Abstract
Posaconazole, a broad-spectrum triazole antifungal agent, is approved for the prevention of invasive aspergillosis and candidiasis in addition to the treatment of oropharyngeal candidiasis. There is evidence of efficacy in the treatment and prevention of rarer, more difficult-to-treat fungal infections. Posaconazole oral suspension solution has shown limitations with respect to fasting state absorption, elevated gastrointestinal pH and increased motility. The newly approved delayed-release oral tablet and intravenous solution formulations provide an attractive treatment option by reducing interpatient variability and providing flexibility in critically ill patients. On the basis of clinical experience and further clinical studies, posaconazole was found to be a valuable pharmaceutical agent for the treatment of life-threatening fungal infections. This review will examine the development history of posaconazole and highlight the most recent advances.
Keywords: antifungal, immunosuppression, invasive fungal infection, pharmacokinetics, pharmacology, posaconazole, triazole
Invasive fungal infections (IFIs) are problematic for critically ill patients, with increased risks of morbidity and mortality. Prophylactic treatment is often advantageous because delayed antifungal treatment has been shown to increase mortality rates [1,2]. The Infectious Disease Society of America guidelines for the use of antimicrobial agents in neutropenic patients with cancer suggest that antifungal prophylaxis be used in high-risk patients, including those undergoing hematopoietic stem-cell transplantation (HSCT) or intensive chemotherapy for leukemia [3]. Unfortunately, safety and tolerability concerns often reduce the use of antifungals in the clinic.
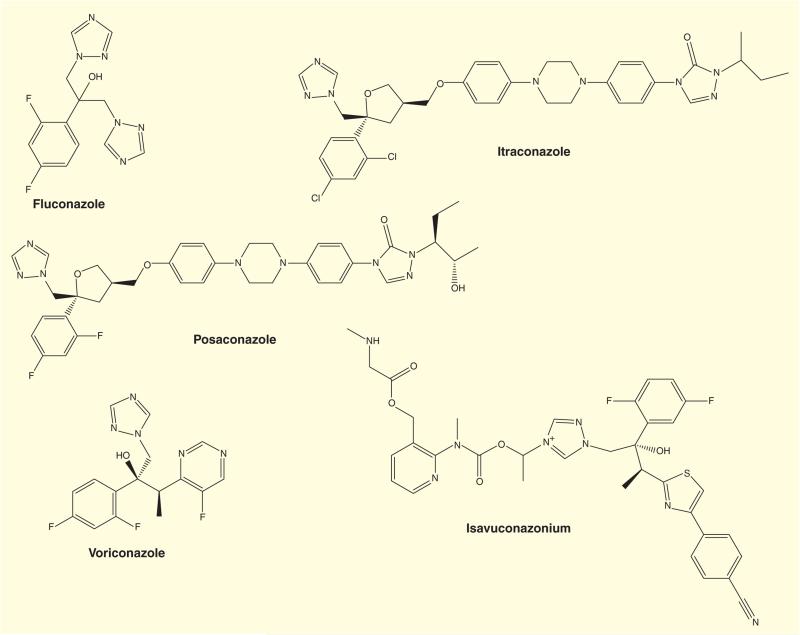
The currently available systemic triazoles are divided into two groups: the first generation (fluconazole and itraconazole) and the second generation (voriconazole, posaconazole and isavuconazonium; Figure 1). Although these agents all possess the same mechanism of action, each has differing antifungal activity, efficacy, pharmacokinetics and safety profiles, leading to unique therapeutic niches [4,5].
Figure 1.
Chemical structures of common triazole anti-fungal drugs and isavuconazonium.
Posaconazole is a triazole antifungal that boasts an extended-spectrum of activity for prophylaxis and treatment of IFIs. Posaconazole has demonstrated efficacy as an antifungal prophylactic in HSCT recipients with graft versus host disease (GVHD) and in neutropenic patients with hematologic malignancy. In addition, posaconazole has been an effective salvage therapy option for patients who are nonresponsive to standard antifungal therapies [6], Overall, posaconazole covers a wide array of IFIs, including aspergillosis, candidiasis, fusariosis, mucormycosis, cryptococcosis, chromoblastomycosis, mycetoma and coccidioidomycosis [7]. Compared with the older azoles (fluconazole, itraconazole and voriconazole), posaconazole has a more favorable safety profile [8]. Furthermore, posaconazole's activity extends beyond that of other azoles, including voriconazole, for instance, that does not cover mucormycosis [9,10].
Chemical development
Posaconazole is designated chemically as 4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)tetrahydro-5-(1H-1,2,4-triazol-1-ylmethyl)-3-furanyl]methoxy]phenyl]-1-piperazinyl]phenyl]-2-[(1S,2S)-1-ethyl-2-hydroxypropyl]-2,4-dihydro-3H-1,2,4-triazol-3-one. Its empirical formula is C37H42F2N8O4 with a molecular weight of 700.8. It is synthesized solely as the (R,R,S,S) enantiomer via a three-step synthesis followed by a micronization step to enhance the dissolution rate. Posaconazole is structurally comparable with itraconazole (Figure 1). Modifications, including fluorine in place of chlorine and a furan ring in place of the dioxolane ring, result in an extended spectrum of antifungal activity [7].
Pharmacology
Posaconazole, in addition to other triazoles, block ergosterol synthesis through 14 α-demethylase (CYP51) inhibition. Ergosterol depletion prevents proper fungal cell wall construction and causes the accumulation of methylated sterol precursors leading to cell death [11]. Posaconazole undergoes negligible oxidative Phase I metabolism (<2%); its metabolism is facilitated instead through a Phase II biotransformation via uridine disphosphate-glucuronosyltransferase enzyme pathway. No relevant active metabolites have been identified for posaconazole [12]. Posaconazole is both a substrate and an inhibitor of the p-glycoprotein efflux transporter [13].
The Biopharmaceutics Classification System of the US FDA Center for Drug Evaluation and Research lists posaconazole as a Class II compound, indicating that it is well absorbed but dissolves slowly (high permeability/low solubility) [14]. The apparent volume of distribution of posaconazole ranges from 5 to 25 l/kg, indicating extensive distribution and tissue penetration [7]. Posaconazole is highly protein bound (>98%), predominately to albumin in a concentration-dependent fashion.
Posaconazole is predominately eliminated in the feces (77% of the radiolabeled dose), primarily eliminated as parent drug (66% of the radiolabeled dose) in healthy volunteers [15]. Fourteen percent of the radiolabeled dose is excreted in urine, primarily in the form of glucuronide conjugates. The mean half-life (t1/2) of posaconazole ranges from 25 to 35 h [16].
Posaconazole has shown in vitro activity against a wide variety of fungal pathogens, including Aspergillus spp., Candida spp., Coccidioides immitis and Fonsecaea pedrosoi. In addition, some species of Fusarium, Rhizopus and Mucor are sensitive to posaconazole [17]. Clinical Aspergillus fumigatus isolates have been identified that demonstrate resistance to posaconazole, specifically those that harbor CYP51 mutations [18].
There are no established pharmacokinetic guidelines with respect to plasma posaconazole for breakthrough IFIs [19]. A posaconazole concentration target greater than 0.50 mg/l is recommended for prophylactic treatment, with others suggesting a concentration target greater than 0.70 mg/l. Cardiothoracic transplant patients having posaconazole levels consistently exceeding 0.50 mg/l had therapeutically successful outcomes [20]. One report suggests that values exceeding 0.70 mg/l do not provide any further reduction in the clinical failure rate, as demonstrated in two randomized, active-controlled clinical studies using posaconazole oral suspension [21].
Oral suspension formulation
The posaconazole oral suspension solution has been reviewed extensively [7,22]; its use has been eclipsed by the newer formulations. Briefly, there are several challenges with respect to the posaconazole oral suspension formulation. One, this formulation is limited by saturable absorption. In a clinical study involving healthy men, posaconazole oral suspension given 400 mg every 12 h or 200 mg every 6 h resulted in a 98 and 220% increase in bioavailability, respectively, compared with 800 mg given as a single dose [23]. Second, there is also significant pharmacokinetic variability in regard to nutrition as bioavailability increases at a low gastric pH along with high-fat meals. [24,25]. Acidic carbonated beverages have been shown to increase the bioavailability of posaconazole oral suspension [26].
