applicable), time since irradiation and age at irradiation of each animal. For animals coming from UMD or UIC, all treatments were approved by their respective animal oversight committees. In this study, all available animals (n = 59) were given the opportunity to engage in the ORT and/or SVD+R tasks, and were included if they consistently participated in the task on a daily basis. In the SVD+R task, the focus of the current report, 8 animals (5 irradiated, 3 control) of 49 were sufficiently engaged to be selected for the study. The 5 experimental animals received single-dose total-body irradiation (3.1–4.3 years since irradiation) at doses ranging from 6.75 to 8.05 Gy, and the remaining 3 were age-matched controls. At the WFSM, monkeys were given access to chow customized to resemble nutritionally the typical American’s diet (TestDiet® no. 5L0P; Purina®, St. Louis, MO), supplemented with fresh fruit or vegetables daily, with water available ad libitum. Monkey no. “I-3” was a type 2 diabetic and received standard, commercially available chow (LabDiet no. 5038; Purina) instead of TestDiet no. 5L0P. Fruits and vegetables were not given until testing was completed for the day. Monkeys were housed either alone or in isosexual pairs. Research at WFSM was approved by the Institutional Animal Care and Use Committee (IACUC) and conformed to all state and federal laws. The institution is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All research and animal care follows the guidelines set forth in the Guide for the Care and Use of Laboratory Animals (36).
FIG. 1.
Flow chart showing the progression of cognitive testing within the cohort of animals given the opportunity to participate.
TABLE 1.
Demographics of Rhesus Macaques Performing the Visual Discrimination Task
| NHP* | Dose (Gy) | Age (years) | Age at irradiation (years) | Postirradiation (years) |
|---|---|---|---|---|
| I-1 | 7.55 | 12.7 | 4.4 | 8.3 |
| I-2 | 7.55 | 11.4 | 3.1 | 8.3 |
| I-3 | 6.75 | 11.6 | 4.3 | 7.3 |
| I-4 | 7.20 | 10.7 | 3.5 | 7.2 |
| I-5 | 8.05 | 10.7 | 3.3 | 7.4 |
| C-1 | 11.6 | |||
| C-2 | 13.3 | |||
| C-3 | 11.7 |
”I” indicates irradiated animals and “C” indicates control animals.
Testing Apparatus
The apparatus (see Fig. 2) was custom designed and fabricated for use with our pen-housed rhesus monkeys so that they could be tested in their home environment. Home environment testing prior to daily feeding allowed us to assess cognitive function without the use of dietary or water restriction, and without the use of restraint chairs or testing chambers. It was comprised of a stainless steel frame and removable sliding door held in place with removable steel pins. Sets of metal brackets affixed to the floor of the apparatus accommodated up to three stainless-steel boxes with hinged lids, which were held in place with removable pins. The apparatus easily attached to the pen of the working monkey by sliding into stainless steel fittings located on the outside front of the pens, allowing the monkey sufficient room to perform the task from his perch. Because one animal (no. I-3) was housed in a cage not compatible with the testing apparatus, so he could receive daily insulin injections and blood glucose monitoring, a separate, portable cage was modified to accommodate the apparatus. This testing cage was designed so that the animal could easily move into it from his home cage for testing. No other deviations in standard testing procedures were made. During both phases of testing, rewards were given in the form of palatable, nutritious pellets (Fruit Crunchies, 190 mg pellets, cat. no. F05798-1; Bio-Serv Inc., Frenchtown, NJ). Approximately 10 pellets were available in a given trial for the animals to retrieve in the event they selected the correct box.
FIG. 2.
Panel A: rhesus macaque performing a simple visual discrimination trial. Panel B: Custom-built simple visual discrimination apparatus.
