Abstract
To interact with the world, we have to make sense of the continuous sensory input conveying information about our environment. A recent surge of studies has investigated the processes enabling scene understanding, using increasingly complex stimuli and sophisticated analyses to highlight the visual features and brain regions involved. However, there are two major challenges to producing a comprehensive framework for scene understanding. First, scene perception is highly dynamic, subserving multiple behavioral goals. Second, a multitude of different visual properties co-occur across scenes and may be correlated or independent. We synthesize the recent literature and argue that for a complete view of scene understanding, it is necessary to account for both differing observer goals and the contribution of diverse scene properties.
Interacting with real-world scenes
Making a cup of tea is an easy task that requires minimal concentration, yet the composition of behaviors involved is deceptively complex: recognizing the room next door as a kitchen, navigating to it while manoeuvring around obstacles, locating and handling objects (e.g., teabag, kettle), and manipulating those objects until they are in a correct state (e.g., filling the kettle). In addition, it requires knowledge of relative locations and future destinations within the environment (e.g., take the kettle to the mug that is currently out of sight). Such interactions with the environment require the selective processing of task-relevant information, as well as the continual storage and retrieval of information from memory. Despite the seeming simplicity of the task, a multitude of scene properties across a range of different dimensions need to be processed, requiring the engagement of distributed brain regions.
The diversity of processes and goals engaged has led research on visual scene perception to progress in many directions over the past 50 years without necessarily much overlap. In the present article, we review this literature under the overarching context of scene understanding (Box 1). We argue that such a broad perspective is necessary to produce a comprehensive, theoretical framework to help elucidate the underlying cognitive and neural mechanisms. In particular, we focus on four of the most commonly studied behavioural goals – categorization, search, navigation and action – and consider the natural overlap among scene properties and the neural mechanisms involved.
Box 1. Scene Understanding.
There is no single way to define a scene, and previous work has often focused on two key considerations [e.g., 82,6,128]. The first consideration is primarily stimulus-based, focusing on the perceived properties of an image. Very generally, any collection of objects or shapes (e.g. a texture, or an array of search items) can be considered a scene. However, real-world scenes differ from such stimuli because they typically contain a large variety of items that are arranged in a meaningful manner, containing a spatial layout that organizes the scene into foreground objects and background elements (e.g., walls, ground plane). As such, a scene is often defined as consisting of a specific viewpoint of a real-world environment (e.g. a beach photograph). Such stimulus-based definitions are most commonly adopted in studies on scene recognition and categorization. To the extent that these tasks require processing an image as a single nameable entity, one could argue that processing of a scene stimulus is not unlike processing an object. However, an important second distinction that has been made is that observers act upon objects but act within scenes [82,6]. In this light, the second way we can consider scenes is as a 3D environment the observer is embedded in and interacts with. Under this interaction-based view, those aspects of a scene allowing the observer to carry out specific behavioural goals, e.g. locomotion or motor interaction, become critical. Daily tasks are generally comprised of several smaller goals occurring in quick succession, or potentially overlapping in time, while the world gradually unfolds around us. The interaction-based view on scenes thus incorporates not only what is visible, but also the memorized (or predicted) arrangement of elements that are involved in a larger, continuous environment.
These considerations are not mutually exclusive: many of the visual properties identified under the scene-as-stimulus view are relevant for interacting with scenes, and in turn individual behavioural goals that involve interaction with scenes may require the representation of different scene properties. The framework we propose explicitly incorporates both of these considerations under the umbrella term of “scene understanding”, which emphasizes both how individual goals affect one another and the respective weighting of visual properties.
Toward a Comprehensive Framework for Scene Understanding
There are two major challenges to producing a comprehensive theoretical framework that would outline the cognitive and neural mechanisms of scene understanding. The first is that while the physical characteristics of our surrounding environment are generally stable, our immediate goals are not. At any given moment, different visual aspects of an environment will be prioritized based on our current goal (Figure 1A; see also Box 2). However, the dynamic nature of scene understanding is often neglected as studies typically focus on individual tasks. The diagnostic (see Glossary) scene properties isolated in these studies may differ across task, making it hard to determine the extent to which such findings generalize to scene understanding in the real world.
Figure 1. Observer goals and scene properties.
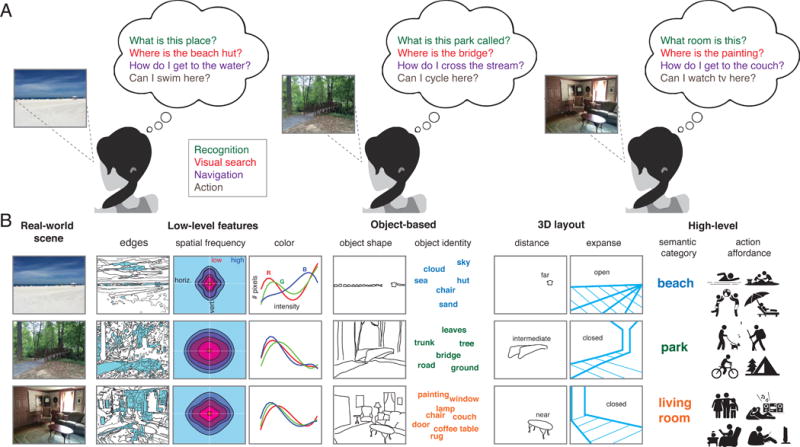
A) Examples of possible observer goals in scene understanding. In this article, we focus on four general task domains that involve scene understanding: 1) recognition, i.e. determining whether a visual scene belongs to a certain category (e.g., beach scene), or depicts a particular place (the park, my living room); 2) visual search, which involves locating specific objects or other scene elements; 3) navigation, which involves determining both the navigability of the immediate space and one’s position relative to an unseen location; and 4) action goals, which may involve navigation but also encompass a broader set of activities such as cooking or playing baseball. B) Examples of scene properties that may be relevant for constructing mental representations necessary to achieve various observer goals. Properties that can be computed from scene images with relatively simple computational models, such as edges, spatial frequency and color, are considered ‘low-level features’. More complex properties are the scene’s constituent objects and 3D properties reflective of the layout of the scene, or the distance of the observer to salient elements in scenes. Finally, semantic category and action affordances can be seen as ‘high-level’ features of scenes that are not easily computed from scenes but may inform multiple observer goals. Note that scenes may differ from one another at multiple levels; for example, the beach scene can be distinguished from the park and living room based on virtually all dimensions, whereas the park and living room image share some but not all properties. Due to the inherent correlations between scene features, assessing their individual contributions to scene representations is challenging [125].
Box 2. Goal driven models of visual processing.
The importance of ethological goals has been emphasized in computational work employing hierarchical convolutional neural networks (CNNs) [129]. These models, which in essence are a generalized form of principles first proposed by Hubel and Wiesel, have garnered increasing attention because they approach human levels of performance on tasks such as object recognition for real-world images [129,130] and show a degree of correspondence between individual layers of the networks and different levels of neural visual object processing [131–133]. CNNs that perform better on object-recognition tasks are found to be better predictors of neuronal spiking data in monkey inferotemporal cortex [134], suggesting the importance of a goal driven approach for creating a model of a given sensory system. Although CNNs have primarily been trained on object recognition tasks, some have focused on scene categorization [135], also revealing a correspondence to human MEG data [136]. Importantly, performance on scene-related recognition tasks was found to be better for a network trained on scene-centric compared to one trained on object-centric data [137]. Further, the estimated receptive fields of a scene network appeared to exhibit features consistent with properties such as surface layout, and comparison with an object network showed stronger representational similarity with scene size [136].
While we have so far emphasized the importance of the task goal in training CNNs, this is not to say that there is no generalization, that the features developed for one task are not also applicable to other tasks. For example, a CNN optimized for object classification showed some generalization to other recognition tasks such as attribute detection (e.g. presence of specific part or material) and scene recognition [138]. Similarly, a CNN trained to perform scene categorization appeared to develop object detectors [137]. An issue that is so far unexplored is whether the degree to which CNNs trained for different tasks develop similar feature representations depends on the inherent correlations in the visual input (Figure 1B), i.e. whether generalization across tasks may occur because of shared information between features in the real-world. One possibility suggested by findings from CNNs is that different processing pathways in the brain could have developed as a direct result of the differential task constraints imposed by the required classification of the respective visual input [127,134,136].