Delayed-release oral tablet formulation
The FDA approved a delayed-release oral posaconazole tablet in November 2013. This tablet was largely designed to overcome the absorption limitations associated with the oral suspension as seen in the clinic and in previous studies. The current posaconazole delayed-release tablet is designed to reduce active drug release at low gastric pH, while increasing release at the elevated pH of the intestine with an absolute bioavailability of 54% for the oral delayed-release tablet. It is suggested that the oral delayed-release tablet be taken with food, although it is not known if the oral bioavailability of the tablet improves under fed conditions [16].
A single- and multiple-dose study was performed in healthy subjects to ascertain optimal dosing and assess pharmacokinetics of this posaconazole tablet formulation [27,28]. During the single 100-mg dose study, the delayed-release tablet formulation had a higher maximum (peak) serum concentration (Cmax) compared with the oral suspension solution in the fasted state (0.39 vs 0.08 mg/l, respectively). In the fed state, the delayed-release tablet formulation still maintained a higher Cmax compared with the oral suspension formulation (0.33 vs 0.24 mg/l, respectively). For the multidose study, subjects were randomized to one of two cohorts. Cohort 1 consisted of either placebo or posaconazole 200 mg single dose on day 1, a 5-day washout period, and then 200 mg BID on day 6, 200 mg QD on days 7–14 and 200 mg BID on days 15–22. Cohort 2 received 400 mg, as opposed to 200 mg, and had the same schedule as cohort 1 until day 14. Median time to maximum concentration (Tmax) was 4 h for the 200-mg dose and 5 h for the 400-mg dose, while mean t1/2 following was similar for both doses (25 and 26 h for the 200- and 400-mg dose, respectively). With the 400-mg dose, the delayed-release tablet exhibited linear pharmacokinetics and steady-state concentrations were achieved after 7 days. Greater intersubject variability was noted in exposure values for the 400-mg dose compared with the 200-mg dose (54 vs 32%, respectively).
A Phase Ib, multicenter dose-determining trial was conducted to examine the pharmacokinetics of posaconazole tablets in patients with acute myeloid leukemia (AML) or myelodysplasia [29]. The two cohorts were administered posaconazole tablets, 200 or 300 mg daily. The 300-mg cohort reached the predefined steady-state concentration goal of 0.50 and 2.50 mg/l in 97% of patients, whereas 79% of patients reached the goal in the 200-mg cohort.
A Phase III multicenter trial used the 300-mg delayed-release posaconazole oral tablet to further examine the pharmacokinetics in AML or myelodysplasia patients along with recent HSCT recipients [30]. During the 28-day trial period, the predefined steady-state concentration goal, set at 0.50–3.75 mg/l, was achieved in 96% of patients, with 81% falling in the range between 0.50 and 2.50 mg/l. These concentration goals are in line with targeted recommendations for breakthrough IFIs.
Intravenous formulation
An intravenous posaconazole formulation developed as an aqueous solution containing the solubilizer sulfobutyl ether beta-cyclodextrin has also been approved for marketing in the USA [31]. Initially, a single-center, two-part rising single- and multiple-dose study in healthy adults was performed to evaluate the pharmacokinetics and safety of intravenous posaconazole [32]. For the first part of the study, six cohorts covered a single-dose posaconazole range from 50 to 300 mg by 30 min peripheral infusion. Intravenous posaconazole showed a greater-than-dose-proportional increase in exposure, whereby Cmax values ranged from 0.31 to 2.84 mg/l for the 50- and 300-mg single-dose administration, respectively. Part 2 was terminated early due to unacceptable rates of infusion site reactions.
To expand the pharmacokinetic and safety profile of intravenous posaconazole, a two-part study was performed, one part classified as Phase Ib and the other as Phase III to bridge the new posaconazole intravenous solution to the previously approved posaconazole suspension [31]. The primary purpose of the Phase Ib trial was to identify the posaconazole dose that would attain an exposure target of 0.50–2.50 mg/l. A single-dose and two multiple-dose cohorts were established for the study, with the multiple dose cohorts used for evaluating the 200 or 300 mg once daily dose after a twice-daily loading dose on the first day. Subjects attaining steady-state exposure goals were 94 and 95% for the 200- and 300-mg dosing cohorts, respectively. Mean concentration average was 1.19 and 1.43 mg/l for the 200- and 300-mg dosing cohorts on day 14, respectively. Intravenous posaconazole demonstrated a similar safety profile to the oral suspension formulation. A 300-mg QD dose was recommended for the Phase III study.
The pharmacokinetics and safety of the posaconazole intravenous formulations have also been investigated [33]. Patients with AML, myelodysplastic syndrome or HSCT were enrolled into a study that further examined the posaconazole intravenous formulation [34]. Posaconazole was intravenously administered 300 mg twice daily on day 1 and 300 mg once daily for 4–13 days afterward. Then, they were switched to posaconazole oral suspension 600 or 800 mg in divided doses for up to 23 days for a total treatment period of 28 days. The intravenous formulation resulted in higher trough concentrations of posaconazole than either of the dosages of oral suspension. Thus, this result shows that the posaconazole intravenous solution can be dosed at a level to reach satisfactory exposure for the treatment or prophylaxis of fungal infections.
Safety & tolerability
In general, posaconazole has a very good safety and tolerability profile [35,36]. Courtney et al. demonstrated the tolerability of oral suspension posaconazole in doses up to 400 mg twice daily in a Phase I study in healthy subjects [37]. Adverse effects were mild, such as fatigue and dry mouth. In the later phase clinical trials, posaconazole was also particularly well tolerated. The main side effects experienced by the participants were gastrointestinal distress (nausea, vomiting and diarrhea), neutropenia and elevated liver enzymes [34,38,39]. Patients suffering from mucositis, diarrhea or in the early post-transplant period in hematopoietic stem cell transplant therapy had reduced posaconazole levels when administered the oral suspension solution [19]. Overall, posaconazole has favorable safety profile compared with other currently approved systemic triazole antifungals (Table 1) [8].
Table 1.
Comparison of triazole adverse events.
| Fluconazole | Itraconazole | Voriconazole | Posaconazole | |
|---|---|---|---|---|
| Gastrointestinal distress | + | ++ | − | + |
| Liver dysfunction | + | ++ | ++ | − |
| Dermatological toxicity | + | + | + | − |
| Ocular toxicity | − | − | ++ | − |
Other than infusion site reactions, the intravenous formulation of posaconazole had few additional adverse effects. Infusion site reactions, specifically thrombophlebitis, were clinically acceptable at 30 min compared with 90 min for single-dose peripheral administration [32]. However, a decrease in infusion time from 90 to 30 min did not reduce infusion site reactions for multiple-dose administrations. It is recommended that infusion be performed by central line when multiple-dose posaconazole administration is necessary.
Early pooled analysis of 18 controlled studies in healthy volunteers and patients receiving posaconazole oral suspension solution indicated low potential to prolong the corrected QT (QTc) interval, among other side effects [40]. An initial report identified minimal safety concerns regarding elevated hepatic function tests and QTc prolongation for the posaconazole delayed-release oral tablets [29]. However, an additional study has shown that the oral delayed-release tablet increases hepatic enzyme levels in addition to prolonging the QTc interval [41]. As such, patients need to be monitored of their hepatic enzyme levels and QTc intervals.
