Abstract
Over the past 5 years our understanding of the seminal role of hypertension (HTN) in the pathogenesis of the cardiorenal metabolic syndrome (CRS) has evolved significantly (1). In the United States, more than 50 million people have blood pressure (BP) at or above 120/80mmHg (2). All components of the CRS, including the cardiovascular disease (CVD) and chronic kidney disease (CKD) components are inexorably linked to metabolic abnormalities and obesity (1–4). Recent statistics show that the prevalence of CKD in US adults ≥30 years is estimated to rise from the current 13.2% to 14.4% in 2020 and 16.7% in 2030 (3). One of the major drivers for all components of the CRS, including CKD, is obesity (body mass index ≥30 kg/m2) which now exceeds 30% in adults in the United States (4). Current estimates also show that many of those with HTN and CRS show some degree of systemic and cardiovascular (CV) insulin resistance, which supports a critical role in the pathogenesis of HTN and other components of the CRS (5,6). Several pathophysiologic factors, in addition to insulin resistance, participate in the link between HTN and CRS. These include inappropriate activation of the renin angiotensin aldosterone system (RAAS), oxidative stress, enhanced sympathetic nervous system activation and systemic and CV tissue inflammation. The goal of this review is to update recent literature with a focus more on the function of insulin resistance, obesity and RAAS-mediated oxidative stress on endothelial dysfunction and the pathogenesis of HTN.
Keywords: Obesity, Insulin resistance, Angiotensin II, Adipocyte, Hypertension
PATHOPHYSIOLOGY
HTN, in the setting of obesity and the CRS, is partly due to an expanded plasma volume resulting in an increase in cardiac output (5, 6). A second important factor in the pathogenesis of HTN coupled with the CRS and obesity, is increased peripheral vascular resistance (5, 6). Expanded plasma volume and hyperinsulinemia leads to increased renal filtration which affects renal sodium handling and promotes renal dysfunction characterized early by albuminuria (5, 6). The increase in vascular resistance impairs blood flow to skeletal muscle tissue which leads to more insulin resistance and hyperinsulinemia, creating a vicious cycle that promotes more volume expansion and renal hyperfiltration (7). In obesity-related hypertension, the expanded intravascular blood volume and increased peripheral vascular resistance, over time, lead to both concentric and eccentric left ventricular hypertrophy and impaired cardiac diastolic relaxation (6–9).
The contribution of Renal Sodium Handling
One of the more salient features common to both HTN and other components of the CRS is the impaired handling of sodium. Early studies showed a direct association between increased insulin and sodium absorption through increased nephron sodium transporters. This leads to a decrease in sodium excretion and thus an increased intravascular volume (6). There is also increasing evidence that insulin resistance in CV tissues contributes to impaired cardiac and vascular relaxation and increased CV stiffness (5, 6). More contemporary studies have delved further into this topic elucidating the role that inflamed adipose tissue (e.g. in visceral and perivascular fat) may play in HTN associated with the CRS (10–13). This inflammation of adipose tissue likely contributes RAAS activation related to increased proinflammatory adipokine secretion. The resulting systemic activation reduced activation of nitric oxide (NO) synthase and increased destruction of NO with resultant reductions in bioavailable NO in CV tissue (10–13).
The Role of the Renin-Angiotensin Aldosterone System
In states of insulin resistance such as obesity, an activated systemic RAAS is critical to the pathogenesis of HTN and other components of the CRS (6). Increasingly, it is apparent that expanded inflamed visceral and perivascular adipocyte tissue is key to driving RAAS activation. Adipocyte production of angiotensinogen may contribute up to 30% of circulating angiotensinogen (12). The notion that adipocyte production of angiotensinogen contributes to an activated RAAS is strengthened by observations of angiotensinogen knockout mice being immune to developing obesity, insulin resistance and HTN (14,15). In other murine studies ablation of adipose-derived angiotensinogen, no obesity-related HTN developed. However, some mice did go on to develop obesity (16). This evolving research underlines the important link between adipocyte-derived angiotensinogen and HTN, particularly in the context of the CRS (17).
There is a burgeoning body of evidence indicating that adipocytes are an important source of extra-adrenal-derived aldosterone (18). This concept is supported by the observation that obese persons, especially females, have increased circulating levels of aldosterone (6). Recent studies have shown that aldosterone-induced mineralocorticoid receptor (MR) activation in vascular tissue can itself be an instigating factor in the promotion of vascular stiffness by promotion of oxidative stress, inflammation, maladaptive immune modulation and fibrosis (19,20). Therefore, this MR activation may be a therapeutic target to prevent the evolution of vascular stiffness and HTN in diet-induced obesity.