Object Retrieval Test
Although participation in the ORT was not considered a necessary prerequisite to SVD+R, ORT was administered prior to the SVD+R task. The ORT has been described elsewhere (37). Briefly, testing was conducted using a modified version of the manual testing apparatus for the current experiment. In this version, instead of metal boxes, the frame of the apparatus held a clear, Plexiglas cube with one open side in the center of the apparatus. On a given trial, the cube was baited with a food reward and rotated such that the opening faced one of three different positions, with the reward located in one of three positions within the cube. The location of the cube’s open side and the reward within it varied across trials. When a visual barrier (i.e., a metal partition) was lifted, providing the animal access to the apparatus, the monkey had 3 min to take the food reward (the reward was almost always taken immediately). If the monkey took the reward without hitting the closed sides of the cube within 3 min, the trial was scored as a correct response. Conversely, if the animal failed to retrieve the reward within 3 min, or touched any of the closed sides of the cube prior to retrieving the reward, the trial was scored as an incorrect response. Because the speed of the monkey was too great to prohibit access to the reward after an error, the animal was allowed to retrieve the food regardless of whether a correct or incorrect response was made. Subsequent to food retrieval, the partition was lowered and the cube immediately rebaited for the next trial. The intertrial interval was the amount of time it took for the experimenter to perform these actions. The animals were tested approximately twice weekly for up to 20 sessions, or until criterion (i.e., 85% correct trials in a session) was met.
Simple Visual Discrimination and Reversal
Familiarization
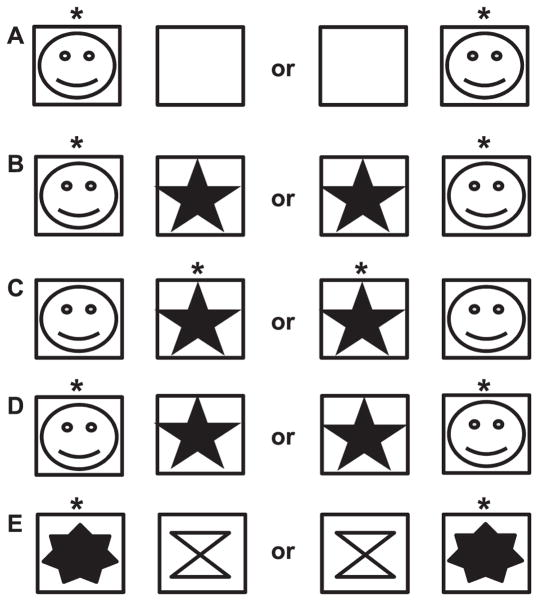
Although some of the animals had prior experience participating in the ORT, all were naïve to the SVD+R used in this study (see Fig. 1). Animals were guided through task familiarization by gradually shaping appropriate responses to the testing apparatus (see Fig. 2) and visual stimuli. Because the testing apparatus was unfamiliar to the animals, they were first trained to open the hinged lids of the goal boxes and retrieve the food reward. The initial configuration of the apparatus consisted of the frame and a single goal box placed in the center of the apparatus. Once the animal was visually attending to the experimenter and apparatus, it was allowed to observe the experimenter opening the lid of a goal box and baiting it with pellets. Subsequently, the animal was able to retrieve the reward. This was repeated over several days until the animal reliably retrieved rewards without guidance from the experimenter. Next, this step was repeated, but a metal partition was put in place whereby the animal could not see the goal box while it was being baited. Once the experimenter fit the box with pellets, the partition was lifted, and the animal was able to retrieve the reward. This step familiarized the animals to the presence of the partition. Once animals were responding as consistently in the presence of the partition as without, the lid of the goal box was fitted with a novel clipart image. Because many animals are sensitive to subtle changes in their environment, a period of several days was often necessary to restore task performance to pre-visual stimulus levels. Once the animals were desensitized to the visual stimulus (i.e., responding returned to pre-stimulus levels), the testing apparatus was fitted with two goal boxes (see Fig. 3A). Only one of the boxes was fitted with a clipart image, which was the same as used in previous steps. In each trial presentation, the box fitted with the image was randomly predetermined, and only that box contained the reward. Sessions comprised of 30 trials were conducted 3–5 times per week in all animals during the familiarization phase. The animals were given 15 s to respond in each trial. Failure to attempt a response across five consecutive trials terminated the session. Responses to each presentation were recorded. Once an animal achieved 85% accuracy in three of four consecutive sessions, task familiarization was complete.
FIG. 3.
Diagram of the SVD+R procedure. Each pair of boxes indicates the possible positions of the stimuli and reward. The squares represent the reward goal boxes. Row A: Familiarization phase with only 1 stimulus. Row B: First 2-stimulus testing phase. Row C: Reversal. Row D: Re-reversal. Row E: New stimulus set is given.