The second challenge arises from the complexity of the input. Any scene can be described at multiple levels, from basic stimulus properties such as edges, spatial frequency, and color, to more complex, high-level characteristics such as object identity, spatial layout and action affordance (Figure 1B). Though individually each property might predict a given behavior, their inherent co-occurrence across scene categories [1] is often not considered: properties can either be correlated (e.g., beach scenes will generally contain large open spaces, far away objects, low spatial frequencies, etc.) or independent (e.g. both city scenes and forests may be characterized by high spatial frequency, vertical edges, etc.). It is therefore difficult, and even potentially misguided, to tease out specific contributions of any individual property to scene understanding without considering potential interactions.
These challenges are intimately linked: many different properties can be processed to complete a single goal, and conversely a single property can be used to facilitate many different goals. The diagnostic value of a visual property depends on a combination of the current goal and prior experience of the observer, as well as its availability within the scene and relationship to other properties [2,3]. In order to determine the cognitive and neural processes enabling scene understanding, it is critical to clarify how observer goals affect the weighting of different properties. In the next section, we will bring together research that addresses four of the main goals of scene understanding, and discuss how the potential utility of multiple properties varies across them.
Goal 1: What is this scene?
Scenes can be categorized at varying levels of hierarchical detail. Relative to the basic level, most commonly used in discourse (e.g., a city) [4], we can also make more generalizable, superordinate judgments (the scene is outdoors), or more detailed, subordinate judgments (a gothic city), or even identify the scene as a particular place (I am approaching North Bridge, Edinburgh). Understanding the meaning of an environment is important as it can facilitate the selection of subsequent behaviors such as actions (approach a crosswalk), searching (the time can be found by looking at the clock tower), deducing relative locations (the University is 10 minutes South), and so on.
The most heavily researched concept in scene categorization is gist, the initial representation of a scene that can be obtained in a brief glance. Studies typically make use of backwards-masking, or rapid serial visual presentation of, scene images and record performance as a function of presentation duration (note that this does not necessarily reflect the timing of relevant brain processes [5]). A large body of work suggests that as little as a ~13ms presentation duration allows for an initial scene percept [6], potentially including conceptual meaning [e.g., 7] [but see, 8]. Though this duration by itself has limited ecological validity compared to the gradually changing, predictable world we experience, it serves as an important demonstration that an initial conceptual representation of a scene requires only a small subset of available visual properties to be processed. This epoch is too short to make a saccade, so only a single percept of a scene is afforded.
Global analysis of low-level features can facilitate the initial representation of a scene. For example, spectral features [9] and summary statistics of local contrast [10] can characterize the spatial properties of a scene: an efficient low-dimensionality resource bypassing computationally expensive processes of recognizing and integrating local components such as objects. Such global properties can accurately predict human scene recognition performance [11] and are processed early enough to facilitate the gist recognition epoch [12,13]. Other features include properties such as contour junctions [14], and color [15,16], which can facilitate initial scene understanding, although research suggests color facilitation occurs after initial processing of spatial structure [17]. Furthermore, some higher-level object information is available very rapidly [18,19] and objects and their co-occurrence may be diagnostic (e.g. the presence of a sink and an oven are highly predictive that the scene is a kitchen) [20].
While these properties and many more provide a quick summary of a scene’s meaning, gist perception is limited for two reasons. First, interpreting the rapidly extracted gist depends on stored representations of typically occurring patterns [21], developed over experiences (e.g., a couch is commonly found in a living room). When scenes are less typical, such as when they contain inconsistent objects [e.g., a boulder in a living room, 3], or contain atypical action relationships between individuals [22], the scene requires longer to process. Scene processing is therefore not entirely stimulus-driven, but is dependent on matching a percept to prior experiences. Secondly, scene recognition extends beyond gist, as we often interact with our environment at greater levels of detail. The more detailed the judgment, the longer the scene needs to be examined [23,24] as viewers supplement gist with goal-driven diagnostic details [2]. Thus, when the scene is an infrequently experienced situation or does not provide enough information relative to the viewer’s goal, additional information must be acquired.
Goal 2: Where is X?
To gain information beyond gist – whether to support detailed recognition, search, or something else – eye movements are essential. This is necessitated by the retina’s inhomogeneity: the central ~2° (fovea) of the visual field is processed in high resolution, but acuity drops off in the surrounding parafoveal (~4.5° into the periphery) and peripheral regions [25]. Appreciating the surrounding scene with full acuity would take roughly 16 minutes for the fovea to be directed to each location in our environment [26]. To overcome this limitation, the visual system directs eye movements in an efficient manner by integrating low-resolution peripheral information with the current goal and knowledge of the environment [27,28], constrained by eye movement tendencies that produce stereotypical scan patterns [29]. Information falling within the foveal or parafoveal regions is optimized for detailed processing, while information in the periphery informs efficient saccadic distribution [27].
Several factors determine where the eyes are guided during scene viewing. Eye movements are strongly biased to direct fixations toward objects instead of backgrounds [30,31], with a preferred landing position within objects [32,33]. However, determining which objects in a scene are fixated, in what order, and for how long, relies heavily on the interplay between the viewer’s goals and available visual information. When the viewer’s goal is non-specific (e.g., memorizing a scene), image-based properties can predict where people fixate: edge density, visual clutter and homogenous segments predict fixation probability, while luminance and contrast play more minor roles [34]. The features used to select fixation sites are also determined by distance from the previous fixation, with shorter saccades (<8°) relying more on specific image features, particularly high-spatial frequencies, compared to longer saccades [35]. Fixation locations in free-viewing tasks can also be predicted based on eye movement tendencies, which act independently of the scene percept yet outperform some image-based models in predicting fixation locations [29].
When there is a more specific top-down goal (e.g., where is the kettle?) the visual system can utilize various scene properties depending on how diagnostic they are for that particular goal. Viewers might rely on matching low-level scene features, such as color [36] or shape [37], with target properties. High-level factors can also bias gaze by using the semantic relationship between gist and object meaning [38,39], as well as the relationships between objects [31,40–42], the spatial dependency between objects [43] or an object’s relationship to the spatial layout [44]. These various guidance factors can be combined to direct attention to the most likely target location [45,46, see Figure 2B].
Figure 2. Mapping properties to goals.
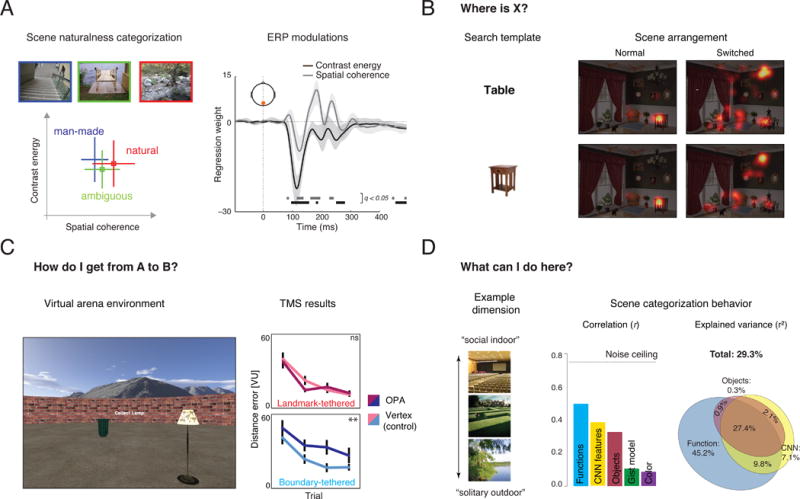
A) Recognizing a scene. Scenes that were easily categorized as man-made or natural resided at opposite ends of a low-level feature space described by two summary statistics of local contrast (see Box 3): contrast energy and spatial coherence, while ambiguous scenes were found in the middle of the space. These statistics also modulate evoked EEG responses in early stages of visual processing. Redrawn from data published in [13]. B) Locating information within a scene. Fixation density heat map during a visual search task. Participants combined precise search templates (object image, bottom row) and reliable spatial expectations (normal vs. switched arrangements, columns) to improve oculomotor efficiency. Reproduced, with permission, from [46]. C) Navigating through scenes. A virtual arena paradigm used to test contributions of landmark and boundary information. Participants learn target locations, which are tethered to a boundary or landmark location. Disruption of OPA with transcranial magnetic stimulation affected navigation with respect to boundaries but not with respect to landmarks. Modified with permission from [70]. D) Actions afforded by a scene. An empirically derived scene function feature space (containing, for example, a dimension separating solitary outdoor activities from social indoor activities) correlated more strongly with scene categorization behaviour than various other models of scene properties, including object labels, CNN representations, and low-level feature models. The variance explained by the function space was partly unique and partly shared with the other models. Modified, with permission, from [78].