Activation of the sympathetic nervous system (SNS)
Multiple studies have supported the role of sympathetic nervous system (SNS) activation in obesity-related HTN. These studies have shown that there is amplified sympathetic milieu in obese patients (21–24). One of the mechanisms responsible for SNS-induced increases in blood pressure is via increases in the hormone leptin which may drive SNS activation (25). Indeed leptin deficiency, in concert with diminished SNS activation, has been associated with a propensity to postural hypotension (26). Chronically high levels of leptin have also been shown to reduce natriuresis and lead to decreases in vascular bioavailable NO (27–29). Thus, hyperinsulinemia, activation of the RAAS, the SNS, and hyperleptinemia may all act in a positive feedback way to promote HTN associated with obesity, insulin resistance and the CRS (30).
Activation of RAAS can also work in a positive feedback loop with an activated SNS as indicated (Fig. 1 and Fig. 2). For example, increased renal sympathetic nerve traffic promotes juxtaglomerular cell renin production and activated RAAS promotes SNS activation. Activated RAAS effects on SNS activation include inhibition of norepinephrine reuptake in the pre-synaptic sympathetic nerve terminals (31). Another contributor to increased sympathetic tone in obese patients is sleep disordered breathing and obstructive sleep apnea (OSA), both of which are seen in many patients with CRS (1, 3). Thus, the RAAS and the SNS work in a positive feedback loop to increase HTN in patients with obesity, insulin resistance and the other components of the CRS (32–35).
Fig. 1.
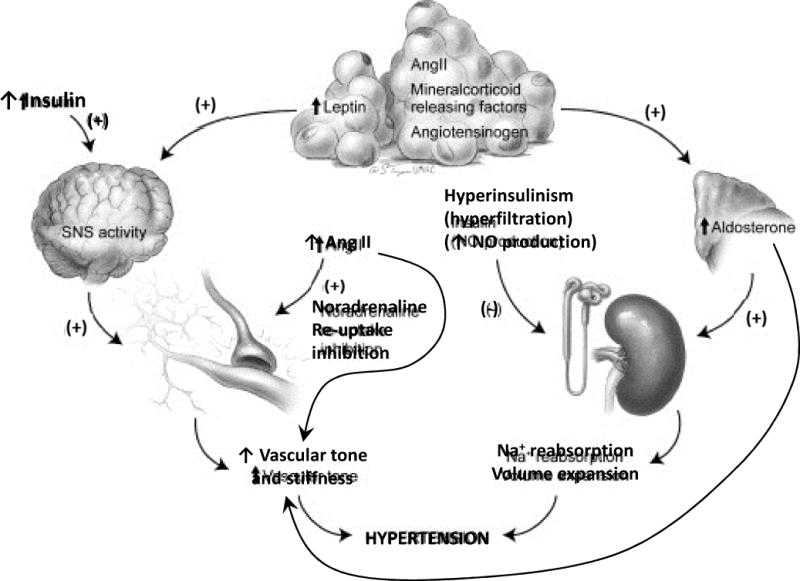
Coordinated influence of obesity, insulin resistance, activation of the RAAS and the SNS in the pathophysiology of hypertension in the CMS.
Adapted from Manrique C, Lastra G, Gardner M, et al. The Renin Angiotensin Aldosterone System in Hypertension: Roles of Insulin Resistance and Oxidative Stress. Med Clin North Am 2009;93(3):569–82; with permission.
Fig. 2.