Training
Training for the SVD+R followed an A-B-A sequence, with “A” indicating the initial response contingency and “B” indicating the reversal contingency. As in the last step of the training phase, the animals were presented with the testing apparatus configured with two goal boxes. However, in the acquisition phase, a novel clipart image was affixed to the lids of both goal boxes. These two randomly selected visual stimuli were used throughout the entire phase. To ameliorate response fatigue, sessions comprised of 30 trials were conducted 2–3 times weekly. The goal box containing the reward in each trial was randomly determined prior to the beginning of the session, and the stimulus marking the reward was determined prior to each testing phase (Fig. 3B). The criterion for completion of SVD acquisition was 90% accuracy in four consecutive sessions. Subsequently, animals began VDR testing. The task requirements were nearly identical to SVD; however, the animals were now required to choose the goal box marked by the stimulus opposite of that which previously signaled the presence of a reward (Fig. 3C). The stimulus previously marking the presence of a food reward now signaled the absence of the reward. The criterion for completion of VDR acquisition was 90% accuracy in four successive sessions. Subsequently, the response contingency for the stimulus pair was again reversed (or returned to the original contingency), with the animal being tested until criterion (Fig. 3D). At this point, a new, randomly selected stimulus pair was given (Fig. 3E).
Statistical Analysis
To best depict the animals’ progress to criterion by group, cognitive performance on the SVD+R tasks is illustrated using reverse Kaplan-Meier survival curves, and data are presented by task phase (familiarization, initial training and reversal). Graphical depictions of individual cognitive performance are presented as sessions to criterion. Fisher’s exact tests were used to analyze group differences on willingness to participate in two separate tasks. All statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS, version 23.0; IBM™, North Castle, NY).
RESULTS
Willingness to Test
The assessment of animals proceeded in a stepwise fashion and is shown in Fig. 1. Within a three-year span, we attempted to administer ORT, SVD+R or both tasks to 59 animals (44 irradiated, 15 control) to determine their suitability for extensive cognitive testing. Animals failing to approach or manipulate the apparatus during familiarization (n = 17; irradiated n = 15, control n = 2) were removed from the testing schedule. Ten animals that participated in the ORT did not receive a chance to participate in SVD+R due either to morbidity or mortality. Of the 49 animals receiving a chance to engage in both tasks, all 12 control animals (100%) participated in at least one session of either task when presented to them, compared to only 23 of 37 irradiated animals (62.16%), Fisher’s exact test, P = 0.01. Overall, there was no difference between irradiated and control groups in the number of animals to complete at least one session in both tasks (27.03% irradiated and 25.00% control), P = 0.99. Furthermore, there were no differences between irradiated and control animals in willingness to participate in either SVD+R (P = 0.73) or ORT (P = 0.19).
Simple Visual Discrimination and Reversal
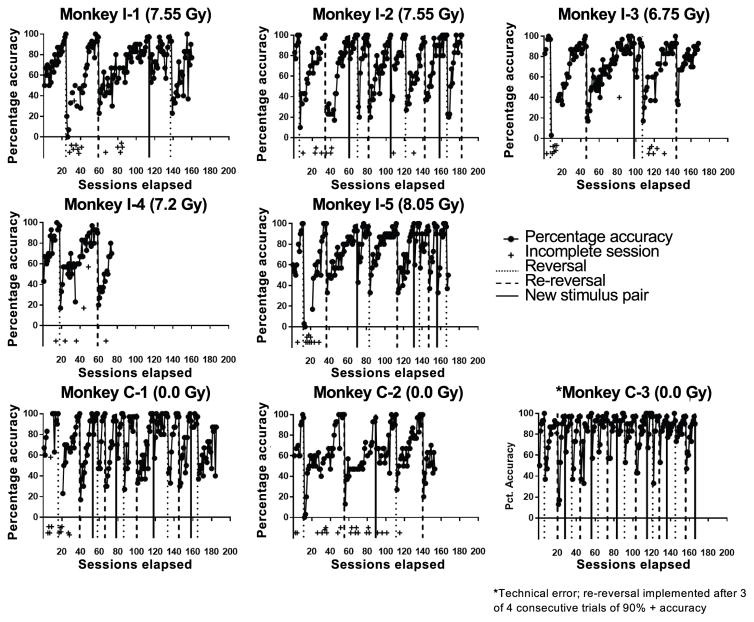
Eight animals (5 irradiated, 3 control) showed the degree of consistency necessary in their willingness to test to allow for longitudinal cognitive assessment. Due to the small sample size, data are qualitatively described. Individual cognitive performances for animals in both groups are shown in Fig. 4. Not only were animals in both groups able to perform the task with a high degree of accuracy, their performance decreased dramatically when the rewarded stimulus was changed (i.e., a reversal or new stimulus set was imposed). This suggests that the animals in both groups learn the task. However, there appeared to be high individual variability in the speed of acquisition among animals.