Gaze allocation is thus the result of a bi-directional relationship between scene properties, ranging from low-level features to high-level semantics, and the viewer’s goal. However, as mentioned above, there is not necessarily a one-to-one mapping of visual properties to a goal. The diagnosticity of a particular property is also dependent on availability. Looking for a kettle in a kitchen would rely primarily on semantic knowledge (e.g., kettles are typically found on the stove), yet if the scene does not provide clear semantic cues, gaze guidance can be driven by episodic memory instead [e.g., where did I last see the kettle?, 47]. Similarly, if the target has no specific spatial dependency, other factors dominate [48]: searching for a fly would rely on low-level feature matching; looking for a banana in a well-lit room would primarily rely on color, while in the same dimly lit room it would rely more on shape.
Additionally, as the representation of a scene changes over time, so too does the information gathered by eye movements. Once recognized, the scene category rarely changes without significant locomotion from the viewer; thus gist becomes a less relevant guiding factor over time [49,50] and other properties become more pertinent – e.g., fixated objects stored in short- and long-term memory [51–53] – enabling the development of more detailed scene representations [2], improved action efficiency [54,55], and so forth.
Goal 3: How do I get from A to B?
Accomplishing a particular goal may require moving from one location to another [e.g., 56,57]. Scene understanding therefore not only entails what a scene is or where specific scene elements are, but also involves representing the information that enables navigation through them.
We discuss two forms of navigation here. The first is how we move from one point to another in vista space [58], which we refer to here as navigability, and is generally concerned with paths and obstacles [11]: for instance, crossing from one side of North Bridge to the other while avoiding buses. This relies on a dynamic representation of our position within a stable spatial layout, and prioritizes updating the location and movement of discrete objects more than their meaning. Clear paths and obstacles can potentially be processed from the same global properties that facilitate the initial labelling of a scene through spatial characteristics [11,12]. As observers move through the world, they can regularly update their spatial location and continue to sample information concerning the state and position of obstacles. For instance, some of the most commonly fixated obstacles when moving through an environment are people [59], who are generally fixated at a distance to determine their heading and avoid collisions [55,59]. Similarly, eye movements are made to gauge the distance of approaching objects [e.g., a car, 60]. Viewers also sample ground information in a goal-driven manner, directing fixations ahead to check for changing terrain, as well as to closer regions that will be stepped on, and surface transitions to avoid [e.g., a curb, 61].
The second form of position-based scene understanding is knowing where you are relative to an unseen location in environmental space [58], which we refer to here as navigation. For example, identifying your location as on North Bridge, Edinburgh, and knowing your relative direction and distance from the University. Navigation relies more on the meaning of a scene and less on the dynamic position of its elements. An observer’s position during navigation can be discerned from a process relying on path-integration – the integration of an observer’s translations and rotations from a start point in order to estimate the current position [62] – working in concert with two informative scene properties. The first is landmarks, which are persistent visual stimuli that have both distinct perceptual features and occur at decision points along a route [63–65]. Three types of information are needed to utilize a landmark: it needs to be recognized (e.g., clock tower), its position relative to other points needs to be retrieved from long-term spatial knowledge (e.g., the clock tower is six blocks from the University), and the heading of the viewer relative to the landmark needs to be determined (e.g., the clock tower is in front of me). At this point a route can be planned [e.g., carry on straight ahead, 66]. The second informative property is scene boundaries, which are extended surfaces that separate one environment from another. Unlike the single point of a landmark, boundaries are made up of multiple points from which directions can be discerned [67]. Though typically thought of as large structures (e.g., walls), a boundary need not necessarily restrict movement and even a subtle geometric property – such as a small ridge – is enough to act as an informative spatial cue [68]. The relative contributions of landmarks and boundaries to navigation can be directly compared using a virtual arena paradigm, in which target location is tethered to a landmark or boundary which participants learn over time [e.g., 69, 70, see Figure 2C].
Locomotion through an environment is experienced as continuous episodes of immediate spaces (walk across the bridge, down the street, around the corner, etc.). Thus, more than recognition or searching for items, the behavioral goal of movement is made up of a sequence of smaller goals. At each new stage, the internal scene representation must change with the observer’s needs, whether it is a decision based more on physical locations in vista space (e.g., head to the gap between obstacles), or recognition in environmental space (e.g., recognize the landmark and interpret location), or the two simultaneously. As such, navigation is an ecologically relevant behavioral goal that emphasizes the dynamic nature of scene understanding.
Goal 4: What can I do here?
Navigation can be seen as one example of a behavioral goal that highlights the strong link between vision and action in scene understanding. This functional perspective on vision was long ago recognized in theoretical frameworks emphasizing scenes as environments that provide possibilities for action, i.e. ‘affordances’ [4,71]. Action affordance has shown to be an important factor in how we understand objects [72], and object affordance influences how we search for items in visual scenes [73]. While several studies have considered how visual scenes serve as a context facilitating recognition of both objects [18,74–76] and actions [77], the idea that affordances determine how we understand the scene itself is relatively unexplored. However, a recent study has shown that descriptions of actions which might occur in a scene predict their categorization better than objects or visual features. In other words, a kitchen scene is understood as a kitchen “because it is a space that affords cooking” [78, p93] (Figure 2D).
An unresolved question is how such action affordances might be computed from a scene. While action descriptions explained most of the variance in the behavioral categorization [78], some of this variance was shared with objects and visual features. Presumably, action affordances can be deduced from a scene by a non-linear combination of multiple scene properties, which may include those depicted in Figure 1B, as well as representations stored in memory, potentially including complex sociocultural aspects of scenes [79]. Another novel line of research suggests that our ability to make physical inferences about our visual environment, e.g., predict possible movements by objects in scenes [80], involves cognitive mechanisms also used in action planning [81]. As such, the concept of action affordances highlights the importance of considering multiple scene properties simultaneously, allowing for potential combination of these properties with action goals. Generally speaking, action affordances could serve as a useful broader concept highlighting the interactive components of scene understanding, and encompassing more complex functional scene properties such as navigability, which ‘afford’ movement within a space.
Mapping Properties to Goals
Based on the discussion of these four main goals of scene understanding, it should be clear that the goals themselves are not mutually exclusive. For example, recognition facilitates search and navigation processes; navigation sometimes requires searching for specific information (e.g. objects, boundaries); scene affordance must consider navigability within a space, and so forth. Similarly, informative properties overlap various goals: spatial layout facilitates the early stages of recognition as well as navigability, edge information can help recognition and obstacle detection, etc. This means that there is no simple way to map between goals and properties. In this context, elucidating the neural mechanisms of scene processing can provide additional insight by demonstrating which properties are represented in different parts of the brain.
The Neural Mechanisms of Scene Understanding
In general, visual scene processing in humans has been characterized by a trio of scene-selective regions: occipital place area (OPA), parahippocampal place area (PPA) and retrosplenial complex (RSC), on the lateral occipital, ventral temporal and medial parietal cortical surfaces, respectively [82]. Studies in non-human primates have also reported scene-selective regions [83–85] as well as regions responsive to spatial landscapes [86]. Much of the research on humans has focused on establishing what specific properties each scene-selective region is sensitive to. For example, responses in PPA have been reported to reflect a wide range of properties, including, i) low-level properties, such as spatial frequency [87–90], orientation [91], texture [92], rectilinearity [93] [, but see, 94], and contour junctions [95]; ii) object properties, such as identity [96], size [97], space diagnosticity [98], co-occurrence [99], and object ensembles [92]; iii) 3D layout, such as size of a space [100], spatial expanse [i.e., open or closed, 96,101,102], distance [102], and boundaries [103]; and iv) high-level properties, such as semantic category [104,105], contextual associations [106,107], and knowledge of scene correspondences [108]. Sensitivity to some of these properties is shared by both OPA and RSC, but in contrast to PPA, they show greater sensitivity to egocentric distance [109] and sense [i.e., left versus right mirror views, 110]. Further, OPA has been associated with the local elements of scenes [111] and transcranial magnetic stimulation over OPA selectively impairs scene discrimination and categorization [112], as well as disrupting navigation relative to boundaries [70, Figure 2C]. RSC may have a particular sensitivity to landmarks [64,113,114], and, in addition to visual responsiveness, RSC (and to some extent anterior PPA) has been associated with spatial memory and imagery, particularly in the context of navigation [108,115–117]. In fact, there may be separate perceptual and memory scene networks [118,119] and systematic organization [e.g., 95,99] within medial parietal cortex, with posterior regions showing more visual selectivity and anterior regions more related to memory [120]. Finally, there are basic response characteristics of the three scene-selective regions that may help inform their functional role and the critical scene properties they represent. In particular, these regions show a peripheral visual field bias with relatively large population receptive fields that make them well placed to capture summary information across large portions of the field of view [120–122]. Further, OPA and PPA show retinotopic biases to the lower and upper visual field, that may make them particularly well suited for capturing information relevant for navigability or processing landmarks, respectively [121].