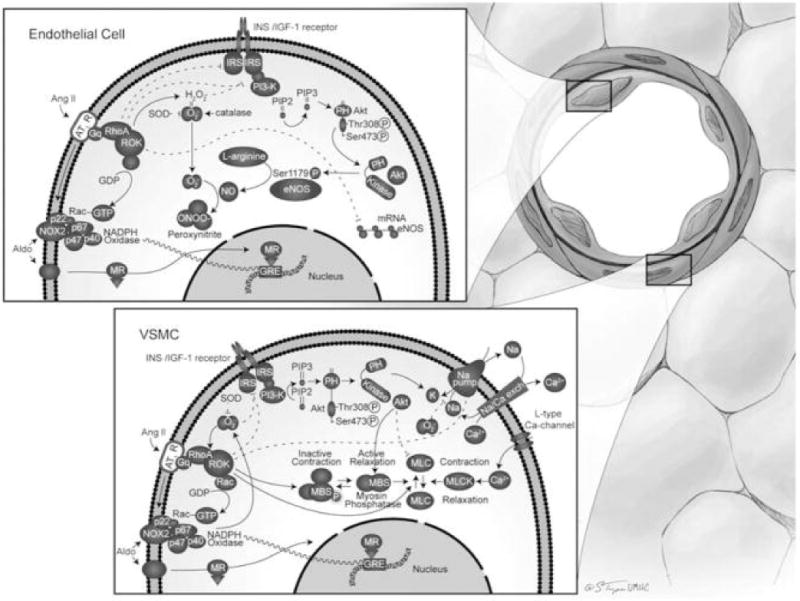
(Upper inset) Vascular effects of insulin (INS) or insulin like growth factor (IGF)-1 and Counter regulatory effects of AT1R and MR activation in endothelial cells. Insulin actions on the blood vessel are partially mediated by increased production of NO through phosphorylation and secondary activation of endothelial NO synthase (eNOS). AT1R activation decreases the availability of NO by way of the induction of insulin resistance, diminishing eNOS mRNA stability, and promoting NADPH oxidase-induced ROS production. Mineralocorticoids also activate NAPDH oxidase with secondary O2– production and consequent generation of peroxinitrite (ONOO–). Akt, PI3K/protein kinase B; GRE, glucocorticoid response element; Gq, G_q subunit; IRS, insulin receptor substrate; NOX2, catalytic subunit of NADPH oxidase; p22, p47, p40, and p67, subunits of NADPH oxidase; PH, pleckstrin homology domain; PIP, phosphatidylinositol phosphate; PIP2, phosphatidylinositol bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; ROK, Rho kinase; SOD, superoxide dismutase. (Lower inset) Opposing effects of ANG II and aldosterone (Aldo) versus insulin/IGF-1 on VSMCs. Insulin and IGF-1 cause VSMC relaxation, whereas ANG II and mineralocorticoids cause contraction. MBS, myosin-bound serine; MLC, myosin light chain; MLCK, MLC kinase; Na/Ca exch, Na_/Ca2_ exchanger.
From Manrique C, Lastra G, Gardner M, et al. The Renin Angiotensin Aldosterone System in Hypertension: Roles of Insulin Resistance and Oxidative Stress. Med Clin North Am 2009;93(3):569–82; with permission.
THE ROLE OF BLOCKING RAAS IN THE CRS
Accumulating evidence has shown the benefits of RAAS blockade in correcting many of the maladaptive aspects of the CRS, especially in patients with insulin resistance and obesity. To this point, multiple studies using ACE inhibitors and Angiotensin II-receptor blockers (ARBs) have shown their benefits in the treatment of HTN, congestive heart failure and coronary artery disease, as well as prevention of CVD and CKD in Type II diabetics (36,37). The TROPHY study, where obese patients were randomized in a double blinded protocol to groups receiving increasing doses of hydrochlorothiazide (12.5, 25 and 50mg) versus lisinopril (10, 20 and 40mg) with a diastolic goal of <90 mmHg, showed some evidence of greater reduction of blood pressure with lisinopril. The statistically significant results for obese patients receiving lisinopril showed 60% had achieved the blood pressure goal compared to 43% taking HCTZ. Metabolically, it was also noted that the patients in the HCTZ arm of the study had less optimal metabolic profiles, plasma glucose levels that were significantly higher and reduced plasma potassium when compared with the lisinopril arm (38). Another sub-analysis of patients with the metabolic syndrome in the Treat to Target survey compared irbesartan by itself and in combination with hydrochlorothiazide. Findings included significant reductions in blood pressure and metabolically, irbesartan was found to alleviate the undesirable effects of the HCTZ in the combination group. Moreover, there were also statistically significant improvements noted in other parameters of the CRS including the waist circumference in both men and women (39). The concept that RAAS inhibitors can improve the negative effects of the CRS was shown in a trial comparing HCTZ monotherapy versus valsartan monotherapy versus a combination of the two in patients with the metabolic syndrome. The significant results of this study showed an increase in the A1C and triglycerides solely in the HCTZ only arm of the study. This once again solidified the notion that the use of an RAAS antagonist was protective against the insulin-resistance properties of the diuretic, when used concurrently (40).