Drug interactions
The other triazole antifungals have numerous drug–drug interactions because they inhibit the p-glycoprotein transporter in addition to CYP P450 enzymes, thus resulting in increased concentration of other drugs [42]. However, posaconazole and fluconazole are less potent inhibitors than voriconazole and itraconazole. Posaconazole inhibits CYP3A4 and p-glycoprotein, whereas other triazoles may also affect CYP2C9 and CYP2C19 [22]. This has important implications for therapy selection. In the GVHD patient population, immunosuppressive drugs, cyclosporine and tacrolimus, are commonly used; as they are CYP 3A4 substrates, posaconazole will likely increase their plasma concentration, potentially resulting in toxicity (Figure 2). The coadministration of posaconazole with several CYP3A4 substrates is contraindicated, including substrates known to prolong the QTc interval (i.e., terfenadine, cisapride), in addition to HMG-CoA reductase inhibitors (i.e., statins) and ergot alkaloids [16].
Figure 2.
Key patient populations with posaconazole drug interactions.
Reduced posaconazole oral suspension exposure is noted with the concurrent use of metoclopramide, phenytoin or rifampin, and the H2 ranitidine [19]. Because the posaconazole oral suspension formulation is more readily absorbed at a lower pH, proton pump inhibitors and cimetidine have been shown to decrease the area under the curve of posaconazole [7,43]. Other histamine H2 receptor antagonists and antacids had no effect on the area under the curve.
In contrast to the oral suspension formulation, the absorption profile of the delayed-release posaconazole tablet is not affected by gastric acidity or motility. To investigate, healthy volunteers were randomized to groups to receive a single 400-mg dose of the delayed-release posaconazole tablet alone; with metoclopramide to affect gastric motility; or with an antacid (aluminum and magnesium hydroxide), ranitidine or esomeprazole to affect gastric acidity in a crossover trial [44]. Exposure, Tmax and t1/2 were comparable whether posaconazole was administered alone or in combination with medications that affect gastric pH and motility. Additional studies demonstrated increased plasma concentrations associated with the delayed-release oral tablet as leukemia patients transitioned from posaconazole oral suspension to tablets had significantly higher posaconazole concentrations (median, suspension 0.75 mg/l, tablet 1.91 mg/l without clinically relevant hepatotoxicity) [45].
Use in special populations
Posaconazole pharmacokinetics are comparable regardless of gender and are not significantly affected by ethnicity [16]. Furthermore, posaconazole pharmacokinetics do not differ significantly with age. Posaconazole is designated pregnancy category C, such that no adequate clinical studies have examined this population [16]. Posaconazole administration has led to skeletal malformations in rats at relative concentrations lower than human therapeutic dosing. Furthermore, posaconazole administration produced higher rates of bone resorption to occur in rabbits, with higher dosages causing a reduction in body weight gain and a reduction in liter size in females. Preclinical animal models also suggest that posaconazole may excrete into breast milk of lactating females. Prophylaxis studies indicate that mean steady-state posaconazole average concentration is consistent in the pediatric population with that of adults [16].
For prophylaxis of candidiasis, posaconazole has been indicated in several clinical contexts in pediatric patients [46]. For allogenic HSCT, posaconazole oral suspension (200 mg TID) is recommended for patients greater than grade II GVHD who are at least 13 years of age. Posaconazole oral suspension (200 mg TID) is recommended for AML and recurrent leukemia patients ages 13 and older after the last dose of chemotherapy until neutrophil recovery. Delayed-release posaconazole tablets are now indicated for patients 13 years or older. However, the use of posaconazole intravenous injection is not recommended for patients under the age of 18 due to preclinical safety concerns [16].
No posaconazole pharmacokinetic effects have been seen in patients administered 400 mg single-dose posaconazole oral suspension with mild-to-moderate renal impairment; thus, no further dose adjustment requirements are recommended for patients with mild-to-moderate renal impairment [16]. However, close monitoring for breakthrough IFI is recommended for patients with severe renal impairment (eGFR: <20 ml/min). Similar studies have not been conducted with the oral delayed-release posaconazole tablets; however, no dose adjustments are recommended for mild-to-moderate renal impairment. Posaconazole intravenous injection should be avoided in patients with moderate or severe renal impairment as excess accumulation of the intravenous vehicle, sulfobutylether-β-cyclodextrin, may be problematic. Accordingly, the serum creatinine levels of these patients should be closely monitored [16].
No posaconazole oral suspension dose adjustments are recommended for patients with mild-to-severe hepatic impairment [16]. Mean area under the curve values of a single dose of 400-mg posaconazole oral suspension range from 21 to 43% higher for patients with hepatic impairments. Respective Cmax and t1/2 values for patients with hepatic impairments vary compared with normal individuals. Similar studies have not been performed with the oral delayed-release tablets or intravenous injection; however, no dose adjustments are recommended for either of these formulations in patients with mild-to-severe hepatic impairment [16].
Immunosuppressant drugs, specifically cyclosporine and tacrolimus, need to have levels monitored when given in combination with posaconazole [47]. Although concurrent use of posaconazole and sirolimus is contraindicated by the manufacturer, a recent study has shown that posaconazole can be given in combination with sirolimus in a liver solid organ transplant patient. [48]. It is imperative that sirolimus concentration levels are monitored when given in conjunction with posaconazole.
Clinical efficacy studies
Phase II clinical studies
Two main Phase II trials were completed in the clinical development program of posaconazole. For the indication of IFI prophylaxis, study P018893 was designed to establish the dose by comparing the pharmacokinetic and pharmacodynamic properties of different posaconazole dosing strategies [16,49,50]. This study focused on the treatment of patients with azole-refractory IFIs or patients experiencing febrile neutropenia and requiring empiric antifungal therapy. Ninety-eight patients were randomized to receive posaconazole as an oral suspension between 800 and 1600 mg every day divided into different dosing schedules. Patients continued to receive the drug for a maximum of 6 months or until febrile neutropenia resolved. Posaconazole was well tolerated and was comparably effective at all dosing schedules in the treatment of the fungal infections. These results agreed with previous pharmacokinetic data, suggesting that the absorption of posaconazole is saturable and therefore limited. Beyond a certain threshold, increasing the dose does not affect posaconazole exposure preventing additional therapeutic benefit and worsened adverse effects.
For the indication of oropharyngeal candidiasis (OPC) treatment, study C/I96-209 was used to elucidate the dose while analyzing the pharmacologic profile [16,51]. This trial involved 463 HIV-infected patients with OPC. The goal of this trial was to compare treatment using various doses of posaconazole with an established dose of fluconazole, which is routinely used for this indication. Patients were randomized to receive either of the agents with posaconazole at the doses of interest and fluconazole at 200 mg once followed by 100 mg daily. Treatment was continued for a total of 14 days. Posaconazole was well tolerated and showed similar efficacy at the dose levels and compared with fluconazole. On the basis of these data, posaconazole 100 mg once daily dose was selected.
Phase III clinical studies
IFI prophylaxis
The approval of posaconazole by the FDA and the EMA was based on several pivotal Phase III trials as shown in Table 2. Ullmann et al. compared the efficacy of posaconazole versus fluconazole in preventing IFIs in a multicenter, randomized, double-blind trial [39]. Six hundred patients with HSCT and GVHD or patients who were being treated with highly immunosuppressive agents were randomized to receive either agent for 16 weeks. One cohort received posaconazole as a 200-mg oral suspension three-times daily with placebo capsules once daily, whereas the second cohort received fluconazole as a 400-mg encapsulated tablet once daily with placebo oral suspension three-times daily. Posaconazole was shown to be noninferior to fluconazole in the prevention of IFIs (5.3 vs 9.0%) and was shown to be superior in the treatment of invasive aspergillosis specifically (2.3 vs 7.0%). Between both groups, adverse events were similar (36 vs 38%, respectively) as was the rate of discontinuation due to adverse events (34 vs 38%, respectively).
Table 2.