FIG. 4.
Individual assessment profiles of the 8 animals that participated readily enough in the tasks to be assessed longitudinally. The first two rows represent the five irradiated animals. The bottom row represents the three control animals. The “+” indicates that either the session was terminated prior to completion, or more than half of the trials were not attempted: i.e., for “+” located below the x-axis, the session was terminated prior to completion and fewer than 15 trials were attempted; and for “+” located at the animal’s overall accuracy for that trial as a function of number of trials attempted, the session was terminated prior to completion, but 15 or more trials were attempted. *Technical error wherein a reversal was given after only three consecutive 90% accuracy sessions instead of four. Dotted vertical lines indicate point of reversal, hashed lines indicate point of re-reversal and solid lines indicate completion of stimulus pair.
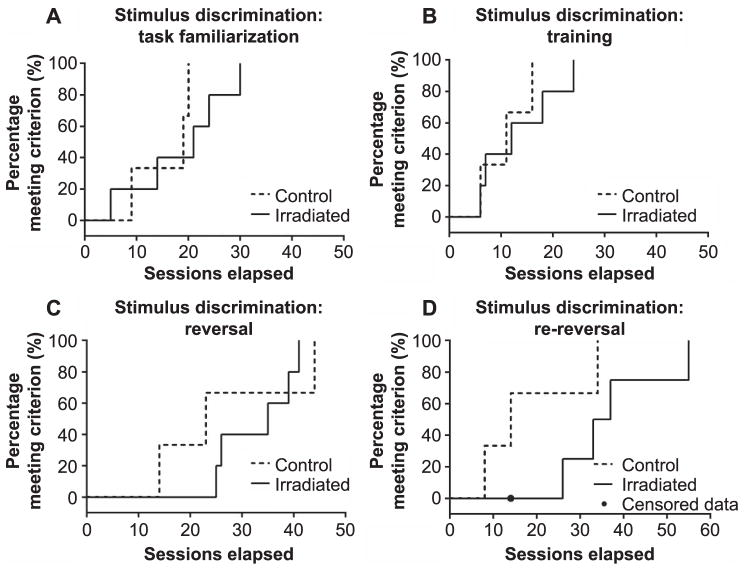
Although both groups were able to readily learn the task, the degree of cognitive flexibility, or the ability to extinguish an old association in favor of a new one (34), differed somewhat among the groups. The number of trials required to meet criterion in each phase for control animals C-1 and C-3 tended to decrease with experience, whereas in the irradiation group, this appears to be the case only for animal no. I-2. Reverse Kaplan-Meier curves reflecting the number of sessions required to reach criterion for all animals in the two groups are shown in Fig. 5A–D for each phase of the study (familiarization, first training phase, reversal, re-reversal). Group differences appeared to be minimal in the familiarization (Fig. 5A) and first training (Fig. 5B) phases. However, visual inspection of the data suggests that all animals had more difficulty reaching criterion subsequent to reversal and to re-reversal, but that this difference may be more pronounced in irradiated animals (see Fig. 5C and D) relative to control animals.
FIG. 5.
Reverse Kaplan-Meier survival curves showed the percentage of animals that accomplished 85% accuracy on three or four successive familiarization sessions (panel A). Percentage of animals that achieved 90% accuracy in four consecutive sessions of the first training phase (panel B), reversal (panel C) or re-reversal (panel D) to the original task training requirements. For irradiated animals, n = 5. For control animals, n = 3.
DISCUSSION
Rhesus macaques that previously received TBI between 6.75 and 8.05 Gy were trained on and evaluated using SVD+R, and their performance was compared to that of age-matched nonirradiated animals. In this study, irradiated animals were less likely to engage in cognitive testing than control animals. Although the propensity to engage in cognitive testing is influenced by host factors rather than the test itself, one possible explanation for the reluctance of irradiated animals in our cohort to participate in cognitive tasks is a radiation-induced attentional deficit; however, further testing is required to assess this inference. Attentional deficits have been reported in long-term human survivors of low-grade glioma who received radiotherapy (38). In addition, adult survivors of pediatric lymphoid malignancies have been reported to demonstrate impairments in both attentional fluctuations and sustained attention (35). It may also be the case that lack of experience in cognitive testing, fear or lack of socialization with the experimenters contributed to poorer performance on the cognitive tasks. These animals were sampled opportunistically from a limited pool of subjects, similar to that which is done for human studies conducted in a clinical setting.