The utility of direct renin inhibitors and mineralocorticoid receptor antagonists (MRA) in treating HTN in CRS
A caveat needs to be made when considering the role of direct renin inhibitors and MRAs in populations with obesity and the metabolic syndrome, as these components have yet to be studied comprehensively. The ALTITUDE study which compared the addition of the renin inhibitor (Aliskiren) versus placebo as an adjunct to an ACE inhibitor (ACE-I) or angiotensin receptor blocker (ARB) definitively showed that there was no benefit to adding Aliskiren to previously established therapy. In fact, the study even had to be stopped prematurely due to greater cardiovascular events reported in the Aliskiren arm of the study (41). There are future data expected on the role of direct renin inhibitors and their role in the management of HTN in the CRS as a compendium both from the aforementioned ALTITUDE study and the ASTRONAUT study (42). Thus, there is no evidence currently showing the benefit of using combination RAAS blockade with ARBs, plus renin or ACE inhibitors.
There is a growing body of evidence that MRA is efficacious in treating hypertensive populations with obesity and the CRS (3–5). This approach appears to be especially noteworthy in those patients with resistant HTN. There are studies currently being carried out in this field. These studies are further bolstered by ongoing work which shows the direct co-relation of targeting the endothelial MR to ameliorate its effect on vascular stiffness (43).
REVIEW OF THE MOST CURRENT GUIDELINES
The most recent antihypertensive guidelines which focus specifically on the subset of patients with obesity and insulin resistance come from the 2013 European Society of Hypertension (ESH) and the European Society for Cardiology (ESC). The mainstay of initial treatment options continues to be lifestyle modification with a greater emphasis on weight loss. The recommendation for initial pharmaceutical intervention is with either a RAAS inhibitor or a calcium channel blocker as the CRS is considered a prediabetic state and some of the other readily available options tend to exacerbate insulin resistance in this subset of patients with HTN (44).
Unfortunately, the recently published JNC 8 guidelines, also known as the 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults, made no particular recommendations for HTN management in patients with obesity and CRS (45). This was in comparison to the JNC 7 guidelines which had recommendations for patients with the CRS which focused mainly on lifestyle modification, though no mention was made about pharmaceutical interventions (46).
A similar trend is noted with the National Institute for Health and Care Excellence (NICE) Hypertension guidelines from 2011, published in collaboration with the British Hypertension Society as well as with the Canadian Hypertension Education Program Recommendations. Both groups of guidelines tended to focus primarily on the concept of lifestyle interventions while not making any comment about pharmaceutical interventions with this subset of patients (47,48).
CONCLUSION
There are multiple factors linking HTN and the CRS. Currently, there is mounting evidence showing that RAAS and SNS activation are interactive factors that promote HTN as well as other components of the CRS. Agents which block the RAAS have been the mainstay of management with most physicians who treat this subset of patients. Even with the advent of new medications, most physicians continue to use these pharmaceutical classes due to their safety profile and long term success.
However, the optimal pharmacological management of HTN in patients with obesity and the CRS are yet to be definitively delineated. The majority of the HTN guidelines have tended to overlook the pharmacologic management of this subgroup of patients with some focusing only on lifestyle modification interventions while others have tended not to refer to them at all. This clearly shows a dearth in information about HTN management in this population and more research into the associated role of the RAAS and blockade of this system should yield more definitive material regarding optimizing HTN treatment in this growing population.
Key Points.
The seminal role of hypertension (HTN) in the pathogenesis of the cardiorenal metabolic syndrome (CRS) has significantly evolved over the past five years. The physiology of this is rooted in the concept that hypertension in the setting of obesity and the CRS is partly due to the excess body mass leading to an expanded plasma volume resulting in an increase in cardiac output.
Impaired handling of sodium is another of the more salient features common to both HTN and the CRS. A review of the literature which portrays that in states of insulin resistance such as with obesity, an activated systemic RAAS appears to play an important role in the pathogenesis of HTN and other components of the CRS.
Evidence shows the benefits of RAAS blockade in correcting many of the maladaptive aspects of the CRS, especially in patients with insulin resistance and obesity.
Currently, there are inadequate guidelines for the optimal pharmacological management of HTN in patients with obesity and the CRS and the inherent need for them be more clearly delineated.
Acknowledgments
The authors would like to thank Brenda Hunter for her editorial assistance.