Pivotal Phase III Trials.
| Indication | Population | Primary Endpoint | Dose/duration | Efficacy | Adverse events | Ref. |
|---|---|---|---|---|---|---|
| IFI prophylaxis | 600 patients with HSCT and GVHD | Incidence of proven or probable IFI | • POS 200 mg TID + placebo capsules OR • FLU 400 mg QD + placebo suspension |
(Incidence of IFI) POS: 5.3% FLU: 9.0% |
POS: 36% FLU: 38% |
[39] |
| IFI prophylaxis | 602 patients undergoing chemotherapy for AML or MDS | Incidence of proven or probable IFI | • POS 200 mg TID OR • FLU 400 mg QD or ITR 200 mg BID |
(Incidence of IFI) POS: 2% FLU/ITR: 8% |
POS: 52% FLU/ITR: 59% |
[52] |
| IFI prophylaxis | 213 patients with AML, MDS, or HSCT | PK of POS IV | POS 300 mg IV BID × 1, 300 mg QD × 4-13 days, then POS 600-800 mg in divided doses QD for up to 23 days |
(Clinical failure: death, IFI, Discontinuation) POS: 31% |
POS: 99%† | [33,34] |
| OPC treatment | 350 patients with HIV infection and OPC | Clinical success (cure or improvement after 28 days) | • POS 200 mg × 1, 100 mg × 13 days, OR • FLU 200 mg ×1, 100 mg × 13 days |
(Clinical success) POS: 91.7% FLU: 92.5% |
POS: 64% FLU: 68% |
[53] |
| OPC treatment | 199 patients with HIV and azole-refractory OPC or EC | Clinical success (cure or improvement after 28 days) | • POS 400 mg BID × 3 days, POS 400 mg QD × 25 days OR • POS 400 mg BID × 28 days |
(Clinical success) Group 1: 75.3% Group 2: 74.7% |
Combined: 49% | [38] |
| IFI treatment | 330 patients with IFIs resistant to standard therapy | Global response status (clinical cure or improvement) | POS 200 mg QID while hospitalized, then 400 mg BID up to 1 year | (Global response status) 50% | POS: 38%† | [17] |
All studies involved the posaconazole suspension unless otherwise noted.
Adverse event calculation was pooled from this study and additional one(s).
AML: Acute myeloid leukemia; BID: Twice daily; EC: Esophageal candidiasis; FLU: Fluconazole; GVHD: Graft vs host disease; HIV: Human immunodeficiency virus; HSCT: Hematopoietic stem cell graft; IFI: Invasive fungal infection; ITR: Itraconazole; IV: Intravenous; MDS: Myelodysplastic syndromes; OPC: Oropharyngeal candidiasis; POS: Posaconazole; QD: Once daily; QID: Four times a day; TID: Thrice daily.
Cornely conducted a study comparing the efficacy of posaconazole with fluconazole and itraconazole for IFI prophylaxis in patients treated for cancer who were projected to experience neutropenia [52]. Six hundred and two patients were randomized and received 200-mg posaconazole three-times daily as an oral suspension or one of the alternate azoles for up to 12 weeks during their rounds of chemotherapy; the choice of the alternate azole (400-mg fluconazole once daily or itraconazole 200 mg twice daily) was made by each investigator. Patients were monitored for fungal infections by a blinded independent data review committee. Posaconazole was shown to be superior in preventing IFIs compared with the alternate azoles (2 vs 8%, respectively). In addition, the mean time to IFI was longer with posaconazole (41 vs 25 days, respectively). Finally, the posaconazole group experienced lower mortality during the treatment period (16 vs 22%, respectively). Adverse effects were comparable for patients taking each agent.
OPC treatment
The efficacy of posaconazole versus fluconazole for patients with HIV/AIDS in the treatment of OPC has been evaluated [53]. Three hundred and fifty patients were randomized and received 200-mg posaconazole or fluconazole oral suspension on the first day as the loading dose, followed by 100 mg every day of the same drug for a total of 14 days. Blinded evaluators investigated each patient for clinical success, which was specified as cure or improvement of OPC. At the end of the trial, clinical success was seen in both groups at similar rates (91.7% for posaconazole and 92.5% for fluconazole). Of the patients considered clinically successful 42 days after trial completion, 31.5% of the posaconazole patients relapsed compared with 38.2% of the fluconazole patients. Also at the 42-day follow-up, mycological eradication was greater in patients of the posaconazole arm (35.6 vs 24.2%, respectively). Adverse events were comparable in each group.
To investigate the role of posaconazole in azole-refractory OPC, Skriest analyzed patients infected with HIV with oropharyngeal or esophageal candidiasis resistant to standard azole treatments of fluconazole and itraconazole [38]. Patients received 400 mg twice daily for 3 days followed by 400 mg daily for 25 days or 400 mg twice daily for 28 days. Seventy five percent of the 176 patients experienced clinical success, defined as cure or improvement. Treatment success was relatively invariable between the groups taking different regimens. Four weeks after the last dose, 74% of 80 patients who had experienced a clinical response had relapsed (80% of patients on the daily dosing regimen, 68% of patients on the twice daily dosing regimen).
Treatment of other approved fungal infections
Additional studies were conducted to support the approval of posaconazole for the treatment of a broader range of fungal infections in Europe [17]. Three hundred and thirty patients with varying fungal infections, which were resistant to or intolerant of standard therapy, including other azoles, echinocandins and amphotericin, were administered posaconazole 200 mg four-times daily while hospitalized and 400 mg twice daily on an outpatient basis for up to 1 year. Overall 50% of patients responded to posaconazole. The investigators analyzed clinical success rates for each fungal infection individually, with the following infections showing the best efficacy with posaconazole: aspergillosis (42%), fusariosis (39%), chromoblastomycosis or mycetoma (82%), coccidioidomycosis (69%).
Catanzaro et al. investigated the role of posaconazole in treating patients with coccidioidomycosis [54]. In 20 treatment-naïve patients with nonmeningeal disseminated or chronic pulmonary coccidioidomycosis, the study investigators administered 400 mg of posaconazole daily. Eighty five percent of the patients experienced a response, defined as a 50% or greater reduction in the mycoses study group score from baseline. Stevens et al. further analyzed the use of posaconazole in the treatment of treatment-resistant coccidioidomycosis [55]. Fifteen patients with refractory coccidioidomycosis were recruited for this trial. Patients were administered 800 mg of posaconazole in divided doses daily for up to a year. At the end of the treatment period, 73% of the patients responded to treatment: four showed a complete eradication of disease and seven showed a partial resolution. Posaconazole was well tolerated in both trials.
Off-label indications
Posaconazole has also been studied for infections that are rarer, often as salvage therapy in smaller, less controlled trials. While clinical experience with posaconazole is limited with these indications, there is evidence to support utilization. van Burik et al. retrospectively analyzed the use of posaconazole in mucormycosis [56]. Information from 91 patients was included in this study. Patients were treated with posaconazole, 80% for at least 30 days. Sixty percent of patients responded to treatment. Restrepo et al. investigated posaconazole's utility as salvage treatment of histoplasmosis [57]. Six patients who had failed other treatments were placed on posaconazole 800 mg per day in divided doses. All patients responded to treatment within a month as demonstrated by clinical improvement.
Posaconazole has also demonstrated good activity in the treatment of fungal infection of the CNS. Pitisuttithum et al. studied 39 patients with CNS fungal infections [58]. Most of these patients had refractory disease (95%) or an HIV infection (74%). The infections were caused by a variety of fungi including Cryptococcus, Aspergillus and a variety of rarer fungi (Pseudallescheria boydii, C. immitis, Histoplasma capsulatum, Ramichloridium mackenziei, Apophysomyces elegans and Basidiomycetes sp.). Patients were administered the posaconazole oral suspension at 800 mg per day in divided doses for at least a month and up to 1 year. Clinical responses were seen in 48% of the patients infected with Cryptococcus and in 50% of the patients with the other fungal infections. This trial provides evidence that posaconazole can be useful as salvage therapy for a wider variety of fungal infections and in the CNS.