While these studies have provided much insight into the neural processing of scenes, it is clear that each region is sensitive to multiple scene properties and determining specific critical properties for each region is difficult. In part, this may reflect the two challenges we have highlighted. First, most studies have focused on individual tasks only, and their results may be specific to that task. Indeed, a recent study [123] carefully and systematically manipulated multiple features of computer-generated scenes and compared brain representation of these features across multiple tasks revealing dynamic coding of scene properties (Figure 3A). For example, differentiation of spatial boundary in PPA was affected by a task instruction requiring participants to attend to either texture or spatial layout. Task effects have also been reported to change sensitivity to diagnostic properties in EEG signals for real-world scenes [124].
Figure 3. Investigating multiple properties and goals.
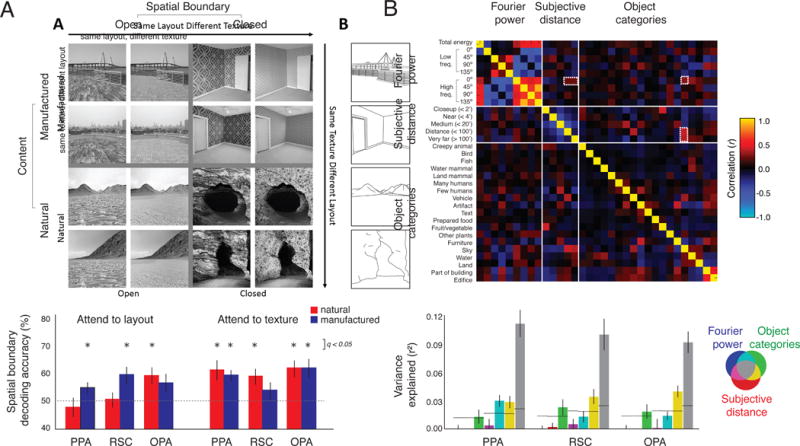
A) Dynamic coding of scene properties. Four different scene categories were created by systematically varying spatial boundary (open vs. closed) and scene content (natural vs. manufactured), and each category contained twelve unique structural layouts and twelve textures. Both scene content and a task manipulation (attend to layout or texture) modulated whether spatial boundary could be decoded from fMRI responses across multiple scene-selective areas. Modified, with permission, from [123]. B) Correlations between scene properties. Three models of scene properties were compared in terms of their inter-correlations and ability to predict fMRI responses in scene-selective cortex: 1) Fourier power at four major orientations, subdivided in low versus high frequencies, as well as a total energy measure; 2) Subjective distance to salient objects in the scene, divided in five different bins; and 3) Object labels, binned in 19 categories. Dashed white outlines indicate an example of high feature correlation between models: pictures containing sky tend to have far distance ratings and relatively high spatial frequency in the horizontal dimension, potentially due to the presence of a thin horizon line and tiny objects in faraway scenes (e.g., beaches; see also Figure 1B). As a result, most of the variance in response magnitude in scene-selective areas is shared across models: Venn diagram colors indicate variance explained by each model and their combinations, and grey shows shared variance across all three models. Adapted, with permission, from [125].
Second, the different properties identified in different studies may in fact be correlated, and reflect sensitivity to the same underlying dimension. One approach to address this problem is to use large numbers of naturalistic images and model multiple dimensions simultaneously. For example, a comprehensive analysis [125; Figure 3B] revealed that the dimensions of spatial frequency, subjective distance and object category all explained variance in scene-selective regions. However, most of the variance explained was shared across the models suggesting that, for example, the apparent sensitivity to scene category could just as easily be interpreted as reflecting differences in spatial frequency. The difficulty of this approach, however, is that there are many possible models that could be tested. Further, these models might differ in their biological plausibility (see Box 3) and we should also be careful not to assume that any sensitivity to low level properties explains away the sensitivity to high level properties – the interaction between low and high level features may actually be informative about how the brains transforms the retinal input into a task-relevant representation [126].
Box 3. Biological plausibility of scene properties.
An important factor to consider when comparing multiple models of scene properties is whether and how these properties can be computed plausibly by the visual system. One example highlighting this issue is the role of spatial frequency in visual scene perception. In the influential Spatial Envelope model [9], spatial frequency regularities of scenes are quantified based on principal components of their power spectra. However, it has long been recognized that the brain cannot perform a computation akin to a whole-scene Fourier transformation necessary to derive the principal components [139,140]. Visual processing in the brain occurs via conversion of light intensities at the retina to local contrast responses in small receptive fields in the lateral geniculate nucleus and primary visual cortex: neurons in these areas thus only “see” a small part of the visual scene. While higher-order visual regions have larger receptive fields (which are thought to integrate the information being fed from preceding regions), recent reports of extensive retinotopic biases in high-level, category-selective regions [e.g., 122] question the idea these areas are capable of representing whole-scene information. Importantly, some global scene properties reflected in the spatial frequency decomposition, such as naturalness, are also reflected in the local contrast distribution, which thus potentially forms a more biologically plausible image statistic that can be computed across a selected portion of the visual field [10,141]. Direct comparison of statistics derived from the Fourier transform versus the local contrast distribution has shown that the latter better predicts visual evoked responses in humans to natural scenes [13,142].
This issue is also relevant considering that the Fourier transformation is commonly used in cognitive neuroscience experiments as an image manipulation tool, e.g. to demonstrate spatial frequency biases in higher-order visual regions with filtered stimuli that contain exclusively high or low spatial frequencies [e.g., 143]. However, brain responses obtained using manipulated images do not necessarily generalize to intact natural scenes [144,145], and it is important not to attribute the operation performed to achieve an image manipulation to a neural computation without considering its biological plausibility. Instead, it might be more useful to think of image manipulation as emphasizing specific aspects that are more or less diagnostic for a given task [146,147].
Despite the many goals of scene understanding, many of the neuroimaging studies we have discussed emphasize recognition or use simple tasks not necessarily related to real-world goals such as passive fixation [e.g., 95,99], orthogonal tasks [e.g., changes in the fixation cross, 102], simple discrimination [e.g., 1-back repetition, 100,111], or familiarity judgments [108]. The major exceptions are the increasing number of studies that focus on navigation [e.g., 108,113] and such an approach is necessary to help elucidate the neural mechanisms of scene understanding. The distinction we have highlighted between recognition and interaction is reminiscent of the division of cortical visual processing into dorsal and ventral pathways which have been characterized as reflecting separate processing of dynamic spatiotemporal relationships and stable visual qualities, respectively [127]. However, the role of the dorsal pathway in scene understanding has been relatively little explored and, navigation aside, there has been little focus on more immediate interactions with the environment such as guidance of eye movements in scenes, which might depend heavily on the dorsal pathway.
Moving forward, we suggest that to build on the current literature and further elucidate the neural mechanisms underlying scene understanding research should continue to emphasize: the use of naturalistic images (reflecting the diverse properties and their correlations in real-world scenes), meaningful tasks (that reflect real world goals), generalizability across tasks, simultaneous consideration of multiple scene properties (avoiding a priori assumptions about specific properties) and an understanding of how those scene properties relate to each other. While integrating all these elements together is certainly ambitious, we believe it paves the way forward for elucidating the neural representation of scenes.