Although the number of animals used did not allow for statistical comparison, with the exception of the reversal, the control group tended to complete each phase of the task more quickly than did the irradiated group. Both groups, however, demonstrated the ability to learn the task. The broadest performance difference between the two groups was demonstrated at the re-reversal phase, which suggests that the control animals learned to adapt to the task’s changing rules while the irradiated animals lacked the cognitive flexibility necessary to do so. Similar increased perseverative errors were reported in a study of juvenile and adult macaques that were exposed to radiation in utero (39). In addition, survivors of pediatric cranial lymphoid malignancies showed deficits in cognitive flexibility 25 years after receiving radiotherapy (35). A published study of a rodent model showed that single-fraction cranial irradiation in rats led to an increase in impulsivity in cognitive tasks (40). Such an effect could lead to the perseverative errors observed in the current study. Moreover, it has been reported that exploratory behavior in mice is inhibited after irradiation (41), and spatial memory is reduced in Morris water maze (42) and Barnes maze (43) tasks after single-dose cranial radiation exposure.
The current study demonstrated the utility of evaluating long-term cognitive function after irradiation. Due to the global proliferation of nuclear arms and the recently realized potential of radiological disasters such as in Fukushima, the domain of radiation-induced cognitive dysfunction is more relevant than ever. Given that these effects can only be ethically studied in humans retrospectively, it is of critical importance to have a model in which radiation-induced cognitive sequelae can be objectively studied. Using the SVD+R task, we detected potentially important preliminary group differences, suggesting that several years after TBI, nonhuman primates suffer from late-delayed cognitive impairments. Limited sample size is a problem inherent in primate research, and precluded statistical analysis in this study. Nevertheless, the data from this work suggest that there are differences between irradiated and control animals, especially in cognitive flexibility. As our cohort grows, we will gain a broader perspective on the late cognitive effects of irradiation.
Based on the data, the majority of incomplete sessions occurred prior to the 40th session of the SVD+R. This suggests that if a more extensive familiarization period been implemented, a more complete data set would have been obtained. Regardless, despite the small sample size, all available animals were given an opportunity to participate in both tasks, and all animals that consistently participated during training were included. Moreover, animal no. I-4 suffered mortality unrelated to the experimental procedure midway through the assessment period, thus decreasing our sample size to four irradiated animals. There are no data to suggest that his cognitive performance was affected by factors related to mortality.
While efforts were made to keep the housing area where the testing occurred quiet during assessments, it is possible that activity in neighboring pens could have affected cognitive performance. In an effort to increase the animals’ attention to the task and decrease the influence to external stimulation, we initially attempted to conducted cognitive tests in an operant chamber. However, animals were reluctant to enter the chamber and refused to interact with the screen. We thus opted to reconfigure our paradigm to a cage-side setting more suitable for this particular group of animals. Future cohorts may be conditioned more readily to accept cage-side testing apparatus as part of enrichment programs across participating institutions to potentially increase the proportion of animals willing to participate in cognitive testing. Another limitation of our study was that performing the test five days per week led to disinterest by the animals in the task. Therefore, testing was done no more than three times per week to maintain the highest rate of participation. Furthermore, during the ORT, we discovered that varying the types of rewards often diminished participation (i.e., animals often held out for a more favorable reward). Thus, we chose flavored pellets in the SVD+R task. Finally, as often as possible, we chose to test in the afternoons, long after the buildings had been cleaned, allowing time for the animals to be calm before testing. By employing our testing paradigm in this way, we successfully avoided having to restrict food or implement chair testing. These strategies may benefit other research programs where cognitive testing is performed and help limit consumption of their resources, and will also allow us to more easily implement different cognitive tests in the future with the current cohort.
Acknowledgments
We thank Dr. Cynthia Lees, Jean Gardin, Chrystal Bragg, Stacey Combs, Matthew Dwyer and Russell O’Donnell for their assistance in daily animal care and data collection. This work was supported by the National Institutes of Health (NIH grant no. U19-AI67798 awarded to Dr. Nelson Chao, PI at Duke University; JMC: primate core leader).