Postmarketing surveillance
The FDA required pediatric studies to be completed secondary to the approval of posaconazole. Completing one study that addressed the use of posaconazole in this population, Döring et al. compared itraconazole, voriconazole and posaconazole in pediatric patients with allogenic HSCT as an oral antifungal prophylactic agent [59]. One hundred and fifty patients between the age of 7 months and 18 years were divided evenly into three cohorts to receive 5 mg/kg twice daily itraconazole, 100 mg twice daily (or 200 mg twice daily if body weight >40 kg) voriconazole or 200 mg thrice daily posaconazole. Patients also received antiviral and antibacterial prophylaxis for other possible infections. At the end of the observation period (maximum of 220 days after HSCT), there were no deaths due to IFIs or even any cases of ‘proven’ or ‘probable’ IFIs in the patients. There were several ‘possible’ infections: two in the itraconazole group, three in the voriconazole group and none in the posaconazole group. The differences between ‘proven’, ‘probable’, and ‘possible’ were analyzed secondary to the National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria. Ultimately, the three agents were considered noninferior to each other in terms of efficacy. Comparing the safety profiles, the adverse effect ratio was comparable between all three groups, with increased liver enzymes representing one of the most common events.
Regulatory affairs
Posaconazole has been approved to treat fungal infections in the USA and EU with the trade name Noxafil®. Posaconazole is marketed as Posanol in Canada [16]. Labeled indications in the USA and Europe are listed in Text Boxes 1 & 2. The FDA approved posaconazole for prophylaxis of the Aspergillus and Candida IFIs in immunocompromised patients of at least 13 years of age in September 2006, and it was approved for the treatment of OPC, including those cases refractory to other azole antifungals, in October 2006 [60,61]. For both indications, posaconazole was approved as a 40 mg/ml oral suspension. Posaconazole was subsequently approved as a delayed-release tablet in addition to an intravenous solution for the indication of invasive Aspergillus and Candida infection prophylaxis in November 2013 and March 2014, respectively [62,63]. In the USA, posaconazole's patent is scheduled to expire in 2019.
The EMA has approved posaconazole as an oral suspension and as a gastro-resistant tablet for treatment of a wider variety of IFIs (aspergillosis, fusariosis, chromoblastomycosis and mycetoma, coccidioidomycosis, and OPC) including those cases refractory to standard antifungal therapy. In addition, posaconazole is approved for the prevention of IFIs in patients who receive immunosuppressive therapy after receiving HSCT or chemotherapy for AML or any other myelodysplastic syndrome [64]. For all of these indications, posaconazole was approved as an oral suspension and as a gastro-resistant tablet in October 2005.
The FDA specified a number of post-marketing commitments from posaconazole. For the IFI indication, the FDA required a pediatric study in patients aged 0 months to 12 years based on the Pediatric Research Equity Act. For the OPC indication, a pediatric study is also required but in patients aged 0 months to 16 years. In addition, the FDA requested a study among patients receiving IFI prophylaxis at risk for low absorption to explore alternate dosing strategies and the utility of therapeutic drug monitoring (TDM) [60,61]. These studies were originally required by 2011. Since then, the FDA has granted a deferral extension, and the studies will need to be completed by 2019 with the exception of the study involving alternate dosing strategies and TDM. The FDA has released the company from the obligation to complete that study [65]. For the new formulations, pediatric studies are also required and will need to be completed within the next 10 years (Figure 3) [62,63].
Figure 3. Regulatory history of posaconazole.
Adapted from references [49,51,62–65].
DRT: Delayed release tablet; EMA: European medicines agency; FDA: Food and drug administration; IFI: Invasive fungal infections; IV: Intravenous; OPC: Oropharyngeal candidiasis; PREA: Pediatric Research Equity Act.
Conclusion
Posaconazole is a useful antifungal agent. This drug has shown efficacy in the role of IFI prophylaxis, the treatment of OPC and salvage treatment for refractory fungal infections. Overall, posaconazole has a favorable safety and tolerability profile. However, the initially approved oral suspension formulation has a wide interpatient variability with respect to absorption. The systemic availability of the oral suspension can be improved through administration with a high-fat meal, nutritional supplement or acidic beverage in addition to divided daily doses. The suspension has shown a reduction in systemic availability when administered with proton pump inhibitors in addition to pharmacotherapeutics that increase gastric motility. Both diarrhea and mucositis have also been shown to reduce the bioavailability of posaconazole.
The administration of multiple daily doses with a full meal or nutritional supplement can be difficult for patients who have difficulties in swallowing. As such, the recent approval of a delayed-release tablet that requires administration only once daily and does not appear to be clinically affected by food, alterations in gastric pH or motility represents a new option for the prophylaxis of IFI. As it achieves consistently higher average serum concentrations, the delayed-release tablet is a better option than suspension for both prophylaxis and treatment of IFI. An intravenous formulation has been recently developed and approved, which provides even greater access to patient populations.
Five-year view
Unlike in the USA, posaconazole has not been approved in the EU as a delayed-release tablet or as a solution for intravenous administration. However, Merck has received ‘positive opinions’ from the Committee for Medicinal Products for Human Use inside the EMA [66,67]. This signifies that European Commission will review the new drug formulations further and likely approve it in the near future, following the precedent set by the FDA.
Posaconazole has been increasingly studied for other fungal infections beyond those infections for which it was originally approved by the major regulatory bodies. Elewski et al. investigated posaconazole in the treatment of toenail onychomycosis and showed improved cure rates compared with the commonly used antifungal agent terbinafine [68]. Posaconazole has also shown some potential for use in Chagas disease and chronic granulomatous disease [69,70], which is a significant finding as both of these conditions currently have limited therapeutic options. With further investigation, posaconazole may be readily used for these indications in the future.
Furthermore, the effect of posaconazole in pediatric patients will become clearer as the Pediatric Research Equity Act required studies are completed in the next few years. Favorable outcomes are expected given the positive safety data of the posaconazole oral suspension in pediatric patients. Thus, the use of the posaconazole formulations will likely increase over time in this subset of patients.
TDM has been increasingly suggested for posaconazole oral suspension therapy, as interpatient concentration variability is high as is the risk for underexposure. Prophylactic target posaconazole concentrations range from at least 0.5 to 0.7 mg/l. As such, a general consensus has yet to be determined. A recent report from Hoenigl et al. suggests the need for TDM because personal on-site education of low posaconazole plasma concentrations can lead to >40% increase in sufficient plasma concentrations [71]. These findings are consistent with a previously reports that found that cases of breakthrough infections were all associated with low posaconazole plasma concentrations [19,21,72]. Bryant et al. (2011) was one of the first studies to report on TDM of prophylactic posaconazole in patients with AML or myelodysplastic syndrome [73]. In this study, greater than 7% of patients did not reach the lower target limit of 0.7 mg/l. Of the 21 patients, three developed ‘proven’ or ‘possible’ fungal infections; all three patients having posaconazole concentrations <0.5 mg/l. Because posaconazole is liable to interact with many other pharmaceutical agents, several sources recommend TDM for posaconazole during the duration of multimodal therapy. This may be particularly useful in cases where posaconazole is used with an agent with whom it has a drug–drug interaction, which is common, as many agents used for cancer, HIV and transplants are CYP3A4 substrates, for example, tacrolimus and cyclosporine. With time, a consensus will be reached concerning TDM, the appropriate concentration goals for effective posaconazole treatment.
Also on the horizon, isavuconazonium is a novel triazole agent, which has recently been approved by the FDA for the treatment of aspergillosis and mucormycosis [74]. Preliminary data from Phase III studies suggest that it may have similar efficacy as voriconazole with fewer adverse effects in treating IFIs [75,76]. Isavuconazonium had a relatively quick regulatory timeline because the prognosis of mucormycosis is so poor at the moment that the FDA labeled isavuconazonium as an orphan drug and as a Qualified Infectious Disease Product for expedited review [77]. Following the approval of isavuconazonium, it will likely influence both the European and the US markets. It will present a good option for mucormycosis and may, in fact, be used instead of posaconazole for some patients suffering from invasive aspergillosis.
Expert commentary
Posaconazole has been recognized by the guidelines for quite a few therapeutic uses beyond its FDA approved indications. The Infectious Disease Society of America has included them in their guidelines for aspergillosis, cryptococcosis, candidiasis, skin and soft tissue infections, and antimicrobial prophylaxis in neutropenia [3–5,78,79]. The Infectious Disease Society of America guidelines on skin and soft tissue infections specifically recommend posaconazole for the treatment of aspergillus, mucormycosis and fusariosis. Posaconazole is also recommended for the general prophylaxis and treatment of IFIs in the respective guidelines for patients affected by HIV, hematopoietic cell transplantation and cancer (Figure 4) [80–82].