Concluding Remarks and Future Directions
Scene understanding entails representing information about the properties and arrangement of the world to facilitate ongoing needs of the viewer. By focusing on four major goals of scene understanding – recognizing the environment, searching for information within the environment, moving through the environment, and determining what actions can be performed – we have demonstrated how different goals use similar properties and, conversely, how many properties can be used for different goals. Further, the brain regions implicated in scene processing appear to represent multiple different properties and might be capable of supporting multiple goals. While studying single tasks and the contribution of isolated properties has elucidated important sub-components of scene understanding, we advocate a more comprehensive scene understanding framework. Allowing for dynamic representation of multiple scene properties across multiple tasks opens up many exciting new research questions (see Outstanding Questions) for which experiments will be required that combine strong, hypothesis-driven manipulations of top-down goals with sophisticated, data-driven measurements of scene properties. While it is challenging to adopt real-world goals for experimentation in a laboratory setting, we believe that as a whole, the research community has developed the tools enabling the power of multiple approaches to be combined in order to help understand how we make sense of real-world scenes.
Outstanding Questions Box.
To what extent are a combination of properties that facilitate a single goal (e.g., edges for scene recognition), generalizable to other tasks (e.g., edges for navigation)?
To what extent do co-occurring features within a scene category interact, or contribute independently to scene understanding?
Can multiple visual goals be carried out simultaneously, or do they interfere with each other? If simultaneously, can a single property be used for multiple purposes or can simultaneous goals occur when they require different features? If they interfere, is a new internal representation generated each time the goal momentarily changes?
Is the neural representation of scenes modulated by the degree of co-occurrence between different levels of description (e.g., do typical scene exemplars have higher consistency between features and therefore more reliable neural representations?)
What is the nature of the stored representation that a recognized scene is matched with? Is it based on physical characteristics or a set of rules? Does this change with recognized hierarchical level of detail?
How do the representations in scene-selective cortex compare with the representations in other regions implicated in navigation, such as hippocampus and entorhinal cortex?
Trends Box.
We are not simply passive viewers of scenes, but active participants within them, and understanding a scene entails processing factors such as what actions can be performed, where items are likely to be, how we can move through it, etc.
Scene understanding has developed many parallel research fields, looking at recognition, search, navigation, and action affordance among others.
The multitude of different properties present in scenes, and the inherent correlations among them, make establishing the critical features for any given goal or for any given brain region extremely challenging.
The research field has arrived at a stage where observer goals should be integrated, and the relationship between co-occurring properties explored, so as to start building a more generalized framework.
Acknowledgments
GLM and CIB are supported by the intramural research program of NIMH (ZIAMH002909). IIAG is supported by a Rubicon Fellowship from the Dutch Organization of Scientific Research (NWO). We thank Wilma Bainbridge, Michelle Greene, Antje Nuthmann and Edward Silson for commenting on earlier versions of this manuscript.
Glossary
- Convolutional Neural Network
A computer vision model with a multi-layer architecture performing hierarchical computations that can be trained to perform classification of visual images
- Diagnosticity
The relative usefulness of a specific subset of perceptual information in facilitating an observer’s goal. For example, an oven is highly diagnostic in helping to categorize a scene as a kitchen, while an apple is less so
- Environmental Space
A physical space that is too large to be appreciated without locomotion, requiring the integration of information over time. Examples include buildings, neighborhoods, golf courses, etc
- Fixation
A period of relative eye movement stability, usually on an object so that its image falls on the fovea, allowing for the perception of local details
- Gist
The perceptual and semantic information comprehended in a single glance of a scene (generally ranging from 13–250ms in presentation duration). The content of gist usually includes a conceptual understanding of a scene (e.g., birthday party), the spatial layout of the environment, and recognizing a few objects
- Saccade
A ballistic eye movement that quickly moves the fovea from one fixated location to another. The eyes rotate up to speeds of 500° per second, and no visual information is extracted during this time
- Scene Selectivity
By comparing the response elicited by visually presented scenes with that for objects or faces, researchers have identified three scene-selective cortical regions, termed the parahippocampal place area, the occipital place area, and the retrosplenial complex
- Spatial Frequency
A measure of how often sinusoidal components of image structure repeat across units of distance, which captures the level of detail present in a scene per degree of visual angle. A scene with small details and sharp edges contains more high spatial frequency information than one composed of large coarse stimuli
- Vista Space
A physical space that can be viewed in its entirety from a single location without locomotion. Examples include a classroom, town square, field, etc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torralba Antonio, Oliva A. Statistics of natural image categories. Netw Comput Neural Syst. 2003;14:391–412. [PubMed] [Google Scholar]
- 2.Malcolm GL, et al. Beyond gist: strategic and incremental information accumulation for scene categorization. Psychol Sci. 2014;25:1087–1097. doi: 10.1177/0956797614522816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene MR, et al. What you see is what you expect: rapid scene understanding benefits from prior experience. Atten Percept Psychophys. 2015;77:1239–1251. doi: 10.3758/s13414-015-0859-8. [DOI] [PubMed] [Google Scholar]
- 4.Tversky B, Hemenway K. Categories of environmental scenes. Cogn Psychol. 1983;15:121–149. [Google Scholar]
- 5.VanRullen R. Four common conceptual fallacies in mapping the time course of recognition. Front Psychol. 2011;2:1–6. doi: 10.3389/fpsyg.2011.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliva A. Scene Perception. New Vis Neurosci. 2013 doi: 10.1111/1467-9280.02459. [DOI] [Google Scholar]
- 7.Potter MC, et al. Detecting meaning in RSVP at 13 ms per picture. Atten Percept Psychophys. 2014;76:270–9. doi: 10.3758/s13414-013-0605-z. [DOI] [PubMed] [Google Scholar]
- 8.Maguire JF, Howe PDL. Failure to detect meaning in RSVP at 27 ms per picture. Attention, Perception, Psychophys. 2016 doi: 10.3758/s13414-016-1096-5. [DOI] [PubMed] [Google Scholar]
- 9.Oliva A, Torralba A. Modeling the shape of the scene: A holistic representation of the spatial envelope. Int J Comput Vis. 2001;42:145–175. [Google Scholar]
- 10.Scholte HS, et al. Brain responses strongly correlate with Weibull image statistics when processing natural images. J Vis. 2009;9:1–15. doi: 10.1167/9.4.29. [DOI] [PubMed] [Google Scholar]
- 11.Greene MR, Oliva A. Recognition of natural scenes from global properties: Seeing the forest without representing the trees. Cogn Psychol. 2009;58:137–179. doi: 10.1016/j.cogpsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene MR, Oliva A. The briefest of glances: The time course of natural scene understanding. Psychol Sci. 2009;20:464–472. doi: 10.1111/j.1467-9280.2009.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groen IIA, et al. From image statistics to scene gist: evoked neural activity reveals transition from low-level natural image structure to scene category. J Neurosci. 2013;33:18814–24. doi: 10.1523/JNEUROSCI.3128-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walther DB, Shen D. Nonaccidental properties underlie human categorization of complex natural scenes. Psychol Sci. 2014;25:851–60. doi: 10.1177/0956797613512662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliva A, Schyns PG. Diagnostic colors mediate scene recognition. Cogn Psychol. 2000;41:176–210. doi: 10.1006/cogp.1999.0728. [DOI] [PubMed] [Google Scholar]