Funding. The research of the authors is supported by funding from the National Institutes of Health (R01-HL73101 and R01-HL107910 to J.R.S.) and the Department of Veterans Affairs Biomedical Laboratory Research and Development Merit (0018 to J.R.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Ronco C. The cardiorenal syndrome: basis and common ground for a multidisciplinary patient-oriented therapy. Cardiorenal Med. 2011;1:3–4. doi: 10.1159/000323352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.http://www.cdc.gov/nchs/data/nhis/earlyrelease/earlyrelease201602_06.pdf
- 3.Hoerger Thomas J, PhD, Simpson Sean A, MA, Yarnoff Benjamin O, PhD, Pavkov Meda E, MD, PhD, Burrows Nilka Ríos, MPH, MT, Saydah Sharon H, PhD, Williams Desmond E, MD, PhD, Zhuo Xiaohui., PhD The Future Burden of CKD in the United States: A Simulation Model for the CDC CKD Initiative. doi: 10.1053/j.ajkd.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, et al. Obesity related hypertension: pathogenesis, cardiovascular risk and treatment-a position paper of the obesity society and the American society of hypertension. Obesity. 2013;21(1):8–24. doi: 10.1002/oby.20181. [DOI] [PubMed] [Google Scholar]
- 5.Aroor AR, Demarco VG, Jia G, Sun Z, Nistala R, Meininger GA, Sowers JR. The role of tissue Renin-Angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front Endocrinol (Lausanne) 2013 Oct 29;4:161. doi: 10.3389/fendo.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014(10):364–76. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manrique C, Sowers JR. Insulin resistance and skeletal muscle vasculature: Significance, assessment and therapeutic modulators. Cardiorenal Med. 2014;4:244–256. doi: 10.1159/000368423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messerli FH, Christie B, DeCarvalho JG, Aristimuno GG, Suarez DH, Dreslinski GR, Frohlich ED. Obesity and essential hypertension. Hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Arch Intern Med. 1981;141(1):81–5. doi: 10.1001/archinte.141.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Frohlich ED, Messerli FH, Reisin E, Dunn FG. The problem of obesity and hypertension. Hypertension. 1983;5(5 Pt 2):III71–8. doi: 10.1161/01.hyp.5.5_pt_2.iii71. [DOI] [PubMed] [Google Scholar]
- 10.De Faria AP, Modolo R, Fontana V, Moreno H. Adipokines: novel players in resistant hypertension. J Clin Hypertens. 2014;16(10):754–9. doi: 10.1111/jch.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahmouni K. Obesity associated hypertension: recent progress in deciphering the pathogenesis. Hypertension. 2014;64:215–21. doi: 10.1161/HYPERTENSIONAHA.114.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padilla J, Vierira-Potter VJ, Jia G, Sowers JR. Role of perivascular adipose tissue on vascular reactive oxygen species in type 2 diabetes: A give and take relationship. Diabetes. 2015;64:1904–06. doi: 10.2337/db15-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, et al. The adipose tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J BiochemCell Biol. 2003;35:807–25. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 14.Massiera F, Seydoux J, Geloen A, et al. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology. 2001;142:5220–5. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- 15.Yvan-Charvet L, Even P, Bloch-Faure M, et al. Deletion of the angiotensin type 2 receptor reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54:991–9. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]
- 16.Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, et al. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60:1524–30. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol, Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 18.Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–78. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 19.Bender SB, Mcgraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62(2):313–9. doi: 10.2337/db12-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia G, Habibi J, Aroor AR, et al. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res. 2016;118:935–943. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troisi RJ, Weiss ST, Parker DR, Sparrow D, Young JB, Landsberg L. Relation of obesity and diet to sympathetic nervous system activity. Hypertension. 1991;17:669–77. doi: 10.1161/01.hyp.17.5.669. [DOI] [PubMed] [Google Scholar]
- 22.Huggett RJ, Burns J, Mackintosh AF, Mary DASG. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension. 2004;44:847–52. doi: 10.1161/01.HYP.0000147893.08533.d8. [DOI] [PubMed] [Google Scholar]