Figure 4. Posaconazole therapy algorithm.
Unless otherwise noted, listed dosages are for the posaconazole oral suspension.
All other indications shown are off-label uses.
Adapted from references [5,16,78,79].
†Denotes FDA approval for the posaconazole oral suspension.
‡Denotes FDA approval for all available posaconazole formulations.
b.i.d.: Twice daily; DRT: Delayed-release tablet; iv.: Intravenous solution; q.d.: Once daily; q.i.d.: Four-times a day; SUS: Suspension; t.i.d.: Thrice daily.
Numerous favorable pharmacoeconomic analyses have been published for posaconazole [83–86]. In these studies, the usage of posaconazole was primarily studied for the prevention of IFIs secondary to HSCT or secondary to febrile neutropenia in AML or myelodysplastic syndrome. Lyseng-Williamson (2011) specifically suggested that posaconazole was the dominant option compared with standard antifungal therapy in many markets if not simply a cost-effective choice [86]. Compared with the other azole antifungals, posaconazole occupies a unique place in therapy. It can be used in fungal infections resistant to fluconazole and itraconazole. Posaconazole can also be used for prophylaxis of a wider variety of fungal infections than voriconazole. As one of the newest agents in a long-standing class of pharmaceuticals, it will be some time before prescribing healthcare providers accumulate enough clinical experience with this agent and become accustomed to it. However, the addition of the delayed-release tablet and the intravenous formulation, as options will certainly accelerate this process as it allows for more convenient dosing.
Box 1. Labeled FDA Indications for use.
Injection, delayed-release tablets and oral suspension
Prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as HSCT recipients with graft versus host disease or those with hematologic malignancies with prolonged neutropenia from chemotherapy
Oral suspension
Treatment of oropharyngeal candidiasis, including oropharyngeal candidiasis refractory to itraconazole and/or fluconazole
Box 2. Labeled EMA indications for use.
Fungal infections in adults
Invasive aspergillosis in patients with disease that is refractory to amphotericin B or itraconazole or in patients who are intolerant of these medicinal products
Fusariosis in patients with disease that is refractory to amphotericin B or in patients who are intolerant of amphotericin B
Chromoblastomycosis and mycetoma in patients with disease that is refractory to itraconazole or in patients who are intolerant of itraconazole
Coccidioidomycosis in patients with disease that is refractory to amphotericin B, itraconazole or fluconazole or in patients who are intolerant of these medicinal products
OPC: as first-line therapy in patients who have severe disease or are immunocompromised, in whom response to topical therapy is expected to be poor
Refractoriness is defined as progression of infection or failure to improve after a minimum of 7 days of prior therapeutic doses of effective antifungal therapy
Prophylaxis of IFIs in the following patients:
Patients receiving remission-induction chemotherapy for acute myelogenous leukemia or myelodysplastic syndromes expected to result in prolonged neutropenia and who are at high risk of developing IFIs
Hematopoietic-stem-cell-transplant recipients who are undergoing high-dose immunosuppressive therapy for graft-versus-host disease and who are at high risk of developing IFIs
Key issues.
Invasive fungal infections (IFIs) are a significant cause of morbidity and mortality for immunocompromised patients; unfortunately, the clinical usefulness of many antifungal agents is limited by safety and tolerability.
Posaconazole is a relatively safe and well-tolerated broad-spectrum azole antifungal agent.
Newer posaconazole formulations, oral tablet and intravenous, increase its availability to a larger patient population.
Posaconazole is a cost–effective and dominant option compared with standard antifungal therapy in many markets.
Posaconazole continues to be studied for additional fungal infections beyond original approval, including toenail onychomycosis, Chagas disease and chronic granulomatous disease.
Acknowledgments
J Moore and J Healy were supported by National Institutes of Health Postdoctoral training grant no. T32GM008562. W Kraft's employer has received research grant support from Merck for clinical trials in which W Kraft was the Principal Investigator.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43(1):25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 2.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49(9):3640–5. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 4.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the infectious diseases society of America. Clin Infect Dis. 2009;48(5):503–35. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the infectious diseases society of America. Clin Infect Dis. 2008;46(3):327–60. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Williams K. Posaconazole salvage treatment for invasive fungal infection. Mycopathologia. 2014;178(3-4):259–65. doi: 10.1007/s11046-014-9792-y. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Theuretzbacher U, Clancy CJ, et al. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clin Pharmacokinet. 2010;49(6):379–96. doi: 10.2165/11319340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8••.Chau MM, Kong DC, van Hal SJ, et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy 2014. Int Med J. 2014;44(12b):1364–88. doi: 10.1111/imj.12600. [Clinical guidelines for optimized antifungal regimens with safety and toxicity profiles.] [DOI] [PubMed] [Google Scholar]
- 9.Marty FM, Cosimi LA, Baden LR. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N Eng J Med. 2004;350(9):950–2. doi: 10.1056/NEJM200402263500923. [DOI] [PubMed] [Google Scholar]
- 10.Rogers TR. Treatment of zygomycosis: current and new options. J Antimicrob Chemother. 2008;61(Suppl 1):i35–40. doi: 10.1093/jac/dkm429. [DOI] [PubMed] [Google Scholar]
- 11.Torres HA, Hachem RY, Chemaly RF, et al. Posaconazole: a broad-spectrum triazole antifungal. Lancet Infect Dis. 2005;5(12):775–85. doi: 10.1016/S1473-3099(05)70297-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Kumari P, Laughlin M, et al. Use of high-performance liquid chromatographic and microbiological analyses for evaluating the presence or absence of active metabolites of the antifungal posaconazole in human plasma. J Chromat A. 2003;987(1-2):243–8. doi: 10.1016/s0021-9673(02)01599-6. [DOI] [PubMed] [Google Scholar]
- 13.Nagappan V, Deresinski S. Reviews of anti-infective agents: posaconazole: a broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007;45(12):1610–17. doi: 10.1086/523576. [DOI] [PubMed] [Google Scholar]
- 14.Gubbins PO, Krishna G, Sansone-Parsons A, et al. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob Agents Chemother. 2006;50(6):1993–9. doi: 10.1128/AAC.00157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieter P, Flannery B, Musick T, et al. Disposition of posaconazole following single-dose oral administration in healthy subjects. Antimicrob Agents Chemother. 2004;48(9):3543–51. doi: 10.1128/AAC.48.9.3543-3551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Product monograph – Noxafil (Posaconazole) Merck & Co. Inc.; Whitehouse Station, NJ: 2014. [12 February 2015]. Available from: www.merck.com/product/usa/pi_circulars/n/noxafil/noxafil_pi.pdf. [Google Scholar]
- 17.Noxafil:EPAR-Scientific Discussion. Agency EM. European Medicines Agency; London, UK: 2005. [10 October 2014]. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000610/WC500037781.pdf. [Google Scholar]
- 18.Mavridou E, Bruggemann RJ, Melchers WJ, et al. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob Agents Chemother. 2010;54(2):860–5. doi: 10.1128/AAC.00931-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolton MJ, Ray JE, Chen SC, et al. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob Agents Chemother. 2012;56(11):5503–10. doi: 10.1128/AAC.00802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields RK, Clancy CJ, Vadnerkar A, et al. Posaconazole serum concentrations among cardiothoracic transplant recipients: factors impacting trough levels and correlation with clinical response to therapy. Antimicrob Agents Chemother. 2011;55(3):1308–11. doi: 10.1128/AAC.01325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang SH, Colangelo PM, Gobburu JV. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther. 2010;88(1):115–19. doi: 10.1038/clpt.2010.64. [DOI] [PubMed] [Google Scholar]