- 16.Goffaux V, et al. Diagnostic colours contribute to the early stages of scene categorization: Behavioural and neurophysiological evidence. Vis cogn. 2005;12:878–892. [Google Scholar]
- 17.Castelhano MS, Henderson JM. The influence of color on the activation of scene gist. J Exp Psychol Hum Percept Perform. 2008;34:660–675. doi: 10.1037/0096-1523.34.3.660. [DOI] [PubMed] [Google Scholar]
- 18.Davenport JL, Potter MC. Scene consistency in object and background perception. Psychol Sci. 2004;15:559–564. doi: 10.1111/j.0956-7976.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- 19.Joubert O, et al. Processing scene context: Fast categorization and object interference. Vision Res. 2007;47:3286–3297. doi: 10.1016/j.visres.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Gagne CR, MacEvoy SP. Do simultaneously viewed objects influence scene recognition individually or as groups? Two perceptual studies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene MR, et al. Visual Noise from Natural Scene Statistics Reveals Human Scene Category Representations. 2014:5134. [Google Scholar]
- 22.Glanemann R, et al. Rapid apprehension of the coherence of action scenes. Psychon Bull Rev. 2016 doi: 10.3758/s13423-016-1004-y. [DOI] [PubMed] [Google Scholar]
- 23.Kadar I, Ben-Shahar O. A perceptual paradigm and psychophysical evidence for hierarchy in scene gist processing. J Vis. 2012;12:1–17. doi: 10.1167/12.13.16. [DOI] [PubMed] [Google Scholar]
- 24.Fei-Fei L, et al. What do we perceive in a glance of a real-world scene? J Vis. 2007;7:1–29. doi: 10.1167/7.1.10. [DOI] [PubMed] [Google Scholar]
- 25.Rayner K, et al. Masking of foveal and parafoveal vision during eye fixations in reading. J Exp Psychol Hum Percept Perform. 1981;7:167–179. doi: 10.1037//0096-1523.7.1.167. [DOI] [PubMed] [Google Scholar]
- 26.Tatler BW, et al. Looking at Domestic Textiles: An Eye-Tracking Experiment Analysing Influences on Viewing Behaviour at Owlpen Manor. Text Hist. 2016;47:94–118. [Google Scholar]
- 27.Nuthmann A. How do the regions of the visual field contribute to object search in real-world scenes? Evidence from eye movements. J Exp Psychol Hum Percept Perform. 2014;40:342–360. doi: 10.1037/a0033854. [DOI] [PubMed] [Google Scholar]
- 28.Castelhano MS, et al. Viewing Task Influences on Eye Movements During Scene Perception. J Vis. 2009;9:1–15. doi: 10.1167/9.3.6. [DOI] [PubMed] [Google Scholar]
- 29.Tatler BW, Vincent BT. The prominence of behavioural biases in eye guidance. Vis cogn. 2009;17:1029–1054. [Google Scholar]
- 30.Malcolm GL, Shomstein S. Object-Based Attention in Real-World Scenes. J Exp Psychol Gen. 2015;144:257–263. doi: 10.1037/xge0000060. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, et al. Predicting human gaze beyond pixels. J Vis. 2014;14:28. doi: 10.1167/14.1.28. [DOI] [PubMed] [Google Scholar]
- 32.Foulsham T, Kingstone A. Optimal and preferred eye landing positions in objects and scenes. Q J Exp Psychol (Hove) 2013;66:1707–28. doi: 10.1080/17470218.2012.762798. [DOI] [PubMed] [Google Scholar]
- 33.Pajak M, Nuthmann A. Object-based saccadic selection during scene perception: Evidence from viewing position effects. J Vis. 2013;13:1–21. doi: 10.1167/13.5.2. [DOI] [PubMed] [Google Scholar]
- 34.Nuthmann A, Einhauser W. A new approach to modeling the influence of image features on fixation selection in scenes. Ann N Y Acad Sci. 2015;1339:82–96. doi: 10.1111/nyas.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatler BW, et al. The long and the short of it: Spatial statistics at fixation vary with saccade amplitude and task. Vision Res. 2006;46:1857–1862. doi: 10.1016/j.visres.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Nuthmann A, Malcolm GL. Eye-guidance during scene search: The role color plays in central and peripheral vision. J Vis. 2016;16(2):1–16. doi: 10.1167/16.2.3. [DOI] [PubMed] [Google Scholar]
- 37.Reeder RR, Peelen MV. The contents of the search template for category-level search in natural scenes. J Vis. 2013;13:1–13. doi: 10.1167/13.3.13. [DOI] [PubMed] [Google Scholar]
- 38.Eckstein MP, et al. Attentional Cues in Real Scenes, Saccadic Targeting, and Bayesian Priors. Psychol Sci. 2006;17:973–980. doi: 10.1111/j.1467-9280.2006.01815.x. [DOI] [PubMed] [Google Scholar]
- 39.Pereira EJ, Castelhano MS. Peripheral Guidance in Scenes: The Interaction of Scene Context and Object Content. J Exp Psychol Hum Percept Perform. 2014;40 doi: 10.1037/a0037524. Advance online publication. [DOI] [PubMed] [Google Scholar]
- 40.Mack SC, Eckstein MP. Object co-occurrence serves as a contextual cue to guide and facilitate visual search in a natural viewing environment. J Vis. 2011;11:1–9. doi: 10.1167/11.9.9. [DOI] [PubMed] [Google Scholar]
- 41.Hwang AD, et al. Semantic guidance of eye movements in real-world scenes. Vision Res. 2011;51:1192–1205. doi: 10.1016/j.visres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coco MI, et al. The interplay of bottom-up and top-down mechanisms in visual guidance during object naming. Q J Exp Psychol. 2014;67:1096–120. doi: 10.1080/17470218.2013.844843. [DOI] [PubMed] [Google Scholar]
- 43.Wu CC, et al. The roles of scene gist and spatial dependency among objects in the semantic guidance of attention in real-world scenes. Vision Res. 2014;105:10–20. doi: 10.1016/j.visres.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 44.Castelhano MS, Heaven C. Scene context influences without scene gist: Eye movements guided by spatial associations in visual search. Psychol Bull Rev. 2011;18:890–896. doi: 10.3758/s13423-011-0107-8. [DOI] [PubMed] [Google Scholar]
- 45.Spotorno S, et al. How context information and target information guide the eyes from the first epoch of search in real-world scenes. J Vis. 2014;14(2):1–21. doi: 10.1167/14.2.7. [DOI] [PubMed] [Google Scholar]
- 46.Spotorno S, et al. Disentangling the effects of spatial inconsistency of targets and distractors when searching in realistic scenes. J Vis. 2015;15:1–21. doi: 10.1167/15.2.12. [DOI] [PubMed] [Google Scholar]
- 47.Võ MLH, Wolfe JM. The interplay of episodic and semantic memory in guiding repeated search in scenes. Cognition. 2013;126:198–212. doi: 10.1016/j.cognition.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehinger KA, et al. Modelling search for people in 900 scenes: A combined source model of eye guidance. Vis cogn. 2009;17:945–978. doi: 10.1080/13506280902834720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navalpakkam V, Itti L. Modeling the influence of task on attention. Vision Res. 2005;45:205–231. doi: 10.1016/j.visres.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 50.Hillstrom AP, et al. The effect of the first glimpse at a scene on eye movements during search. Psychon Bull Rev. 2012;19:204–210. doi: 10.3758/s13423-011-0205-7. [DOI] [PubMed] [Google Scholar]
- 51.Draschkow D, et al. Seek and you shall remember: Scene semantics interact with visual search to build better memories. J Vis. 2014;14:10–10. doi: 10.1167/14.8.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Josephs EL, et al. Gist in time: Scene semantics and structure enhance recall of searched objects. Acta Psychol (Amst) 2016;169:100–108. doi: 10.1016/j.actpsy.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olejarczyk JH, et al. Incidental memory for parts of scenes from eye movements. Vis cogn. 2014;22:975–995. [Google Scholar]
- 54.Hayhoe MM, Ballard D. Modeling task control of eye movements. Curr Biol. 2014;24:R622–R628. doi: 10.1016/j.cub.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jovancevic J, et al. Control of attention and gaze in complex environments. J Vis. 2006;6:1431–1450. doi: 10.1167/6.12.9. [DOI] [PubMed] [Google Scholar]
- 56.Ehinger KA, Wolfe JM. When is it time to move to the next map? Optimal foraging in guided visual search. Atten Percept Psychophys. 2016 doi: 10.3758/s13414-016-1128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfe JM. When is it time to move to the next raspberry bush? Foraging rules in human visual search. J Vis. 2013;13:10. doi: 10.1167/13.3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montello DR. Scale and multiple psychologies of space. In: Frank AU, Campari I, editors. Spatial information theory: A theoretical basis for GIS. Springer; 1993. pp. 312–321. [Google Scholar]
- 59.Foulsham T, et al. The where, what and when of gaze allocation in the lab and the natural environment. Vision Res. 2011;51:1920–1931. doi: 10.1016/j.visres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Zito GA, et al. Street crossing behavior in younger and older pedestrians: an eye- and head-tracking study. BMC Geriatr. 2015;15:176. doi: 10.1186/s12877-015-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marigold DS, Patla AE. Gaze fixation patterns for negotiating complex ground terrain. Neuroscience. 2007;144:302–313. doi: 10.1016/j.neuroscience.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Loomis JM, et al. Human Navigation by Path Integration. Wayfinding behavior: Cognitive mapping and other spatial processes. 1999:125–151. [Google Scholar]