References
- 1.Bunn M. Testimony of Matthew Bunn for the Committee on Homeland Security and Governmental Affairs. United States Senate; 2008. The risk of nuclear terrorism – and next steps to reduce the danger. ( http://bit.ly/2cA19wn) [Google Scholar]
- 2.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–95. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 3.Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev. 2008;14:238–42. doi: 10.1002/ddrr.26. [DOI] [PubMed] [Google Scholar]
- 4.Doyle DM, Einhorn LH. Delayed effects of whole brain radiotherapy in germ cell tumor patients with central nervous system metastases. Int J Radiat Onc Biol Phys. 2008;70:1361–4. doi: 10.1016/j.ijrobp.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Szerlip N, Rutter C, Ram N, Yovino S, Kwok Y, Maggio W, et al. Factors impacting volumetric white matter changes following whole brain radiation therapy. J Neurooncol. 2011;103:111–9. doi: 10.1007/s11060-010-0358-7. [DOI] [PubMed] [Google Scholar]
- 6.Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong CL, Gyato K, Awadalla AW, Lustig R, Tochner ZA. A critical review of the clinical effects of therapeutic irradiation damage to the brain: the roots of controversy. Neuropsychol Rev. 2004;14:65–86. doi: 10.1023/b:nerv.0000026649.68781.8e. [DOI] [PubMed] [Google Scholar]
- 8.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 7. Philadelphia: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 9.Caveness WF. Pathology of radiation damage to the normal brain of the monkey. Natl Cancer Inst Monogr. 1977;46:57–76. [PubMed] [Google Scholar]
- 10.Bakken TE, Miller JA, Luo R, Bernard A, Bennett JL, Lee C-K, et al. Spatiotemporal dynamics of the postnatal developing primate brain transcriptome. Hum Mol Genet. 2015;24:4327–39. doi: 10.1093/hmg/ddv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt V, Pankau B, Fischer J. Old world monkeys compare to apes in the primate cognition test battery. PLoS One. 2012;7:e32024. doi: 10.1371/journal.pone.0032024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shively CA, Clarkson TB. The unique value of primate models in translational research. Am J Primatol. 2009;71:715–21. doi: 10.1002/ajp.20720. [DOI] [PubMed] [Google Scholar]
- 13.Nagahara AH, Bernot T, Tuszynski MH. Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol Aging. 2010;31:1020–31. doi: 10.1016/j.neurobiolaging.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Wu Y, Min F, Li Z, Huang J, Huang R. A nonhuman primate model of Alzheimer’s disease generated by intracranial injection of amyloid-42 and thiorphan. Metab Brain Dis. 2010;25:277–84. doi: 10.1007/s11011-010-9207-9. [DOI] [PubMed] [Google Scholar]
- 15.Emborg ME. Nonhuman primate models of Parkinson’s disease. ILAR J. 2007;48:339–55. doi: 10.1093/ilar.48.4.339. [DOI] [PubMed] [Google Scholar]
- 16.Willard SL, Shively CA. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis) Am J Primatol. 2012;74:528–42. doi: 10.1002/ajp.21013. [DOI] [PubMed] [Google Scholar]
- 17.Roitberg BZ, Emborg ME, Sramek JG, Palfi S, Kordower JH. Behavioral and morphological comparison of two nonhuman primate models of Huntington’s disease. Neurosurgery. 2002;50:137–46. doi: 10.1097/00006123-200201000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Blackman RK, MacDonald AW, III, Chafee MV. Effects of ketamine on context-processing performance in monkeys: a new animal model of cognitive deficits in schizophrenia. Neuropsychopharmacology. 2013;38:2090–100. doi: 10.1038/npp.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer’s type: individual differences in acquisition of a visuospatial paired-associate learning task in rhesus monkeys. Behav Brain Res. 2004;149:123–33. doi: 10.1016/s0166-4328(03)00214-6. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda S, del Zoppo GJ. Models of focal cerebral ischemia in the nonhuman primate. ILAR J. 2003;44:96–104. doi: 10.1093/ilar.44.2.96. [DOI] [PubMed] [Google Scholar]