- 22.Lipp HP. Clinical pharmacodynamics and pharmacokinetics of the antifungal extended-spectrum triazole posaconazole: an overview. Br J Clin Pharma. 2010;70(4):471–80. doi: 10.1111/j.1365-2125.2010.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezzet F, Wexler D, Courtney R, et al. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin Pharmacokinet. 2005;44(2):211–20. doi: 10.2165/00003088-200544020-00006. [DOI] [PubMed] [Google Scholar]
- 24.Krishna G, Moton A, Ma L, et al. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother. 2009;53(3):958–66. doi: 10.1128/AAC.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishna G, Ma L, Vickery D, et al. Effect of varying amounts of a liquid nutritional supplement on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob Agents Chemother. 2009;53(11):4749–52. doi: 10.1128/AAC.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walravens J, Brouwers J, Spriet I, et al. Effect of pH and comedication on gastrointestinal absorption of posaconazole: monitoring of intraluminal and plasma drug concentrations. Clin Pharmacokinet. 2011;50(11):725–34. doi: 10.2165/11592630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27••.Krishna G, Ma L, Martinho M, et al. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J Antimicrob Chemother. 2012;67(11):2725–30. doi: 10.1093/jac/dks268. [Clinical studies examining the pharmacokinetics and safety of the posaconazole oral tablet formulation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishna G, Ma L, Martinho M, et al. Single-dose Phase I study to evaluate the pharmacokinetics of posaconazole in new tablet and capsule formulations relative to oral suspension. Antimicrob Agents Chemother. 2012;56(8):4196–201. doi: 10.1128/AAC.00222-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duarte RF, Lopez-Jimenez J, Cornely OA, et al. Phase Ib study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob Agents Chemother. 2014;58(10):5758–65. doi: 10.1128/AAC.03050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornely OA, Duarte RF, Haider S, et al. Phase III pharmacokinetics (PK) and safety study of posaconazole (POS) tablet in patients at risk for invasive fungal infection.. 23rd European congress of clinical microbiology and infectious diseases; Berlin, Germany. 2013. [Google Scholar]
- 31••.Maertens J, Cornely OA, Ullmann AJ, et al. Phase IB study of the pharmacokinetics and safety of posaconazole intravenous solution in patients at risk for invasive fungal disease. Antimicrob Agents Chemother. 2014;58(7):3610–17. doi: 10.1128/AAC.02686-13. [Clinical studies examining the pharmacokinetics and safety of the posaconazole intravenous formulation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kersemaekers WM, van Iersel T, Nassander U, et al. Pharmacokinetics and safety study of posaconazole intravenous solution administered peripherally to healthy subjects. Antimicrob Agents Chemother. 2015;59(2):1246–51. doi: 10.1128/AAC.04223-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NDA 205-596 - Posaconazole Injection, Noxafil. FDA Center for Drug Evaluation and Research. FDA/Center for Drug Evaluation and Research; Washington, DC: 2014. [15 December 2014]. Available from: www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205596Orig1s000MedR.pdf. [Google Scholar]
- 34.Cornely OA, Haider S, Grigg A, et al. Phase III Pharmacokinetics (PK) and Safety Study of Posaconazole (POS) IV in Patients (Pts) at Risk for Invasive Fungal Infection (IFI).. Interscience conference of antimicrobial agents chemother; Denver, CO. 2013. [Google Scholar]
- 35.Chandrasekar P. Prophylaxis against Aspergillus is not perfect: problems and perils in stem cell transplantation. Med Mycol. 2009;47(Suppl 1):S349–54. doi: 10.1080/13693780802239105. [DOI] [PubMed] [Google Scholar]
- 36.Jacinto PL, Chandrasekar P. Safety of posaconazole. Expert Opin Drug Saf. 2013;12(2):265–74. doi: 10.1517/14740338.2013.765857. [DOI] [PubMed] [Google Scholar]
- 37.Courtney R, Pai S, Laughlin M, et al. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob Agents Chemother. 2003;47(9):2788–95. doi: 10.1128/AAC.47.9.2788-2795.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skiest DJ, Vazquez JA, Anstead GM, et al. Posaconazole for the treatment of azole-refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clin Infect Dis. 2007;44(4):607–14. doi: 10.1086/511039. [DOI] [PubMed] [Google Scholar]
- 39.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356(4):335–47. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 40.Moton A, Krishna G, Wang Z. Tolerability and safety profile of posaconazole: evaluation of 18 controlled studies in healthy volunteers. J Clin Pharma Ther. 2009;34(3):301–11. doi: 10.1111/j.1365-2710.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 41.Pettit NN, Han Z, delaCruz J, et al. Posaconazole (PCZ) Tablet Formulation Therapeutic Drug Monitoring (TDM) and Toxicity Analysis.. Interscience conference on antimicrob agents chemother; Washington, DC. 2014. [Google Scholar]
- 42.Neofytos D, Avdic E, Magiorakos AP. Clinical safety and tolerability issues in use of triazole derivatives in management of fungal infections. Drug Healthc Patient Saf. 2010;2:27–38. doi: 10.2147/dhps.s6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cojutti P, Candoni A, Simeone E, et al. Antifungal prophylaxis with posaconazole in patients with acute myeloid leukemia: dose intensification coupled with avoidance of proton pump inhibitors is beneficial in shortening time to effective concentrations. Antimicrob Agents Chemother. 2013;57(12):6081–4. doi: 10.1128/AAC.01586-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraft WK, Chang PS, van Iersel ML, et al. Posaconazole tablet pharmacokinetics: lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob Agents Chemother. 2014;58(7):4020–5. doi: 10.1128/AAC.02448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung DS, Tverdek FP, Kontoyiannis DP. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother. 2014;58(11):6993–5. doi: 10.1128/AAC.04035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hope WW, Castagnola E, Groll AH, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18(Suppl 7):38–52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 47.Sansone-Parsons A, Krishna G, Martinho M, et al. Effect of oral posaconazole on the pharmacokinetics of cyclosporine and tacrolimus. Pharmacotherapy. 2007;27(6):825–34. doi: 10.1592/phco.27.6.825. [DOI] [PubMed] [Google Scholar]
- 48.Dahlan R, Patel A, Haider S. Successful use of posaconazole to treat invasive cutaneous fungal infection in a liver transplant patient on sirolimus. Can J Infect Dis Med Microbiol. 2012;23(2):e44–7. doi: 10.1155/2012/272197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.NDA 22-003 – Prophylaxis of invasive aspergillus and candida infections medical review. FDA Center for Drug Evaluation and Research; Washington, DC: 2006. [8 October 2014]. Available from: www.accessdata.fda.gov/drugsatfda_docs/nda/2006/022003s000_Noxafil_MedR.pdf. [Google Scholar]
- 50.PK/PD Study of posaconazole for empiric treatment of invasive fungal infections in neutropenic patients or treatment of refractory invasive fungal infections (Study P01893) (COMPLETED) ClinicalTrials.gov; Washington, DC: 2013. [8 October 2014]. Available from: http://clinicaltrials.gov/show/NCT00034671. [Google Scholar]
- 51.NDA 22-027 – Invasive fungal infection and refractory invasive fungal infection medical review. FDA center for drug evaluation and research; Washington, DC: 2006. [8 October 2014]. Available from: www.accessdata.fda.gov/drugsatfda_docs/nda/2006/022027_noxafil_toc.cfm. [Google Scholar]