- 63.Miller J, Carlson L. Selecting landmarks in novel environments. Psychon Bull Rev. 2011;18:184–191. doi: 10.3758/s13423-010-0038-9. [DOI] [PubMed] [Google Scholar]
- 64.Auger SD, et al. Retrosplenial Cortex Codes for Permanent Landmarks. PLoS One. 2012;7:e43620. doi: 10.1371/journal.pone.0043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stankiewicz BJ, Kalia AA. Acquisition of structural versus object landmark knowledge. J Exp Psychol Hum Percept Perform. 2007;33:378–390. doi: 10.1037/0096-1523.33.2.378. [DOI] [PubMed] [Google Scholar]
- 66.Epstein RA, Vass LK. Neural systems for landmark-based wayfinding in humans. Philos Trans R Soc B. 2014;369:1–7. doi: 10.1098/rstb.2012.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mou W, Zhou R. Defining a Boundary in Goal Localization: Infinite Number of Points or Extended Surfaces. J Exp Psychol Learn Mem Cogn. 2013;39:1115–1127. doi: 10.1037/a0030535. [DOI] [PubMed] [Google Scholar]
- 68.Lee SA, Spelke ES. Young children reorient by computing layout geometry, not by matching images of the environment. Psychon Bull Rev. 2011;18:192–8. doi: 10.3758/s13423-010-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doeller CF, et al. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc Natl Acad Sci U S A. 2008;105:5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Julian JB, et al. The Occipital Place Area Is Causally Involved in Representing Environmental Boundaries during Navigation. Curr Biol. 2016 doi: 10.1016/j.cub.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gibson JJ. The Ecological Approach To Visual Perception. Taylor & Francis Group; 1986. [Google Scholar]
- 72.Bainbridge WA, Oliva A. Interaction envelope: Local spatial representations of objects at all scales in scene-selective regions. Neuroimage. 2015;122:408–416. doi: 10.1016/j.neuroimage.2015.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castelhano MS, Witherspoon RL. How You Use It Matters: Object Function Guides Attention During Visual Search in Scenes. Psychol Sci. 2016;1:16. doi: 10.1177/0956797616629130. [DOI] [PubMed] [Google Scholar]
- 74.Oliva A, Torralba A. The Role of Context In Object Recognition. Trends Cogn Sci. 2007;11:520–527. doi: 10.1016/j.tics.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 75.Wokke ME, et al. Conflict in the kitchen : Contextual modulation of responsiveness to affordances. Conscious Cogn. 2016;40:141–146. doi: 10.1016/j.concog.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Bar M, et al. Top-down facilitation of visual recognition. Proc Natl Acad Sci. 2006;103:449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wurm MF, Schubotz RI. What’s she doing in the kitchen? Context helps when actions are hard to recognize. Psychon Bull Rev. 2016 doi: 10.3758/s13423-016-1108-4. [DOI] [PubMed] [Google Scholar]
- 78.Greene MR, et al. Visual scenes are categorized by function. J Exp Psychol Gen. 2016;145:82–94. doi: 10.1037/xge0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rietveld E, Kiverstein J. A Rich Landscape of Affordances. Ecol Psychol. 2014;26:325–352. [Google Scholar]
- 80.Battaglia PW, et al. Simulation as an engine of physical scene understanding. Proc Natl Acad Sci U S A. 2013;110:18327–32. doi: 10.1073/pnas.1306572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fischer J, et al. The functional neuroanatomy of intuitive physical inference. Prep. 2016 doi: 10.1073/pnas.1610344113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Epstein RA. Neural systems for visual scene recognition. In: Kveraga K, Bar M, editors. Scene vision: making sense of what we see. MIT Press; 2014. pp. 105–134. [Google Scholar]
- 83.Kornblith S, et al. A network for scene processing in the macaque temporal lobe. Neuron. 2013;79:766–781. doi: 10.1016/j.neuron.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lafer-Sousa R, Conway BR. Parallel, multi-stage processing of colors, faces and shapes in macaque inferior temporal cortex. Nat Neurosci. 2013;16:1–13. doi: 10.1038/nn.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verhoef BE, et al. Functional architecture for disparity in macaque inferior temporal cortex and its relationship to the architecture for faces, color, scenes, and visual field. J Neurosci. 2015;35:6952–68. doi: 10.1523/JNEUROSCI.5079-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaziri S, et al. A channel for 3D environmental shape in anterior inferotemporal cortex. Neuron. 2014;84:55–62. doi: 10.1016/j.neuron.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rajimehr R, et al. The “parahippocampal place area” responds preferentially to high spatial frequencies in humans and monkeys. PLOS Biol. 2011;9:e1000608. doi: 10.1371/journal.pbio.1000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watson DM, et al. Patterns of response to visual scenes are linked to the low-level properties of the image. Neuroimage. 2014;99:402–410. doi: 10.1016/j.neuroimage.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 89.Watson DM, et al. Patterns of neural response in scene-selective regions of the human brain are affected by low-level manipulations of spatial frequency. Neuroimage. 2016;124:107–117. doi: 10.1016/j.neuroimage.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 90.Kauffmann L, et al. The Neural Bases of the Semantic Interference of Spatial Frequency-based Information in Scenes. J Cogn Neurosci. 2015;27:2394–2405. doi: 10.1162/jocn_a_00861. [DOI] [PubMed] [Google Scholar]
- 91.Nasr S, Tootell RBH. A Cardinal Orientation Bias in Scene-Selective Visual Cortex. J Neurosci. 2012;32:14921–14926. doi: 10.1523/JNEUROSCI.2036-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cant JS, Xu Y. Object Ensemble Processing in Human Anterior-Medial Ventral Visual Cortex. J Neurosci. 2012;32:7685–7700. doi: 10.1523/JNEUROSCI.3325-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nasr S, et al. Thinking Outside the Box: Rectilinear Shapes Selectively Activate Scene-Selective Cortex. J Neurosci. 2014;34:6721–6735. doi: 10.1523/JNEUROSCI.4802-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bryan PB, et al. Rectilinear Edge Selectivity Is Insufficient to Explain the Category Selectivity of the Parahippocampal Place Area. Front Hum Neurosci. 2016;10:137. doi: 10.3389/fnhum.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choo H, Walther DB. Contour junctions underlie neural representations of scene categories in high-level human visual cortex. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 96.Harel A, et al. Deconstructing visual scenes in cortex: Gradients of object and spatial layout information. Cereb Cortex. 2013;23:947–957. doi: 10.1093/cercor/bhs091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Konkle T, Oliva A. A Real-World Size Organization of Object Responses in Occipitotemporal Cortex. Neuron. 2012;74:1114–1124. doi: 10.1016/j.neuron.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mullally SL, Maguire EA. A New Role for the Parahippocampal Cortex in Representing Space. J Neurosci. 2011;31:7441–7449. doi: 10.1523/JNEUROSCI.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stansbury DE, et al. Natural Scene Statistics Account for the Representation of Scene Categories in Human Visual Cortex. Neuron. 2013;79:1025–1034. doi: 10.1016/j.neuron.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park S, et al. Parametric Coding of the Size and Clutter of Natural Scenes in the Human Brain. Cereb Cortex. 2015;25:1792–1805. doi: 10.1093/cercor/bht418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park S, et al. Disentangling Scene Content from Spatial Boundary: Complementary Roles for the Parahippocampal Place Area and Lateral Occipital Complex in Representing Real-World Scenes. J Neurosci. 2011;31:1333–1340. doi: 10.1523/JNEUROSCI.3885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kravitz DJ, et al. Real-world scene representations in high-level visual cortex: it’s the spaces more than the places. J Neurosci. 2011;31:7322–33. doi: 10.1523/JNEUROSCI.4588-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferrara K, Park S. Neural representation of scene boundaries. Neuropsychologia. 2016;89:180–190. doi: 10.1016/j.neuropsychologia.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walther DB, et al. Simple line drawings suffice for functional MRI decoding of natural scene categories. Proc Natl Acad Sci. 2011;108:9661–9666. doi: 10.1073/pnas.1015666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Walther DB, et al. Natural Scene Categories Revealed in Distributed Patterns of Activity in the Human Brain. J Neurosci. 