- 21.Howell LL, Murnane KS. Nonhuman primate neuroimaging and the neurobiology of psychostimulant addiction. Ann N Y Acad Sci. 2008;1141:176–94. doi: 10.1196/annals.1441.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- 23.Hanbury DB, Robbins ME, Bourland JD, Wheeler KT, Peiffer AM, Mitchell EL, et al. Pathology of fractionated whole-brain irradiation in rhesus monkeys (Macaca mulatta) Radiat Res. 2015;183:367–74. doi: 10.1667/RR13898.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lonser RR, Walbridge A, Vortmeyer AO, Pack SD, Nguyen TT, Gogate N, et al. Induction of glioblastoma multiforme in nonhuman primates after therapeutic doses of fractionated whole-brain radiation therapy. J Neurosurg. 2002;97:1378–89. doi: 10.3171/jns.2002.97.6.1378. [DOI] [PubMed] [Google Scholar]
- 25.Curran CR, Young RW, Davis WF. The performance of primates following exposure to pulsed whole-body gamma-neutron radiation. Bethesda: Armed Forces Radiobiology Research Institute; 1973. AFRRI Report No. SR73-1. [Google Scholar]
- 26.Turbyfill CL, Roudon RM, Kieffer VA. Behavior and physiology of the monkey (Macaca mulatta) following 2500 rads of pulsed mixed gamma-neutron radiation. Aerosp Med. 1972;43:41–5. [PubMed] [Google Scholar]
- 27.Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol. 2009;85:539–73. doi: 10.1080/09553000902985144. [DOI] [PubMed] [Google Scholar]
- 28.Friedman HR, Selemon LD. Fetal irradiation interferes with adult cognition in the nonhuman primate. Biol Psychiatry. 2010;68:108–11. doi: 10.1016/j.biopsych.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw EG, Robbins ME. Biological bases of radiation injury to the brain. In: Meyers CA, Perry JR, editors. Cognition and cancer. Cambridge: Cambridge University Press; 2008. pp. 83–96. [Google Scholar]
- 30.Robbins ME, Bourland JD, Cline JM, Wheeler KT, Deadwyler SA. A model for assessing cognitive impairment after fractionated whole-brain irradiation in nonhuman primates. Radiat Res. 2011;175:519–25. doi: 10.1667/RR2497.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L, Olson J, D’Agostino R, Jr, Linville C, Nicolle MM, Robbins ME, et al. Aging masks detection of radiation-induced brain injury. Brain Res. 2011;1385:307–16. doi: 10.1016/j.brainres.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rapp PR. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta) Behav Neurosci. 1990;104:876–84. doi: 10.1037//0735-7044.104.6.876. [DOI] [PubMed] [Google Scholar]
- 33.Zeamer A, Decamp E, Clark K, Schneider JS. Attention, executive functioning and memory in normal aged rhesus monkeys. Behav Brain Res. 2011;219:23–30. doi: 10.1016/j.bbr.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci. 2013;7:201. doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuitema I, de Sonneville L, Kaspers G, van der Pal H, Uyttebroeck A, van den Bos C, et al. Executive dysfunction 25 years after treatment with cranial radiotherapy for pediatric lymphoid malignancies. J Int Neuropsychol Soc. 2015;21:657–69. doi: 10.1017/S1355617715000788. [DOI] [PubMed] [Google Scholar]
- 36.Guide for the care and use of laboratory animals. 8. Washington, D.C: National Academies Press; 2011. ( http://bit.ly/1nv823I) [PubMed] [Google Scholar]
- 37.Rutten K, Basile JL, Prickaerts J, Blokland A, Vivian JA. Selective PDE inhibitors rolipram and sildenafil improve object retrieval performance in adult cynomolgus macaques. Psychopharmacology. 2008;196:643–8. doi: 10.1007/s00213-007-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douw L, Klein M, Fagel SSAA, van den Heuvel J, Taphoorn MJB, Aaronson NK, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long term follow-up. Lancet Neurol. 2009;8:810–8. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 39.Selemon LD, Friedman HR. Motor stereotypies and cognitive perseveration in non-human primates exposed to early gestational irradiation. Neuroscience. 2013;248:213–24. doi: 10.1016/j.neuroscience.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis CM, DeCicco KL, Roma PG, Hienz RD. Individual differences in attentional deficits and dopaminergic protein levels following exposure to proton radiation. Radiat Res. 2014;181:258–71. doi: 10.1667/RR13359.1. [DOI] [PubMed] [Google Scholar]
- 41.Naylor AS, Bull C, Nilsson MKL, Zhu C, Björk-Eriksson T, Eriksson PS, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105:14632–7. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]