- 52.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Eng J Med. 2007;356(4):348–59. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 53.Vazquez JA. Posaconazole for the management of mucosal candidiasis. Future Microbiol. 2007;2(3):245–56. doi: 10.2217/17460913.2.3.245. [DOI] [PubMed] [Google Scholar]
- 54.Catanzaro A, Cloud GA, Stevens DA, et al. Safety, tolerance, and efficacy of posaconazole therapy in patients with nonmeningeal disseminated or chronic pulmonary coccidioidomycosis. Clin Infect Dis. 2007;45(5):562–8. doi: 10.1086/519937. [DOI] [PubMed] [Google Scholar]
- 55.Stevens DA, Rendon A, Gaona-Flores V, et al. Posaconazole therapy for chronic refractory coccidioidomycosis. Chest. 2007;132(3):952–8. doi: 10.1378/chest.07-0114. [DOI] [PubMed] [Google Scholar]
- 56.van Burik JA, Hare RS, Solomon HF, et al. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61–5. doi: 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 57.Restrepo A, Tobon A, Clark B, et al. Salvage treatment of histoplasmosis with posaconazole. J Inf. 2007;54(4):319–27. doi: 10.1016/j.jinf.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Pitisuttithum P, Negroni R, Graybill JR, et al. Activity of posaconazole in the treatment of central nervous system fungal infections. J Antimicrob Chemother. 2005;56(4):745–55. doi: 10.1093/jac/dki288. [DOI] [PubMed] [Google Scholar]
- 59.Doring M, Blume O, Haufe S, et al. Comparison of itraconazole, voriconazole, and posaconazole as oral antifungal prophylaxis in pediatric patients following allogeneic hematopoietic stem cell transplantation. Eur J Clin Microbiol Infect Dis. 2014;33(4):629–38. doi: 10.1007/s10096-013-1998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.NDA 22-003 Approval. FDA Center for Drug Evaluation and Research; Washington, DC: 2006. [15 October 2014]. Available from: www.accessdata.fda.gov/drugsatfda_docs/appletter/2006/022003ltr.pdf. [Google Scholar]
- 61.NDA 22-027 Approval. FDA Center for Drug Evaluation and Research; Washington, DC: 2006. [15 October 2014]. Available from: www.accessdata.fda.gov/drugsatfda_docs/appletter/2006/022027s000ltr.pdf. [Google Scholar]
- 62.NDA 205053 Approval. FDA center for drug evaluation and research; Washington, DC: 2013. [15 October 2014]. Available from: www.accessdata.fda.gov/drugsatfda_docs/appletter/2013/205053Orig1s000ltr.pdf. [Google Scholar]
- 63.NDA 205596 Approval. FDA Center for Drug Evaluation and Research; Washington, DC: 2014. [15 October 2014]. Available from: www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/205596Orig1s000ltr.pdf. [Google Scholar]
- 64.Summary of Product Characteristics. European Medicines Agency; London, UK: 2010. [15 October 2014]. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000610/WC500037784.pdf. [Google Scholar]
- 65.Postmarket Requirements and Commitments. FDA Center for Drug Evaluation and Research; Washington, DC: 2014. [15 October 2014]. Avaialbe from www.accessdata.fda.gov/scripts/cder/pmc/index.cfm. [Google Scholar]
- 66.CHMP issues positive opinion for tablet formulation of merck's NOXAFIL (posaconazole) Merck Newsroom; Whitehouse Station: 2014. [23 October 2014]. Available from: www.mercknewsroom.com/news-release/corporate-news/chmp-issues-positive-opinion-tablet-formulation-mercks-noxafilposaconaz. [Google Scholar]
- 67.CHMP issues positive opinion for intravenous (IV) Formulation of Merck's NOXAFIL® (posaconazole) Merck Newsroom; Whitehouse Station: 2014. [23 October 2014]. Available from: www.mercknewsroom.com/news-release/research-and-development-news/chmp-issues-positive-opinion-intravenous-iv-formulation-m. [Google Scholar]
- 68.Elewski B, Pollak R, Ashton S, et al. A randomized, placebo- and active-controlled, parallel-group, multicentre, investigator-blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br J Dermatol. 2012;166(2):389–98. doi: 10.1111/j.1365-2133.2011.10660.x. [DOI] [PubMed] [Google Scholar]
- 69•.Molina I, Gomez i Prat J, Salvador F, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370(20):1899–908. doi: 10.1056/NEJMoa1313122. [Examines potential benefit of posaconazole administration for Chagas disease.] [DOI] [PubMed] [Google Scholar]
- 70.Welzen ME, Bruggemann RJ, Van Den Berg JM, et al. A twice daily posaconazole dosing algorithm for children with chronic granulomatous disease. Pediatr Infect Dis J. 2011;30(9):794–7. doi: 10.1097/INF.0b013e3182195808. [DOI] [PubMed] [Google Scholar]
- 71.Hoenigl M, Duettmann W, Raggam RB, et al. Impact of structured personal on-site patient education on low posaconazole plasma concentrations in patients with haematological malignancies. Int J Antimicrob Agents. 2014;44(2):140–4. doi: 10.1016/j.ijantimicag.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 72.Hoenigl M, Raggam RB, Salzer HJ, et al. Posaconazole plasma concentrations and invasive mould infections in patients with haematological malignancies. Int J Antimicrob Agents. 2012;39(6):510–13. doi: 10.1016/j.ijantimicag.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Bryant AM, Slain D, Cumpston A, et al. A post-marketing evaluation of posaconazole plasma concentrations in neutropenic patients with haematological malignancy receiving posaconazole prophylaxis. Int J Antimicrob Agents. 2011;37(3):266–9. doi: 10.1016/j.ijantimicag.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 74.NDA 207501 Approval. FDA Center for Drug Evaluation and Research; Washington, DC: 2015. [17 March 2015]. Available from: www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/207501Orig1s000ltr.pdf. [Google Scholar]
- 75.New drug against invasive mold disease as effective as existing drugs with fewer adverse effects.. Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. 2014; [13 October 2014]. Available from: www.icaac.org/index.php/newsroom/92-icaac-2014/newsroom/310-new-drug-against-invasive-mold-disease-as-effective-as-existing-drugs-with-fewer-adverse-events. [Google Scholar]
- 76.Patterson T, Selleslag D, Mullane K, et al. A Phase III, randomized, double-blind, non-inferiority trial to evaluate efficacy and safety of isavuconazole versus voriconazole in patients with invasive mold disease (SECURE): Outcomes in Neutropenic Patients IDWeek. 2014 Abstract 1210. [Google Scholar]
- 77•.Isavuconazole. Basilea Pharmaceutica. Basilea Pharmaceutica Ltd; Switzerland: 2014. [15 March 2015]. Available from: www.basilea.com/Portfolio/Isavuconazole/ [Potential future direction of azole antifungal therapeutics.] [Google Scholar]
- 78.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–52. doi: 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- 80.Masur H, Brooks JT, Benson CA, et al. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(9):1308–11. doi: 10.1093/cid/ciu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.National comprehensive care network; Abington PA: 2014. [8 December 2014]. Clinical practice guidelines in oncology: Prevention and treatment of cancer-related infections. Available from: www.nccn.org/professionals/physician_gls/PDF/infections.pdf. [Google Scholar]
- 83.Al-Badriyeh D, Slavin M, Liew D, et al. Pharmacoeconomic evaluation of voriconazole versus posaconazole for antifungal prophylaxis in acute myeloid leukaemia. J Antimicrob Chemother. 2010;65(5):1052–61. doi: 10.1093/jac/dkq076. [DOI] [PubMed] [Google Scholar]
- 84.de la Camara R, Jarque I, Sanz MA, et al. Economic evaluation of posaconazole vs fluconazole in the prevention of invasive fungal infections in patients with GVHD following haematopoietic SCT. Bone Marrow Transplant. 2010;45(5):925–32. doi: 10.1038/bmt.2009.272. [DOI] [PubMed] [Google Scholar]
- 85.Heimann SM, Cornely OA, Vehreschild MJ, et al. Treatment cost development of patients undergoing remission induction chemotherapy: a pharmacoeconomic analysis before and after introduction of posaconazole prophylaxis. Mycoses. 2014;57(2):90–7. doi: 10.1111/myc.12105. [DOI] [PubMed] [Google Scholar]
- 86••.Lyseng-Williamson KA. Posaconazole: a pharmacoeconomic review of its use in the prophylaxis of invasive fungal disease in immunocompromised hosts. Pharmacoeconomics. 2011;29(3):251–68. doi: 10.2165/11206800-000000000-00000. [Provides a cost–benefit analysis of posaconazole to other antifungal regimens.] [DOI] [PubMed] [Google Scholar]