2009;29:10573–10581. doi: 10.1523/JNEUROSCI.0559-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baumann O, Mattingley JB. Functional Organization of the Parahippocampal Cortex: Dissociable Roles for Context Representations and the Perception of Visual Scenes. J Neurosci. 2016;36:2536–2542. doi: 10.1523/JNEUROSCI.3368-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aminoff EM, et al. The role of the parahippocampal cortex in cognition. Trends Cogn Sci. 2013;17:379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marchette SA, et al. Outside Looking In: Landmark Generalization in the Human Navigational System. J Neurosci. 2015;35:14896–908. doi: 10.1523/JNEUROSCI.2270-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Persichetti AS, Dilks DD. Perceived egocentric distance sensitivity and invariance across scene-selective cortex. Cortex. 2016;77:155–163. doi: 10.1016/j.cortex.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dilks DD, et al. Mirror-Image Sensitivity and Invariance in Object and Scene Processing Pathways. J Neurosci. 2011;31:11305–11312. doi: 10.1523/JNEUROSCI.1935-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kamps FS, et al. The occipital place area represents the local elements of scenes. Neuroimage. 2016;132:417–424. doi: 10.1016/j.neuroimage.2016.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dilks DD, et al. The Occipital Place Area Is Causally and Selectively Involved in Scene Perception. J Neurosci. 2013;33:1331–1336. doi: 10.1523/JNEUROSCI.4081-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Auger SD, et al. A central role for the retrosplenial cortex in de novo environmental learning. Elife. 2015;4 doi: 10.7554/eLife.09031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Troiani V, et al. Multiple object properties drive scene-selective regions. Cereb Cortex. 2014;24:883–897. doi: 10.1093/cercor/bhs364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marchette SA, et al. Anchoring the neural compass: coding of local spatial reference frames in human medial parietal lobe. Nat Neurosci. 2014;17:1598–1606. doi: 10.1038/nn.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vass LK, Epstein RA. Abstract Representations of Location and Facing Direction in the Human Brain. J Neurosci. 2013;33:6133–6142. doi: 10.1523/JNEUROSCI.3873-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vass LK, Epstein RA. Common Neural Representations for Visually Guided Reorientation and Spatial Imagery. Cereb Cortex. 2016 doi: 10.1093/cercor/bhv343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baldassano C, et al. Two distinct scene processing networks connecting vision and memory. bioRxiv. 2016 doi: 10.1101/057406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baldassano C, et al. Differential connectivity within the Parahippocampal Place Area. Neuroimage. 2013;75:236–245. doi: 10.1016/j.neuroimage.2013.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Silson EH, et al. Scene-selectivity and retinotopy in medial parietal cortex. Front Hum Neurosci. 2016;10:1–17. doi: 10.3389/fnhum.2016.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Silson EH, et al. A Retinotopic Basis for the Division of High-Level Scene Processing between Lateral and Ventral Human Occipitotemporal Cortex. J Neurosci. 2015;35:11921–11935. doi: 10.1523/JNEUROSCI.0137-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Silson EH, et al. Evaluating the correspondence between face-, scene-, and object-selectivity and retinotopic organization within lateral occipitotemporal cortex. 2016;16:1–21. doi: 10.1167/16.6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lowe MX, et al. Feature diagnosticity and task context shape activity in human scene-selective cortex. Neuroimage. 2016;125:681–692. doi: 10.1016/j.neuroimage.2015.10.089. [DOI] [PubMed] [Google Scholar]
- 124.Groen IIA, et al. The time course of natural scene perception with reduced attention. J Neurophysiol. 2015 doi: 10.1152/jn.00896.2015. [DOI] [PubMed] [Google Scholar]
- 125.Lescroart MD, et al. Fourier power, subjective distance, and object categories all provide plausible models of BOLD responses in scene-selective visual areas. Front Comput Neurosci. 2015;9:1–20. doi: 10.3389/fncom.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Groen IIA, et al. Contributions of low- and high-level properties to neural processing of visual scenes in the human brain. Philos Trans R Soc B. doi: 10.1098/not. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kravitz DJ, et al. The ventral visual pathway: An expanded neural framework for the processing of object quality. Trends Cogn Sci. 2013;17:26–49. doi: 10.1016/j.tics.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Henderson JM, Hollingworth A. High-level scene perception. Annu Rev Psychol. 1999;50:243–271. doi: 10.1146/annurev.psych.50.1.243. [DOI] [PubMed] [Google Scholar]
- 129.Yamins DLK, DiCarlo JJ. Using goal-driven deep learning models to understand sensory cortex. Nat Neurosci. 2016;19:356–365. doi: 10.1038/nn.4244. [DOI] [PubMed] [Google Scholar]
- 130.Kriegeskorte N. Deep Neural Networks: A New Framework for Modeling Biological Vision and Brain Information Processing. Annu Rev Vis Sci. 2015;1:417–446. doi: 10.1146/annurev-vision-082114-035447. [DOI] [PubMed] [Google Scholar]
- 131.Güçlü U, van Gerven MaJ. Deep Neural Networks Reveal a Gradient in the Complexity of Neural Representations across the Ventral Stream. J Neurosci. 2015;35:10005–10014. doi: 10.1523/JNEUROSCI.5023-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khaligh-Razavi SM, et al. Deep Supervised, but Not Unsupervised, Models May Explain IT Cortical Representation. PLoS Comput Biol. 2014;10:e1003915. doi: 10.1371/journal.pcbi.1003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cichy RM, et al. Comparison of deep neural networks to spatio-temporal cortical dynamics of human visual object recognition reveals hierarchical correspondence. Sci Rep. 2016;6:27755. doi: 10.1038/srep27755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yamins DLK, et al. Performance-optimized hierarchical models predict neural responses in higher visual cortex. Proc Natl Acad Sci U S A. 2014;111:8619–24. doi: 10.1073/pnas.1403112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou B, et al. Learning Deep Features for Scene Recognition using Places Database. 2014:487–495. [Google Scholar]
- 136.Cichy RM, et al. Dynamics of scene representations in the human brain revealed by magnetoencephalography and deep neural networks. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou B, et al. Object Detectors emerge in Deep Scene CNNs. International Conference on Learning Representations 2015 [Google Scholar]
- 138.Razavian AS, et al. CNN Features off-the-shelf: an Astounding Baseline for Recognition. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition Workshops. 2014:806–813. [Google Scholar]
- 139.Graham N. Does the brain perform a Fourier analysis of the visual scene? Trends Neurosci. 1979;2:207–208. [Google Scholar]
- 140.Field DJ. Relations between the statistics of natural images and the response properties of cortical cells. J Opt Soc Am. 1987;4:2379–94. doi: 10.1364/josaa.4.002379. [DOI] [PubMed] [Google Scholar]
- 141.Ghebreab S, et al. A biologically plausible model for rapid natural image identification. Advances in Neural Information Processing Systems. 2009:629–637. [Google Scholar]
- 142.Groen IIA, et al. Spatially pooled contrast responses predict neural and perceptual similarity of naturalistic image categories. PLoS Comput Biol. 2012;8:e1002726. doi: 10.1371/journal.pcbi.1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Canário N, et al. Distinct preference for spatial frequency content in ventral stream regions underlying the recognition of scenes, faces, bodies and other objects. Neuropsychologia. 2016;87:110–119. doi: 10.1016/j.neuropsychologia.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 144.Talebi V, Baker CL. Natural versus Synthetic Stimuli for Estimating Receptive Field Models: A Comparison of Predictive Robustness. J Neurosci. 2012;32:1560–1576. doi: 10.1523/JNEUROSCI.4661-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.David SV, et al. Natural Stimulus Statistics Alter the Receptive Field Structure of V1 Neurons. J Neurosci. 2004;24:6991–7006. doi: 10.1523/JNEUROSCI.1422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oliva A, Schyns PG. Coarse blobs or fine edges? Evidence that information diagnosticity changes the perception of complex visual stimuli. Cogn Psychol. 1997;34:72–107. doi: 10.1006/cogp.1997.0667. [DOI] [PubMed] [Google Scholar]
- 147.Awasthi B, et al. Distinct spatial scale sensitivities for early categorization of faces and places: neuromagnetic and behavioral findings. Front Hum Neurosci. 2013;7:1–11. doi: 10.3389/fnhum.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]