- 23.Raumouni K, Correla MI, Haynes WG, Mark AL. Obesity associated hypertension: new insights into mechanisms. Hypertension. 2005;25:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 24.Haynes WG, Morgan DA, Walsh SA, et al. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–8. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 26.Bravo PE, Morse S, Borne DM, Aguilar EA, Reisin E. Leptin and hypertension in obesity. Vasc Health Risk Manag. 2006;2(2):163–9. doi: 10.2147/vhrm.2006.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan DA, Thedens DR, Weiss R, et al. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1730–6. doi: 10.1152/ajpregu.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper SA, Whaley-Connell A, Habibi J, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293:H2009–23. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 29.Kazumi T, Kawaguchi A, Katoh J, Iwahashi M, Yoshino G. Fasting insulin and leptin levels are associated with systolic blood pressure independent of percentage body fat and body fat mass index. J Hypertens. 1999;17:1451–5. doi: 10.1097/00004872-199917100-00013. [DOI] [PubMed] [Google Scholar]
- 30.Grassi G. Renin-angiotensin-sympathetic crosstalks in hypertension: reappraising the relevance of peripheral interactions. J Hypertens. 2001;19:1713–6. doi: 10.1097/00004872-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Raasch W, Betge S, Dendorfer A, et al. Angiotensin converting enzyme inhibition improves cardiac neuronal uptake of noradrenaline in spontaneously hypertensive rats. J Hypertens. 2001;19:1827–33. doi: 10.1097/00004872-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Troisi RJ, Weiss ST, Parker DR, Sparrow D, Young JB, Landsberg L. Relation of obesity and diet to sympathetic nervous system activity. Hypertension. 1991;17:669–77. doi: 10.1161/01.hyp.17.5.669. [DOI] [PubMed] [Google Scholar]
- 33.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, et al. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–3. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 34.Huggett RJ, Burns J, Mackintosh AF, Mary DASG. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension. 2004;44:847–52. doi: 10.1161/01.HYP.0000147893.08533.d8. [DOI] [PubMed] [Google Scholar]
- 35.Gillespie EL, White CM, Kardas M, et al. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care. 2005;28:2261–6. doi: 10.2337/diacare.28.9.2261. [DOI] [PubMed] [Google Scholar]
- 36.Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 1. A meta-analysis of randomized clinical trials. Diabetes Metab. 2004;30:487–96. doi: 10.1016/s1262-3636(07)70146-5. [DOI] [PubMed] [Google Scholar]
- 37.Reisin E, Weir MR, Falkner B, Hutchinson HG, Anzalone DA, Tuck ML. For the Treatment in Obese Patients with Hypertension (TROPHY) study group. Hypertension. 1997;30(1):140–5. doi: 10.1161/01.hyp.30.1.140. [DOI] [PubMed] [Google Scholar]
- 38.Kintscher U, Bramlage P, Paar WD, Thoenes M, Unger T. Irbesartan for the treatment of hypertension in patients with the metabolic syndrome: a sub analysis of the treat to target post authorization survey. Prospective, observational two armed study in 14, 200 patients. Cardiovasc Diabetol. 2007;6:12. doi: 10.1186/1475-2840-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zappe DH, Sowers JR, Hsueh WA, Haffner SM, Deedwania PC, Fonseca VA, et al. Metabolic and antihypertensive effects of combined angiotensin receptor blocker and diuretic therapy in Prediabetic hypertensive patients with the cardiometabolic syndrome. J Clin Hypertens. 2008;10(12):894–903. doi: 10.1111/j.1751-7176.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of Aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–13. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 41.Friedrich S, Schmieder RE. Review of direct renin inhibition by Aliskiren. J Renin-Angiotensin-Aldosterone Syst. 2013;14(3):193–6. doi: 10.1177/1470320313497328. [DOI] [PubMed] [Google Scholar]
- 42.The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) 2013 ESH/ESC guidelines for the management of arterial hypertension. J Hypertens. 2013;31:1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 43.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, et al. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circ Res. 2016 Mar 18;118(6):935–43. doi: 10.1161/CIRCRESAHA.115.308269. Epub 2016 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancia G1, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013 Jul;31(7):1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 45.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence based guideline for the management of high blood pressure in adults: report from the panel members appointment to the eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 46.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. National heart, lung and blood institute joint national committee on prevention, detection, evaluation and treatment of high blood pressure; national high blood pressure education program coordinating committee. The seventh report of the joint national committee on prevention, detection, evaluation and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 47.National Clinical Guideline Centre. Hypertension: the clinical management of primary hypertension in adults: update of clinical guidelines 18 to 34. Royal College of Physicians; (UK): 2011. [PubMed] [Google Scholar]
- 48.Dasqupta K, Quinn RR, Zarnke KB, Rabi DM, Ravani P, Daskalopoulou SS, et al. The 2014 Canadian hypertension education program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention and treatment of hypertension. Can J Cardiol. 2014;30(5):485–501. doi: 10.1016/j.cjca.2014.02.002. [DOI] [PubMed] [Google Scholar]


